Abstract
Purpose
We aimed to determine if there were gender differences in lean body mass (LBM) in patients with RA when compared with sex- and race- specific National Health and Nutrition Examination Survey (NHANES) reference data, and investigated the impact of sex differences in risk factors for LBM deficits.
Methods
DXA measures of whole body LBM and appendicular LBM (arms and legs, ALM) were obtained on a total of 190 subjects from two independent cohorts (141 from San Francisco (SF) , 49 from Philadelphia (PA)), expressed as indices adjusted for height (LBMI and ALMI, kg/m2), and converted to sex- and race- specific Z-scores relative to age based on NHANES data. Sarcopenia was defined using four different sex-specific definitions. Multivariable linear and logistic regression adjusted analyses for disease activity, disease duration, physical activity, CCP seropositivity, fat mass index, and glucocorticoid use.
Results
While there were significant differences between the two cohorts, ALMI Z-scores were significantly lower in men compared to women in both (SF: -1.43 v. -0.43, p<0.0001; PA: -0.83 v. -0.06, p=0.03). Observed gender differences were significant after adjustment in multivariable analyses within both cohorts. Odds of sarcopenia were 3 to 8 times greater in men in the SF cohort. Men in the PA cohort also had a higher, but non-significant, risk of sarcopenia.
Conclusion
RA is associated with significant LBM deficits, with greater deficits observed in men. Future study may help elucidate the mechanisms driving greater deficits among men.
Keywords: Rheumatoid Arthritis, Body Composition, Lean Body Mass, Sarcopenia
Abnormal body composition, characterized by lean body mass deficits and excess fat mass, has been reported among individuals with rheumatoid arthritis (RA). Initial reports focused on depletion of lean body mass and cachexia.(1-5) More recent research called attention to excess fat mass or obesity in RA.(6-8) Body composition alterations are clinically important because they have been associated with greater disability and poor long-term outcomes.(5, 9-13) More specifically, low lean body mass has been linked to risk of poor health outcomes, disability, and overall mortality in the general population and in patients with RA.
Previous studies have documented sex differences in body composition phenotypes of RA.(14-16) For example, we previously reported that men with RA were at greater risk of obesity, compared with women.(12) Similarly, two previous studies demonstrated significantly lower estimates of lean body mass in men with RA compared to matched controls, but this association was absent in women.(10, 17) Despite these observations, assessment of sex differences in the extent of lean mass deficits among men and women has been limited by the lack of robust sex-specific reference ranges and consideration of sex differences in risk factors for abnormal body composition.
Whole body dual energy absorptiometry (DXA) measures of body composition were obtained in ∼20,000 adults in the National Health and Nutrition Examination Survey (NHANES) and sex- and race- specific reference curves relative to age are available.(18) The primary objective of this analysis was to assess lean body mass in participants with RA from two existing independent cohorts, compared with these robust population-based reference data. A secondary objective was to examine sex differences in lean body mass deficits and risk of sarcopenia (based on published criteria), independent of disease-related risk factors for lean body mass deficits.
Methods
Data Sources
These analyses employed two independent datasets assembled for other primary purposes. A brief overview of each dataset is provided. The internal review board at each institution approved the studies and all subjects gave written informed consent.
1. University of California San Francisco (UCSF) Cohort
The UCSF cohort was developed to study the impact of body composition on disability in RA. Details of the cohort have previously been published.(12) Briefly, the majority of the research participants in this dataset were drawn from the University of California, San Francisco (UCSF) RA Panel Study. After telephone interviews in the study years 2007–2009, RA Panel participants who lived in the greater San Francisco area were recruited for in-person assessments, including measurement of body composition. Exclusion criteria were non–English speaking, age <18 years, current daily oral prednisone dose >50 mg, current pregnancy, uncorrected vision problems that interfered with reading, and patients who had undergone joint replacement within 1 year. Three subjects were recruited but were not included in this analysis due to an outdated consent in one and improperly stored data in two.
2. University of Pennsylvania (UPenn) Cohort
The UPenn cohort was developed as a pilot study to evaluate alterations in body composition and bone structure in patients with RA.(19) Subjects composing the UPenn cohort were recruited from the University of Pennsylvania Rheumatology practices and consisted of individuals with RA, ages 18-70 years, who met 2010 American College of Rheumatology criteria. Subjects with Juvenile Idiopathic Arthritis (or another inflammatory arthritis), active cancer, a history of chronic diseases known to affect bone health (e.g. chronic kidney disease, liver disease, malabsorption syndromes), or pregnancy were excluded. One RA subject was excluded because her weight exceeded the limit for the DXA machine.
Key Study Measures
Body composition, including regional body fat and lean mass, were assessed in both cohorts with whole-body DXA. Outcome measures included whole-body lean body mass and appendicular lean body mass (sum of lean mass in the arms and legs).
For the UCSF subjects, a Lunar Prodigy DXA system (software version 9.3) was used. In vivo coefficients of variation for measurement of lean mass by the Lunar Prodigy have been estimated at 1% or less.(20) Body composition measures for the UCSF subjects were adjusted based on the method by Shepherd et al. to facilitate comparison to NHANES data that were generated on Hologic equipment.(21) Subjects from the UPenn cohort underwent whole-body DXA assessment using a Hologic densitometer (Delphi Systems, Hologic, Inc., Bedford, MA) and therefore did not require the Shepherd adjustment. The in vitro coefficient of variation for Hologic measurement of lean mass was less than 0.6% and the in vivo coefficient of variation in adults was less than 1%.(22)
Study Covariates
In both cohorts, age, race/ethnicity, disease duration, and smoking status were obtained by self-report. Height was measured with a wall-mounted stadiometer. Disease activity was measured using the patient-reported Rheumatoid Arthritis Disease Activity Index (RADAI) in the UCSF cohort (23), and with the Disease Activity Score 28 (DAS28) with C-reactive protein (CRP) in the UPenn cohort. Anti-cyclic citrullinated peptide (CCP) antibody titers were analyzed by commercial laboratories. CCP seropositivity was defined as a value above the normal reference range. Self-reported physical activity was obtained using the International Physical Activity Questionnaire (IPAQ)(24, 25) in the UCSF cohort and the Multi-Ethnic Study of Atherosclerosis (MESA) questionnaire(26) in the UPenn cohort. For both groups, moderate or vigorous activity (IPAQ) or intentional exercise (MESA) was quantified in metabolic equivalent (MET)-hours per week.
Statistical Analysis
Statistical analyses were performed using Stata 11 (StataCorp, College Station, TX) and SAS 9.3 (Cary, NC). Characteristics of men and women within each cohort were compared using chisquare analyses for categorical variables and t-tests for continuous variables. Height-adjusted indices were created for total lean body mass (LBMI, kg/m2), appendicular lean body mass (ALMI, kg/m2), and fat mass (FMI, kg/m2). The results in the RA participants were converted to sex- and race-specific Z-scores (standard deviation scores) using NHANES reference curves.(18) The National Center for Health Statistics previously released whole-body DXA data from an NHANES population-based sample that was acquired with modern fan beam scanners in 15 counties across the United States from 1999 through 2004. The NHANES dataset was partitioned by gender and race and DXA whole-body measures of FMI, LBMI, and ALMI were analyzed to provide age, gender, and race-specific reference ranges. The NHANES reference curves were generated using the LMS method. This method normalizes the underlying reference data by dividing age into groups and then applying a power transformation that extends one tail of the distribution and contracts the other, eliminating skewness in the body composition result. A smooth curve is fitted to the normalizing power transformation for each age group, generating an optimum “L” (power) curve that normalizes the dependent measure, e.g. lean mass, over the entire age range. The procedure also fits Median (M) and coefficient of variation (S) curves, and these three curves (L, M, and S) fully describe the distributions of the reference data. The generated Z-score represents the number of standard deviations above or below average an individual is compared to population-based controls of the same age range, sex and race. This methodology helps deal with the heteroskedasticity and skew in body composition data and allows quantification of relative deficits according to the age-, sex-, and race-specific average and the variability among NHANES reference data.
T-tests were used to assess sex differences in lean body mass indices (LBMI and ALMI), Z-scores, and risk factors within each cohort. Multivariable linear regression analyses evaluated sex differences after adjustment for a-priori hypothesized potential confounders based on Table 1. These included CCP seropositivity, disease activity [RADAI or DAS28(CRP)], RA disease duration, oral glucocorticoid use, FMI Z-score, and physical activity.
Table 1.
Characteristics of UCSF and UPenn cohorts.
| UCSF Cohort (N=141) | UPenn Cohort (N=49) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Men | Women | p | Men | Women | p | |
| N | 56 | 85 | -- | 11 | 38 | -- |
| Age, (yrs)* | 56.6 (11.1) | 59.0 (10.5) | 0.2 | 50.9 (13.4) | 51.2 (13.4) | 0.9 |
| Race | ||||||
| Black, % (n) | 8.9 (5) | 1.2 (1) | 0.04 | 27.3 (3) | 39.5 (15) | 0.5 |
| Latino, % (n) | 10.7 (6) | 12.9 (11) | 0.8 | 0 (0) | 0 (0) | |
| Body mass index (BMI)* | 28.6 (6.6) | 26.2 (5.4) | 0.02 | 26.1 (5.2) | 31.3 (8.9) | 0.07 |
| Disease duration (yrs)* | 16.1 (8.1) | 21.6 (12.3) | 0.0008 | 15.4 (12.0) | 12.7 (10.7) | 0.5 |
| Current Smoking, % (n) | 7.1 (4) | 4.7 (4) | 0.7 | 18.2 (2) | 15.4 (6) | 0.8 |
| CCP Positive, % (n) | 100 (56) | 81 (69) | 0.0002 | 81.8 (9) | 74.4 (29) | 0.6 |
| Disease activity* | ||||||
| DAS28(CRP) | --- | --- | --- | 2.89 (1.04) | 3.21 (1.21) | 0.4 |
| RADAI | 2.7 (1.8) | 2.5 (1.7) | 0.4 | --- | --- | --- |
| Glucocorticoid use, % | 33.9 (19) | 36.5 (31) | 0.9 | 27.3 (3) | 43.6 (17) | 0.3 |
| HAQ Score* | 0.95 (0.65) | 0.94 (0.69) | 1 | 0.74 (0.83) | 0.93 (0.67) | 0.4 |
| Physical activity | ||||||
| IPAQ† | 0 (0, 7.3) | 0 (0, 10.0) | 0.5 | --- | --- | --- |
| Intentional exercise§ | --- | --- | --- | 8.75 (0, 33) | 21 (1.75, 59.5) | 0.4 |
Mean (SD)
Moderate and vigorous MET hours/week from International Physical Activity Questionnaire (IPAQ) for UCSF; presented as median (inter-quartile range)
Intentional exercise MET hours/week from the Multi-Ethnic Study of Atherosclerosis (MESA) for UPenn; presented as median (inter-quartile range)
UCSF= University of California San Francisco; UPenn= University of Pennsylvania;
DAS28= Disease Activity Score 28;
RADAI = Rheumatoid Arthritis Disease Activity Index (self-reported);
CCP= Cyclic Citrullinated Peptide Antibody;
HAQ= Health Assessment Questionnaire
Sarcopenia was defined based on four published sex-specific definitions of low ALMI based on previous studies. Baumgartner defined sarcopenia as an ALMI (kg/m2) more than 2 SD below the mean in a young reference population (18-40 years of age) from the Aging Process Study and the Rosetta Study (Men: 7.26, Women: 5.45). (27) Coin defined sarcopenia as present if ALMI was 1 standard deviation (SD) below the mean for young adults (18-40 years of age) living in the Mediterranean area (Men: 7.59, Women: 5.47). (28) Newman defined low ALMI as a value lower than the 20th percentile among 70-79 year olds in the Health ABC Study (Men: 7.23, Women: 5.67). (29) Finally, we used NHANES data to establish cutoffs based on a ALMI 1 SD below the mean among young adults (20-40 years) from NHANES (Men: 7.07, Women: 6.32). Logistic regression was used to assess the odds of sarcopenia in men compared to women within each cohort. For the UCSF cohort, multivariable analyses also compared the odds of sarcopenia adjusting for CCP seropositivity, FMI, physical activity, and oral glucocorticoid use. Race was not included in these models because none of the African American participants in the UCSF cohort met sarcopenia criteria. Disease duration, RADAI, and current smoking were not significantly associated with sarcopenia in bivariate analyses. The number of subjects in the UPenn cohort was insufficient for multivariable analysis.
Results
Study Sample Characteristics
Characteristics of the two cohorts are shown in Table 1. Briefly, the UCSF cohort was older [58.6 (10.8) v. 51.1 (13.4) p<0.001), had fewer black subjects (4% v. 37%, p<0.001) and a greater number of Latino subjects (12% v. 0%, p=0.01), had lower BMI [29.5 (7.5) v. 27.1 (6.2) p=0.02], had longer disease duration [19.4 (11.1) v. 13.5 (11.0) p=0.002], and had a greater proportion of subjects who were seropositive (89% v. 78% p=0.054).
Within the UCSF cohort, the proportion of participants of black race was greater in men than women (9% v. 1%, p=0.04), disease duration was shorter in men (15.7 vs. 21.4 years, p=0.0008), and mean (SD) BMI was greater in men (28.7 [6.6] v. 26.1 [5.4], p=0.02). The proportion of men that were anti-cyclic ctrullinated peptide (CCP) antibody positive was significantly greater than the proportion of women who were CCP positive (100% v. 86%, p<0.001). Otherwise, there were no significant differences in disease or sociodemographic characteristics between men and women. There were no significant differences between men and women in the current use [Men: 33% v. Women: 37%, p=0.7] or the median daily dose [0 (0, 2.5) v. 0 (0, 2.1), p=0.8] of oral glucocorticoids. The current use of methotrexate was similar for men and women (Men: 83% v. Women: 89%, p=0.54), but men were more likely to report current use of a biologic therapy (Men: 55% v. Women 38%, p=0.06).
Within the UPenn cohort, mean (SD) BMI tended to be lower among men (26.1 [5.2] v. 31.3 [8.9], p=0.07), and FMI was significantly lower among men (7.5 [3.5] v. 13.3 [5.5], p=0.002). There were no other significant differences in demographics or disease characteristics between men and women in the UPenn cohort (Table 1). Women reported a greater number of years of exposure to prednisone [Median 1 (0.1, 3) v. 0 (0, 0) p<0.001]. However, there were no differences in the median daily dose of prednisone between men [0 (0,2.5)] and women [0 (0, 5)] (p=0.3). The current use of methotrexate [Men: 64% v. Women: 64% (p=1)] and biologic therapies [Men: 73% v. Women: 62% (p=0.5)] was also similar.
Similar to what would be expected in healthy adults, men with RA had significantly greater LBMI and ALMI compared to women in the UCSF cohort (Table 2). Absolute differences in LBMI and ALMI between men and women in the UPenn cohort were similar to those observed in the UCSF cohort, but were not statistically significant.
Table 2.
Comparison of lean mass estimates between men and women from the UCSF and UPenn cohorts.
| UCSF cohort | UPenn cohort | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Men(n = 56) | Women(n = 85) | p | Men(n = 11) | Women(n = 38) | p | |
| Lean/Fat mass indices | ||||||
| LBMI | 17.3 (2.8) | 15.6 (2.0) | 0.0002 | 18.3 (2.0) | 17.0 (2.6) | 0.1 |
| ALMI | 7.6 (1.4) | 6.6 (0.9) | <0.001 | 8.1 (1.0) | 7.3 (1.3) | 0.09 |
| FMI | 11.3 (5.0) | 10.4 (4.1) | 0.23 | 7.5 (3.5) | 13.3 (5.5) | 0.002 |
Data presented as mean (standard deviation).
P-value from t-tests comparing men and women within cohorts.
UCSF= University of California San Francisco; UPenn= University of Pennsylvania;
ALMI= Appendicular lean mass index (Appendicular Leankg / heightm2);
LBMI= Total lean body mass index (Lean Body Masskg / heightm2);
FMI= Fat mass index (Fat masskg / heightm2)
Results for comparison to national reference ranges (NHANES)
Comparison of both the UCSF and UPenn cohorts to the population-based NHANES data revealed substantial lean body mass deficits for men (i.e., Z-scores were negative, p<0.001) (Table 3). In the UCSF cohort, the mean Z-scores were also below zero among women (p<0.001), indicating average values below the normative mean, although less so than for the men. Total LBMI and ALMI Z-scores were significantly lower for men than women in the UCSF cohort in both unadjusted and adjusted analyses (p<0.0001).
Table 3.
Unadjusted and multivariable adjusted lean body mass index (LBMI) and appendicular lean mass index (ALMI) Z-scores* for men and women
| UCSF Cohort | UPenn Cohort | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Men | Women | p† | Men | Women | p | |
| N | 56 | 85 | 11 | 38 | ||
| Unadjusted | ||||||
| LBMI Z-Score | -1.39 (-1.74, -1.04) | -0.34 (-0.54, -0.14) | <0.0001 | -0.74 (-1.36, -0.12) | -0.07 (-0.41, 0.27) | 0.07 |
| ALMI Z-Score | -1.43 (-1.79, -1.07) | -0.47 (-0.65, -0.29) | <0.0001 | -0.83 (-1.43, -0.23) | -0.06 (-0.39, 0.27) | 0.03 |
| FMI Z-Score | 0.63 (0.27, 0.99) | -0.51 (-0.74, -0.29) | <0.0001 | -0.35 (-1.36, 0.66) | 0.22 (-0.14, 0.57) | 0.2 |
| Adjusted§ | ||||||
| LBMI Z-Score | -1.37 (-1.63, -1.11) | -0.28 (-0.49, -0.07) | <0.0001 | -0.47 (-0.85, -0.08) | -0.14 (-0.35, 0.06) | 0.1 |
| ALMI Z-Score | -1.30 (-1.56, -1.05) | -0.09 (-0.29, 0.12) | <0.0001 | -0.57 (-0.93, -0.20) | -0.14 (-0.33, 0.05) | 0.04 |
Data presented as mean or adjusted mean (95% CI).
p-values from comparisons between men and women within cohorts.
Adjusted means:
For UCSF: Adjusted mean calculated from multivariable linear regression controlling for CCP seropositivity, RA disease duration, physical activity, oral glucocorticoid use, fat mass index (FMI) Z-score, and self-reported disease activity (RADAI).
For UPenn: Adjusted mean calculated from multivariable linear regression controlling for race, CCP seropositivity, RA disease duration, physical activity, oral glucocorticoid use, fat mass index (FMI) Z-Score, and DAS28(CRP).
UCSF= University of California San Francisco; UPenn = University of Pennsylvania;
ALMI= Appendicular lean mass index (Appendicular lean masskg / heightm2);
LBMI= Total lean body mass index (Lean body masskg / heightm2)
Within the UPenn cohort, the mean LBMI and ALMI Z-scores were substantially below the normative mean among men (p=0.01) (Table 3). However, LBMI and ALMI Z-scores among women with RA were not significantly different from the normal age- and sex- specific norms (p>0.7). Significant differences in ALMI Z-scores between men and women were also evident in the UPenn cohort, as in the UCSF cohort. Adjusting for hypothesized potential confounders including RA disease duration, disease activity, CCP antibody seropositivity, oral glucocorticoid use, FMI Z-score, and reported physical activity partially attenuated the observed differences between men and women in the UPenn cohort (Table 3). Gender differences in ALMI Z-scores remained significant after adjustment in both cohorts.
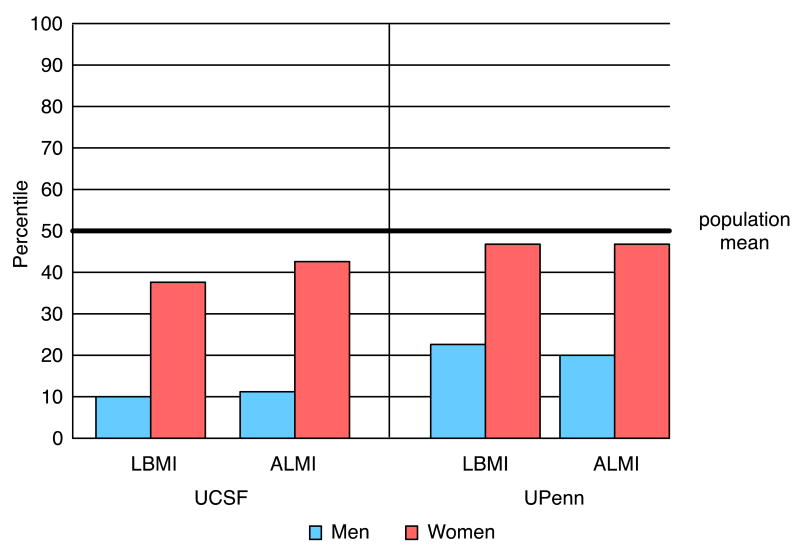
On average, LBMI for men was at the 10th and 23rd percentiles, and ALMI at the 11th and 20th percentiles (UCSF and UPenn, respectively; Figure 1). In the UCSF cohort, women were at the 37th percentile for LBMI and 44th percentile for ALMI. UPenn women were at the 47th percentile for both LBMI and ALMI.
Figure 1.
Percentiles of lean body mass and appendicular lean mass indices of men and women with RA, based on mean group Z-score compared to age-, sex-, and race-specific norms from NHANES.
LBMI= Lean Body Mass Index Z-Score; ALMI= Appendicular Lean Mass Index Z-score;
UCSF= University of California San Francisco; UPenn= University of Pennsylvania
Presence of Sarcopenia in Men and Women
Within the UCSF cohort, 37-57% of the men met criteria for sarcopenia, depending on the definition (Table 4). Among women, the proportions were smaller (13-20%). The unadjusted odds of sarcopenia in the UCSF cohort were between 3 and 8 times greater for men compared to women, depending on which of the four definitions of sarcopenia was used. Within the smaller UPenn cohort, the odds of sarcopenia for men compared to women ranged from 1.2 to 4; however, the confidence intervals did not exclude 1. In the UCSF cohort, adjustment for covariates yielded even greater odds of sarcopenia for men, ranging from 7.3 to 22.2. The increase in the odds of sarcopenia among men after adjustment was primarily the result of the adjustment for the greater fat mass seen in the men, which would be expected to be associated with greater ALMI and LBMI Z-scores in this group. Within the UPenn cohort there were too few total subjects with sarcopenia to adequately adjust for potential confounders.
Table 4.
Prevalence of sarcopenia in men and women and unadjusted and adjusted odds of sarcopenia among men compared to women.
| UCSF Cohort | UPenn Cohort | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Men | Women | p† | Men | Women | p | |
| All Subjects | ||||||
| N | 56 | 85 | 11 | 38 | ||
| Sarcopenia*, % (n) | ||||||
| Coin | 57.1 (32) | 14.1 (12) | <0.001 | 18.2 (2) | 5.3 (2) | 0.2 |
| Baumgartner | 51.8 (29) | 12.9 (11) | <0.001 | 18.2 (2) | 5.2 (2) | 0.2 |
| Newman | 51.8 (29) | 20.0 (17) | <0.001 | 18.2 (2) | 15.8 (6) | 0.9 |
| NHANES | 37.5 (21) | 15.3 (13) | 0.003 | 18.2 (2) | 10.5 (4) | 0.5 |
| Odds Ratio for Men (95% CI) | Odds Ratio for Men (95% CI) | |||||
|
|
|
|||||
| Unadjusted | ||||||
| Coin (27) | 8.1 (3.6, 18.2) | -- | <0.001 | 4.0 (0.5, 32.4) | 0.2 | |
| Baumgartner (28) | 7.2 (3.2, 16.4) | -- | <0.001 | 4.0 (0.5, 32.4) | 0.2 | |
| Newman (29) | 4.3 (2.1, 9.1) | -- | <0.001 | 1.2 (0.2, 6.9) | 0.9 | |
| NHANES | 3.3 (1.5, 7.4) | -- | 0.003 | 1.9 (0.3, 12.0) | 0.5 | |
| Adjusted§ | ||||||
| Coin | 12.6 (4.8, 33.2) | -- | <0.0001 | -- | -- | |
| Baumgartner | 11.2 (4.3, 29.2) | -- | <0.0001 | -- | -- | |
| Newman | 5.9 (2.5, 13.8) | -- | <0.0001 | -- | -- | |
| NHANES | 4.1 (1.7, 10.0) | -- | 0.002 | -- | -- | |
Sarcopenia as previously defined according to previously published sex-specific cutoffs of ALMI. (27-29) NHANES sex-specific definition defined as 1 SD below mean for 20-40 year olds.
p-value from chi-square analyses comparing prevalence of sarcopenia between men and women within cohorts, and from bivariate and multivariate logistic regression analyses.
For UCSF: Adjusted for CCP seropositivity, physical activity, oral glucocorticoid use, and fat mass index. Models did not include race because none of the African American participants met sarcopenia criteria.
UCSF= University of California San Francisco; UPenn = University of Pennsylvania;
CI= Confidence Interval
Discussion
This is the first study to examine gender variations in lean body mass deficits in patients with RA. We evaluated gender differences in a cohort of subjects with detailed body composition measures compared to published national reference ranges. These novel observations were confirmed in an analysis using a second, independent cohort. Gender differences were observed in both cohorts even though there were differences in the two cohorts in terms of the selection of subjects, racial/ethnic backgrounds, body mass index, age, and disease duration. We found that the independent UCSF and UPenn studies observed that men's height-adjusted lean mass was at the 11th and 20th percentile, respectively, compared to age-, sex-, and race-specific national averages (i.e., 50th percentile). In contrast, women's height-adjusted lean mass was only slightly lower than age- and sex-specific norms, at the 44th and 47th percentiles, respectively. Furthermore, men had a greater odds of sarcopenia compared to women utilizing several distinct sex-specific definitions of sarcopenia. These dramatic differences were not explained by group differences in disease characteristics such as disease activity, disease duration, reported physical activity, or CCP seropositivity. Previous studies of sarcopenia using these definitions, primarily in the elderly, have not observed similar gender differences.(27, 28, 30)
While previous studies found statistical differences in appendicular lean mass between individuals with RA only among men,(10, 17) ours is the first study to specifically test the hypothesis that men have greater deficits in muscle than women.
The observation that men have greater lean mass deficits is clinically important since sarcopenia is associated with falls, fracture, and early death among the elderly, and with greater disability.(10, 31) Therefore, identification of those at risk who are most likely to benefit from interventions to reduce or reverse muscle deficits is critical. Our findings also support the hypothesis that there may be distinct mechanisms by sex that either accelerate or offer protection from muscle deficits; better delineation of these mechanisms may offer avenues for effective treatments. Since the clinical implications of greater loss of muscle in men remains unknown, future studies will need to clarify the contribution of muscle deficits to the risk of important adverse outcomes within men and women.
Previous work has shown that body composition alterations during abnormal states or aging can be sex-specific. For example, men have been shown to have greater declines in muscle mass with aging compared to women.(32) Females may also have attenuated muscle loss during disuse.(33) Finally, the distribution of changes in muscle and fat composition during active weight loss is sex-specific.(34) This study is the first to call attention to the possibility that RA could have a greater disease impact on muscle outcomes in men.
The mechanisms underlying these gender differences are not clear. Female sex hormones have been shown to ameliorate arthritis in mouse models.(35) Pikwer et al. also recently showed that hormonal changes in men may precede the development of RA, and perhaps pre-dispose to disease.(36) Hormonal pathways important in regulating muscle mass in men may be differentially dysregulated in RA, resulting in a greater impact of the disease on maintenance of muscle mass among men.(37) For example, testosterone levels are a main regulator of muscle mass in men. Levels of testosterone are lower in men with RA and increase in correlation with improvements in disease activity, suggesting that alterations in this pathway could help to explain the greater relative deficits in men.(38)
Men have also been shown to produce greater TNF- α and Interleukin (IL)-6 in response to inflammatory stimuli.(39) Thus differences in body composition phenotypes could potentially be explained by differences in cytokine production as the result of genetic and chromosomal differences. Finally, women with RA score higher on subjective assessments of disease symptoms(40), so men who develop RA might have differences in disease presentation that result in under-recognition of severe and active disease.
There are several limitations to consider in interpreting our findings. First, the crosssectional study design does not enable us to evaluate temporal associations. Our knowledge of the clinical characteristics of subjects was limited to those reported here so it is possible that unmeasured differences in disease severity, comorbid conditions, or treatments could have influenced our findings. For example, we do not have access to information regarding lifetime use of glucocorticoids. While the use of NHANES reference ranges is a critical strength of this study, it is also important to emphasize that we do not have access to patient-specific data on the control population. In addition, DXA results from UCSF subjects were analyzed using earlier versions of the Lunar Prodigy software than the versions used in the adjustment published by Shepherd.(21) However, this should not have impacted the comparison between men and women. Furthermore, the analysis of the risk of sarcopenia (which supports the main conclusion) is not dependent on comparison to NHANES and, in addition, the confirmatory findings in the UPenn cohort do not carry this concern.
These limitations notwithstanding, there are important strengths to this study. Firstly, findings were replicated using two independent cohorts. While there were some differences in the UCSF and UPenn subjects likely attributable to the selection of subjects in these independent cohorts, the finding that men have more significant deficits was observed in both groups. Thus, these observations are unlikely to be due to primarily to selection bias within a single study. Secondly, we used objective measures of body composition, and referenced to nationally representative age-, sex-, and race-norms. Lastly, although we did not have exhaustive information regarding RA or general medical history, we did have access to a number of important covariates such as disease duration, disease activity, current glucocorticoid use, CCP antibody status, and self-reported physical activity.
In conclusion, these observations suggest that men with RA have greater diseaserelated loss of lean mass than women with RA. Taken in context with previous findings that men with RA had greater levels of fat, our results indicate that disease-related body composition alterations overall are greater in men with RA. Future study should evaluate for sex-differences in the dysregulation of pathways important for muscle homeostasis including sex hormones (testosterone), secretion and signaling of growth hormone, IGF-1, and other regulators such as myostatin.(41)
Significance and innovation.
Men with rheumatoid arthritis fall further outside normal reference ranges for appendicular lean mass compared to women with the disease.
Men with rheumatoid arthritis are also more likely to be sarcopenic based on previously defined sex-specific definitions.
Gender differences in the risk of sarcopenia are not dependent on group differences in demographics or disease characteristics.
Acknowledgments
Funding: At UCSF, this research was supported by NIH/NIAMS grant P60 AR053308 and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131, and by the Rosalind Russell Medical Research Center for Arthritis. At UPenn, research was supported by the University of Pennsylvania Clinical and Translational Research Center (UL1 RR024134). Dr. Baker is supported by a Veterans Affairs Clinical Science Research and Development Career Development Award. Dr. Ibrahim is supported by a K24 Award (K24 AR055259) from the National Institutes of Musculoskeletal and Skin Disorders. Dr. Leonard is also supported by a K24award (K24 DK076808).
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol. 1992;19(10):1505–10. [PubMed] [Google Scholar]
- 2.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85(1):89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 2009;11(2):108. doi: 10.1186/ar2658. Epub 2009 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers GD, Deighton CM, Rennie MJ, Booth AH. Rheumatoid cachexia: a clinical perspective. Rheumatology (Oxford) 2008;47(8):1124–31. doi: 10.1093/rheumatology/ken146. [DOI] [PubMed] [Google Scholar]
- 5.Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol. 2008;37(5):321–8. doi: 10.1080/03009740802055984. [DOI] [PubMed] [Google Scholar]
- 6.Giles JT, Allison M, Blumenthal RS, Post W, Gelber AC, Petri M, et al. Abdominal adiposity in rheumatoid arthritis: Association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 2010;62(11):3173–82. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 2011;50(3):450–62. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- 8.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66(10):1316–21. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165(14):1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 10.Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59(6):807–15. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer HR, Fontaine KR, Bathon JM, Giles JT. Muscle density in rheumatoid arthritis: Associations with disease features and functional outcomes. Arthritis Rheum. 2012;2012(5):34464. doi: 10.1002/art.34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz PP, Yazdany J, Trupin L, Schmajuk G, Margaretten M, Barton J, et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(1):62–70. doi: 10.1002/acr.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nevill AM, Jamurtas AZ, Koutedakis Y, et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin Rheumatol. 2009;28(4):439–44. doi: 10.1007/s10067-008-1073-z. [DOI] [PubMed] [Google Scholar]
- 14.Morgacheva O, Furst DE. Women are from venus, men are from Mars: do gender differences also apply to rheumatoid arthritis activity and treatment responses? J Clin Rheumatol. 2012;18(5):259–60. doi: 10.1097/RHU.0b013e31825833e0. [DOI] [PubMed] [Google Scholar]
- 15.Jawaheer D, Olsen J, Hetland ML. Sex differences in response to anti-tumor necrosis factor therapy in early and established rheumatoid arthritis -- results from the DANBIO registry. J Rheumatol. 2012;39(1):46–53. doi: 10.3899/jrheum.110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jawaheer D, Olsen J, Lahiff M, Forsberg S, Lahteenmaki J, da Silveira IG, et al. Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clin Exp Rheumatol. 2010;28(4):454–61. [PMC free article] [PubMed] [Google Scholar]
- 17.Book C, Karlsson MK, Nilsson JA, Akesson K, Jacobsson LT. Changes in body composition after 2 years with rheumatoid arthritis. Scand J Rheumatol. 2011;40(2):95–100. doi: 10.3109/03009742.2010.507215. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JF, Feldt JMV, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in Muscle Mass, Muscle Density and Modified Association with Fat in Rheumatoid Arthritis. Arthritis Care & Research. 2014 doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity (Silver Spring) 2012;20(1):30–9. doi: 10.1038/oby.2011.211. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res. 2012;27(10):2208–16. doi: 10.1002/jbmr.1654. [DOI] [PubMed] [Google Scholar]
- 22.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34(6):1044–52. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Fransen J, Langenegger T, Michel BA, Stucki G. Feasibility and validity of the RADAI, a self-administered rheumatoid arthritis disease activity index. Rheumatology (Oxford) 2000;39(3):321–7. doi: 10.1093/rheumatology/39.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 25.Brown WJ, Trost SG, Bauman A, Mummery K, Owen N. Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport. 2004;7(2):205–15. doi: 10.1016/s1440-2440(04)80010-0. [DOI] [PubMed] [Google Scholar]
- 26.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–54. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 28.Coin A, Sarti S, Ruggiero E, Giannini S, Pedrazzoni M, Minisola S, et al. Prevalence of Sarcopenia Based on Different Diagnostic Criteria Using DEXA and Appendicular Skeletal Muscle Mass Reference Values in an Italian Population Aged 20 to 80. J Am Med Dir Assoc. 2013;14(7):507–12. doi: 10.1016/j.jamda.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27(3):387–99. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, et al. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab. 2013 doi: 10.1007/s00774-013-0468-3. [DOI] [PubMed] [Google Scholar]
- 33.Miles MP, Heil DP, Larson KR, Conant SB, Schneider SM. Prior resistance training and sex influence muscle responses to arm suspension. Med Sci Sports Exerc. 2005;37(11):1983–9. doi: 10.1249/01.mss.0000176302.99185.be. [DOI] [PubMed] [Google Scholar]
- 34.Tang M, Leidy HJ, Campbell WW. Regional, but not total, body composition changes in overweight and obese adults consuming a higher protein, energy-restricted diet are sex specific. Nutr Res. 2013;33(8):629–35. doi: 10.1016/j.nutres.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Inoue E, Imai Y. Female sex hormones ameliorate arthritis in SKG mice. Biochem Biophys Res Commun. 2013;434(4):740–5. doi: 10.1016/j.bbrc.2013.03.111. [DOI] [PubMed] [Google Scholar]
- 36.Pikwer M, Giwercman A, Bergstrom U, Nilsson JA, Jacobsson LT, Turesson C. Association between testosterone levels and risk of future rheumatoid arthritis in men: a population-based case-control study. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202781. [DOI] [PubMed] [Google Scholar]
- 37.Tengstrand B, Carlstrom K, Fellander-Tsai L, Hafstrom I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J Rheumatol. 2003;30(11):2338–43. [PubMed] [Google Scholar]
- 38.Tengstrand B, Carlstrom K, Hafstrom I. Gonadal hormones in men with rheumatoid arthritis--from onset through 2 years. J Rheumatol. 2009;36(5):887–92. doi: 10.3899/jrheum.080558. [DOI] [PubMed] [Google Scholar]
- 39.Lefevre N, Corazza F, Duchateau J, Desir J, Casimir G. Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock. 2012;38(1):37–42. doi: 10.1097/SHK.0b013e3182571e46. [DOI] [PubMed] [Google Scholar]
- 40.Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med. 2012;10:82. doi: 10.1186/1741-7015-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med. 2011;106(1):102–8. doi: 10.1016/j.rmed.2011.07.016. [DOI] [PubMed] [Google Scholar]