Abstract
Objective
Juvenile Fibromyalgia (JFM) is characterized by chronic musculoskeletal pain and marked reduction in physical activity. Despite recommendations for exercise to manage JFM pain, exercise adherence is poor. Due to pain and activity avoidance, adolescents with JFM are at risk for altered joint mechanics that may make them susceptible to increased pain and reduced tolerance for exercise. The primary aim of this study was to assess functional deficits in patients with JFM compared to healthy controls using objective biomechanical assessment.
Methods
Female adolescent patients with JFM (n=17) and healthy controls (n=14) completed biomechanical assessments including gait analysis and tests of lower extremity strength (isokinetic knee extension/flexion, hip abduction) and functional performance (Drop Vertical Jump) along with self-report measures of disability (Functional Disability Inventory), pain intensity, depressive symptoms (Children’s Depression Inventory), and fear of movement (Tampa Scale of Kinesiophobia).
Results
Patients with JFM demonstrated mild deficiencies in walking gait and functional performance (p’s <. 05), significantly lower left knee extension and flexion strength (19–26% deficit) and bilateral hip abduction strength (33–37%) compared to healthy controls (p’s < .008). Patients with JFM reported significantly higher functional disability, pain intensity, depressive symptoms, and fear of movement relative to controls (p’s < 0.01).
Conclusions
This study showed that adolescents with JFM exhibited objective alterations in biomechanics, and self-reported fear of movement which may reinforce their activity avoidance. Interventions for JFM should include a focus on correcting functional deficits and instilling greater confidence in adolescents with JFM to engage in exercise to improve functional outcomes.
Keywords: Juvenile Fibromyalgia, chronic pain, biomechanics, gait analysis, neuromuscular training, neuromuscular training, fear of movement
Juvenile Fibromyalgia (JFM) is a chronic pain condition characterized by widespread musculoskeletal pain and several associated symptoms that contribute to significant functional disability (1, 2) that persists over time (3). Regular participation in moderate to vigorous physical activity and muscle strengthening exercises at least two times a week is recommended for pain management in patients with JFM, but adherence to these recommendations is poor (4, 5). In fact, a majority of pediatric and adult patients with fibromyalgia have been found to engage in extended periods of inactivity (6, 7), and based on objective activity monitoring, adolescents with JFM were found to remain very sedentary even after completing cognitive-behavioral treatment that significantly reduced their self-reported functional disability (8). Prolonged engagement in sedentary behavior can contribute to physical deconditioning and loss of confidence in patients’ ability to engage in physical activity (9). In particular, fear of movement is a prevalent concern for adults with fibromyalgia and is associated with poorer physical performance, muscle weakness, and increased pain sensitivity (10, 11). As such, pain-related fear may reinforce patients’ activity avoidance, exacerbate pain, and contribute to functional deficits, which can further contribute to physical deconditioning (11, 12).
Due to their reduced participation in physical activities, adolescents with JFM may be susceptible to functional deficits that affect the fundamental movements necessary to re-engage in physical activity. For example, adults with fibromyalgia and youth with rheumatic conditions are weaker in lower extremity strength compared to healthy controls (13–16). Such biodynamic deviations can alter their joint mechanics, exacerbate chronic pain, and consequently increase their risk of falls as demonstrated in adults with fibromyalgia (9, 17–19) and adolescents with other rheumatic or chronic pain conditions (20–22). The increased risk for functional deficits and pain flares with physical activity for patients with JFM can interfere with their ability to safely engage in exercise, and contribute to a downward progression of disability, pain-related fear, and activity avoidance into adulthood. There is a need to better understand the types of biomechanical and biodynamic deviations prevalent in adolescents with JFM so that tailored exercise-based treatments can be designed to meet their specific needs.
The use of three-dimensional (3-D) biomechanical analyses to assess a range of functional abilities, from walking gait to more dynamic maneuvers like jumping, can provide valuable insight regarding the nature of functional deficits associated with musculoskeletal pain conditions such as JFM. Biomechanical assessments of gait are increasingly used to identify functional deficits in patients with a variety of health conditions (23, 24). In fact, quantitative data from gait analyses has guided the development of targeted interventions resulting in improved postoperative gait in children requiring orthopedic surgery (25). On tasks requiring higher levels of skill and coordination, the drop vertical jump test (DVJ) commonly has been used to identify poor neuromuscular control (26) and predict risk of lower extremity injury (27, 28). Biomechanical and biodynamic assessments provide an ideal methodology to better understand functional deficits associated with high levels of physical impairment in JFM.
The primary aim of this study was to objectively assess a spectrum of functional abilities progressing in difficulty from walking gait and strength to functional performance. We hypothesized that adolescents with JFM would demonstrate altered gait and deficiencies in lower extremity strength and performance compared to healthy controls. Patients with JFM also were expected to demonstrate higher levels of self-reported functional disability, pain intensity, depressive symptoms, and pain-related fear of movement than healthy controls.
Patients and Methods
Participants
Participants included patients with JFM and healthy controls 12–18 years of age. Patients were eligible if they were diagnosed with JFM by a pediatric rheumatologist or pain physician using Yunus and Masi (1) and American College of Rheumatology (ACR) criteria (29). Healthy control participants were eligible if they had no chronic illness or chronic pain condition. Exclusion criteria included: diagnosis of a comorbid rheumatic disease (e.g., juvenile arthritis, systemic lupus erythematous), and untreated major psychiatric diagnosis (e.g., major depression, bipolar disorder, psychoses) or documented developmental delay.
Patients with JFM were recruited from outpatient pediatric specialty clinics at a large US Midwest Children’s Hospital. Eligible patients were introduced to the study by their treating physician, and if they expressed interest, a trained research coordinator explained the study to the patient and parent/primary caregiver in greater detail. Healthy controls (matched by age and sex) from a list of research volunteers were contacted by phone by a research coordinator. If they were unable to reach the family by phone, a letter was sent to the home describing the study and requesting their participation and a phone number to call if they were interested. Parents provided written informed consent and adolescents provided written assent. Institutional Review Board approval was obtained prior to study initiation.
Biomechanical and Biodynamic Assessments
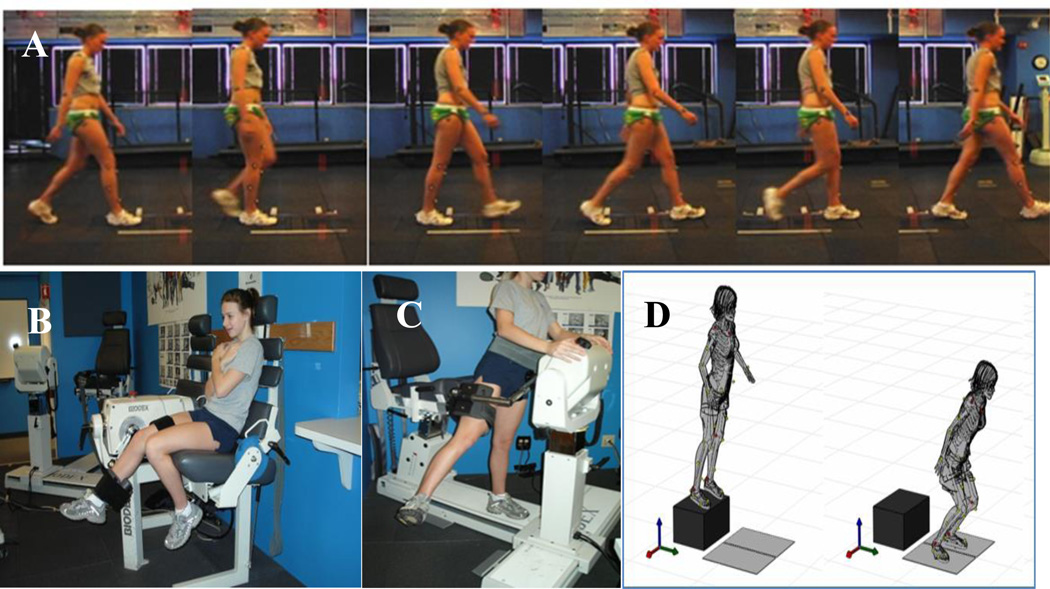
The following assessments represent a spectrum of functional tasks progressing in difficulty, starting with walking gait, followed by strength, and functional performance (see Figure 1).
Figure 1.
Biomechanical and biodynamic assessments of A) walking gait, B) isokinetic knee strength, C) hip abduction strength, and D) Drop Vertical Jump.
Gait analysis
A walking gait analysis was collected during two walking conditions: 1) self-selected pace and 2) standardized pace (1.2 m/s) at 240 Hz using a 10 camera, real-time, high speed, 3-D motion analysis system (Raptor-E, Motion Analysis Corp., Santa Rosa, CA). For the self-selected pace condition, participants were simply instructed to walk at their normal pace and were given no corrective feedback. During the standardized pace condition, participants were given feedback to either walk slightly faster or slower depending on their self-selected pace. Their pace was standardized by measuring the time it took them to walk a known distance (3m). Participants walked across embedded force plates (AMTI, Watertown, MA) sampling at 1200 Hz in the floor of the laboratory. Three foot strikes on each side were recorded for each walking condition.
Knee Strength
Isokinetic knee strength was assessed using the Biodex System II (Shirley, NY). Participants were positioned in the dynamometer chair in a seated position, with their hips at approximately 90° and with their knee aligned with the axis of rotation of the machine. The lower leg was strapped to the dynamometer arm approximately 2 inches above the lateral malleolus. A thigh strap and waist strap were also used to further ensure security of the testing position. Participants performed 5 continuous repetitions of maximal knee extension/flexion at an isokinetic speed for 300°/sec for practice. Starting position was 90° knee flexion; participants extended their knee to full extension and actively flexed the knee back to the starting position. A short break (approximately 60 seconds) was provided after the practice trials. Participants then performed 10 continuous repetitions with maximal effort on each leg. Peak torques (Nm/kg) of knee extension and knee flexion were recorded.
Hip Strength
Hip abduction strength was assessed using the Biodex System III. Participants faced the dynamometer head with the center of their hip aligned with the axis of rotation. The testing leg was strapped to the dynamometer arm just above the knee. Participants were instructed to kick out to the side as hard as possible 5 times and the testing speed was set to 120°/sec. Isokinetic strength assessments at the hip have been performed at various speeds (30°/sec to 210°/sec) in the side lying position (30–34). After pilot testing, 120°/sec was determined to be a more comfortable and functional speed in the standing position. Five repetitions were deemed an appropriate amount to capture maximal effort, while minimizing fatigue of the supporting limb. Intra-rater reliability for this testing was demonstrated to be good to excellent (35). Participants practiced with ample rest prior to the actual test on each leg. The peak torque (Nm/kg) of 5 repetitions was recorded.
Functional Performance
The 3-D motion analysis system also was used to assess landing techniques during the drop vertical jump test (DVJ) (36). Demonstrations of how to complete the DVJ were followed by practice trials. Participants were allowed as many practice trials as needed (typically 2–3) to be comfortable with the test. During the DVJ, participants stood on top of a 31 cm box, with their feet approximately shoulder width apart. They were instructed to drop off the box with both feet at the same time, land on the force plates in front of them, and then immediately jump from both feet to perform a maximal effort vertical jump to reach an overhead target. Participants were provided rest breaks between trials; however, most participants were ready for the next trial immediately following successful practice completion.
Self-report measures
Functional Disability
The Functional Disability Inventory (FDI) is a well-validated 15-item self-report inventory developed to assess perceived difficulty in the performance of daily activities in home, school, recreational, and social domains due to pain (37). Participants rated how much difficulty they have performing each of the activities on a 5-point Likert scale (0 = no trouble to 4 = impossible). Total scores range from 0 to 60, where higher scores indicate greater disability. Clinical reference points for the FDI are: 0–12 No/Mild disability,13–29 Moderate Disability, and 30–60 Severe Disability (38). The FDI has been found to have high internal consistency, moderate to high test-retest reliability, and good predictive validity (37, 39).
Pain Intensity
Participants marked their average pain intensity levels in the past week on a 0–10 cm VAS scale, anchored by “no pain” and “pain as bad as it can be.” The pain intensity VAS is one of the most widely used scales for pain assessment and has been validated for use with children over the age of 5 years with adequate reliability and validity (40, 41). Clinical cut-offs for pain intensity include: 0–0.4 No pain, 0.5–4.4 mild, 4.5–7.4 moderate, and 7.5–10 severe (42).
The current study was part of a larger ongoing study of youth with JFM. Due to study protocol changes, only a subset of patients with JFM completed the following self-report measures which were added later in the study.
Depressive Symptoms
The Children’s Depression Inventory (CDI) is a well-validated 27-item self-report of depressive symptoms in children and adolescents with moderate test-retest reliability (43). Participants chose one of three responses for each item that best described their symptoms for the past two weeks. Total scores range from 0 to 54 with higher scores indicating greater severity of depressive symptoms. Clinical cut-offs for total CDI scores are: No/Minimal Depressive Symptoms = <10, Mild = 10–19, Moderate = 20–28, Severe >28 (43). The CDI was completed by 11 of 17 JFM patients and all 14 healthy controls.
Fear of movement
The Tampa Scale of Kinesiophobia (TSK) is a 17-item self-report originally developed to assess the fear of movement related to chronic lower back pain (44). A short form of the scale (11 items) with improved psychometric properties, adequate reliability, and norms across a variety of pain conditions has been published (45) and was used in this study. It comprises two subscales that assess activity avoidance and somatic focus, with higher total and subscale scores indicating greater fear of movement, activity avoidance, and somatic focus (range 11–44). The TSK was completed by 8 of 17 JFM participants and all 14 healthy controls.
Procedure
Participants completed a comprehensive assessment of walking gait, lower extremity strength, and functional performance with a trained exercise physiologist at the hospital’s Sports Medicine and Human Performance Laboratory. Self-report measures of functional disability, pain intensity, depressive symptoms, and fear of movement were administered by a trained research coordinator.
For biomechanical assessments, participants wore 43 retro-reflective markers placed on standardized locations with a minimum of three markers per segment, including bilateral lower extremities (e.g., foot, shank, thigh) and trunk (e.g., pelvis, thorax) (20). A static trial was collected first in which the participant stood still, aligned with the laboratory coordinate system, in order to measure the participants’ neutral (zero) alignment. All biomechanical kinematic measures were calculated relative to this position.
Data Analyses
Descriptive statistics of the sample, strength, and self-report measures were calculated using SPSS software version 21. All biomechanical data reduction was performed using custom Matlab scripts (Mathworks, Natick, MA). Kinematic analyses and strength data were inherently standardized for body weight, so no additional adjustments for weight differences were needed. Basic kinetic data including gait speed and stride length were computed for both groups.
Kinematic measures were calculated for the right leg from the gait trials using the self-selected and standardized pace condition. For each of the 3 trials, the gait cycle—beginning with the heel strike on the force plate and ending with the successive heel strike—was determined, averaged, and normalized to 101 data points to allow for comparison between groups. Heel strike was determined in Visual3D using a proprietary algorithm (i.e., force > 20N) (46). Peak values were determined during the first 60% of stance comparable to previous gait studies (47). Three patients with JFM were excluded from the gait analysis due to invalid motion capture, via either poor marker recognition or model visualization.
For each of the 3 DVJ trials, the stance phase—beginning with the participant’s initial contact with the force plate and ending at the point the participant left the force plate—was determined, averaged, and normalized to 101 data points. Four patients with JFM declined to complete the DVJ, primarily due to increased pain and discomfort during the practice trial. Given the high volume of data extracted during the 3-D motion analysis of gait and DVJ, kinematic waveforms depicting gait cycle and stance phase, respectively, for both groups were the most efficient and effective method to illustrate the data. Graphical representations of gait and DVJ data illustrate the mean performance (represented by solid/dashed lines) and (+/−) standard errors (represented by shaded areas) for patients with JFM and healthy controls. Non-overlapping standard errors represent significant group differences with 95% confidence (p < 0.05 level).
Lastly, JFM and healthy control groups were compared using independent t-tests for measures of basic kinetic data, peak values for gait cycle, strength, and self-report measures of physical and psychosocial functioning. Bonferroni correction was used to adjust for the risk of inflated error given multiple comparisons and limited sample size. Therefore, for peak values for gait cycle, group differences were considered significant at p <0.006, for lower extremity strength measures at p < 0.008, and for self-report measures at p < 0.01.
Results
Sample Characteristics
The sample consisted of 17 patients with JFM and 14 healthy controls. All participants were female and primarily Caucasian (77%). Patients with JFM were on average 15.94 (SD = 1.95) years of age, 160.88 cm (SD = 6.17) in height, and 71.23 kg (SD = 21.08) in weight. Healthy control participants were 14.71 (SD = 1.68) years on average, 159.4 cm (SD = 8.32) in height, and 56.36 kg (SD = 15.07). No significant differences were found in adolescent age, race, or height between patients with JFM and controls. However, body weight was significantly higher in patients with JFM compared to healthy controls (t = 2.21; p < .05). Four patients who declined to complete the DVJ reported significantly higher baseline average pain intensity (M = 7.88, SD = 1.71) than those patients with JFM who completed the test (M = 5.49, SD = 1.66, t (15) = 2.39, p < .05). Notably, there were no significant differences in body weight (kg) between patients who completed the DVJ (M = 67.28, SD = 20.37) and those who did not (M = 84.05, SD = 20.55; t = 1.44, p = 0.17). No differences in demographic variables or functional disability were found. Subgroup analyses of patients with JFM (n=8) and controls (n=8) with normal range body mass index demonstrated differences in kinematic, strength, and psychosocial measures consistent with the full sample, suggesting deficits were not solely due to BMI differences.
Biomechanical and Biodynamic Assessments
Walking Gait
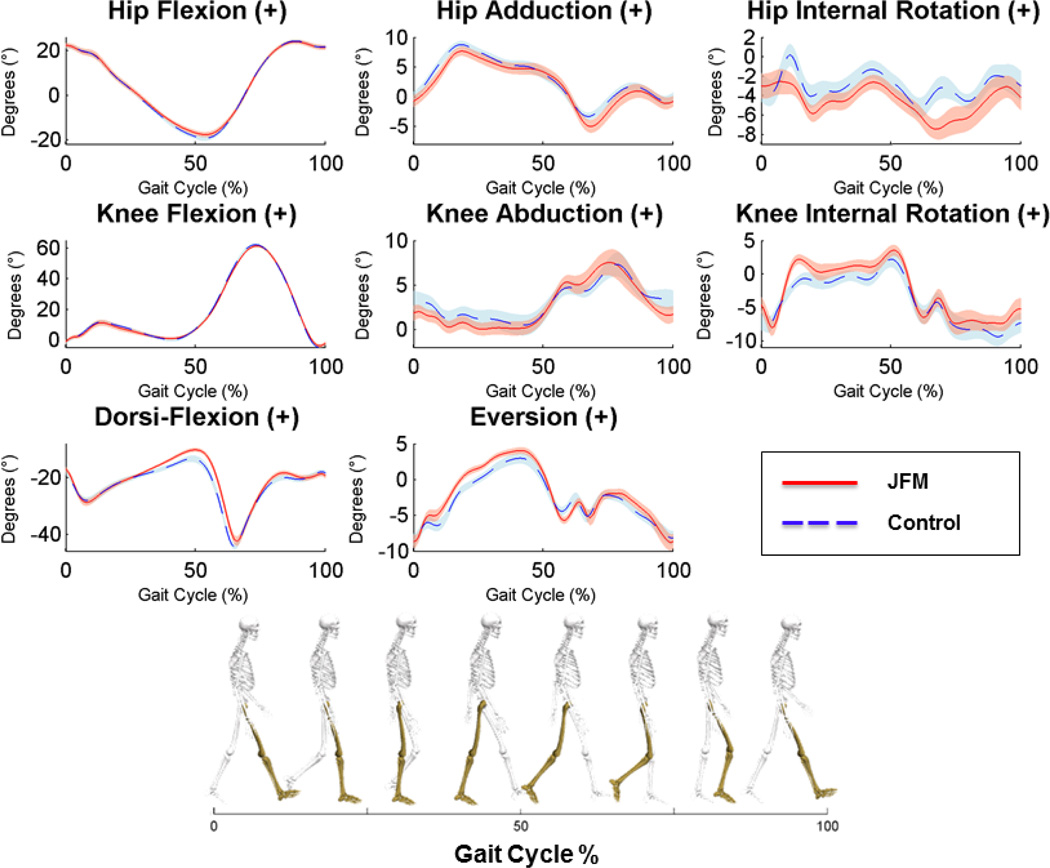
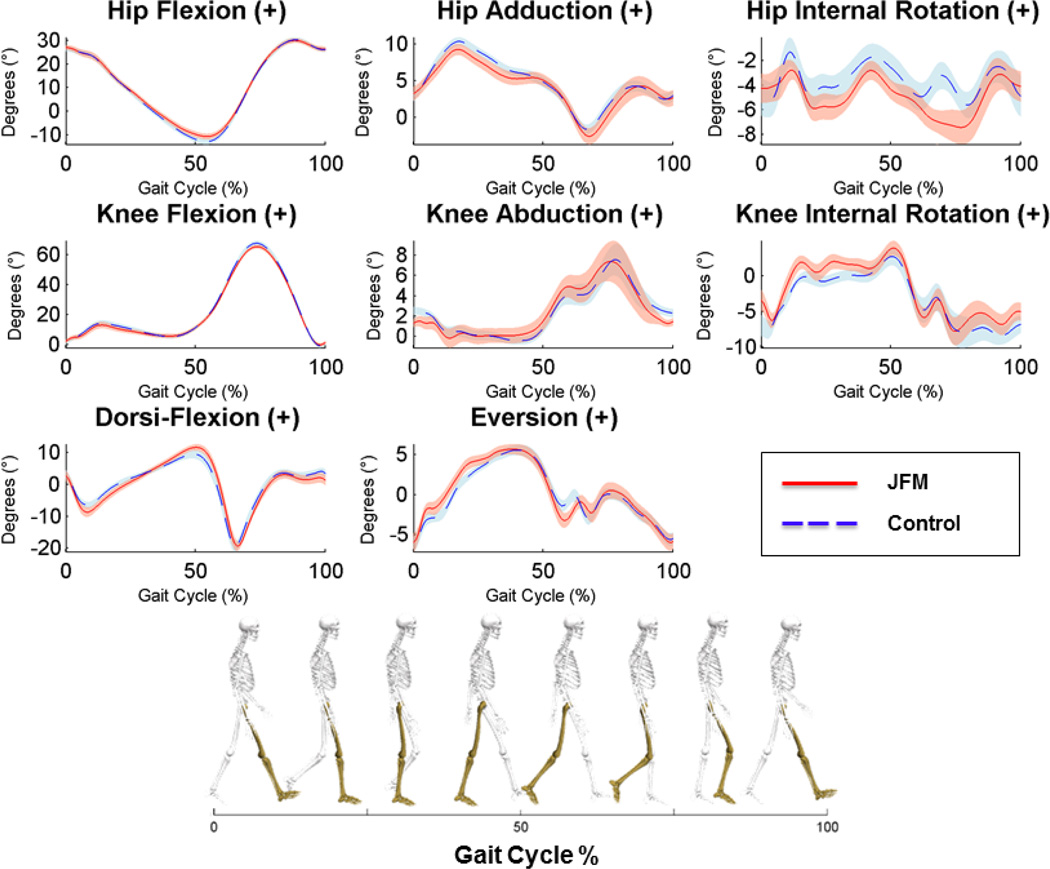
There was no significant group difference in walking speed during the self-selected pace; however, patients with JFM had significantly shorter strides during the self-selected pace (p < 0.05, Suppl Table 1). Graphical representation of the biomechanical gait analyses are presented in Figures 2 and 3. During the standardized pace, patients with JFM exhibited mild yet significant alterations in increased dorsiflexion, increased eversion, and differences in increased internal rotation at the knee during early stance portion of the gait cycle trended toward significance (Suppl Table 1). Similarly, during the self-selected pace, patients with JFM exhibited mild alterations in eversion and trending differences in increased internal rotation at the knee (Figure 3, Suppl Table 1).
Figure 2.
Time-series plots of kinematic variables collected during the standardized gait cycle for JFM patients and controls.
Solid/dashed lines represent mean performance and the surrounding shaded areas illustrate the (+/−) standard error for patients with JFM (depicted in pink) and healthy controls (in blue). Non-overlapping standard errors represent significant group differences. The left column displays angular motion in the sagittal plane at the hip, knee, and ankle joints, respectively, from top to bottom. The middle column displays angular motion in the coronal plane, and the right column displays angular motion in the transverse plane. Hip, Knee, and Dorsi-Flexion can be visualized as a bend in each joint, reducing the angle between the long bones of the lower extremities. Hip Adduction is illustrated as the thigh moves toward the midline of the body. Knee Abduction can be visualized as the knee joint moves toward the midline of the body in a “knock-kneed” position. Eversion of the foot occurs when the foot rotates toward the midline of the body, in a more flat-footed position. Hip and Knee Internal Rotation can be visualized as a twisting of the joint in the direction of the midline of the body.
Figure 3.
Time-series plots of kinematic variables collected during the self-selected gait cycle for JFM patients and controls.
Solid/dashed lines represent mean performance and the surrounding shaded areas illustrate the (+/−) standard error for patients with JFM (depicted in pink) and healthy controls (in blue). Non-overlapping standard errors represent significant group differences. The left column displays angular motion in the sagittal plane at the hip, knee, and ankle joints, respectively, from top to bottom. The middle column displays angular motion in the coronal plane, and the right column displays angular motion in the transverse plane. Hip, Knee, and Dorsi-Flexion can be visualized as a bend in each joint, reducing the angle between the long bones of the lower extremities. Hip Adduction is illustrated as the thigh moves toward the midline of the body. Knee Abduction can be visualized as the knee joint moves toward the midline of the body in a “knock-kneed” position. Eversion of the foot occurs when the foot rotates toward the midline of the body, in a more flat-footed position. Hip and Knee Internal Rotation can be visualized as a twisting of the joint in the direction of the midline of the body.
Knee and Hip Strength
Following Bonferroni correction, results of isokinetic knee strength revealed that patients with JFM demonstrated significantly lower peak torques in left knee extension (18–22% deficit; p’s < .008) and knee flexion (20–24% deficit; p’s < .008) compared to healthy controls (Table 1). Right knee strength showed similar trends in group differences, but was non-significant following α-correction. More pronounced deficiencies (34–38%; p’s ≤ .001) in bilateral hip abduction strength were noted for patients with JFM compared to controls (Table 1).
Table 1.
Descriptive statistics of isokinetic knee extension and flexion strength and hip abduction strength for patients with JFM and healthy controls.
| JFM | Healthy Controls | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Knee Extension (0–1.2) | ||||||
| Right | 0.95 | 0.25 | 1.15 | 0.18 | 2.60 | .015 |
| Left | 0.89 | 0.20 | 1.15 | 0.19 | 3.57 | .001* |
| Knee Flexion (0–1.2) | ||||||
| Right | 0.62 | 0.19 | 0.82 | 0.22 | 2.65 | .013 |
| Left | 0.64 | 0.15 | 0.80 | 0.17 | 2.87 | .008* |
| Hip Abduction (0–1) | ||||||
| Right | 0.65 | 0.27 | 0.99 | 0.25 | 3.51 | .001* |
| Left | 0.59 | 0.23 | 0.97 | 0.29 | 4.02 | <.001* |
p-level remains significant after Bonferroni correction
Note: Strength measures represent peak torques (Nm/kg)
Functional Performance
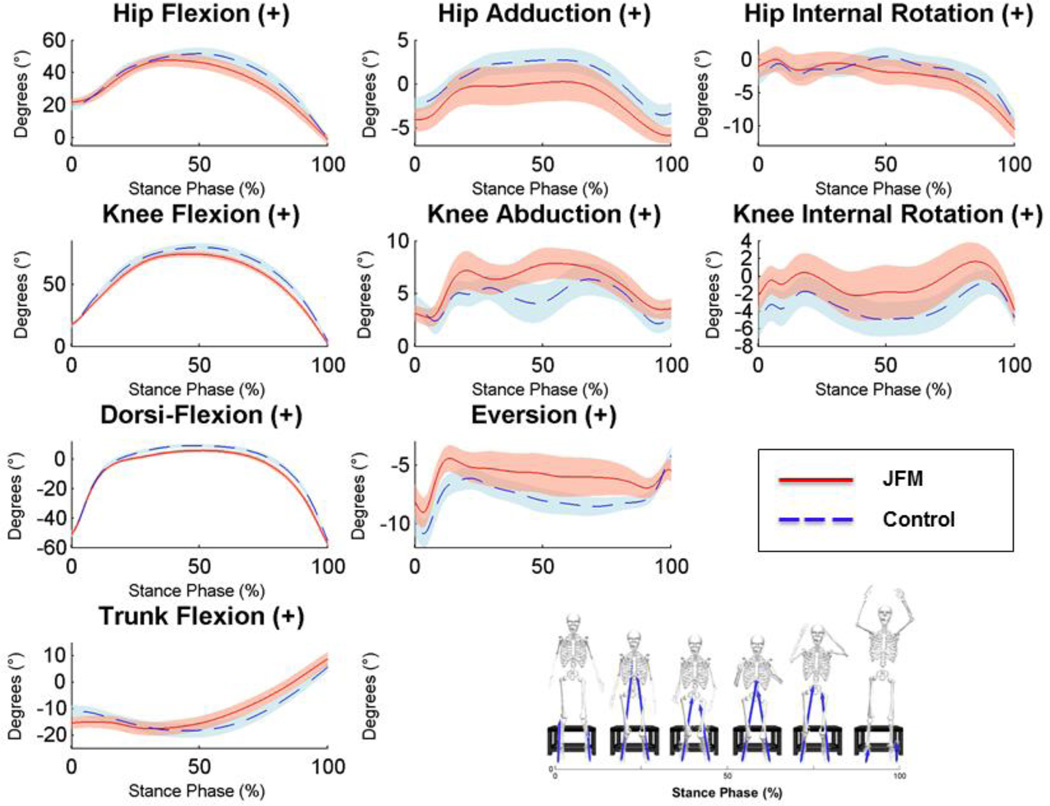
The 3-D motion analysis system also assessed landing techniques during the DVJ. As illustrated in Figure 4, adolescents with JFM exhibited significantly reduced ankle dorsiflexion, excessive knee abduction, as well as reduced trunk flexion upon landing relative to controls.
Figure 4.
Time-series plots of kinematic variables collected during the stance phase of the drop vertical jump.
Solid/dashed lines represent mean performance and the surrounding shaded areas illustrate the (+/−) standard error for patients with JFM (depicted in pink) and healthy controls (in blue). Non-overlapping standard errors represent significant group differences. The left column displays angular motion in the sagittal plane at the hip, knee, ankle, and the trunk, respectively, from top to bottom. The middle column displays angular motion in the coronal plane, and the right column displays angular motion in the transverse plane. Hip, Knee, and Dorsi-Flexion can be visualized as a bend in each joint, reducing the angle between the long bones of the lower extremities. Hip Adduction is illustrated as the thigh moves toward the midline of the body. Knee Abduction can be visualized as the knee joint moves toward the midline of the body in a “knock-kneed” position. Eversion of the foot occurs when the foot rotates toward the midline of the body, in a more flat-footed position. Hip and Knee Internal Rotation can be visualized as a twisting of the joint in the direction of the midline of the body. Trunk Flexion is a reduction in the angle of the torso relative to the pelvis.
Physical and Psychosocial Functioning
Patients with JFM reported significantly poorer physical and psychosocial functioning compared to healthy controls (Table 2). In particular, considerably large group differences were found, such that adolescents with JFM reported functional disability within the moderate range and moderate average pain intensity relative to controls. Additionally, youth with JFM reported marked fear of movement and mild levels of depressive symptoms compared to their peers.
Table 2.
Descriptive statistics of functional disability, average pain intensity, depressive symptoms, and fear of movement for patients with JFM and healthy controls.
| JFM | Healthy Controls | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Functional Disability (0–60) | 23.82 | 10.17 | 3.79 | 4.21 | 6.89 | <.001* |
| Average Pain Intensity (0–10) | 6.05 | 1.93 | 0.44 | 0.72 | 10.29 | <.001* |
| Depressive Symptoms (0–54) | 12.73 | 8.04 | 4.14 | 3.82 | 3.53 | .002* |
| Fear of Movement (11–44) | 30.25 | 5.55 | 17.71 | 3.87 | 6.24 | <.001* |
p-level remains significant after Bonferroni correction
Note: All healthy control participants (n=14) and a subset of patients with JFM reported on depressive symptoms (n =11) and fear of movement (n=8) because measures were included later in the protocol.
Discussion
To our knowledge, this is the first study to objectively assess and document functional deficits in adolescents with JFM, and is an essential first step to inform the development of the most appropriate and effective exercise-based interventions to manage pain in JFM. The findings indicate that youth with JFM demonstrate significant functional deficits across a spectrum of functional abilities from walking gait to more complex functional performance, and these deficits are evident beyond group differences in BMI. In particular, adolescents with JFM demonstrated altered walking gait, strength deficiencies in knee extension/flexion and hip abduction, and reduction in functional performance. Youth with JFM exhibited relatively mild deficiencies on functional tasks that were less challenging (i.e., walking gait), and additional deficits emerged as the functional tasks increased in difficulty (i.e., DVJ) (48–50). As noted in the kinematic plots, qualitative assessments of the time series joint mechanics indicate that patients with JFM demonstrated increased knee abduction and knee internal rotation angle during the DVJ which is associated with increased load to the passive structures of the knee (51).
Specific deficiencies in walking gait included reduced stride length during the self-selected pace and alterations in dorsiflexion, eversion, and internal rotation at the knee during early stance at both the self-selected and standardized pace. When the constraint of walking speed was removed, adolescents with JFM showed a reduction in stride length relative to controls. Identifying the specific reasons for these alterations (e.g., pain, fear of movement, strength deficits) in future studies will help better understand the atypical movement strategies in patients with JFM.
Additional deficiencies in functional performance (DVJ) suggested reduced flexion at the knee and ankle, which can be visualized in a more upright landing and countermovement during the stance phase. Patients with JFM demonstrated increased knee abduction indicative of entering into a knock-kneed positioning while landing or performing the countermovement, which may place patients at increased risk for injury (27, 28). In addition, patients with JFM appeared to land with decreased trunk flexion and increased hip flexion at initial contact, potentially limiting the height of the vertical jump. These deficits noted in higher level tasks of functional performance may be indicative of a reduction in proximal stabilization in patients with JFM. That is, hip strength may be a critical modulator of lower extremity alignment and load during dynamic tasks in JFM (52, 53). This is consistent with the lower extremity and hip strength weaknesses found in adults with FM (13–15), in which increased lower extremity pain has been significantly associated with deficits in hip strength (15).
Along with the objectively measured functional alterations and differences in body mass, adolescents with JFM demonstrated marked self-reported fear of movement along with higher functional disability, pain intensity, and more depressive symptoms compared to healthy controls, which may also contribute to the observed differences in biomechanics evidenced by youth with JFM. Given that JFM is not associated with any known joint or tissue damage which might cause deficits in biomechanics, it is likely that these alterations are a response to pain, pain-related fear, and overall deconditioning. Notably, these patterns are comparable to functional limitations and psychosocial functioning evidenced by adults with fibromyalgia. Specifically, adult patients who had less confidence in movement or exhibited high levels of pain-related fear had significantly poorer postural control and physical performance (9, 11). Moreover, adults with FM have demonstrated decreased tolerance for physical activity (54), reduced physical performance, are more susceptible to neuromuscular fatigue, and are at increased risk for falls (9, 11, 19). Findings from the current study suggest that the functional deficits in youth with JFM appear to be milder than those prevalent in adults with fibromyalgia. However, a negative cycle of pain-related fear, activity avoidance, disability, and pain (10) common in other chronic pain conditions, in which fear has been associated with decreased speed in both preferred and fast walking, weakened muscle strength, and reduced performance on physical tasks in adults (55–57), may lead to a downward spiral in physical activity over time. Early intervention to correct functional deficits is, therefore, of great importance.
Results of this study support the use of biomechanical analyses of gait and functional movements as well as strength measures to guide targeted interventions that effectively address neuromuscular deficits associated with JFM. Integrative neuromuscular training programs have been shown to improve deficits in patients who have lower extremity injuries (21, 26, 58–62). Similarly, biomechanical measures can be used as an innovative method to assess the effectiveness of integrative training programs in improving physical functioning in patients with JFM (63, 64). For youth with JFM, an integrative neuromuscular program should involve specialized instruction in fundamental movements and strength building while minimizing pain flare-ups and delayed-onset muscle soreness, which can contribute to reduced physical performance and activity avoidance (63, 65). Such a treatment approach may improve the motor skills and confidence that youth with JFM require to engage in activity while simultaneously addressing psychological contributors to enhance motivation and adherence, thus affording them the opportunity to integrate regular exercise into their lifestyle and achieve better pain management.
The interpretation of study findings should be considered within the context of some limitations. The study results serve as the first step in documenting preliminary evidence of functional deficiencies in adolescents with JFM, and additional studies are needed to explore the reasons for these deficits. It is important for future studies to include larger samples to allow for more complex statistical tests of underlying mechanisms of biomechanical alterations and functional outcomes, along with the ability to control for differences in body mass index and depressive symptoms. Future studies may also consider collecting pain intensity ratings during functional tasks in an effort to more directly assess the potential impact of pain on patients’ performance. Also, these findings are specifically relevant for patients with JFM and may not be generalizable to other chronic pain conditions. Despite these limitations, results from biomechanical assessments appear to be a useful, objective method to guide the development of tailored exercise training as well as serve as objective outcomes for evaluating interventions.
In summary, the use of biomechanical assessment provides a novel and objective methodology to inform researchers and clinicians of functional deficits that may contribute to increased pain and exercise intolerance. During adolescence, patients with JFM appear to exhibit emerging deficiencies in the fundamental movements needed to confidently engage in activity coupled with psychological characteristics, including fear of movement and depressive symptoms, which may inhibit exercise adherence. Early interventions focused on correcting these functional deficits and instilling greater confidence in patients to engage in more vigorous activity (e.g, combined integrative neuromuscular training and cognitive-behavioral therapy) hold great promise in improving outcomes for patients with JFM and clearly need further investigation (63, 64).
Supplementary Material
Significance and Innovations.
Adolescents with Juvenile Fibromyalgia (JFM) demonstrated mild deficiencies in walking gait and significantly lower extremity strength and functional performance compared to healthy controls.
Adolescents with JFM reported increased fear of movement, higher functional disability, pain intensity, and more depressive symptoms compared to healthy controls.
Altered joint mechanics indicate that youth with JFM may demonstrate deficits in their fundamental movements that may be the result of pain avoidance during physical activity.
Interventions focused on correcting functional deficits and instilling greater confidence in patients to engage in activity are needed.
Acknowledgments
Funding: National Institutes of Arthritis and Musculoskeletal and Skin Diseases grant (NIAMS R21-AR063412-01) to Susmita Kashikar-Zuck, Ph.D, Division of Behavioral Medicine and Clinical Psychology, and Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center
References
- 1.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis & Rheumatism. 1985;28(2):138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis and Rheumatism. 2008;59(10):1392–1398. doi: 10.1002/art.24099. [DOI] [PubMed] [Google Scholar]
- 3.Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, et al. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 2010;49(11):2204–2209. doi: 10.1093/rheumatology/keq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Pain Society. Guideline for the management of fibromyalgia syndrome pain in adults and children. Glenview, IL: American Pain Society; 2005. [Google Scholar]
- 5.Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow-up. J Clin Rheumatol. 2011;17(2):64–68. doi: 10.1097/RHU.0b013e31820e7ea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc. 2011;43(5):905–912. doi: 10.1249/MSS.0b013e3181fca1ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashikar-Zuck S, Flowers SR, Verkamp E, Ting TV, Lynch-Jordan AM, Graham TB, et al. Actigraphy-based physical activity monitoring in adolescents with juvenile primary fibromyalgia syndrome. J Pain. 2010;11(9):885–893. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res. 2013;65(3):398–405. doi: 10.1002/acr.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones K, King L, Mist S, Bennett R, Horak F. Postural control deficits in people with fibromyalgia: a pilot study. Arthritis research & therapy. 2011;13(4):R127. doi: 10.1186/ar3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of behavioral medicine. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 11.Turk DC, Robinson JP, Burwinkle T. Prevalence of fear of pain and activity in patients with fibromyalgia syndrome. J Pain. 2004;5(9):483–490. doi: 10.1016/j.jpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Nijs J, Roussel N, Van Oosterwijck J, De Kooning M, Ickmans K, Struyf F, et al. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: state of the art and implications for clinical practice. Clin Rheumatol. 2013;3:3. doi: 10.1007/s10067-013-2277-4. [DOI] [PubMed] [Google Scholar]
- 13.Maquet D, Croisier J-L, Renard C, Crielaard J-M. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69(3):293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 14.Panton LB, Kingsley JD, Toole T, Cress ME, Abboud G, Sirithienthad P, et al. A comparison of physical functional performance and strength in women with fibromyalgia, age-and weight-matched controls, and older women who are healthy. Physical therapy. 2006;86(11):1479–1488. doi: 10.2522/ptj.20050320. [DOI] [PubMed] [Google Scholar]
- 15.Góes SM, Leite N, Shay BL, Homann D, Stefanello JM, Rodacki AL. Functional capacity, muscle strength and falls in women with fibromyalgia. Clinical Biomechanics. 2012;27(6):578–583. doi: 10.1016/j.clinbiomech.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Giannini MJ, Protas EJ. Comparison of peak isometric knee extensor torque in children with and without juvenile rheumatoid arthritis. Arthritis & Rheumatism. 1993;6(2):82–88. doi: 10.1002/art.1790060207. [DOI] [PubMed] [Google Scholar]
- 17.Jones KD, Horak FB, Winters-Stone K, Irvine JM, Bennett RM. Fibromyalgia is associated with impaired balance and falls. J Clin Rheumatol. 2009;15(1):16–21. doi: 10.1097/RHU.0b013e318190f991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russek LN, Fulk GD. Pilot study assessing balance in women with fibromyalgia syndrome. Physiother Theory Pract. 2009;25(8):555–565. doi: 10.3109/09593980802668050. [DOI] [PubMed] [Google Scholar]
- 19.Bachasson D, Guinot M, Wuyam B, Favre-Juvin A, Millet GY, Levy P, et al. Neuromuscular fatigue and exercise capacity in fibromyalgia syndrome. Arthritis Care Res. 2013;65(3):432–440. doi: 10.1002/acr.21845. [DOI] [PubMed] [Google Scholar]
- 20.Ford KR, Myer GD, Melson PG, Darnell SC, Brunner HI, Hewett TE. Land-Jump Performance in Patients with Juvenile Idiopathic Arthritis (JIA): A Comparison to Matched Controls. Int J Rheumatol. 2009;2009:478526. doi: 10.1155/2009/478526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myer GD, Brunner HI, Melson PG, Paterno MV, Ford KR, Hewett TE. Specialized neuromuscular training to improve neuromuscular function and biomechanics in a patient with quiescent juvenile rheumatoid arthritis. Phys Ther. 2005;85(8):791–802. [PubMed] [Google Scholar]
- 22.Carry PM, Kanai S, Miller NH, Polousky JD. Adolescent patellofemoral pain: a review of evidence for the role of lower extremity biomechanics and core instability. Orthopedics. 2010;33(7):498–509. doi: 10.3928/01477447-20100526-16. [DOI] [PubMed] [Google Scholar]
- 23.Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait & Posture. 2003;18(1):114–125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 24.Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R. Sagittal gait patterns in spastic diplegia. Journal of Bone & Joint Surgery, British Volume. 2004;86-B(2):251–258. doi: 10.1302/0301-620x.86b2.13878. [DOI] [PubMed] [Google Scholar]
- 25.Filho MCdM, Yoshida R, Carvalho WdS, Stein HE, Novo NF. Are the recommendations from three-dimensional gait analysis associated with better postoperative outcomes in patients with cerebral palsy? Gait & Posture. 2008;28(2):316–322. doi: 10.1016/j.gaitpost.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in "high-risk" versus "low-risk" athletes. BMC Musculoskelet Disord. 2007;8(39):39. doi: 10.1186/1471-2474-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 28.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1078. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin WA, Weinhandl JT, Kernozek TW, Cobb SC, Keenan KG, O'Connor KM. The effects of single-leg landing technique on ACL loading. Journal of biomechanics. 2011;44(10):1845–1851. doi: 10.1016/j.jbiomech.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Kea J, Kramer J, Forwell L, Birmingham T. Hip abduction-adduction strength and one-leg hop tests: test-retest reliability and relationship to function in elite ice hockey players. Journal of Orthopaedic & Sports Physical Therapy. 2001;31(8):446–455. doi: 10.2519/jospt.2001.31.8.446. [DOI] [PubMed] [Google Scholar]
- 32.Bertocci GE, Munin MC, Frost KL, Burdett R, Wassinger CA, Fitzgerald SG. Isokinetic performance after total hip replacement. American journal of physical medicine & rehabilitation. 2004;83(1):1–9. doi: 10.1097/01.PHM.0000098047.26314.93. [DOI] [PubMed] [Google Scholar]
- 33.Johnson ME, Mille M-L, Martinez KM, Crombie G, Rogers MW. Age-related changes in hip abductor and adductor joint torques. Archives of physical medicine and rehabilitation. 2004;85(4):593–597. doi: 10.1016/j.apmr.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Masuda K, Kikuhara N, Takahashi H, Yamanaka K. The relationship between muscle cross-sectional area and strength in various isokinetic movements among soccer players. Journal of sports sciences. 2003;21(10):851–858. doi: 10.1080/0264041031000102042. [DOI] [PubMed] [Google Scholar]
- 35.Brent JL, Myer GD, Ford KR, Paterno MV, Hewett TE. The effect of sex and age on isokinetic hip-abduction torques. Journal of sport rehabilitation. 2013;22(1) doi: 10.1123/jsr.22.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Medicine and Science in Sports and Exercise. 2003;35(10):1745–150. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 37.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of Pediatric Psychology. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, et al. Clinical utility and validity of the Functional Disability Inventory (FDI) among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–1607. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1–2):77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 42.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. The Journal of Pain. 2003;4(7):407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs M. Children's Depression Inventory Available from Multi-Health systems, Inc. North Tonawanda, N.Y. 14120-2060: 908 Niagara Falls Blvd.; 1992. [Google Scholar]
- 44.Miller RP, Kori SH, Todd DD. The Tampa Scale for Kinesiophobia. Unpublished Report. 1991 [Google Scholar]
- 45.Roelofs J, Sluiter JK, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, et al. Fear of movement and (re)injury in chronic musculoskeletal pain: Evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain. 2007;131(1–2):181–190. doi: 10.1016/j.pain.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Stanhope S, Kepple T, McGuire D, Roman N. Kinematic-based technique for event time determination during gait. Medical and Biological Engineering and Computing. 1990;28(4):355–360. doi: 10.1007/BF02446154. [DOI] [PubMed] [Google Scholar]
- 47.Ferber R, McClay Davis I, Williams DS, 3rd, Laughton C. A comparison of within- and between-day reliability of discrete 3D lower extremity variables in runners. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(6):1139–1145. doi: 10.1016/S0736-0266(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 48.Hewett TE, Myer GD, Ford KR, Heidt RS, Colosimo AJ, McLean SG, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. The American Journal of Sports Medicine. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 49.Myer GD, Ford KR, Divine JG, Wall EJ, Kahanov L, Hewett TE. Longitudinal assessment of noncontact anterior cruciate ligament injury risk factors during maturation in a female athlete: A case report. Journal of athletic training. 2009;44(1):101. doi: 10.4085/1062-6050-44.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quatman CE, Kiapour A, Myer GD, Ford KR, Demetropoulos CK, Goel VK, et al. Cartilage pressure distributions provide a footprint to define female anterior cruciate ligament injury mechanisms. The American journal of sports medicine. 2011;39(8):1706–1713. doi: 10.1177/0363546511400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hewett TE, Myer GD. The mechanistic connection between the trunk, hip,. knee, and anterior cruciate ligament injury. Exercise and sport sciences reviews. 2011;39(4):161–166. doi: 10.1097/JES.0b013e3182297439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myer GD, Chu DA, Brent JL, Hewett TE. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clinics in sports medicine. 2008;27(3):425–448. doi: 10.1016/j.csm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Gier M, Peters ML, Vlaeyen JWS. Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain. 2003;104(1–2):121–130. doi: 10.1016/s0304-3959(02)00487-6. [DOI] [PubMed] [Google Scholar]
- 55.Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, Al-Zaabie N, Nelson RM. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. International Journal of Rehabilitation Research. 2003;26(2):101–108. doi: 10.1097/00004356-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Al-Obaidi SM, Nelson RM, Al-Awadhi S, Al-Shuwaie N. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine. 2000;25(9):1126–1131. doi: 10.1097/00007632-200005010-00014. [DOI] [PubMed] [Google Scholar]
- 57.Geisser ME, Haig AJ, Wallbom AS, Wiggert EA. Pain-related fear, lumbar flexion, and dynamic EMG among persons with chronic musculoskeletal low back pain. The Clinical journal of pain. 2004;20(2):61–69. doi: 10.1097/00002508-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Lephart SM, Abt JP, Ferris CM, Sell TC, Nagai T, Myers JB, et al. Neuromuscular and biomechanical characteristic changes in high school athletes: a plyometric versus basic resistance program. Br J Sports Med. 2005;39(12):932–938. doi: 10.1136/bjsm.2005.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- 60.Myer GD, Ford KR, McLean SG, Hewett TE. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. Am J Sports Med. 2006;34(3):445–455. doi: 10.1177/0363546505281241. [DOI] [PubMed] [Google Scholar]
- 61.Myer GD, Ford KR, Brent JL, Hewett TE. The Effects of Plyometric versus Dynamic Balance Training on Power, Balance and Landing Force in Female Athletes. J Strength Cond Res. 2006;20(2):345–353. doi: 10.1519/R-17955.1. [DOI] [PubMed] [Google Scholar]
- 62.Myer GD, Stroube BW, Dicesare CA, Brent JL, Ford KR, Heidt RS, Jr, et al. Augmented Feedback Supports Skill Transfer and Reduces High-Risk Injury Landing Mechanics: A Double-Blind, Randomized Controlled Laboratory Study. Am J Sports Med. 2013 doi: 10.1177/0363546512472977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas SM, Sil S, Kashikar-Zuck S, Myer GD. Can Modified Neuromuscular Training Support the Treatment of Chronic Pain in Adolescents? Strength & Conditioning Journal. 2013;35(3):12–26. [Google Scholar]
- 64.Kashikar-Zuck S, Myer G, Ting TV. Can behavioral treatments be enhanced by integrative neuromuscular training in the treatment of juvenile fibromyalgia? Pain Management. 2012;2(1):9–12. doi: 10.2217/pmt.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trost Z, France CR, Thomas JS. Pain-related fear and avoidance of physical exertion following delayed-onset muscle soreness. PAIN. 2011;152(7):1540–1547. doi: 10.1016/j.pain.2011.02.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.