Abstract
Background
Oxidative stress can result in damage to the brain and other organs. To protect from oxidative damage, the human body possesses molecular defense systems, based on the activity of antioxidants, and enzymatic defense systems, including the enzymes catalase (CAT), superoxide-dismutase (SOD) and glutathione-peroxidase (GPx). While pre-clinical research has shown that stimulant use is associated with oxidative damage, oxidative stress and the antioxidant defense systems have not been evaluated in clinical samples of stimulant-dependent patients.
Objectives
This study aimed to investigate the link between stimulant dependence and oxidative stress.
Methods
Peripheral blood samples from 174 methamphetamine (n=48) and/or cocaine-dependent (n=126) participants as well as 30 normal control participants were analyzed for the enzyme activities of CAT, SOD and GPx in the erythrocytes, and the total antioxidant capacity and the malondialdehyde concentration in the plasma.
Results
We could show an association of stimulant dependence with a depletion of total antioxidant capacity to 54.6±4.7 %, which correlates with a reduced activity of the SOD to 71.3±0.03 % compared to healthy control participants (100 %).
Conclusion
Stimulant-dependent patients had significantly lower antioxidant capacity relative to controls, suggesting that they may be at greater risk for oxidative damage to the brain and other organs.
Keywords: Cocaine, methamphetamine, oxidative stress, superoxide dismutase, total antioxidant capacity
1. Introduction
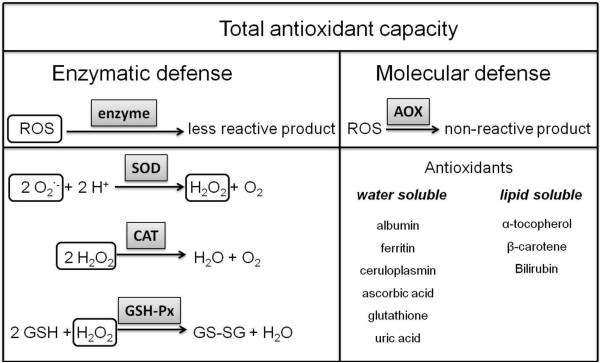
Reactive oxygen species cause oxidative stress in cells and tissues by oxidation of DNA or lipids, leading to a loss of the function of the affected target molecule (Cadet et al.,1999). To protect the cellular targets from oxidative damage, the network of defense mechanisms provides the total antioxidant capacity of the system (Figure 1). This network can be separated into the enzymatic defense, including the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), and the molecular defense, comprising water and lipid soluble antioxidants (Sfrent-Cornateanu et al.,2008). These defense mechanisms prevent the oxidation of lipids, proteins and DNA.
Figure 1.
Overview of the oxidative defense mechanism in human blood and tissues, AOX – antioxidant, GSH-Px – glutathione peroxidase, CAT – catalase, SOD – superoxide dismutase, ROS – reactive oxygen species
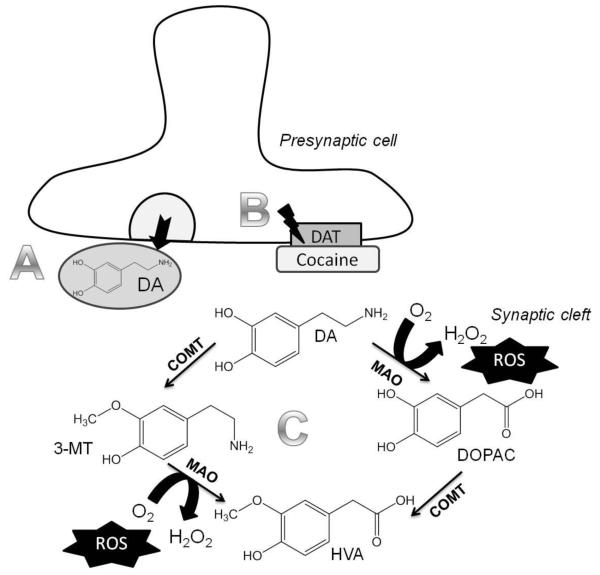
Oxidative stress is defined as the imbalance between the antioxidant defense and the release of reactive oxygen species (ROS). Several substances and substance classes can cause oxidative stress. The influence of stimulant use on oxidative stress has been investigated in cell culture experiments and in animal studies since the mid 1990s (Cerruti et al.,1995). In 2003, it was shown that a single injection of cocaine to a pregnant rat leads to a significant reduction of the antioxidant active forms of glutathione and α-tocopherol in the brain of the fetus (Lipton et al.,2003). Thus, a lack in the antioxidant defense may result in an increase in ROS in the system. In addition, cocaine is known to cause oxidative stress in neuronal cells in culture (Poon et al.,2007). Furthermore, in rats, the formation of ROS in the frontal cortex and the striatum has been shown following intraperitoneal (i.p.) injection of cocaine at a dose of 20 mg/kg body weight (Dietrich et al.,2005). One mechanism of action by which cocaine causes oxidative damage in the central nervous system is linked to its ability to block the reuptake of dopamine from the synaptic cleft into the presynaptic cell via a dopamine transporter (Figure 2). This leads to increasing concentrations of dopamine in the synaptic cleft. At high dopamine concentrations in the synaptic cleft, ROS are released upon its enzymatic degradation (Figure 2) (Smythies and Galzigna,1998). In addition to its effects in the brain, the toxicity of cocaine in other organs, such as the liver and kidneys, is linked to oxidative stress (Valente et al.,2012). Methamphetamine is a psychostimulant that causes oxidative stress in the brain (Yamamoto and Zhu,1998), thereby contributing to an irreversible damage of the dopaminergic brain regions in rats (Ricaurte et al.,1980). Furthermore, it has been shown that methamphetamine alters the intra-cellular calcium signaling, leading to subsequent formation of ROS (Potula et al.,2010).
Figure 2.
Mechanism of increased ROS formation in the synaptic cleft due to cocaine blockage of the dopamine transporter (DAT) in the central nervous system. A. Dopamine (DA) is released from the presynaptic cell into the synaptic cleft. B. Cocaine blocks the reuptake of dopamine into the presynaptic cell, increasing the amount of dopamine in the synaptic cleft. C. Dopamine is degraded in the synaptic cleft by two enzymes, catechol-O-methyl transferase (COMT) and monoamine oxidase (MAO). During the reaction of the MAO, hydrogen peroxide is formed, from which oxygen radicals can form. DOPAC – dihydroxyphenyl acetic acid, 3MT – 3-methoxytyramine, HVA – homovanillic acid
While pre-clinical research has shown that stimulant use is associated with oxidative stress and damage, oxidative stress and the antioxidant defense systems have not been evaluated in clinical samples of stimulant-dependent patients. The goal of this study was to address this research gap by comparing stimulant-dependent patients and control participants on oxidative stress and antioxidant capacity. The lipid peroxidation product malondialdehyde (MDA), which is commonly used as a biomarker of oxidative stress in vivo (Zemel and Sun,2008), indicates whether the antioxidant defense is sufficient to counteract oxidative attack (Lim et al.,2011). In addition to the MDA levels in the plasma, the effects on the antioxidant enzymes SOD, CAT and GSH-Px in erythrocytes as well as the total antioxidant capacity in the plasma were analyzed in this study.
2. Materials and Methods
2.1 Participants
For this study, 174 stimulant-dependent patients, among them 126 cocaine and 48 methamphetamine abusers, who participated in the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) 12-step facilitation for stimulant abusers (STAGE-12) trial (Donovan et al.,2011) were recruited from six different sites of the community treatment programs (CTPs) in Columbus (OH), Dallas (TX), Eugene (OR), Portland (OR), Jacksonville (FL) and Seattle (WA). The participants have been characterized in a previous study (Winhusen,2013). Briefly, all participants were mentally and physically stable enough to participate in the study, and all were seeking outpatient substance use disorder treatment with a diagnosis of stimulant abuse or dependence (Hudziak et al.,1993) and had used stimulants (cocaine or methamphetamine) within 60 days prior to the test. All participants completed one single session in which they gave a urine sample for drug screening, the blood samples were obtained and other baseline characteristics were determined. Of the stimulant abusers, 39 were tested positive for stimulant use and 35 were tested positive for other drugs (marijuana, amphetamines or benzodiazepine). Among the cocaine and methamphetamine abusers 23 and 59, respectively, were abstinent from the stimulant for 30 and more days prior to the visit. The volunteers used methamphetamine for 10.2 ± 7.0 or cocaine for 13.1 ± 7.6 years, respectively (Winhusen et al.,2013).
As control participants, 30 healthy adults, who tested negative for depression, anxiety and attention deficit and hyperactivity disorder (ADHD) were recruited in Columbus (OH). These participants had no history of traumatic brain injuries, HIV, stroke or seizure disorder and were not methamphetamine or cocaine dependent. Since smoking can impact oxidative stress/damage (Sardas et al.,2009), cigarette smokers were oversampled in the normal controls (73%) to approximate the proportion of smokers in the stimulant-dependent group (79%). Further characteristics of the participants are shown in Table 1.
Table 1.
Gender, ethnicity, age and smoking habit distribution in the patients and control subjects.
| Controls (n=30) |
Patients (n=174) |
|
|---|---|---|
| Gender: | ||
| Male | 43.3 % | 31.7 % |
| Female | 56.7 % | 68.3 % |
| Race* (%) | ||
| White | 70.0% | 43.4% |
| African-American | 26.7% | 46.3% |
| Other/Mixed | 3.3% | 10.3% |
| Ethnicity-Hispanic | 3.4 % | 5.7 % |
| Age (years) | 44.5 ± 9.5 | 38.6 ± 9.6 |
| Cigarette smoker | 73.3 % | 79.2 % |
p < 0.05 between control and patients
2.2 Procedure
Blood was drawn at the respective site and immediately separated into plasma and erythrocytes by centrifugation. All blood samples were frozen to −80°C, shipped to Madison, WI on dry ice and immediately stored at −80°C. All samples were analyzed within three months after the blood draw. Analyses of the samples were done blinded, so that control samples did get processed in a similar time manner as patient's samples.
2.3 Total Antioxidant Capacity
To measure the total antioxidant capacity, the plasma samples were analyzed using the Antioxidant Assay Kit from Cayman Chemical Company (Ann Arbor, MI, USA) and following the manufacturer’s protocol. Briefly, plasma samples were thawed on ice and diluted 1:25 with assay buffer. The diluted samples were used to perform the assay based on the oxidation of 2,2-Azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS®) by metmyoglobin. Results are given in mM trolox equivalents, since a trolox standard curve was measured within each assay.
2.4 Enzyme activity assays
The activity of the anti-oxidative enzyme glutathione peroxidase (GPx) was determined using the Glutathione Peroxidase Kit from Cayman Chemical Company (Ann Arbor, MI, USA) following the manufacturer’s protocol. Therefore, the erythorcyte samples were thawed on ice and diluted 1:50 with sample buffer.
The activity of the superoxide dismutase was measured using the SOD Assay Kit-WST from Dojindo Molecular Technologies (Rockville, MD, USA) following the manufacture’s protocol. Here, the erythrocyte samples were thawed on ice and diluted 1:100 in dilution buffer.
The results of the SOD and GPx activities were calculated as kU/g hemoglobin (1000 units/g hemoglobin).
The catalase activity was measured photometrically at 240 nm using a photometer. The erythrocyte samples were diluted 1:5 with ddH2O and then 1:200 in phosphate buffer (20 mM KH2PO4, 30mM Na2HPO4, pH 7). The absorbance was measured after injection of H2O2 directly and 30 s later, against a buffer blank. The catalase activity was calculated in MU/g hemoglobin (106 units/g hemoglobin) using the following formula with V = total volume in the cuvette, v = sample volume in the cuvette, d = dilution factor and c(hb) = concentration of hemoglobin in g/l.
Catalase activity (MU/g hg)
= (V*1000 μM/mM*(E1/E2)*d*2/min)/(v*0.036 L/(mmol*cm)*1000000 U/MU* c(hb) g/L)
2.5 Hemoglobin concentration
The hemoglobin concentration was determined photometrically using the Pointe Scientific hemoglobin reagent (Fisher Scientific, Hampton, NH, USA). The diluted erythrocyte samples were mixed with the reagent to a 1:100 dilution and the absorbance was measured in triplicate at 540 nm in a 96-well plate using a Varioskan Flash Multimode Reader (Fisher Scientific, Madison, WI, USA).
2.6 Lipid Peroxidation
To measure the effect on lipid peroxidation, the secondary reaction product malondialdehyde (MDA) was determined in the plasma samples using the MDA-TBARS Assay Kit from Cayman Biochemicals (Ann Arbor, MI, USA). Briefly, in 5 ml glass vials, 100 μl of standard or sample were mixed with SDS solution and color reagent and boiled for 1 h. After cooling down to room temperature, the sample was centrifuged at 4 °C for 10 min at 1600xg and the absorbance of the supernatant at 540nm was measured in triplicate (150 μl each). Based on the standard curve the concentration of MDA in the sample was determined in μM.
2.7 Statistics
Each sample was measured in triplicate for all assays performed. The means of these replicates were used to compare the results. Outliers within the replicates were excluded using the Nalimov outlier test. All data obtained for patient samples were compared to those of the control subjects using an analysis of variances of Blom-transformed data and a post-hoc Dunnet's test for non-equal variances.
3. Results
3.1 Enzyme activities and total antioxidant capacity of stimulant-dependent vs. control participants
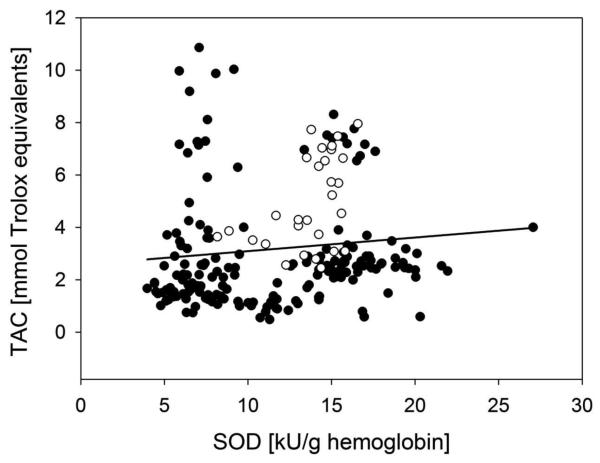
The results for the activities of glutathione peroxidase, superoxide dismutase and catalase, the total antioxidant capacity and the lipid peroxidation were compared between control participants (n = 30) and the stimulant-dependent participants from all sites (n = 176). Table 2 shows the differences in the total antioxidant capacity. Stimulant-dependent patients were significantly depleted (p < 0.001) in their total antioxidant capacity compared to healthy controls. Since no significant impact of the years of stimulant abuse, or days of abstinence on the total antioxidant capacity was determined, all stimulant abusers were combined in the analysis. Additionally, the SOD activity (Table 2) was reduced in the stimulant-dependent patients (10.2 ± 0.43 kU/g hemoglobin) vs. healthy participants (14.3 ± 0.37 kU/g hemoglobin). Table 1 displays the demographics of the study volunteers. Statistical comparison between the control and the stimulant-dependent participants identified no significant difference between the groups regarding gender and age. In contrast, the race distribution differed between the two populations. However, the subset sample size was too small to warrant correlation analysis with the outcome measures. The association between a reduced SOD activity and a depletion of total antioxidant capacity was compared by correlation analysis (Figure 3), resulting in a linear correlation with a significant (p < 0.01) correlation coefficient of 0.278 with a square of the regression coefficient of only 7.7%, presenting a weak correlation. Furthermore, correlation analysis between days of storage and the outcome measures showed a no impact of days of storage on any of the biomarkers measured (r² of days of storage vs. SOD activity = 0.024, vs. GPx activity = 0.033, vs. CAT activity = 0.028, vs. TAC = 0.046).
Table 2.
Activities of the antioxidant enzymes glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) in the blood of healthy volunteers (control), all drug abusing patientst and subgroups of the drug abusing patients depending on their stimulant source. MDA, malondialdehyde; TAC, total antioxidant capacity; TE = trolox equivalents.
| GPx (U/g hg) |
CAT (kU/g hg) |
SOD (kU/g hg) |
MDA (μM) |
TAC (mmol TE) |
|
|---|---|---|---|---|---|
| Control (n = 30) | 24.4 ± 1.39 | 0.52 ± 0.03 | 14.3 ± 0.37 | 3.93 ± 0.77 | 4.37 ± 0.33 |
| Patients (n = 174a) | 22.9 ± 0.52 | 0.52 ± 0.02 | 10.2 ± 0.43* | 3.13 ± 0.39 | 2.33 ± 0.16*** |
| Cocaine dep. (n =124a) |
23.5 ± 0.8 | 0.55 ± 0.03 | 9.39 ± 0.54** | 3.00 ± 0.53 | 2.31 ± 0.24*** |
| METH dep. (n = 48a) |
21.7 ± 0.7 | 0.50 ± 0.02 | 11.6 ± 0.66 | 3.46 ± 0.48 | 2.06 ± 0.23*** |
Exclusion of the users of both stimulants (n = 2) explains difference between all patients and individual groups of cocaine and METH dependent patients.
p < 0.05 vs. control,
p < 0.001 vs. control.
Figure 3.
Spearman correlation of the superoxide dismutase activity (SOD) and the total antioxidant capacity (TAC) of control (empty circles) and patients (full circles) with 95 % confidence intervals (grey lines).
3.2 Enzyme activities and total antioxidant capacity of patients using either cocaine-depenent or methamphetamine-dependent vs. control participants
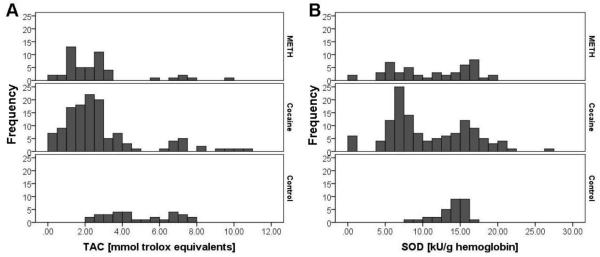
Cocaine and methamphetamine increase the level of ROS by different mechanisms (Dietrich, Mangeol, Revel, Burgun, Aunis and Zwiller,2005, Yamamoto and Zhu,1998), thus, the response of the body to these two drugs may vary as well. To evaluate this possibility, the results were separated according to the type of drug the stimulant-dependent patients used. Here, the total antioxidant capacity of cocaine-dependent and methamphetamine-dependent participants was reduced to 2.31 ± 0.24 and 2.06 ± 0.23 mmol trolox equivalents, respectively, compared to 4.37 ± 0.33 mmol trolox in controls. In addition, the SOD activity of the cocaine-dependent subset was significantly reduced compared to the control group. The histogram for the total antioxidant capacity (Figure 4A) clearly shows the difference between the control participants and the stimulant-dependent participants, independent of the choice of stimulant. In comparison, in the histogram of the SOD activity (Figure 4B), a trend towards the same direction is noticeable for both subgroups of stimulant-dependent participants, while only the cocaine-dependent participants demonstrated a significantly decreased enzyme activity compared to the controls (Table 2).
Figure 4.
Histogramms for the frequencies of (A) total antioxidant capacity (TAC) and (B) superoxide dismutase (SOD) activity, separated by the stimulant source compared to the control
3.3 Malondialdehyde formation of stimulant-dependent vs. healthy control participants
For the formation of the secondary product of lipid peroxidation, MDA, a marker for oxidative stress in the lipid metabolism, no significant differences were detected between the control group and the entire patients group (Table 2), or between the cocaine- and methamphetamine-dependent subgroups (Table 2).
4. Discussion
The present study, which is the first to compare stimulant-dependent and healthy control participants on antioxidant capacity and oxidative stress, revealed that the stimulant-dependent patients evidenced a significant reduction in total antioxidant capacity.
Stimulant abuse has been associated with oxidative tissue damage. However, the mechanisms by which stimulants lead to the formation of ROS in vivo may vary. Cocaine leads to the formation of ROS via dopamine (Dietrich, Mangeol, Revel, Burgun, Aunis and Zwiller,2005), whereas methamphetamine alters the calcium signaling, also resulting in a formation of ROS (Yamamoto and Zhu,1998). In the present study, both the cocaine-dependent and methamphetamine-dependent participants evidenced a significantly decreased total antioxidant capacity compared to controls, suggesting that depletion of the total antioxidant capacity is independent of the mechanism by which the ROS are formed. The cocaine-dependent participants also evidenced a significant reduction in the SOD activity in erythrocytes, which might have contributed to the lower antioxidant capacity (Figure 1). While only the cocaine-dependent participants showed a significant reduction in SOD activity relative to the controls, the histograms for SOD activity show that the distribution was similar for the cocaine- (n = 124) and methamphetamine-dependent (n = 48) participants; the lack of a significant effect in the methamphetamine-dependent participants may have been due to the smaller sample size for that group. In addition, the correlation between the results of the SOD activity in the erythrocytes and the total antioxidant capacity was weak. These results suggest that a higher number of test participants would be needed to achieve a stronger correlation of the reduced SOD activity with the depleted total antioxidant capacity. While both cocaine-dependent and methamphetamine-dependent patients had significantly lower total antioxidant capacity relative to controls, we did not detect a significant increase in oxidative damage of lipids based on plasma MDA concentration. However, the MDA assay has been widely discussed as not being very sensitive (Lee et al.,2012),and as delivering false positive results, since not only MDA, but other aldehydes as well as sugars can react with the tiobarbituric acid (Kosugi et al.,1987). In addition, MDA is also formed as a byproduct of the cyclooxygenase reaction in platelets (Makris et al.,1985). Furthermore, the defense mechanisms prevent the body from oxidative damage, thus only when the balance between antioxidant defense and oxidative stress is completely shifted towards the oxidative stress, damage of lipids may occur.
The results of the present study can only be compared to the results from animal studies, since no data on the effects of stimulant abuse on oxidative stress in human participants is yet available. Our results are in accordance with previous results in mice, treated with 20 mg/kg cocaine, which showed reduced activities of catalase and glutathione peroxidase compared to the control mice (Labib et al.,2002). The authors concluded that the ROS generated by cocaine metabolites (Boelsterli et al.,1993, Goldlin and Boelsterli,1991) lead to a decrease of enzyme activities of GSH-Px and catalase compared to the saline control (Labib, Turkall and Abdel-Rahman,2002). In the animal studies on oxidative damage caused by cocaine (Labib, Turkall and Abdel-Rahman,2002, Muriach et al.,2010) the SOD, CAT or GPx activities were measured in the target tissues, liver and brain, not in the blood. Although respective human data are lacking, it is known that cocaine abuse leads to an elevated inflammatory state and thereby enhanced oxidative stress status in drug abusing patients (Fox et al.,2012). Since similar pathways regulate the response to inflammation and oxidative stress, an inflammatory response might also have contributed to the reduced activities of SOD in the present study.
The decrease in SOD activity was weakly correlated to depletion in the total antioxidant capacity. Although the limited number of participants studied might be one reason for this weak correlation, the reduction of the total antioxidant capacity of 54.6 ± 4.7 % may not only be explained by the reduction of the enzyme activities, since depletion of water and lipid soluble antioxidants has not been evaluated in this study. The oxidative imbalance could, therefore, also be caused by a reduced oxidative defense due to a lack of antioxidants.
Up to now, no data on the correlation between oxidative stress associated with stimulant abuse and brain damage in healthy participants are available. In contrast, it has been shown that the formation of ROS plays a crucial role in the development Alzheimer's disease, a neurodegenerative disease leading to brain damage. A study on Alzheimer's patients showed that, with the age of the patients, more ROS were formed and, with the progression of the disease, the total plasma antioxidant capacity was depleted (Guidi et al.,2006). These results suggest that a depletion of plasma TAC associated with stimulant-dependence could be linked to an increased risk for oxidative brain damage. In addition, a correlation between a depleted TAC and an increase of oxidative DNA damage in brain tissue of patients with transitional meningioma compared to control patients has been shown (Hanimoglu et al.,2007).
The present findings should be considered in light of several limitations. First, due to concern about participant study burden in this ancillary study, we did not assess for factors that can have a significant impact on oxidative DNA damage and general health such as diet, exercise, chronic stress, trauma, and environment (McEwen,2006, Watters et al.,2008) and so the significant differences observed might be related to these factors. Second, the sample sizes for some of the analyses were small, and, thus, our analyses were likely underpowered. Another important limitation is that this study is correlational in nature and, thus, cause and effect determinations cannot be made. In addition, the study was conducted with a stimulant-dependent sample that abused other substances and, thus, the observed associations cannot be attributed solely to stimulant use. The oxidative imbalance could also be caused by a reduced oxidative defense due to low levels of antioxidants based on poor eating habits of stimulant abusers compared to the control group. Furthermore, stimulants can activate stress pathways, resulting in elevated stress biomarkers (Hamidovic et al.,2010). Thus, dietary intake of antioxidants as well as other life style factors affecting the antioxidative balance, e.g. smoking, as well as stress biomarkers and history of chronic stress and trauma should be evaluated in future studies. Finally, the generalizability of the findings from the stimulant-dependent and normal control comparisons is limited since the normal controls were recruited from a single site and the control sample size was smaller than the case sample size.
In summary, this is the first study to evaluate oxidative stress and antioxidant defense systems in a clinical sample of stimulant-dependent patients. Consistent with pre-clinical research findings demonstrating that stimulants decrease total antioxidant activity, the present study revealed that total antioxidant capacity was significantly lower in both cocaine-dependent and methamphetamine-dependent patients relative to normal controls. This could, in turn, render stimulant-dependent patients at greater risk for oxidative damage to the brain and other organs. Future research to replicate and extend these findings is warranted.
Acknowledgments
Sponsor: National Institute on Drug Abuse; grant number: 3UIO-DA013732-08
Abbreviations
- CAT
catalase
- GSH-Px
glutathione peroxidase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
The authors declare no conflict of interest and no financial disclosure.
5. References
- Boelsterli, Wolf, Goldlin Oxygen free radical production mediated by cocaine and its ethanol-derived metabolite, cocaethylene, in rat hepatocytes. Hepatology. 1993;18:1154–1161. [PubMed] [Google Scholar]
- Cadet, Delatour, Douki, Gasparutto, Pouget, Ravanat, Sauvaigo Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Cerruti, Sheng, Ladenheim, Epstein, Cadet Involvement of oxidative and L-arginine-NO pathways in the neurotoxicity of drugs of abuse in vitro. Clin Exp Pharmacol Physiol. 1995;22:381–382. doi: 10.1111/j.1440-1681.1995.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Dietrich, Mangeol, Revel, Burgun, Aunis, Zwiller Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology. 2005;48:965–974. doi: 10.1016/j.neuropharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Donovan, Daley, Brigham, Hodgkins, Perl, Floyd How practice and science are balanced and blended in the NIDA Clinical Trials Network: the bidirectional process in the development of the STAGE-12 protocol as an example. Am J Drug Alcohol Abuse. 2011;37:408–416. doi: 10.3109/00952990.2011.596970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, D'Sa, Kimmerling, Siedlarz, Tuit, Stowe, Sinha Immune system inflammation in cocaine dependent individuals: implications for medications development. Human Psychopharmacology-Clinical and Experimental. 2012;27:156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldlin, Boelsterli Reactive oxygen species and non-peroxidative mechanisms of cocaine-induced cytotoxicity in rat hepatocyte cultures. Toxicology. 1991;69:79–91. doi: 10.1016/0300-483x(91)90155-t. [DOI] [PubMed] [Google Scholar]
- Guidi, Galimberti, Lonati, Novembrino, Bamonti, Tiriticco, Fenoglio, Venturelli, Baron, Bresolin, Scarpini Oxidative imbalance in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2006;27:262–269. doi: 10.1016/j.neurobiolaging.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Hamidovic, Childs, Conrad, King, de Wit Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug Alcohol Depend. 2010;109:175–180. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanimoglu, Tanriverdi, Kacira, Sanus, Atukeren, Aydin, Tunali, Gumustas, Kaynar Relationship between DNA damage and total antioxidant capacity in patients with transitional meningioma. Clin Neurol Neurosurg. 2007;109:561–566. doi: 10.1016/j.clineuro.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hudziak, Helzer, Wetzel, Kessel, McGee, Janca, Przybeck The use of the DSM-III-R Checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Kosugi, Kato, Kikugawa Formation of yellow, orange, and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid. Anal Biochem. 1987;165:456–464. doi: 10.1016/0003-2697(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Labib, Turkall, Abdel-Rahman Inhibition of cocaine oxidative metabolism attenuates endotoxin potentiation of cocaine mediated hepatotoxicity. Toxicology. 2002;179:9–19. doi: 10.1016/s0300-483x(02)00247-0. [DOI] [PubMed] [Google Scholar]
- Lee, Margaritis, Channon, Antoniades Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Won, Kim, Jang, Jyothi, Dandona, Ha, Kim Antioxidant enzymes induced by repeated intake of excess energy in the form of high-fat, high-carbohydrate meals are not sufficient to block oxidative stress in healthy lean individuals. Br J Nutr. 2011;106:1544–1551. doi: 10.1017/S0007114511002091. [DOI] [PubMed] [Google Scholar]
- Lipton, Gyawali, Borys, Koprich, Ptaszny, McGuire Prenatal cocaine administration increases glutathione and alpha-tocopherol oxidation in fetal rat brain. Brain Res Dev Brain Res. 2003;147:77–84. doi: 10.1016/j.devbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Makris, Tsakiris, Papadopoulos, Ballas The ratio MDA/MDAa as a new index of platelet hyperactivity. Haemostasis. 1985;15:331–336. doi: 10.1159/000215168. [DOI] [PubMed] [Google Scholar]
- McEwen Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriach, Lopez-Pedrajas, Barcia, Sanchez-Villarejo, Almansa, Romero Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem. 2010;114:675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- Poon, Abdullah, Mullan, Mullan, Crawford Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochem Int. 2007;50:69–73. doi: 10.1016/j.neuint.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Potula, Hawkins, Cenna, Fan, Dykstra, Ramirez, Morsey, Brodie, Persidsky Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185:2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte, Schuster, Seiden Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Sardas, Cimen, Karsli, Yurdun, Donbak Comparison of genotoxic effect between smokeless tobacco (Maras powder) users and cigarette smokers by the alkaline comet assay. Hum Exp Toxicol. 2009;28:214–219. doi: 10.1177/0960327108098333. [DOI] [PubMed] [Google Scholar]
- Sfrent-Cornateanu, Mihai, Stoian, Lixandru, Bara, Moldoveanu Antioxidant defense capacity in scleroderma patients. Clin Chem Lab Med. 2008;46:836–841. doi: 10.1515/CCLM.2008.132. [DOI] [PubMed] [Google Scholar]
- Smythies, Galzigna The oxidative metabolism of catecholamines in the brain: a review. Biochim Biophys Acta. 1998;1380:159–162. doi: 10.1016/s0304-4165(97)00131-1. [DOI] [PubMed] [Google Scholar]
- Valente, Carvalho, Bastos, de Pinho, Carvalho Contribution of Oxidative Metabolism to Cocaine-Induced Liver and Kidney Damage. Curr Med Chem. 2012 doi: 10.2174/092986712803988938. [DOI] [PubMed] [Google Scholar]
- Watters, Satia, Kupper Correlates of antioxidant nutrients and oxidative DNA damage differ by race in a cross-sectional study of healthy African American and white adults. Nutr Res. 2008;28:565–576. doi: 10.1016/j.nutres.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen Preliminary evaluation of a model of stimulant use, oxidative damage, and executive dysfunction. American Journal of Drug and Alcohol Abuse. 2013 doi: 10.3109/00952990.2013.798663. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen, Walker, Brigham, Lewis, Somoza, Theobald, Somoza Preliminary evaluation of a model of stimulant use, oxidative damage and executive dysfunction. Am J Drug Alcohol Abuse. 2013;39:227–234. doi: 10.3109/00952990.2013.798663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Zhu The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Zemel, Sun Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr. 2008;138:1047–1052. doi: 10.1093/jn/138.6.1047. [DOI] [PubMed] [Google Scholar]