Abstract
Respiratory syncytial virus (RSV) is an important human pathogen. Expression of virus structural proteins produces self-assembled virus-like nanoparticles (VLP). We investigated immune phenotypes after RSV challenge of immunized mice with VLP containing RSV F and G glycoproteins mixed with F-DNA (FdFG VLP). In contrast to formalin-inactivated RSV (FIRSV) causing vaccination-associated eosinophilia, FdFG VLP immunization induced low bronchoalveolar cellularity, higher ratios of CD11c+ versus CD11b+ phenotypic cells and CD8+ T versus CD4+ T cells secreting interferon (IFN)-γ, T helper type-1 immune responses, and no sign of eosinophilia upon RSV challenge. Furthermore, RSV neutralizing activity, lung viral clearance, and histology results suggest that FdFG VLP can be comparable to live RSV in conferring protection against RSV and in preventing RSV disease. This study provides evidence that a combination of recombinant RSV VLP and plasmid DNA may have a potential anti-RSV prophylactic vaccine inducing balanced innate and adaptive immune responses.
Keywords: RSV, nanoparticle vaccine, prophylactic vaccine, bronchoalveolar cells
Background
The World Health Organization estimates that respiratory syncytial virus (RSV) causes 64 millions of infection and 160,000 deaths globally 1. In the 1960s, a formalin-inactivated RSV vaccine (FI-RSV) induced exacerbated disease during the next winter season, resulting in 80% hospitalizations and two deaths 2,3. Despite the extensive endeavor to develop RSV vaccines, there is no licensed vaccine. Purified RSV fusion (F) or attachment (G) glycoprotein subunit and recombinant vectored RSV vaccines are also known to cause enhanced RSV disease (ERD) 4-6. RSV reinfection is common throughout life, indicating that natural RSV infection fails to establish long-lasting immunity 7,8.
Virus-like nanoparticles (VLP) can be generated through the assembly of structural proteins and lipid bilayer membranes, and are morphologically similar to the virus 9,10. Recent studies reported that a chimeric Newcastle disease virus (NDV) core protein VLP containing the RSV F and G ectodomains was produced by DNA transfection of avian cells 11,12. DNA vaccines encoding either RSV F or G developed IgG2a antibodies, T helper (Th) type 1/Th2 cytokine responses, but potential concerns of pulmonary disease remain controversial 13,14. A combination of RSV F DNA and protein vaccines was shown to induce F-specific antibodies and cytotoxic CD8+ T cell responses in a neonatal mouse model 15.
Recombinant baculovirus-derived VLP vaccines containing the full-length of RSV F or G in a membrane-anchored form are in a range of 60 to 120 nm particles and shown to induce immune responses contributing to clearing lung viral loads 16. DNA vaccines induce host immune responses by transduced cells expressing protein antigens endogenously and the efficacy of DNA vaccines is less likely to be affected by maternal antibodies. Innate and adaptive immune responses contributing to RSV protection remain unknown. We hypothesized that a combination of DNA and VLP vaccines would develop balanced immune responses to ensure virus clearance but not to inflame tissues causing RSV lung disease. In comparison with FI-RSV and live RSV, we investigated bronchoalveolar cellular phenotypes after immunization with a combined vaccine of RSV F DNA and VLP containing RSV F and G glycoproteins (FdFG VLP).
Methods
Preparation of RSV VLPs, RSV F encoding DNA, and FI-RSV
Nanoparticle VLP consisting of an influenza virus matrix (M1) protein core and RSV glycoproteins F (RSV F VLP) or G (RSV G VLP) on the surface were produced using the insect cell expression system and characterized as described 16. Briefly, SF9 insect cells were infected with recombinant baculoviruses expressing M1 and RSV F or RSV G proteins, and RSV VLP nanoparticles released into cell culture media were purified by ultracentrifugation 16. The plasmid DNA encoding RSV F protein (RSV F DNA) was previously described 17 and amplified in E. coli cells and purified using endotoxin-free kits (Qiagen). FI-RSV was prepared by formalin inactivation as described in the supplementary materials.
Immunization and challenge
Female BALB/c mice aged 6 to 8 weeks (Charles River) were immunized intramuscularly with RSV vaccines or infected intranasally with live RSV A2. Groups of mice (n=15) were intramuscularly immunized with RSV vaccines at week 0 and 4. For FI-RSV immunization, 2 μg and 1 μg of FI-RSV were used for prime and boost, respectively. A mixed RSV vaccine designated as FdFG VLP contained RSV F DNA, RSV F VLP and RSV G VLP. The priming dose of FdFG VLP was composed of 10 μg F VLP/10 μg G VLP/50 μg F DNA and the boosting dose was half of each component (5 μg F VLP/5 μg G VLP/25 μg F DNA). For a control group, mice (n=15) were intranasally infected with live RSV A2 strain (1×106 plaque forming units (PFU) for prime, 0.5×106 PFU for boost) at weeks 0 and 4. Serum samples were collected at 3 weeks after prime and boost immunization. At 26 weeks after boost immunization, naïve, immunized, and infected mice were challenged with RSV (1×106 PFU/mouse). Animal experiments in this study were performed under the approval of Georgia State University (GSU) IACUC review boards (IACUC A11026). We followed the guidelines of the GSU IACUC for all animal experiments and husbandry. GSU IACUC operates under the federal Animal Welfare Law and regulations of the Department of Health and Human Services.
Antibody ELISA and RSV neutralizing activity
To determine antigen-specific antibody levels, maxisorp immunoplates were coated with FI-RSV (4 μg/ml), RSV F protein (100 ng/ml, BEI, NIH) or RSV G protein (200 ng/ml, Sino biological Inc.) as an ELISA antigen. RSV-specific neutralizing antibody titers in mouse sera were measured using the red fluorescent monomeric Katushka 2 protein expressing RSV A2 strain (A2-K-line19F) 18,19 as detailed in the supplementary materials.
Lung and BALF samples, flow cytometry analysis, and determination of virus titers
Day 5 post RSV challenge, mice were euthanized to collect bronchoalveolar lavage (BAL) fluids (BALF) and lung samples. BALF samples were obtained by infusing 1 ml of phosphate buffered saline into the lungs via trachea using a 25-gauge catheter 20.
For cell phenotype analysis, the harvested BAL cells from BALF samples (n=5, pooled) were stained with fluorophore-labeled surface markers (CD45, CD11b, CD11c, CD3, CD8, Fc blockers). An antibody cocktail for cell surface staining and intracellular interferon-gamma (IFN- γ) staining were used as described in supplementary methods. The lung homogenates were centrifuged at 2000 rpm for 10 minutes to collect supernatants. The virus titer in supernatants was determined by an immunoplaque assay 21.
Results
A combined VLP and DNA vaccine induces high IgG2a/IgG1 antibody ratios
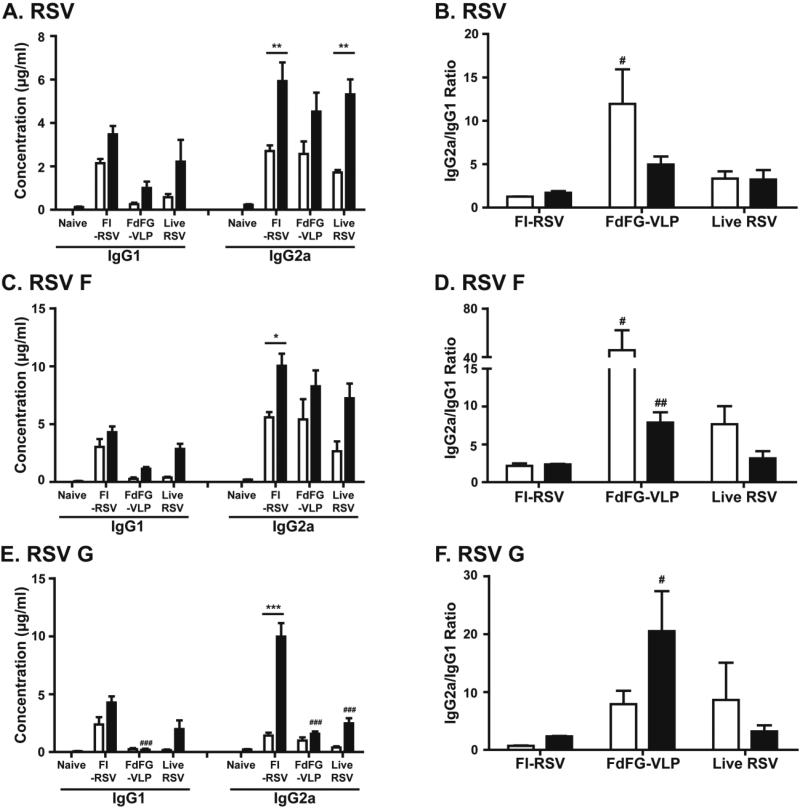
Both F VLP and G VLP were shown to raise similar RSV neutralizing titers and control lung viral loads 16. In addition, antibody responses specific for RSV G central domains were demonstrated to contribute to conferring protection and ameliorating RSV disease 22. We found that FdFG VLP was more effective in inducing higher levels of IgG2a antibodies (Th1 type) whereas F DNA alone was not highly immunogenic (Supplementary Fig. S1). Therefore, to further evaluate the protective immune responses and safety of FdFG VLP in comparison with FI-RSV and live RSV, mice were intramuscularly immunized with FdFGVLP, FI-RSV, or infected with live RSV (Fig. 1). At 3 weeks after prime and boost immunization, RSV specific serum antibodies were determined using FI-RSV as an ELISA coating antigen (Fig. 1A). Highest levels of IgG1 antibody were detected in the group of FI-RSV whereas FdFG VLP immunized mice showed lowest levels of IgG1 isotype antibody (Fig. 1A). The live RSV group showed a similar level of IgG1 and IgG2a antibodies specific for RSV after the 1st infection, which was significantly increased after the 2nd dose of infection (Fig. 1A, B). A higher level of IgG1 antibodies was induced in FI-RSV immunized mice compared to that in FdFG VLP immunized or live RSV infected mice (Fig. 1A, B). As a result, the FdFG VLP group showed the highest ratios of IgG2a/IgG1 in particular after prime immunization (Fig. 1B).
Figure 1. RSV-specific serum IgG isotype antibodies.
(A) RSV specific IgG1 and IgG2a antibodies. (B) Ratios of IgG2a/IgG1 antibodies specific to RSV. (C) IgG1 and IgG2a isotype antibodies specific for purified RSV F protein. (D) Ratios of IgG2a/IgG1 isotype antibodies specific for RSV F protein. (E) IgG1 and IgG2a isotype antibodies specific for purified RSV G protein. (F) Ratios of IgG2a/IgG1 isotype antibodies specific for RSV G protein. * and *** indicates p<0.05 and 0.001, respectively, between prime and boost immunized sera. #, ## and ### mean p<0.05, 0.01 and 0.001, respectively, between the FI-RSV and FdFG VLP groups. Naïve: unimmunized mice. FI-RSV: FI-RSV vaccine. FdFGVLP: a combined vaccine of RSV F DNA, RSV F and G VLP. Live RSV: live RSV A2 strain.
When ELISA was performed using the purified RSV F protein as a coating antigen, IgG1 antibodies were induced at higher levels by prime vaccination with FI-RSV than those by priming with FdFG VLP or infection with live RSV (Fig. 1C). In contrast, FdFG VLP immunization or live RSV infection induced higher levels of IgG2a antibodies specific for RSV F than those of IgG1. Consequently, higher ratios of IgG2a/IgG1 antibodies were induced in the FdFG VLP group, followed by live RSV infection (Fig. 1D).
FI-RSV immunization also induced significantly higher levels of RSV G protein specific IgG1 and IgG2a antibody responses than live RSV infection or FdFG VLP immunization after prime immunization (Fig. 1E). Because of low levels of IgG1 isotype antibodies, relatively higher ratios of IgG2a/IgG1 were observed in the FdFG group (Fig. 1F). The pattern and levels of antibody responses were maintained for over 6 months (data not shown). These results indicate that FdFG VLP vaccine induces IgG2a antibodies predominantly recognizing the RSV F protein antigen. In line with high levels of IgG2a antibody responses, IFN-γ was secreted at higher levels by stimulation of whole splenocytes with live RSV, F or G VLP than the level of IFN-γ with FIRSV (Supplementary Fig. S2).
Control of RSV replication by immunization with RSV vaccines
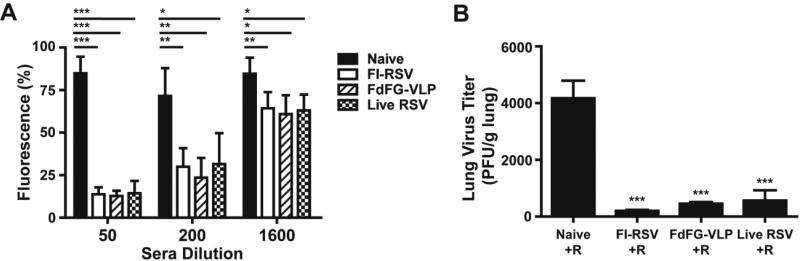
RSV neutralizing activity is considered an important protective immune correlate of RSV vaccines. To determine RSV neutralizing activity of immune sera, we performed neutralizing antibody titration by using the RSV A2 strain expressing red fluorescent monomeric Katushka 2 protein 18,19. Immune sera from the FI-RSV, FdFG-VLP and live RSV immunized mouse groups showed significantly decreased levels of fluorescent intensity compared to naïve mouse sera, indicating the inhibition of RSV infection (Fig. 2A). Up to 200 dilutions, approximately 50% decrease in RSV replication were observed in immune sera and there were no statistical significances among different vaccine immune sera (Fig. 2A). Thus, sera from FI-RSV, FdFG VLP and live RSV immunized mice exhibited high levels of RSV neutralizing activities compared to naïve sera.
Figure 2. FdFG VLP immunization induces RSV neutralizing activity and controls lung viral loads.
(A) RSV neutralizing activity in immunized sera. Data were presented as mean fluorescence percentages ± SEM. *, ** and *** indicates p<0.05, 0.01 and 0.001, respectively, by student t test. (B) Lung RSV titers from naïve and immunized mice after RSV challenge. The results were representative out of 3 independent experiments. *** indicates p<0.001 compared to the Naïve+R group.
To determine whether immunization with FI-RSV or FdFG VLP could protect mice against RSV replication, immunized or previously infected mice were intranasally challenged with RSV (1×106 PFU/mouse) at 26 weeks of post boost immunization. RSV titers were analyzed in the lung samples collected at day 5 post RSV challenge (Fig. 2B). Unimmunized naïve mice that were infected with RSV showed the highest levels of lung viral loads. RSV was detected at significantly lower levels in the lungs from mice that were previously immunized with FI-RSV or FdFG VLP, or previously infected with RSV compared to those of naïve mice (Fig. 2B). Thus, mice that were immunized with FI-RSV or FdFG VLP controlled RSV replication in lungs after RSV challenge infection.
FdFG VLP immunization prevents severe cellular infiltration into airway upon RSV infection
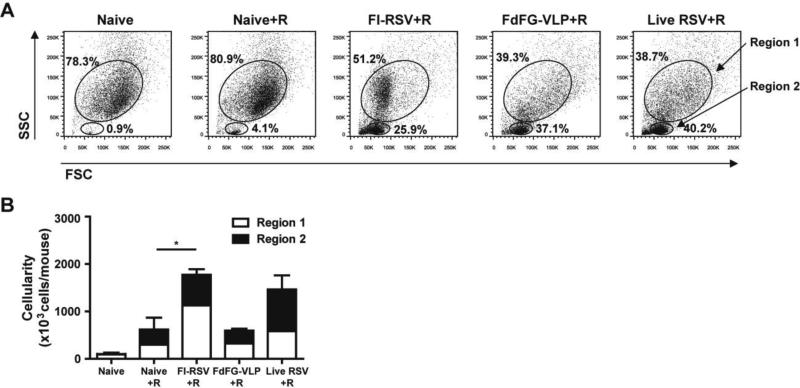
Phenotypes of immune cells contributing to RSV disease or protection are not completely defined. We determined whether FdFG VLP immunization would reduce infiltrating cells into airway upon RSV infection compared to FI-RSV immunization (Fig. 3). Naïve mice without infection exhibited low percentages (data not shown) and cellularity of lymphocytes (Fig. 3B). At day 5 post RSV infection, naïve mice showed a moderate increase in lymphocytes and cellularity (Fig. 3A, B). As expected, FI-RSV immunized mice showed the highest cellular infiltrates with large size cell populations (Region 1 gate, Fig. 3) which include granulocytes, dendritic cells, and monocytes and macrophages in bronchoalveolar cells (Supplementary Table 1, Fig. 3B). FdFG VLP immunized mice showed a lower level of cellularity in bronchoalveolar cells compared to those in FI-RSV immunized mice (Fig. 3B). Live RSV group that was previously infected two times with RSV also caused substantial levels of lymphocytes and granular/myeloid cells, which is higher than those by FdFG VLP immunization (Fig. 3B). The region 1 gated BAL cells of FI-RSV immunized mice were found to be smaller in size than those in naïve, FdFG VLP, or live RSV mice (Fig. 3A) and most cells in the region 1 gate from the FIRSV group are likely to be eosinophils (Fig. 5). Mice with FdFG VLP immunization showed a similar pattern of region 1 large cell populations as RSV-reinfected mice in response to RSV challenge (Fig. 3B).
Figure 3. FdFG VLP immunization lowers bronchoalveolar cellularity compared to FIRSV or live RSV.
(A) Flow cytometry profiles of BAL cells based on forward (size) and side (granularity) scattering. (B) Cellularity of Region 1 and 2 in BAL fluids. Cellularity was presented from the results of total BAL cell numbers per mouse multiplied by percentages of each population. The data are presented as mean ± SEM. * indicates p<0.05.
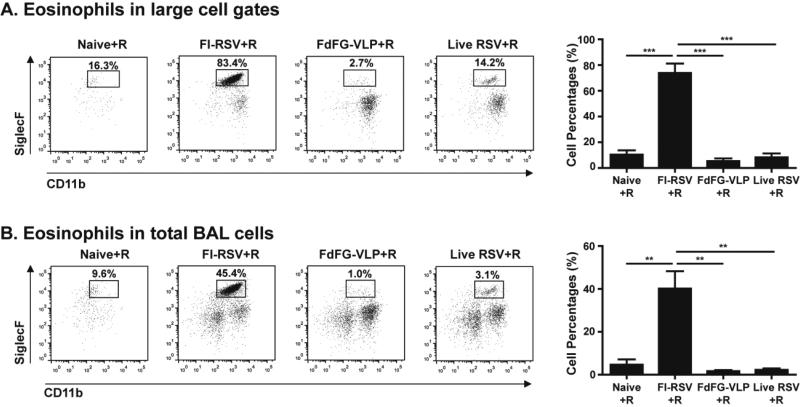
Figure 5. FdFG VLP immunization does not induce eosinophilia upon RSV challenge.
(A) Eosinophils (CD11b+SiglecF+) in CD45+CD11c− large cell gates of BAL cells. (B) Eosinophils (CD11b+SiglecF+) in CD45+CD11c− total BAL cells. The mean percentage data are presented in right panels as mean ± SEM. *, **, and *** indicate p<0.05, 0.01, and 0.001, respectively.
FdFG VLP immunization modulates innate CD11b+ and CD11c+ cells in BALF
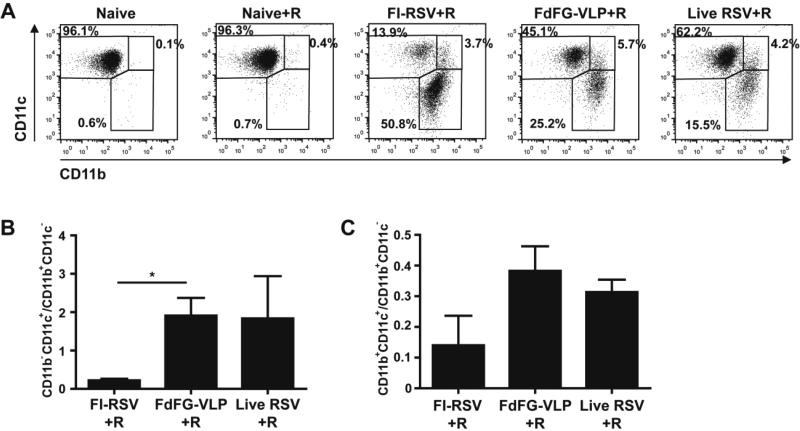
A better understanding of cellular phenotypes in BALF following RSV vaccination would be informative for determining immune responses associated with protection or lung inflammatory disease. The FI-RSV group showed a higher level of myeloid marker CD11b+ cells compared to FdFG VLP or live RSV at day 5 post RSV challenge (Fig. 4A). FdFG VLP immunization of mice induced a moderate range in levels of CD11b+ cells similar to that in the live RSV group after RSV challenge. In contrast, CD11c+ cells were found at higher levels in the FdFG VLP group compared to the FI-RSV group. That is, the FdFG VLP group exhibited a trend of increasing CD11c+ cells and lowering CD11b+ cells, which is similar to a pattern observed with the group of live RSV infection. As a result, the ratios of CD11c+ versus CD11b+ cells were highest in the FdFG VLP group whereas FI-RSV showed the lowest level of CD11c+ cells among the groups after RSV challenge (Fig. 4B, C). CD45+CD11c+ cells appeared to be alveolar macrophages with a F4/80+ phenotype (data not shown) and the majority of CD45+CD11b+CD11c− cells seemed to be eosonophils (Fig. 5). These results suggest that an imbalance in CD11c+ versus CD11b+ cells and cell types in airway might be an important parameter contributing to FI-RSV vaccination-induced lung inflammatory disease with severe infiltrates around the airways and interstitial spaces (Supplementary Fig. S3).
Figure 4. Distribution of CD11b and CD11c positive cells in bronchoalveolar lavage fluids.
(A) Flow cytometry profiles gated on CD11b and CD11c. CD45+ granulocyte/myeloid cells in BAL were gated by CD11b and CD11c expression. (B) CD11b−CD11c+ (upper-left in A)/CD11b+CD11c− (lower-right in A) ratios. (C) CD11b+CD11c+ (upper-right in A)/CD11b+CD11c− (lower-right in A) ratios. The ratios are presented as mean ± SEM. * indicates p<0.05 by student t test.
FdFG VLP immunization does not induce eosinophilia upon RSV infection
Eosinophils were demonstrated to have the phenotypes of CD45+CD11c−CD11b+SiglecF+ and enriched in inflamed lung tissues 23,24. At day 5 post RSV challenge, the FI-RSV immunized group induced prominently a population with CD11b+SiglecF+ cells, which was approximately 83% out of the CD45+CD11c− large cell gated populations (Fig. 5A) and 45% out of total BALF cells (Fig. 5B). High levels of eosinophils showed a correlation with severe infiltrates around the bronchial airways and interstitial spaces from the lung histology of FI-RSV immune mice (Supplementary Fig. S3). A low but distinct population with CD45+CD11c−CD11b+SiglecF+ cells at a level of approximately 14% (Fig. 5A) was also observed in the live RSV group after RSV challenge. Importantly, the group of mice immunized with FdFG VLP vaccine did not show such a distinct population of CD11b+SiglecF+ cells. Unimmunized naïve mice also showed CD11b+SiglecF+ phenotypic cells even at a low level after RSV infection (Fig. 5). Therefore, results in this study provide evidence that FdFG VLP immunization would not induce pulmonary eosinophilia whereas FI-RSV immunization induces severe eosinophilia and live RSV re-infections may induce a low level of eosinophils.
FdFG VLP immunization increases the ratios of adaptive CD8+/CD4+ cells secreting IFN-γ in BAL lymphocytes
Previous studies demonstrated that FI-RSV immunized mice had an increased number of CD4+ T cells infiltrating BALF after RSV challenge, which was shown to be involved in vaccine-enhanced lung disease 25. Thus, it was assumed that a reverse trend would be beneficial in preventing lung disease. CD4+ T cells and CD8+ T cells in BALF were analyzed day 5 post RSV challenge (Fig. 6). FI-RSV immunization induced high CD4+ T cells in BALF after RSV challenge. In contrast, FdFG VLP immunization resulted in approximately 3-fold lower CD4+ cellularity in BALF compared to that induced by FI-RSV immunization or by live RSV infection (supplementary Table 1). Overall, the ratios of CD8+/CD4+ T cells were relatively higher in the FdFG VLP and live RSV group than those in the FI-RSV group (Fig. 6B).
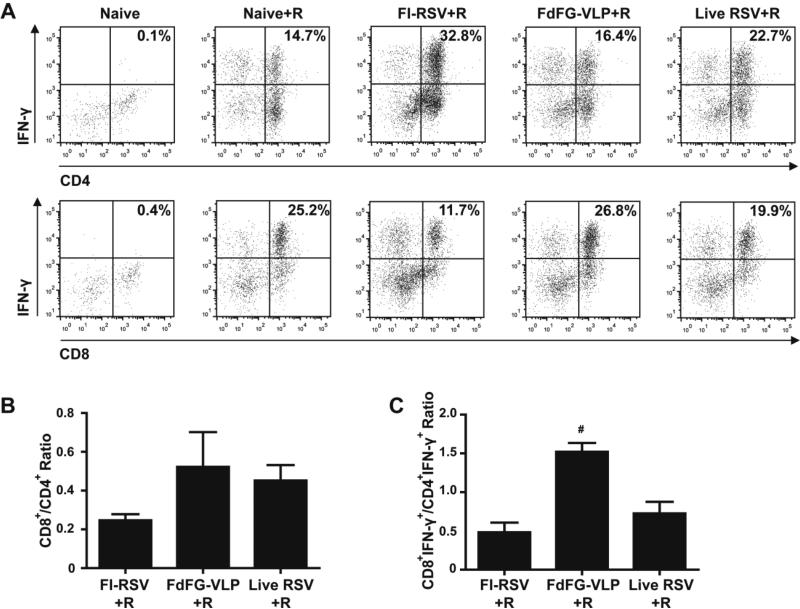
Figure 6. IFN-γ producing lymphocytes in bronchoalveolar lavage fluids.
(A) IFN-γ+ cell percentages in CD4+ and CD8+ lymphocyte (CD3+) populations. The dot plots are representative of three independent intracellular cytokine staining experiments. (B) Ratios of CD8+ to CD4+ T cells in total lymphocyte population. (C) Ratios of IFN-γ producing CD8+ to CD4+ T cells in BAL lymphocytes. Ratios are presented as mean ± SEM. # indicates p<0.05 compared to the FI-RSV+R group.
It was demonstrated that IFN-γ can have both beneficial protective and detrimental systemic disease effects 6, indicating that IFN-γ response should be balanced to avoid disease after RSV challenge. Intracellular IFN-γ cytokine staining of cells in BALF was presented (Fig. 6). FI-RSV immunization induced highest levels of IFN-γ producing CD4+ T cells (32.8% of total CD3+ T cells, Fig. 6A) whereas the FdFG VLP group showed a relatively low level of IFN-γ+ CD4+ T cells (16.4%, Fig. 6A). Since the CD4+ T cellularity in the FI-RSV group showed 3 fold higher than that in the FdFG VLP group (supplementary Table 1), the total IFN-γ producing CD4+ T cells induced by FI-RSV immunization were approximately 5- to 6-fold higher than those by FdFG VLP immunization. Interestingly, live RSV showed an intermediate level of IFN-γ secreting CD4+ T cells (22.7%, Fig. 6A). In line with high levels of IFN-γ producing CD4+ T cells and eosinophils, we found that FI-RSV and live RSV more strongly stimulated in vitro bone marrow derived dendritic cells (BMDCs) to secrete proinflammatory cytokines IL-6 and TNF-α than RSV F or G VLP (Supplementary Fig. S2).
CD8+ T cells were reported more likely to contribute to protection against RSV 26. Contrary to IFN-γ+ CD4+ T cells, a reverse pattern of IFN-γ producing CD8+ T cells between FI-RSV and FdFG VLP groups was observed in T cells from BALF after RSV challenge. The FdFG VLP group showed the highest level of IFN-γ+ CD8+ T cells (26.8% of total CD3+ T cells, Fig. 6A, C). FI-RSV immunization induced a lowest level of IFN-γ+ CD8+ T cells (11.7%, Fig. 6A, C). Interestingly, FI-RSV was not effective in IL-12 Th1 type cytokine production from in vitro BMDC cultures (Supplementary Fig. S2). An intermediate level of IFN-γ+ CD8+ T cells was observed in the live RSV group (19.9%, Fig. 6A, C). Accordingly, the ratios of CD8+ and CD4+
T cells producing IFN-γ were highest in the FdFG VLP group (Fig. 6C), indicating that FdFG VLP immunization can modulate IFN-γ secreting CD4+ and CD8+ T cells infiltrating into airway upon RSV challenge. To further determine whether FdFG VLP could modulate the expression of cytokines in lung microenvironment, we determined IL-4, IL-5, IL-13 Th2 type and IFN-γ Th1 type cytokines in BALF as well as in lung extract (Supplementary Fig. S4) after RSV challenge of immunized mice. Lung extracts from FI-RSV immunized mice showed a trend of increasing Th2 cytokines (IL-4, IL-5, IL-13) whereas FdFG VLP immunization resulted in an increase of IFN- γ production in lung milieu. A similar pattern of cytokines was observed in BALF samples (Supplementary Fig. S4). Therefore, a pattern of increasing Th2 cytokines in lungs of FI-RSV immune mice might have contributed to inflammatory RSV disease.
Discussion
Protective immune correlates are not well understood because there is no licensed RSV vaccine. In particular, cellular phenotypes contributing to protection and disease remain largely unknown after RSV vaccination. Results in this study provide evidence that FdFG VLP could confer protection against RSV by preventing pulmonary eosinophilia and modulating cellular phenotypes as well as cellularity of infiltrates and IFN-γ secreting cells in addition to inducing Th1 type antibodies and cytokines.
FdFG VLP vaccination induced antibodies recognizing RSV, predominantly binding to the RSV F protein antigen and little to the RSV G antigen. After prime immunization with FdFG VLP, higher levels of IgG2a antibodies for RSV F were observed than those for RSV G (Fig. 1C and E), indicating that RSV F is more immunogenic than RSV G and this result is consistent with those in mice that were immunized with NDV VLPs containing both RSV F and G proteins 11. After boost immunization, IgG2a antibodies for RSV G were increased. Meanwhile, IgG1 antibodies specific for RSV F were relatively increased after boost immunization. Accordingly, IgG2a/IgG1 ratios showed an opposite direction between RSV F and RSV G specific antibodies after boost immunization (Fig. 1D, F). Therefore, antibody isotype profiles and distribution between RSV F and G specific antibodies may reflect an intrinsic difference in immunogenicity and protection.
RSV F is known to be an agonist for Toll-like receptor 4 (TLR4) 27. There seems to be a certain correlation between TLR4 polymorphism and RSV disease severity 28. TLR is known to regulate host immune responses against RSV 29. High levels of IgG2a antibodies to RSV F than those to RSV G might be due to an effective stimulation of dendritic cells via TLR4 by F VLP. RSV neutralizing monoclonal antibodies targeting the RSV F protein have been licensed, making the F protein an attractive vaccine target 30. Thus, it might be desirable that FdFG VLP immunization induced antibody immune responses that are predominantly specific for RSV F. Immune responses to RSV G were shown to be effective in controlling lung viral loads 16,12, and also to cause eosinophilia and secrete Th2 cytokines31,32. Also, purified RSV F protein vaccine was shown to induce a Th2-like response33. Higher levels of IgG2a antibodies were induced by immunization with FdFG VLP. Therefore, it should be informative to determine the contributions of each RSV FdFG VLP vaccine component to RSV protection and disease. Inclusion of F DNA in the FG VLP was found to contribute to further increasing IgG2a antibody responses (Supplementary Fig. S1). An enhanced Th1-like response induced by FdFG VLP might be due to the endogenous expression of genetic RSV F DNA vaccine plus intrinsic property of a nano-particulate nature of VLP. In support of this property of VLPs, influenza hemagglutinin proteins presented on VLP induced strong IgG2a isotype and IFN-γ producing T cell responses compared to soluble hemagglutinin proteins20 .
There are some controversies regarding the efficacy of lung viral clearance in FI-RSV immunized animals. Low RSV neutralizing antibodies were reported to be induced by FI-RSV immunization12,34,35. Accordingly, low efficacy of lung viral clearance was shown in FI-RSV immunized mice12,35. In contrast, other previous studies demonstrated that FI-RSV immunized mice or cotton rats controlled RSV lung viral loads after infection36-40. It is not clear yet why lung viral clearance efficacies and RSV neutralizing titers are various among different studies on FI-RSV immunizations in animal models. FI-RSV preparation, doses of FI-RSV vaccines, animal models, and assay methods may influence the outcomes of FI-RSV vaccination efficacy despite its observed histopathology.
In this study, the total cell numbers of granulocyte/myeloid large cell populations and lymphocytes infiltrating BALF were found to be highest in the FI-RSV group. In contrast, FdFG VLP immunization did not result in significant infiltrates of leukocytes into BALF, and their bronchoalveolar cellularity was significantly lower than that by FI-RSV immunization following subsequent RSV infection. The group of mice intramuscularly immunized with FdFG VLP showed lower levels of granulocyte/myeloid cells and lymphocytes infiltrated into BALF upon RSV challenge even compared to those observed in the group of live RSV. Therefore, based on the results in this study, FdFG VLP is less likely to induce inflammatory cellular infiltrates into lungs upon RSV challenge compared to live RSV infection even at 26 weeks after immunization. FI-RSV immunization resulted in granulocyte/myeloid cells (region 1 gated cells in Fig. 3) that were smaller in size as shown by forward light scattering, and the majority (~80%) of this region 1 gated granulocyte/myeoloid cell population was found to have eosinophil phenotypic markers (CD45+CD11c−CD11b+SiglecF+). The marked increase in pulmonary eosinophilia is a hallmark of FI-RSV vaccine-enhanced disease3,41. Also, the ratios of CD11c+ cell phenotypes and CD11b+ myeloid cell phenotypes (CD11c+/CD11b+) were very low in the FI-RSV group. Whereas, the FdFG VLP and live RSV groups of mice showed larger in size but low numbers of BALF granulocyte/myeloid cells that were composed of high levels of CD11c+ phenotypic cells, and low levels of eosinophils. The FdFG VLP group showed even a lower level of eosinophils when compared to that in the live RSV group. The majority of CD11c+ phenotypic cells in mice with FdFG VLP vaccine appeared to be alveolar macrophages with a F4/80+ phenotype (data not shown) but further detailed studies should be carried out to define these cell types. The CD11c+ phenotypic cells were shown to play a crucial role in inducing Th1-polarized adaptive immune responses 42,43. CD11b+ phenotypic cells were reported to promote the recruitment of leukocytes by pro-inflammatory cytokines following infection 44,45. The high levels of CD11b+ cells in BALF may be correlated with a property of FI-RSV in stimulating BMDCs to secrete IL-6 and TNF-α cytokines but not IL-12 or IFN-γ cytokines (Supplementary Fig. S1). In contrast, F VLP was less effective in stimulating BMDCs to secrete IL-6 and TNF-α cytokines. We observed a similar pattern of cellular phenotypes in lungs but less prominent compared to those in BALF (data not shown). Further characterization of these infiltrating cells in BALF and lungs after vaccination and RSV challenge will provide an informative insight into designing a safer vaccine against RSV. The results in this study suggest that modulation of innate immune cells in BALF by FdFG VLP vaccination plays an important role in conferring protection against RSV eosinophilic inflammatory disease.
T cells are known to contribute to RSV disease as well as protection, indicating that a balance between CD4+ T cells and CD8+ T cells is important25,46. It is highly significant to note that, among groups, FdFG VLP immunized mice showed highest levels of BALF CD8+ T cells producing IFN-γ, which resulted in the highest ratio of CD8+/CD4+ T cells making IFN-γ. In contrast, FI-RSV mice exhibited highest levels of BALF CD4+ T cells producing IFN-γ, giving the lowest ratio of CD8+/CD4+ T cells making IFN-γ. Live RSV mice showed over 2 fold less ratio of CD8+/CD4+ T cells making IFN-γ compared to those in FdFG VLP immunized mice. We also found that the cellularity of BALF CD4+ T cells was highest in FI-RSV mice and then followed by live RSV mice whereas FdFG VLP mice showed relatively a low level of CD4+ T cellularity in BALF. In addition to total BALF cellularity, high levels of IL-4 secreting lung and spleen cells were detected in FI-RSV immunized mice but not in FdFG VLP immunized mice (data not shown). In support of results in this study, IFN-γ producing CD8+ T cells were shown to inhibit Th2 responses and pulmonary RSV disease47. Histopathology results of lung tissue sections suggest that FI-RSV immunization induced severe pulmonary inflammation, and moderate lung inflammation was observed with live RSV re-infections and unimmunized mice upon RSV infection (Supplementary Fig. S3). In contrast, FdFG VLP immunization did not induce such pulmonary inflammatory disease (Supplementary Fig. S3). Therefore, in addition to high cellularity in BALF, high levels of eosinophils, IL-4 and IFN-γ producing CD4+ T cells, and Th2 cytokines may be all together contributing to RSV lung disease upon infection, resulting in severe pulmonary histopathology.
In summary, we showed that FdFG VLP nanoparticulate vaccine could provide protection against RSV without causing eosinophilia. Phenotypic analysis of BALF cells suggested that FdFG VLP vaccination induced apparently balanced immune responses of CD11c+ phenotypic cells and IFN-γ producing CD8+ T cells locally. Also, high levels of CD11b+ eosinophils and IFN-γ producing CD4 appear to contribute to FI-RSV vaccination-induced pulmonary RSV disease. These results provide first evidence that RSV vaccines based on VLP in combination with F DNA genetic vaccine can be developed as an effective and safe RSV vaccine inducing protective immunity comparable or better than live RSV.
Supplementary Material
Acknowledgement
The histology core facility in the Center for Inflammation, Immunity, & Infection was supported by Georgia Research Alliance. The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Respiratory Syncytial Virus A2 F soluble protein, NR-28908. The authors thank T. Kang for editing the manuscript.
Funding information: This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.), 1R01AI087798 (MLM), and 1U19AI095227 (MLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflicts of interest.
References
- 1.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. Journal of virology. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 3.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 4.Hancock GE, Heers KM, Smith JD, Scheuer CA, Ibraghimov AR, Pryharski KS. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV). Vaccine. 2001;19:4874–4882. doi: 10.1016/s0264-410x(01)00228-6. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 6.Castilow EM, Olson MR, Meyerholz DK, Varga SM. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. Journal of virology. 2008;82:2196–2207. doi: 10.1128/JVI.01949-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. The Journal of infectious diseases. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 8.Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatric research. 2002;52:363–367. doi: 10.1203/00006450-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus research. 2009;143:140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeltins A. Construction and characterization of virus-like particles: a review. Molecular biotechnology. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. Journal of virology. 2011;85:366–377. doi: 10.1128/JVI.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. Journal of virology. 2010;84:1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, Taylor G. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. The Journal of general virology. 2000;81:2519–2523. doi: 10.1099/0022-1317-81-10-2519. [DOI] [PubMed] [Google Scholar]
- 14.Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. Journal of virology. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez X, Li X, Kovarik J, Klein M, Lambert PH, Siegrist CA. Combining DNA and protein vaccines for early life immunization against respiratory syncytial virus in mice. European journal of immunology. 1999;29:3390–3400. doi: 10.1002/(SICI)1521-4141(199910)29:10<3390::AID-IMMU3390>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. The Journal of infectious diseases. 2011;204:987–995. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. Journal of virology. 2013;87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shcherbo D, Shemiakina II, Ryabova AV, Luker KE, Schmidt BT, Souslova EA, Gorodnicheva TV, Strukova L, Shidlovskiy KM, Britanova OV, Zaraisky AG, Lukyanov KA, Loschenov VB, Luker GD, Chudakov DM. Near-infrared fluorescent proteins. Nature methods. 2010;7:827–829. doi: 10.1038/nmeth.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE, Jr., Moore ML. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology. 2012;434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–175. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirkova T, Boyoglu-Barnum S, Gaston KA, Malik FM, Trau SP, Oomens AG, Anderson LJ. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. Journal of virology. 2013;87:13466–13479. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. European journal of immunology. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 25.Waris ME, Tsou C, Erdman DD, Day DB, Anderson LJ. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. Journal of virology. 1997;71:6935–6939. doi: 10.1128/jvi.71.9.6935-6939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Stokes KL, Currier MG, Sakamoto K, Lukacs NW, Celis E, Moore ML. Vaccine-elicited CD8+ T cells protect against respiratory syncytial virus strain A2-line19F-induced pathogenesis in BALB/c mice. Journal of virology. 2012;86:13016–13024. doi: 10.1128/JVI.01770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature immunology. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 28.Puthothu B, Forster J, Heinzmann A, Krueger M. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Disease markers. 2006;22:303–308. doi: 10.1155/2006/865890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein Klouwenberg P, Tan L, Werkman W, van Bleek GM, Coenjaerts F. The role of Toll-like receptors in regulating the immune response against respiratory syncytial virus. Critical reviews in immunology. 2009;29:531–550. doi: 10.1615/critrevimmunol.v29.i6.40. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. The Journal of infectious diseases. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 31.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. Journal of virology. 2003;77:9831–9844. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 33.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 34.Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, Kleeberger SR, Wills-Karp M, Karron RA. A role for immune complexes in enhanced respiratory syncytial virus disease. The Journal of experimental medicine. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boelen A, Andeweg A, Kwakkel J, Lokhorst W, Bestebroer T, Dormans J, Kimman T. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19:982–991. doi: 10.1016/s0264-410x(00)00213-9. [DOI] [PubMed] [Google Scholar]
- 37.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. Journal of virology. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, Horswood RL, Chanock RM. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. Journal of virology. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamphuis T, Meijerhof T, Stegmann T, Lederhofer J, Wilschut J, de Haan A. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS One. 2012;7:e36812. doi: 10.1371/journal.pone.0036812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. Journal of virology. 2004;78:6024–6032. doi: 10.1128/JVI.78.11.6024-6032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Openshaw PJ, Culley FJ, Olszewska W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine. 2001;20(Suppl 1):S27–31. doi: 10.1016/s0264-410x(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 42.McAleer JP, Zammit DJ, Lefrancois L, Rossi RJ, Vella AT. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. Journal of immunology. 2007;179:6524–6535. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- 43.Gelfand EW. Development of asthma is determined by the age-dependent host response to respiratory virus infection: therapeutic implications. Current opinion in immunology. 2012;24:713–719. doi: 10.1016/j.coi.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villenave R, Shields MD, Power UF. Respiratory syncytial virus interaction with human airway epithelium. Trends in microbiology. 2013;21:238–244. doi: 10.1016/j.tim.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Ruotsalainen M, Hyvarinen MK, Piippo-Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatric pulmonology. 2013;48:633–639. doi: 10.1002/ppul.22692. [DOI] [PubMed] [Google Scholar]
- 46.Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, 3rd, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. Journal of virology. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson MR, Hartwig SM, Varga SM. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. Journal of immunology. 2008;181:7958–7968. doi: 10.4049/jimmunol.181.11.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.