Abstract
The trend in conducting successful biomedical research is shifting from individual academic labs to coordinated collaborative research teams. Teams of experienced investigators with a wide variety of expertise are now critical for developing and maintaining a successful, productive research program. However, assembling a team whose members have the right expertise requires a great deal of time and many resources. To assist investigators seeking such resources, the Indiana Clinical and Translational Sciences Institute (Indiana CTSI) created the Project Development Teams (PDTs) Program to support translational research on and across the Indiana University-Purdue University Indianapolis, Indiana University, Purdue University, and University of Notre Dame campuses. PDTs are multidisciplinary committees of seasoned researchers who assist investigators, at any stage of research, in transforming ideas/hypotheses into well-designed translational research projects. The teams help investigators capitalize on Indiana CTSI resources by providing investigators with, as needed, mentoring and career development; protocol development; pilot funding; institutional review board, regulatory, and/or nursing support; intellectual property support; access to institutional technology; and assistance with biostatistics, bioethics, recruiting participants, data mining, engaging community health, and collaborating with other investigators.
Indiana CTSI leaders have analyzed metrics, collected since the inception of the PDT Program in 2008 from both investigators and team members, and found evidence strongly suggesting that the highly responsive teams have become an important one-stop venue for facilitating productive interactions between basic and clinical scientists across four campuses, have aided in advancing the careers of junior faculty, and have helped investigators successfully obtain external funds.
In 1992, Rosenfield reported a trend in scientific research towards transdisciplinary research teams.1 Over the last several decades, biomedical research has become increasingly dependent on elucidating complex biological and disease processes through sophisticated study designs and novel technologies. The expertise required to conduct such high-impact studies rarely exists in a single laboratory and usually requires the collaboration of investigators and team members with diverse expertise.2 Such fundamental differences in the traditional and contemporary research cultures could become major barriers to developing truly impactful, translational science within and across academic institutions. Any serious attempt at the transformation of the academic research infrastructure and culture must facilitate transdisciplinary collaboration for future research to be successful.
Program Origins and Description
In 2006, as the leaders of the Indiana Clinical and Translational Sciences Institute (Indiana CTSI) were building the infrastructure and resources needed to apply for a Clinical and Translational Science Award (CTSA), they knew the institute required a component that would facilitate collaboration among investigators working in multidisciplinary teams. Specifically, the leaders wanted to address the oft-cited problem that “clinical and basic scientists don't really communicate.”2 Previously, in 2005, the Pediatrics Department at Indiana University (IU) School of Medicine and the IU Simon Cancer Center had established project development teams (PDTs), composed of clinical scientists, basic scientists, and biostatisticians, that were successful in helping investigators design and implement translational research projects. The primary investigator (PI) on the CTSA grant (A.S.) expanded these two existing programs to help facilitate research across all four campuses in the Indiana CTSI (the Indiana University-Purdue University Indianapolis [IUPUI], IU, Purdue University, and University of Notre Dame campuses).
The Indiana CTSI PDTs are multidisciplinary committees composed of seasoned researchers who assist investigators in developing ideas/hypotheses into well-designed translational research projects. Each PDT is coordinated by a chair and a project manager. The chair of each PDT is a senior faculty member who is compensated for 10% of his or her time by the Indiana CTSI to lead the team. This faculty member is responsible not only for selecting the standing members of his or her team (typically 6-8 academics) but also for making recommendations for and inviting ad hoc reviewers when needed. Project managers are staff members who devote between 25 and 50% of their time to the Indiana CTSI, depending on the PDT that they serve. These individuals are responsible for scheduling the investigators and preparing them to present to the teams. They also maintain all communications between the Indiana CTSI and the investigator as well as track the projects from application to grant submission. Most teams include basic and clinical scientists as well as members with other expertise (e.g., intellectual property). The teams function as a “one stop shop” for investigators by providing, as needed, mentoring and career development; protocol development; pilot funding; assistance with biostatistics; institutional review board, regulatory, and/or nursing support; bioethical consults; assistance with recruiting research participants; electronic medical records data mining; intellectual property support; means to engage community health; ways of collaborating with other investigators; and access to over 60 translational technology resources on the IUPUI, IU, Purdue University, and University of Notre Dame campuses. The PDTs are available to help investigators at any stage of their research from preclinical to community engagement. The PDT Program has some similarities to the innovative “Studio” program at the Vanderbilt Institute for Clinical and Translational Research3; however, some key differences set these two programs apart (see List 1).
The purpose of this article is to provide an account of the PDT Program after five years (2008/09 – 2012/13) of existence both to demonstrate its effectiveness and to share our experiences with leaders of other centers who may be interested in developing similar programs.
Overall Purpose for and Process of PDTs
As they were designing the PDT Program, Indiana CTSI leaders considered, based on their own personal experiences, several critical obstacles that investigators interested in building multidisciplinary, translational research projects face: (1) a deficiency of expert guidance and mentorship in multidisciplinary research; (2) a lack of coordinated, easy access to resources and/or to the institutional research infrastructure; (3) insufficient protected time for experts to assist new investigators; (4) a complex web of multiple regulatory submissions; (5) a lack of coordinated access to patient populations and health systems for recruiting research participants; and (6) a scarcity of readily accessible pilot funds for generating preliminary data or addressing critiques in extramural grant applications. Currently, the burden of navigating these multiple barriers lies solely or predominately on the PI. Further, designing multidisciplinary, translational, sound research is time-intensive and very laborious. Often investigators do not know how to navigate the infrastructure of their institutions to maximize their access to assistance, resources, and collaborators. The PDT Program addresses this impediment by bringing necessary resources together in a single venue through which investigators have the opportunity to present their project to a team of academics with diverse expertise.
Although we have described the PDT process elsewhere,4 we have also provided a brief summary here. To initiate PDT assistance, an investigator accesses the Indiana CTSI hub Webpage5 and then clicks on, first, the “Research resources” link and, second, the “Project Development Teams” link. This link provides access to information the investigator needs to submit a simple online application. A well-designed, complete study protocol is not required for application; the investigator can come to a team with anything from a hypothesis to a full grant proposal. No investigator is denied access to PDT assistance; rather, the application serves as a means to understand the investigator's needs and to match him or her with the most appropriate team. The project is assigned to a PDT whose members have the right expertise (Table 1), and if additional expertise is necessary members from other PDTs or ad hoc reviewers may be invited to join the lead team to provide specific expertise. Next, the project manager schedules a meeting for the PDT and the investigator. Approximately one week prior to the meeting, the PDT members receive and review the application and any other materials the investigator provides. Detailed reviews are not required prior to the meeting, so each team member usually spends about an hour preparing. While the members are reviewing the proposal materials, the investigator is putting together his/her 10 – 15 minute presentation. At the review meeting, the PI presents an overview of his or her research proposal to the PDT, and the PDT members provide the PI with advice, guidance, and information about and/or access to necessary resources, including occasionally funding. The one-hour meeting facilitates a dynamic interaction between the investigator and the PDT members. Because the teams consist of experienced researchers as well as representatives from many Indiana CTSI resources and programs, the meeting allows the PI an opportunity to efficiently gather input from multiple individuals to strengthen his or her particular project.
Table 1.
Name and description of each Project Development Team for all members in the Indiana Clinical and Translational Sciences Institute
| Translational research area | Project development team name (Site) | Team focus | Committee specific expertisea |
|---|---|---|---|
| From promising preclinical and animal studies or investigational drugs or devices into practice-based research | Concepts to Clinic (Indiana University School of Medicine) | Supports a wide range of research projects, including those on the path from basic science and/or animal models to all phases of clinical trials | Nephrology, physiology, oncology, pharmacometrics, animal modeling of disease, human genetics, neurology, psychiatry, geriatric medicine, health disparities, clinical pharmacology, pharmacogenomics |
| Human Health and Biomedical Technologies (Purdue University) | Biomedical engineering, biotechnology, nanotechnology, commercialization, basic molecular sciences, and nutritional sciences | Food science, nutrition, molecular biology, metabolomics, technology commercialization, biomedical engineering, complementary and alternative medicine, immunology, bioinformatics, computational modeling, oncology, urology, osteoporosis, community engagement, Phase 0 clinical trials, health care systems, diagnostic pathology | |
| Diagnostic and Therapeutic Development (University of Notre Dame) | Drug, vaccine, biomarker, and diagnostics development | Medicinal chemistry, biochemistry, nanofluidics, analytical chemistry, signal transduction, oncology, infectious disease, immunobiology, vaccine development, drug design, metabolomics, assay development, enzymology | |
| From investigational drugs or devices into practice-based research through to dissemination and implementation research and then back to new hypotheses for novel treatments | Behavior and Population Sciences (Indiana University School of Nursing) | Supports behavioral and population research, including epidemiological research, clinical interventions, and projects requiring providing access to patients, families, and communities. | Nursing, behavioral oncology, health services research, psychology, rehabilitation sciences, radiology, pharmacotherapeutics, psychometrics, sociology, networking, community engagement, women's health, epidemiology, public policy, dentistry, psychiatry |
| Community and Urban Health (Indiana University Purdue University Indianapolis) | Supports translational research relating to improving health outcomes and promoting sustainability in urban environments. | Pediatrics health research, epidemiology, survey research, women's health, neuroscience, psychiatry, visual communications, geography, community health engagement, public health | |
| Networks, Complex Systems, and Health (Indiana University Bloomington) | Supports network science and complex systems research with a health-related focus. | Sociology, social networking analysis, bioinformatics, biological networking, psychiatry, animal modeling of disease, toxicology, carcinogenesis, criminology, health economics, health policy | |
| Research from promising preclinical and animals studies through to dissemination and implementation research and then back to new hypotheses for novel treatments. | Pediatric Sciences and Rare Diseases (Indiana University School of Medicine) | Supports multidisciplinary clinical and translational research projects in pediatric populations | Nutrition, metabolism, endocrinology, angiogenesis, neonatology, global health, cardiology, neuroscience, psychiatry, oncology, health outcomes research |
In addition to the senior researchers on each team, all teams include a member with skill, expertise, and/or knowledge in each of the following: technology transfer, biostatistics, regulatory, nurse coordinator, bioethics, technology core resources, subject recruitment, community engagement, biobank, and bioinformatics
The Benefits of the PDT Program
After five years, the impact of the PDT on research at the Indiana CTSI institutions is emerging. Below, we present the results of surveys of PDT members and of investigators who have brought projects to a PDT. Since the formation of the Indiana CTSI during the 2008-2009 academic year, the PDT Program has reviewed 571 projects along the translational spectrum: 244 T1(translation to humans); 257 T2(translation to patients); and 70 T3(translation to practice and community). Further, the number of PDTs has increased from four to seven (Table 1) in order to provide comprehensive guidance and assistance at each of these different stages of research and to better accommodate researchers at all Indiana CTSI partner institutions.
Team mentoring
The Indiana CTSI has recognized the importance of providing team guidance both to enhance the development of research proposals and to accelerate the process of external funding procurement. As mentioned above, the Indiana CTSI leaders strategically selected members of each PDT from a variety of backgrounds and fields of expertise (e.g., nanotechnology or children's health research) as well as from key institutional programs (e.g., biostatistics or bioethics) across all 4 CTSI campuses. The PDT Program facilitates a comprehensive review of research projects by a multidisciplinary group of well-established researchers and helps PIs avoid pitfalls that may render a project less likely to attain external funding. Specifically, the PDTs have played an active role in the creation of 35 new collaborations between basic and clinical scientists between 2008 and June, 2014. The PDT Program is especially beneficial for junior investigators because it provides networking opportunities and access to potential mentors from a variety of disciplines. Notably, junior investigators are often referred to the PDT Program by their departmental chairs or other senior faculty to receive much-needed input prior to a grant submission.
The PDT Program and its interdisciplinary team mentoring approach have led to protocol design improvements and the streamlining of project milestones. All investigators who have brought research to a PDT, including those who received no pilot funds, have acknowledged these benefits. In fact, program evaluation survey results from 2013 - 2014 indicate that 72% of researchers who did not receive funding but interacted with the PDTs felt that the guidance they received had a positive impact on their research approach, and 73% made changes to their protocols in line with the feedback they received from the team. One junior faculty member conducting research in Kenya submitted a grant application that was initially unsuccessful. She met with the Behavior and Population Sciences PDT by phone while she was in Kenya and then continued to receive assistance via e-mail and other phone conferences. After incorporating much of the multidisciplinary guidance she received from the PDT and reapplying for a National Institutes of Health grant, the investigator's resubmission was funded.
Single point of access to resources
Research projects now encompass multiplexed approaches of data gathering methods. Commonly, a single study proposal will encompass several techniques and/or stages of translational research. Traditionally, the PIs bore all or most of the responsibility for finding needed resources located outside of their laboratory/research group; however, today, given the variety of techniques used in any given study, the number of resource contacts required has increased. Scientists now need to be in contact with basic as well as clinical researchers, bioinformaticians, statisticians, technology transfer agents, bioengineers, regulatory support agents, study coordinators, health economic analysts, biotech company representatives, and bioethicists (just to name a few) to plan a cutting edge translational research approach.
By engaging with the PDT, PIs no longer need to search out resource contacts. If expertise is required outside of the PDT, the project manager or one of the team members makes the initial contact for the PI and continues to be available if future assistance is needed.
The 94 investigators who took advantage of the PDT Program during the 2013 funding period reported that they received 787 “services” or program/resource connections from Indiana CTSI programs. The mean number of program/resource connections was 8.37 and the median was 8 compared to a mean of 1.96 for all investigators6 (median 1). These data suggest the PDT Program is providing an efficient process for investigators to connect with other Indiana CTSI resources.
Responsiveness
Scientific research is a dynamic process that requires flexibility and ongoing problem solving. From the initiation of the PDT Program, responding quickly to evolving investigator needs has been a key goal.
The PDT Program's responsiveness has been demonstrated in both the duration and the number of meetings for each PDT. Initially, each team determined how many times per month and for what duration they would meet. However, adjustments have been made to compensate for the high demand. For example, one of the teams initially needed to review projects only on a biannual basis, but by the end of the second year, that team adopted a rolling review process, now meeting monthly, to expedite the reviews and to enable PIs to meet external grant deadlines. Now the average timeline from application to team meeting for all investigators is approximately 4 weeks.
Further, the amount of time that elapses between the PDT meeting and distribution of the feedback to the PI is on average 12 ± 3 days. Taken together with the time from application to meeting, a PI can request PDT assistance, meet with the team, and receive feedback in approximately 6 weeks.
Breaking down institutional silos
The PDT Program has successfully connected the three major research institutions, located on four campuses, across the state of Indiana. Each campus has unique research strengths, particular patterns of investigator interactions, and a distinct institutional culture. At least one PDT is located on each campus, enabling the teams to leverage the specific research expertise and knowledge of each locale. All PDTs include members from the home campus as well as from the other universities, facilitating institutional interactions. In addition, depending on project goals, investigators can access any PDT (i.e., the one with the most relevant research expertise)—not just the PDTs located on their own campus. To date, 73 investigators have consulted a PDT beyond their campus. More importantly, institutional silos are minimized by seamlessly providing investigators at all campuses wider access to comprehensive expertise, advice, and resources such as access to pilot funding and clinical populations who may serve as participants in research. The broad and robust network of translational research initiated by the PDT Program has allowed a collaborative environment to flourish and has diminished traditional cross-institutional barriers within Indiana.
Data-driven management
From the beginning of the program, the Indiana CTSI Translational Sciences Research Officer (T.J.S.) implemented a data system to track all projects evaluated by the PDTs, using the capabilities of Research Electronic Data Capture (REDCap), built by Vanderbilt,7 for administrative and evaluation purposes. The PDT manager also created a simple entry form using REDCap so that investigators could submit applications and progress reports with minimal effort. Using this system, extensive data are systematically and consistently collected from hundreds of projects, allowing the manager to assess PDT Program progress. Milestones and metrics are developed for each PDT project that receives pilot funding; the program manager evaluates the metrics at designated reporting intervals. This close monitoring allows the program manager to quickly assist with projects that are not achieving milestones as expected. If the intervention is unsuccessful, projects (and their funding) are ended early. The REDCap system also produces information about the individual performance of each of the PDTs, as well as the overall effectiveness of pilot funding, providing a continuous evaluation mechanism for the Indiana CTSI to best manage its pilot investment.
Funding
Overall success in generating extramural funds
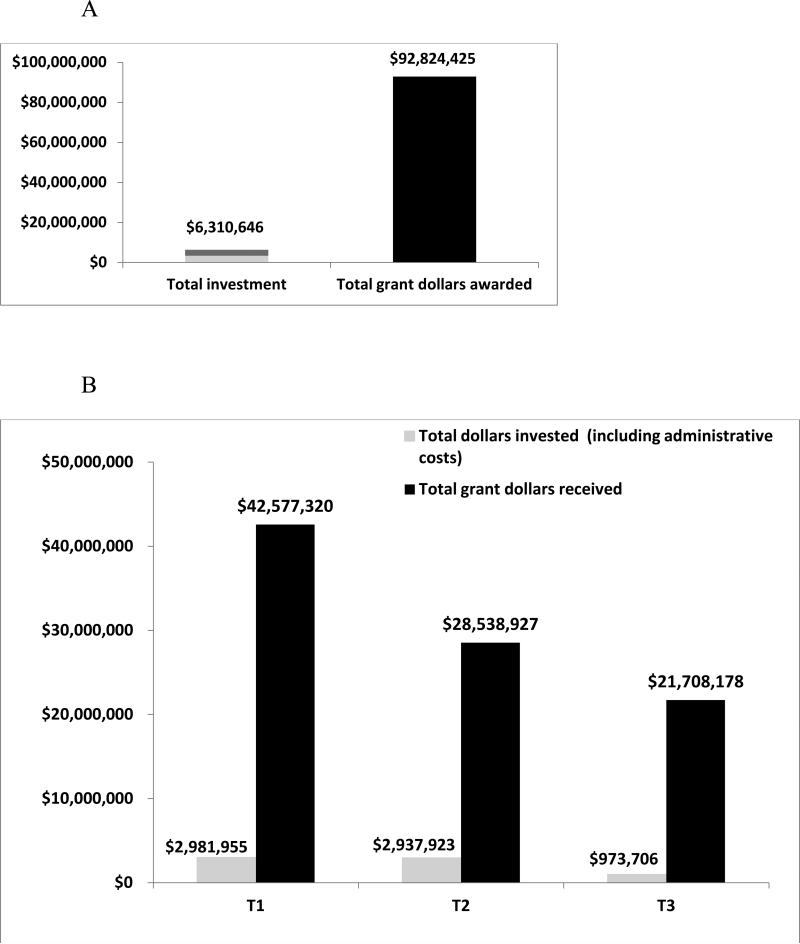
Recently, the Indiana CTSI conducted a return on investment analysis, examining the outcome of grant applications for those individuals receiving pilot funding from the PDT Program. Figure 1A shows that a total of $6,310,646 invested (May 2008- June 2014) in pilot funding and administrative costs (including PDT members’ salary support) realized a return on investment of—for every $1— more than $14 from grants in the amount of $92,824,425. The PDT has also fostered new intellectual property, including 6 licenses granted to industry, 19 disclosures filed, 28 patents (issued or pending), and 10 startup companies formed. Additionally, the PDTs have funded projects across the translational spectrum from T1 – T3. Figure 1B shows the total investment and total return for projects at each phase: T1 (244), T2 (257), and T3 (70).
Assisting junior investigators
Assistant professors have the most difficulty in obtaining external funding for research. The 2005 NIH report, Bridges to Independence, states that many investigators experience a 4- to 7-year lag between taking an academic position and receiving their first research grant.8 For many assistant professors, this lag may mean the difference between staying in research and being forced to leave. Since the publication of Bridges to Independence, research funding opportunities and budgets have diminished,9 resulting in an even more challenging and competitive research environment. Because of the special vulnerability of assistant professors, we examined the impact of the PDT Program on these investigators. Table 2 shows some of the metrics for junior faculty who received pilot funding support from the PDTs from 2008 through 2011. These data indicate that 24 (37%) of the 65 junior investigators who received pilot funding were successful in obtaining additional grant funding within an average of 18 months after their initial meeting with a PDT. This data, coupled with the average years at rank for the investigators (2.5), demonstrates that the PDT Program is able to facilitate the procurement of funding for junior faculty in just about 4 years.
Table 2.
Metrics of the Junior Faculty Using the Indiana Clinical and Translational Science Institute's Project Development Teams (PDTs)
| Metric | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|
| Average years at current rank | 2.7 | 2.1 | 2 | 3.2 |
| Number (% of investigators reviewed by PDTs) of all assistant professor investigators who were awarded pilot funds | 8/20 (40) | 26/56 (46) | 14/42 (33) | 17/35 (49) |
| Number (% of those who received pilot funds) who successfully obtained grant awards | 7/8 (88) | 9/26 (35) | 5/14 (36) | 3/17 (18) |
| Average time in months to grant award from initial PDT meeting | 19.6 | 21.6 | 17.2 | 12 |
PDT Member Satisfaction
The PDT has benefitted the Indiana CTSI (in terms of return on investment) and investigators (in terms of facilitating mentoring and leveraging resources), but also team members. Five percent of each team member's salary supports their work on the team (10% for each team chairperson). Additionally, a recent anonymous survey revealed that members derive satisfaction from being a part of a team (Table 3). The members’ satisfaction helps keep them engaged in the program and wanting to assist investigators.
Table 3.
Summary of the Project Development Team Membership Satisfaction Survey Resultsa
| Survey question | Responses, No. (%)b |
|---|---|
| How long have you been a member? | |
| <1 year | 4 (6) |
| 1 - 2 years | 18 (29) |
| > 2 years | 41 (65) |
| How many hours per week do you average per PDT project? | |
| <2 hours / week | 40 (63) |
| 2 - 4 hours / week | 19 (30) |
| 4 - 6 hours / week | 4 (6) |
| Did you feel the PDT you served on worked well as a team? | |
| Yes | 58 (91) |
| Somewhat | 6 (9) |
| Did you feel that your input made a difference to investigators? | |
| Yes | 50 (78) |
| Somewhat | 10 (16) |
| Not sure | 3 (5) |
| Absolutely not | 1 (1) |
| Have you learned from your fellow PDT members & grown as a faculty and/or researcher? | |
| Yes | 58 (92) |
| Somewhat | 4 (6) |
| Absolutely not | 1 (2) |
| Do you feel you are involved in a worthwhile service to other researchers? | |
| Yes | 60 (94) |
| Somewhat | 3 (5) |
| Not sure | 1 (1) |
| Do you feel you have a mentoring role with junior faculty that they might not otherwise receive? | |
| Yes | 40 (63) |
| Somewhat | 16 (25) |
| Not sure | 6 (9) |
| Absolutely not | 2 (3) |
| Do you enjoy being on the PDT and feel that you are making a difference in helping progress translational research? | |
| Yes | 53 (84) |
| Somewhat | 10 (16) |
| Would you like to continue being part of the pdt process? | |
| Yes | 60 (94) |
| Somewhat | 4 (6) |
Project Development Teams are part of the Indiana Clinical and Translational Science Institute. The results in this table are from a survey of team members conducted in 2013.
Not all respondents answered all questions, and percentages may not equal 100 due to rounding.
Lessons Learned and Next Steps
Since its initiation in May of 2008, the PDT Program has experienced many challenges as well as successes. First, we realized that investigators do not always recognize what is needed for a project to be successful. After the PDT meeting, the overall study design and resources necessary might significantly change, thus requiring additional PDT meetings. We have found that assigning one of the team members to be a primary contact helps to facilitate each investigator's progress between meetings. We also noted that the variation in presentations across investigators resulted in inefficient meetings. In the last year (2013), we have created and implemented a presentation template with specific headings that has greatly improved the flow and productivity of investigator-team discussions.
Probably the greatest lesson learned has been coming to understand the different cultures at each institution. One of the major goals of the PDTs is to break down institutional silos across the state. The fact that the PDTs are focused on themes means that many times we have investigators from one institution coming to a PDT at another institution, and the expectations of the team members on how the process should occur have varied. To aide in continuity, the Indiana CTSI recently assigned a “navigator” to each of the 4 campuses. The navigators stay in constant communication with one another, and at least two navigators sit as members on all PDTs. These connections represent a vital means of more fully promoting productive interactions across the three universities and four campuses.
Going forward, we hope to continue building a strong research community across the state. We understand the PDT Program may not be feasible at all institutions, at least not to the scale at the Indiana CTSI, as significant financial resources and faculty time are required yet, we have helped a variety of CTSA institutions develop and establish a smaller-scale PDT Program and hope to inspire other such collaborative programs.
Summary
The mission of the Indiana CTSI is to successfully transform clinical and translational research and improve health care across Indiana and beyond. The PDT Program has helped accomplish this mission by creating networks of multidisciplinary investigators across the three major research universities within Indiana. Over the five years of its existence, the PDT Program has expanded and continually improved to bridge the myriad disciplines that contribute synergistically to translational research. The PDT Program has accelerated the process of research from initial concept to external funding acquisition facilitating T1 to T3 research using this unique team approach.
List 1 Similarities and Differences Between the University of Vanderbilt School of Medicine's Studio Program and The Indiana Clinical and Translational Sciences Institute's Project Development Team (PDT) Program.
Similarities
Both programs...
Are designed to facilitate the development of improved research and/or science.
Involve team members with a variety of expertise.
Facilitate investigator access to the expert advice needed for developing a project.
Involve investigators-in-training as participating members of the review team, so they may gain experience.
Do not charge investigators for using the service.
Key differences
Membership
- Studio membership is ad hoc, recruited for specific projects (30% of Studio panel members participated in only one Studio panel).
- ○ Has the advantage of customized expertise to match the research.
- ○ Requires additional effort to recruit.
- ○ May add some overhead in terms of time to the meeting as members who are not familiar with one another develop an understanding of shared and divergent ideas about research.
- • The PDT Program uses fixed membership teams.
- ○ Recruitment occurs just once (participation is tracked and membership changes as needed over time).
- ○ Team members develop knowledge of shared and divergent ideas about research, which facilitates their work together.
- ○ Expertise can be added as needed.
Ability to provide funding
Studio panels do not provide pilot grant funds; investigators apply for funding through a separate process.
The PDTs may themselves provide pilot grant funding to projects deemed ready to implement.
Organization of panels
Studio panels are organized by research stage.
PDTs are organized by research stage (preclinical, clinical), by unique projects at each institution (Purdue, Notre Dame, Indiana University), by research area (behavioral, community, urban health), and by population (pediatrics, community and urban health).
Compensation
Studio members are paid an honorarium of $150 and receive a box lunch.
5% of each team member's salary and 10% of each team chairman's salary comes from PDT participation.
Figure 1.
A graph depicting (A) the return on investment (ROI) from May 2008 to June 2014 for 2008 to 2013 Project Development Team (PDT) funded projects; ROI was greater than $14 for every $1 invested, including pilot funding and PDT members’ salaries; and (B) the overall total dollars invested in PDT projects, including administrative costs and the total return on investment in grant dollars represented by translational phase (See Table 1 for translational phase descriptions)
Acknowledgments
Funding/Support: This work was supported by Indiana Clinical and Translational Sciences Award grant UL1 6RR025761 and TR000006 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Other disclosures: None reported.
Ethical approval: Reported as exempt.
Previous poster presentations: (1) Sajdyk TJ, Sors TG, Shekhar A, and Denne SC. “Project Development Teams: The Outcomes and Impacts of Accelerating Translational Science Through Team Mentoring.” Translational Science meeting abstr, Washington, DC: 2013. (2) Sajdyk TJ, Murray M, Hunt J, Shekhar A, and Denne SC. “Project Development Teams: A Novel Procedure for Facilitating T1 to T3 Translational Research in Academia.” Translational Science meeting abstr, Washington, DC: 2012.
Contributor Information
Tammy J. Sajdyk, Indiana Clinical and Translational Sciences Institute, Indiana University, Indianapolis, Indiana..
Thomas G. Sors, Bindley Bioscience Center and research navigator, Purdue University, West Lafayette, Indiana..
Joe D. Hunt, Indiana Clinical and Translational Sciences Institute, Indiana University, Indianapolis, Indiana..
Mary E. Murray, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Indiana..
Melanie E. Deford, Office of Research, University of Notre Dame, South Bend, Indiana..
Anantha Shekhar, Indiana Clinical and Translational Sciences Institute, assistant vice president for research, Indiana University, and professor of psychiatry, Indiana University School of Medicine, Indianapolis, Indiana..
Scott C. Denne, Project Development Team Program and professor of pediatrics, Indiana University School of Medicine, Indianapolis, Indiana..
References
- 1.Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Social science & medicine. 1992;35:1343–1357. doi: 10.1016/0277-9536(92)90038-r. [DOI] [PubMed] [Google Scholar]
- 2.Roberts SF, Fischhoff MA, Sakowski SA, Feldman EL. Perspective: Transforming science into medicine: How clinician-scientists can build bridges across research's “valley of death.”. Academic Medicine. 2012;87:266–270. doi: 10.1097/ACM.0b013e3182446fa3. [DOI] [PubMed] [Google Scholar]
- 3.Byrne DW, Biaggioni I, Bernard GR, et al. Clinical and Translational Research Studios: A multidisciplinary internal support program. Academic Medicine. 2012;87:1052–1059. doi: 10.1097/ACM.0b013e31825d29d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denne S, Sajdyk T, Sorkness CA, Drezner MK, Shekhar A. Utilizing pilot funding and other incentives to stimulate interdisciplinary research. In: Alving B, Dai K, Chan SHH, editors. Translational Medicine—What, Why and How: An International Perspective. Vol. 3. Karger; Basel: 2013. pp. 63–73. [Google Scholar]
- 5.Indiana Clinical and Translational Science Institute [September 4, 2014];Homepage. 2012 https://www.indianactsi.org.
- 6.Hansen D, Shneiderman B, Smith MA. Analyzing Social Media Networks with NodeXL: Insights From a Connected World. Elsevier; 2011. [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health No. Bridges to Independence: Fostering the Independence of New Investigators in Biomedical Research. National Academies Press; Washington (DC): 2005. [PubMed] [Google Scholar]
- 9.Rockey S, Collins F. Rock Talk. National Institutes of Health Extramural Nexus; Sep 24, 2013. [September 4, 2014]. http://nexus.od.nih.gov/all/2013/09/24/one-nation-in-support-of-biomedical-research/. [Google Scholar]