Abstract
Recent findings in diverse organisms strongly support a conserved role for mitochondrial ETC dysfunction in longevity modulation, but the underlying mechanisms are not well understood. One way cells cope with mitochondrial dysfunction is through a retrograde transcriptional reprogramming response. In this review, we primarily focus on the work that has been performed in C. elegans to elucidate these mechanisms. We describe several transcription factors that participate in mitochondria-to-nucleus signaling and discuss how they mediate the relationship between mitochondrial dysfunction and lifespan.
Keywords: mitochondrial electron transport chain, aging, longevity, transcription factors, retrograde signaling
Introduction
Mitochondria are essential for bioenergetics and metabolism and are central to cell viability and survival. A major function of mitochondria is oxidative phosphorylation and ATP production, which occurs through a series of electron transferring reactions via the electron transport chain (ETC) located in the mitochondrial inner membrane. Mitochondria are also major sites of several key processes, including beta oxidation, the tricarboxylic acid cycle, and apoptosis regulation. As a result, mitochondrial function is essential for maintaining cellular homeostasis and survival.
Mitochondrial function has long been linked to aging, and mitochondrial oxidative phosphorylation declines with age in diverse organisms [1]. During oxidative phosphorylation, electrons can leave the ETC and react with oxygen prematurely in the mitochondria to produce toxic reactive oxygen species (ROS). In 1972, Harman proposed the “mitochondrial theory of aging”, which suggests that ROS produced from normal mitochondrial metabolism can cause minor cellular damage, and their accumulation over time drives physiological function decline with age [2,3]. The mitochondrial theory of aging has been well accepted until recent years when cumulative observations from various model organisms started to challenge this theory. For example, inactivation of one of the five C. elegans mitochondrial superoxide dismutases, sod-2, which normally acts to detoxify superoxide, actually prolongs lifespan [4]. Moreover, mild mitochondrial dysfunction has been shown to promote longevity from yeast to mammals [4–11]. Together, these observations suggest that mitochondria influence the longevity of an organism in a more complex way than just via the production of toxic ROS molecules.
The mitochondrial ETC consists of five complexes, which are located in the mitochondrial inner membrane, and their perturbation has been shown to have disparate effects on animal longevity. For example, specific point mutations in several mitochondrial ETC subunits extend lifespan [12,13]. Similarly, genome-wide RNAi screens in C. elegans uncovered many mitochondrial ETC subunits that extend lifespan when attenuated [6,7,14,15]. However, C. elegans with mutations in some ETC subunits can also exhibit shorter lifespans [16]. A threshold model has been proposed to explain the differential longevity effects of different ETC mutations. The model suggests that reducing ETC function up to a certain threshold can be beneficial in some aspects and extend organismal lifespan. However, when ETC function is reduced below this threshold, it is detrimental and shortens the lifespan of an organism [4]. This model is further supported by RNAi experiments where different levels of mitochondrial inhibition were achieved by exposing C. elegans to different concentrations of siRNA against specific mitochondrial subunits; optimal longevity was caused by intermediate levels of mitochondrial inhibition, where as a high level of RNAi-mediated inhibition shortened lifespan [17]. It is interesting to note that in addition to lifespan changes, C. elegans with mutations in or RNAi inhibition of ETC subunits often exhibit other physiological defects, including slower development, slower feeding and defecation rates, and a reduced brood size. Therefore, lifespan extension via mitochondrial dysfunction in C. elegans has a physiological cost.
Similar findings in other organisms support a conserved role for mitochondrial ETC dysfunction in longevity modulation. RNAi inhibition of many different ETC subunits in D. melanogaster robustly extend lifespan with little pleiotropic phenotypes [10]. Additionally, two mouse mutants with compromised mitochondrial ETC function live longer [5,8]. Although the lifespan extension associated with mitochondrial ETC dysfunction is well conserved, the underlying mechanisms are not well understood. In this review, we primarily focus on the work that has been performed in C. elegans to elucidate these mechanisms, as C. elegans has been a leading and robust model system for studying aging and mitochondrial dysfunction. One way cells cope with mitochondrial dysfunction is through a retrograde transcriptional reprograming response, which has been extensively studied in yeast [18,19]. Activation of retrograde signaling has been shown to extend replicative lifespan and delay senescence in yeast, and parallel mitochondria-to-nucleus signaling pathways also exist in C. elegans [11,20]. In this review, we briefly summarize various longevity phenotypes observed in the different C. elegans ETC mutants since they have been recently reviewed [21,22]. Instead, here we focus on describing several transcription factors that participate in this retrograde signaling and ultimately how they mediate the relationship between mitochondrial dysfunction and lifespan.
C. elegans mitochondrial ETC mutants exhibit altered lifespans
Several mutations in different ETC complexes have been isolated in C. elegans, and these mutants exhibit differential lifespans. Therefore, these mutants represent powerful tools for understanding how mitochondrial function regulates organismal lifespan. The clk-1 mutant was the first long-lived mutant to be isolated. Besides a longer lifespan, clk-1 mutants exhibit a slower developmental rate, longer defecation cycle, and reduced brood size [23]. The clk-1 gene encodes a hydroxylase that is required for ubiquinone biosynthesis. During oxidative phosphorylation, ubiquinone transfers an electron from complexes I and II to complex III in the ETC. Therefore, clk-1 mutation does not directly affect mitochondrial ETC complexes but rather the electron transfer efficiency during oxidative phosphorylation. Indeed, Felkai et al. found that electron transport was reduced in mitochondria isolated from clk-1 animals. Consistently, clk-1 overexpression increased mitochondrial activity and shortened lifespan [23]. Excitingly, the role of clk-1 in longevity is conserved, as heterozygous clk-1/MCLK1 mutant mice also exhibit a longer lifespan and attenuated aging phenotypes [8,24].
Two independent genetic screens that aimed to uncover mutants with phenotypes similar to the clk-1 mutant identified two additional long-lived ETC mutants, nuo-6 and isp-1 [12,13]. The isp-1 gene encodes the Rieske iron sulfur protein and is an ETC complex III subunit, and nuo-6 encodes a conserved subunit of mitochondrial ETC complex I. Both genes confer a robustly long life in C. elegans when mutant [12]. Similar to clk-1 mutants, both isp-1 and nuo-6 mutant animals develop slower and exhibit reduced reproductive capacity and other behavioral phenotypes. Since all three of these mutations affect mitochondrial ETC function, it is not surprising that these ETC mutants consume less oxygen. However, while isp-1 mutants exhibit similar ATP levels to wildtype, both clk-1 and nuo-6 mutants actually possess increased ATP levels [13,25]. Additionally, the mechanisms that mediate the long lifespan of these ETC mutants appear to be distinct. For example, both nuo-6 and isp-1 mutants have slightly elevated mitochondrial superoxide, but clk-1 mutants have wildtype levels. Moreover, the antioxidant N-acetyl-cysteine (NAC) can suppress the lifespan extension of both nuo-6 and isp-1 mutants but not of clk-1 mutants. Together these observation suggest that the long-lived phenotypes of nuo-6 and isp-1 mutants depend on mitochondrial superoxide, whereas the longevity of clk-1 mutants might be independent of mitochondrial ROS [26].
As briefly mentioned in the introduction, not all mitochondrial ETC mutations promote longevity. In C. elegans, two mutations in mitochondrial ETC components, gas-1 and mev-1, shorten lifespan. The gas-1 gene encodes a conserved iron protein subunit of complex I of the ETC, and mev-1 encodes succinate dehydrogenase subunit c, which is part of ETC complex II. Similar to the above mentioned long-lived ETC mutants, these short-lived mutants develop slowly and exhibit reproductive defects and slower behavior phenotypes. The gas-1 and mev-1 mutations reduce complex I and complex II activity, respectively, but retain wildtype ATP levels, suggesting that mev-1 mutants either consume less energy or exhibit increased complex I activity to compensate for the complex II defect [27,28]. The short lifespan of mev-1 mutants has been proposed to be caused by increased ROS stress, as mev-1 mutants possess higher mitochondrial superoxide [27,29], and ROS can be produced by complex II of the ETC [27,30]. These observations suggest that the mev-1 mutation may directly increase mitochondrial superoxide. Consistently, as a consequence of increased mitochondrial ROS, more oxidative damage to proteins has been observed in gas-1 and mev-1 mutants [28,31].
Despite the many phenotypic similarities between gas-1 and mev-1 mutants, each mutant possesses some distinct characteristics. For example, gas-1 mutants exhibit a decreased mitochondrial membrane potential and reduced mitochondrial density, suggesting that the short life of these mutants may be due to severely reduced mitochondrial ETC function [31]. Indeed, Pujol et al. demonstrated that the gas-1 mutation caused an over compensation of complex II, which destabilized complex I and thus limited gas-1 mutant lifespan [32]. It is therefore interesting to speculate that in mev-1 mutants, as in gas-1, a compensatory upregulation of complex I or complex III activity might ensue, as RNAi knock down of the ETC complex III component cyc-1 partially restored the lifespan of these animals [20]. In addition to increased oxidative damage, developing mev-1 mutant embryos possess more apoptotic cells and fail to upregulate the anti-apoptotic gene ced-9 under hypoxia [33]. Dysregulation of apoptosis could be a cause of the short life of these mutants. Indeed, deleting the pro-apoptotic gene ced-3 restores mev-1 mutant longevity [33]. However, we did not detect altered apoptosis in adult mev-1 mutants [34].
As elaborated above, the different C. elegans mitochondrial ETC mutants exhibit distinct lifespans. These mitochondrial ETC mutants share some similar characteristics but each has their own unique properties. To date, three genome-wide studies have surveyed the transcriptional changes in response to nuo-6, isp-1, clk-1, gas-1, and mev-1 mutations [20,35,36]. These investigations revealed that a compensatory transcriptional response likely plays an important role in the longevity of these mutants. Interestingly, different transcriptional changes appear to respond to distinct ETC perturbations even when longevity outcomes are similar. For example, microarray analyses indicate that the long-lived isp-1 and clk-1 mutants share similar but also distinct gene expression profiles [20]. The genes fstr-1/2 (F57F4.3/F57F4.1) showed expression changes only in the clk-1 mutant but not in isp-1 mutant, and fstr-1/2 RNAi suppressed the long life of the clk-1 mutant but not of the isp-1 mutant [20]. The comparison of gene expression profiles between isp-1 and nuo-6 mutants revealed a significant overlap between these mutants suggesting an overlapping mechanism that regulates their lifespans. Consistent with the requirement of mitochondrial ROS signaling in mediating the lifespans of isp-1 and nuo-6, many genes that display expression changes in these mutants have also been shown to respond to pro-longevity doses of ROS [36]. Likewise, the short-lived mev-1 and gas-1 mutants also exhibit differential gene expression patterns. These data indicate that different disruptive ETC mutations can induce distinct transcriptional responses with unique physiological consequences [35]. Next, we describe several transcription factors currently known to engage in compensatory transcriptional responses in the various mitochondrial ETC mutants.
HIF-1: hypoxia inducing factor
C. elegans hif-1 encodes the mammalian HIF-1α ortholog, which is a subunit of the HIF-1 transcription factor complex that responds to reduced oxygen levels in the environment. It is important to note that wild C. elegans live in soil, which provides a lower oxygen environment than the atmosphere. Living in such hypoxic conditions has likely driven C. elegans to become tolerant of a wide range of oxygen levels, from 0% to 60% (normoxia is 10–21% oxygen). Although C. elegans can survive in a wide oxygen range, oxygen levels can modulate lifespan, as worms grown in hypoxia live longer, and worms grown in high oxygen exhibit shortened lifespans [37]. Interestingly, hif-1 mutant animals live longer when grown at 25°C. However, hif-1 mutants exhibit a normal lifespan when grown at lower temperatures suggesting that hif-1 modulates longevity in response to some specific environmental cues [38,39].
It is possible that a C. elegans-specific pathway evolved to allow these animals to adapt to a lower oxygen environment. ETC dysfunction might create a stress similar to hypoxia; therefore, dissecting the physiological and metabolic consequences of hypoxia and ETC dysfunction may further our understanding of how the mitochondrial ETC mediates longevity. The link between hypoxia signaling and mitochondrial ETC mutant longevity has been extensively explored in C. elegans. Several mitochondrial ETC mutants are more resistant to chronic oxygen deprivation [40], suggesting that a hypoxia signaling response is activated in the ETC mutants. HIF-1 activity is upregulated in isp-1 and clk-1 mutants, and hif-1 is required for their lifespan extension. Moreover, knocking down several mitochondrial ETC components using RNAi activated the HIF-1 target, nhr-57, indicative of HIF-1 activity [41]. Additionally, stabilizing HIF-1 by inhibiting its negative regulators vhl-1 and egl-9 using RNAi extended the lifespan of wildtype worms but not of the ETC mutants. Therefore, mitochondrial dysfunction-induced activation of HIF-1 activity contributes to the long life of ETC mutants [41].
Under hypoxia, where oxygen is lacking, HIF-1α cannot be hydroxylated by EGL-9, which blocks the subsequent ubiquitination by VHL-1 and therefore remains stable. Consistent with a mitochondrial dysfunction-induced role for HIF-1, it can also be activated and stabilized by ROS in C. elegans [41]. The link between ROS and HIF-1 is conserved in mammals, as human cells exhibit increased ROS levels in response to hypoxia [42–44]. Some ETC mutants, such as isp-1 and nuo-6, possess higher mitochondrial ROS, and this increase is required for their lifespan extension [26]. Increased ROS in the ETC mutants is proposed to stabilize HIF-1 under normoxia. This ROS-mediated HIF-1 stabilization might be explained by changes in the redox state of free iron in the cell. Increased cellular ROS oxidizes Fe2+ to Fe3+ in mammalian cells, which deactivates HIF prolyl hydroxylase activity and thus blocks the degradation of HIF-1α [45]. A recent study also suggested that HIF-1 has a direct role in enforcing ROS production in the ETC mutants, which is necessary for promoting their lifespan [46].
As HIF-1 is a transcription factor, its effect on longevity is likely mediated through its transcriptional targets. Shen et al. identified HIF-1 hypoxia-responsive targets by global gene expression profiling using microarrays [47]. Sixty-three of these targets are predicted to participate in signal transduction, metabolism, transport, and extracellular matrix remodeling. These data support the idea that HIF-1 helps the cell to survive under hypoxia by altering the metabolism and other crucial cellular processes. ETC dysfunction caused by isp-1 or clk-1 mutations and hypoxia were shown to share similar but also distinct HIF-1-mediated transcriptional changes in C. elegans [41]. Supporting the idea that HIF-1 might have differential targets under hypoxic and ETC stresses, we recently identified HIF-1 targets in the isp-1 mutant and showed that HIF-1 regulates distinct target genes under ETC stress [46]. In the isp-1 mutant, HIF-1 might amplify the ROS signal by suppressing the expression of the free iron chelator, ftn-1 and activating the free iron transporter, smf-3 [48]. A more thorough comparison of HIF-1 targets under hypoxia and ETC dysfunction will provide important insights into how HIF-1 modulates lifespan under these stresses.
SKN-1: C. elegans Nrf
C. elegans ortholog NF-E2-related factor (Nrf), skn-1, has also be implicated as a longevity regulator, as it is required to maintain the normal lifespan for C. elegans [49]. Similar to HIF-1, SKN-1 can be activated by ROS and is necessary for the lifespan extension in response to transient increased ROS levels. For example, a low-dose arsenite extends lifespan due to transient induction of ROS, and Schmeisser et al. demonstrated that SKN-1 is required for this extended lifespan [50]. Since the mitochondrial ETC is one of the major sites for ROS production, ETC dysfunctions often perturb ROS production. For example, both long-lived ETC mutants isp-1 and nuo-6 have increased mitochondrial ROS that serve as an important signal to promote organismal longevity [26]. Likewise, inhibiting the ETC complex I in C. elegans using chemicals also yields a ROS-dependent lifespan extension [51]. Schmeisser et al. further demonstrated that this lifespan extension also requires active neuronal SKN-1. Together, these data suggest that SKN-1 likely regulates lifespan in response to reduced mitochondrial function. The role of SKN-1 in longevity in response to specific defects in ETC is less defined due to a collection of inconsistent data across different studies. Both Rea et al. and Tullet et al. demonstrated that SKN-1 is not required for the lifespan extension observed when ETC components are knocked down with RNAi [17,52]. However, Park et al. showed that knocking down skn-1 by RNAi partially suppressed the extended lifespan of clk-1 mutants, suggesting an inhibitory role for SKN-1 in longevity [53] This discrepancy further highlights the hypothesis that defects in different parts of the mitochondrial ETC could be distinct.
SKN-1 is a well conserved transcription factor and participates in many different biological processes, including embryonic development, stress responses, and normal lifespan [49,52,54,55]. SKN-1 activity in response to the environmental and physiological cues is tightly regulated. In mammals, Nrf activity is negatively regulated by Kelch-like ECH-associated protein 1 (Keap1), which sequesters Nrf in the cytoplasm and prepares it for subsequent degradation. Although a clear Keap ortholog in C. elegans has not been identified, C. elegans WDR-23 shares a similar function with Keap, as it also negatively regulates SKN-1 [56]. Interestingly, Paek et al. discovered that a pool of SKN-1 proteins associate with the mitochondrial outer membrane and represent another mechanism that sequesters SKN-1 from the nucleus. When this interaction between SKN-1 and the mitochondrial outer membrane is disrupted, SKN-1 is constitutively active [55]. This finding provides a link between mitochondria and SKN-1 regulation and hints at a possibility that mitochondrial dysfunction might affect SKN-1 activity. Moreover, SKN-1 has been demonstrated to be the downstream target of two MAPKs, PMK-1/P38 and MPK-1/ERK [57,58]. As the activation of the MAPK pathway relies on ATP availability, reduced mitochondrial ETC function likely affects SKN-1 activation through altering the phosphate metabolism balance. Together, current data suggest that mitochondrial function likely regulates the activity of SKN-1. However, further investigation is needed to strengthen the regulatory link between mitochondrial ETC function and SKN-1 activity. As mentioned above, SKN-1 is a transcription factor with many complex roles. The transcriptional targets of SKN-1 in response to oxidative stress, reduced insulin-like pathway signaling, and during development have already been identified [52,59,60]. Identifying SKN-1 targets in response to ETC dysfunction will enhance our understanding of how this transcription factor helps the organism cope with mitochondrial dysfunction.
CEP-1: C. elegans p53
The sole p53 homolog in C. elegans, cep-1, has also been demonstrated to mediate the lifespans of ETC mutants. p53 is a major tumor suppressor with a variety of conserved roles, including its well-characterized pro-apoptotic function. Abrogation of cep-1 on its own has been shown to slightly increase worm lifespan [61], and cep-1 transcripts actually decrease over time in wildtype aging animals, [62], so the relationship between the absence of cep-1 and longevity is unclear. Ventura et al. first observed that cep-1 was required for the different lifespan outcomes of animals that exhibited varying degrees of mitochondrial dysfunction. A mild RNAi inhibition of several mitochondrial components, atp-3, cco-1, and isp-1, prolonged the lifespan of wildtype worms, which was abrogated upon cep-1 mutation. Conversely, cep-1 mutation increased the lifespan of animals that lived shorter lives when these same components were more severely knocked down using more concentrated RNAi [63]. Recently, we demonstrated that cep-1 mutation decreased the lifespan of the long-lived ETC mutants isp-1 and nuo-6 but increased the lifespan of the short-lived ETC mutants mev-1 and gas-1 [34]. These results harken back to the observations by Ventura et al. (2010) that suggested that cep-1 responds to different mitochondrial stress levels in disparate ways. To identify mediators of these distinct responses, we compared the CEP-1-regulated transcriptional profiles of isp-1 and mev-1 mutants, which surprisingly yielded a large overlap [34]. However, a closer analysis revealed that a small subset of genes were, in fact, differentially regulated between these two mutant backgrounds. As this group was enriched for the “aging” Gene Ontology term, we proposed that our analysis successfully identified genes with functions important for longevity. We confirmed that one of these candidates, the iron transporter ferritin (ftn-1), indeed exhibited different CEP-1-mediated regulation between the long-lived and short-lived mutants. Importantly, RNAi-mediated knockdown of ftn-1&ftn-2 (a close homolog of ftn-1) only partially attenuated the lifespan of isp-1 mutants but had no effect on mev-1 lifespan, indicating that cep-1 mediates isp-1 lifespan uniquely via ftn-1 regulation and likely additional channels [34].
Iron homeostasis may represent an important CEP-1-mediated process that potentiates longevity. Neither knockdown or overexpression of ftn-1 in wildtype animals alters lifespan [34,64], so its partial suppression of the long lifespan of isp-1 animals may be due to the already iron-sensitized isp-1 mutant background, as ISP-1 is a Rieske iron sulfur protein. Interestingly, RNAi-mediated knockdown of the nuclear-encoded mitochondrial protein frataxin (frh-1) alone extends the lifespan of wildtype animals [65]. Frataxin mediates iron–sulfur cluster formation as well as mitochondrial iron bioavailability and defective frataxin function is the main cause of Friedreich’s ataxia in humans. Notably, cep-1 mutation can partially suppress the longer lifespan of animals treated with frh-1 RNAi [63], which may indicate that CEP-1 responds to iron homeostasis distress and that this response promotes longevity in an iron-stressed environment. This hypothesis is consistent with the observation that cep-1 is required for the induction of ftn-1 expression upon iron stress (i.e., in isp-1 mutants) but does not affect ftn-1 expression in the absence of iron stress (i.e., in mev-1 mutants) [34]. Apart from iron homeostasis genes, analysis of the other genes that were differentially changed between the CEP-1-regulated transcriptional profiles of isp-1 and mev-1 mutants should uncover aging-related functions for genes that have yet to be ascribed those roles.
In addition to studying CEP-1 targets and their impact on longevity, understanding what occurs upstream of CEP-1 could also uncover how CEP-1 potentiates distinct lifespan outcomes in response to mitochondrial dysfunction. Genes that encode proteins that participate in ROS-generating or ROS-responsive mechanisms represent particularly good candidates for further investigation, as ROS are well-known activators of p53 [66]. ROS can activate p53 indirectly via oxygen radical-induced DNA damage that in turn unleashes p53-mediated pro-apoptosis or pro-antioxidant responses. ROS can also regulate p53 activity directly by interacting with p53’s redox-sensitive Cysteine residues. These residues reside in p53’s DNA-binding domain and affect the ability of p53 to specifically recognize the consensus sequence in its targets [66]. Importantly, the residues that govern DNA binding are conserved between p53 and CEP-1 [67]. While several mitochondrial ETC mutants are known producers of ROS, and therefore p53/CEP-1’s role in mediating their lifespans may not be surprising, how CEP-1 responds to different ETC mutants to yield disparate lifespan outcomes remains to be clarified.
CEH-23: C. elegans homeobox protein
We identified ceh-23 through an RNAi screen for suppressors of the long lifespan of isp-1. Inactivating ceh-23 by mutation or RNAi partially suppresses the long-lived phenotype of isp-1 mutant animals but does not shorten the lifespan of wildtype worms or other mutants that live longer due to perturbations distinct from the mitochondrial ETC. Therefore, ceh-23 mediates part of the lifespan extension in ETC mutants, but its absence does not affect the general health of the organism. More interestingly, inactivating ceh-23 exclusively shortened the lifespan of ETC mutants without affecting their developmental or reproductive phenotypes [68]. Thus, ceh-23 activity uncouples longevity from the other pleiotropic phenotypes associated with mitochondrial ETC inhibition. We found this to be extremely interesting as it provides hope for identifying mechanisms specific to the longevity role of the mitochondrial ETC that are distinct from its essential energetic role. Consistent with a possible central role of CEH-23 in longevity, overexpression of ceh-23 is sufficient to prolong lifespan in wildtype worms [68].
The CEH-23 protein contains a highly conserved homeobox domain. Homeobox proteins are predicted to bind DNA and act as transcription factors [69]. Consistent with a transcription function, CEH-23 is expressed in the nucleus of both neuronal and intestinal cells [68]. Unlike HIF-1 and CEP-1, little is known about how CEH-23 is regulated or the identity of its downstream targets that are important for longevity. The only known role of ceh-23 in C. elegans is a possible function in AIY neuron differentiation. ceh-23 expression is induced by TTX-3 in the AIY neuron, and elevated ceh-23 expression is required to maintain one of the AIY differentiation markers, sra-11 [70]. However, no functional defects in the AIY neurons have been detected in ceh-23 null mutants. Interestingly, ablation of the AIY interneurons shortened the lifespan of wildtype and long-lived daf-2 mutant worms, which positions the AIY neuron as an important determinant of longevity. However, the CEH-23-mediated regulation of SRA-11 is unlikely to be important for the longevity of ETC mutants, as sra-11 mutants do not exhibit a longer lifespan [71]. We previously observed higher ceh-23 expression in the ETC mutants using both a transcriptional reporter and RT-qPCR [68], indicating that ceh-23 might respond to mitochondrial dysfunction. However, it is unclear whether the regulatory circuit observed during AIY neuron differentiation is also responsible for the up-regulation of ceh-23 in ETC mutants or if another signaling pathway activates ceh-23 expression in the ETC mutants. Nevertheless, the neuronal and intestinal expression of CEH-23 is particularly interesting in the context of data indicating that mitochondrial ETC inhibition in either of these tissues alone is sufficient to alter the lifespan of the entire organism [72]. Further elaboration of CEH-23 functions will likely illuminate how neuronal CEH-23 might respond to mitochondrial dysfunction to modulate overall longevity.
UBL-5, DEV-1, ATFS-1: key regulators of mitochondrial unfolded protein response
The lifespan of several ETC mutants is also mediated by the transcription factors UBL-5, DVE-1, and ATFS-1, which govern the mitochondrial unfolded protein response (UPRmt), a mechanism that monitors protein homeostasis and maintains proper protein function, in this case, specifically in the mitochondria [72,73]. Upon mitochondrial dysfunction, UBL-5, a ubiquitin-like protein, and the transcription factor DVE-1 are translocated from the cytoplasm into intestinal nuclei and form a complex [74,75]. Reduced ubl-5 and dve-1 levels via RNAi abrogated the longer lifespans of the ETC mutants isp-1 and clk-1 (albeit dve-1 RNAi also reduced the lifespan of wildtype animals) [72]. These long-lived ETC mutants also rely on the cytoplasmic bZip transcription factor ATFS-1 to mitigate their mitochondrial stress. These mutants do not tolerate the absence of atfs-1 and cannot develop when grown on atfs-1 RNAi [76]. Given that ATFS-1 harbors both a nuclear and mitochondrial localization signal, it is predicted to be a critical toggle between a stressed and unstressed state, where it is shuttled to either the mitochondria or the nucleus depending on the absence or presence of the UPRmt, respectively [76]. A perturbed UPRmt is also deleterious for the short-lived ETC mutant mev-1, where RNAi-mediated knock down of dve-1 and ubl-5 even further reduced the lifespan of these animals [77]. Notably, the UPRmt in C. elegans can be visualized by the induction of hsp-6::gfp and hsp-60::gfp—two mitochondrial chaperones, and all three of these transcription factors are required for this induction [73].
Although UPRmt components clearly contribute to the lifespan-defining mechanisms of ETC mutants, it remains unclear how an activated UPRmt can result in distinct lifespan outcomes in response to different ETC perturbations. For example, while short-lived gas-1 mutants display increased hsp-6::gfp expression, so do long-lived nuo-6 mutants, albeit at lower levels compared to gas-1 [32]. Interestingly, a recent study demonstrated that the degree of hsp-6::gfp induction actually correlated with the extent of lifespan increase in worms inhibited for various mitochondrial ribosomal proteins (mrps) [78]. In this study, RNAi of mrps-5 extended the lifespan of C. elegans mev-1 mutants but not of cco-1 mutants. Because mev-1 mutants did not display a stoichiometric imbalance between the nuclear and mitochondrial-encoded oxidative phosphorylation subunits but cco-1 did, and, consistently, mev-1 RNAi did not induce hsp-6::gfp expression but cco-1 RNAi did, the authors proposed that mrps knockdown extended the lifespan of mev-1 mutants by inducing a UPRmt specifically via perturbing the nuclear- to mitochondrial-encoded protein ratio of the mitochondrial protein complexes [78]. However, other studies suggested that mev-1 mutants did exhibit increased hsp-6::gfp expression [72,79] (Durieux et al. 2011; Runkel et al., 2013). Thus, how important the mitochondrial protein imbalance is in activating the UPRmt and its effects on lifespan merit further investigation.
Several proteins mediate the signaling between unfolded/misfolded mitochondrial proteins and nuclear-encoded gene expression characteristic of the UPRmt. Upon mitochondrial protein stress, the ClpP protease, in concert with its binding partner ClpX, degrade the perturbed mitochondrial proteins [75,80]. The transporter HAF-1 then transports these degraded peptides outside of the mitochondrial matrix [80]. This initiates a yet-to-be thoroughly defined signaling cascade that promotes the relocalization of UBL-5 [74], DVE-1, and ATFS-1 [75,80]. In parallel to the UPRmt pathway, the eIF2α kinase GCN-2 has also been demonstrated to be required for the longevity of clk-1 mutant animals [81]. Interestingly, instead of abolishing hsp-60::gfp expression, which would be consistent with the requirement of GCN-2 in clk-1 mutant lifespan, gcn-2 deletion in clk-1 mutants actually further increased hsp-60::gfpexpression. Even more curious, knocking down the eIF2α phosphatase gsp-1, which acts in antithesis to the gcn-2 kinase, also attenuated the lifespan of clk-1 mutants as well as hsp-60::gfp expression. Therefore, at least in this context, hsp-60::gfp induction (i.e., UPRmt induction) does not simply correlate with a positive outcome (lifespan extension) in the presence of ETC stress.
Additional factors have been documented to induce the UPRmt. For example, paraquat induced both hsp-60::gfp and sod-3::gfp (a marker of ROS accumulation) expression in worms [73,79]. A recent investigation by Runkel et al. into the paraquat-induced UPRmt revealed that ATFS-1 was required for this hsp-60::gfp induction but HAF-1 was not. Further, treating animals with the ROS scavenger NAC reduced the paraquat-induced expression of hsp-60::gfp by 75%, suggesting that, whether directly or indirectly, ROS induces a UPRmt [79]. Interestingly, when animals were treated with acrylamide, a compound that induces cytoplasmic but not mitochondrial ROS, hsp-6::gfp expression barely increased by 1.5-fold, further clarifying that ROS generated in the mitochondria are likely necessary to activate UPRmt [79]. Conversely, hsp-60::gfp expression was actually induced, and not repressed, when clk-1 and isp-1 mutants were treated with the ROS scavenger as corbate in another study [81]. Moreover, NAC treatment did not attenuate the UPRmt and the longevity of mrps-5 mutants, also suggesting that these mechanisms are ROS-independent. Therefore, while it appears that ROS and mitochondrial protein stress share similar stress-response mechanisms, their interactions are not trivial and can even be uncoupled.
The components of the UPRmt summarized above largely work cell-autonomously. Intriguingly, UPRmt can also be induced via non-cell-autonomous signaling that may be distinct from cell-autonomous signals. Knock down of the cytochrome c oxidase-1 cco-1 in the neurons alone induced hsp-60::gfp expression in the intestine and increased organismal lifespan [72]. A “mitokine” signal was proposed to be induced upon mitochondrial dysfunction in neurons and then transmitted to other cells in the animal, including the intestine, to coordinate a systemic compensatory response. Although ubl-5 is required for the long life and UPRmt induction of cco-1 mutants, these phenotypes were not affected when ubl-5 was knocked down in intestinal cells in animals that were also neuronally depleted for cco-1. While a neuronal signal can induce intestinal UPRmt with pro-longevity effects, this signal does not rely on UBL-5, which appears to function exclusively in a cell-autonomous manner in the intestine [72]. Further elaboration of the signaling components mediating this non-cell-autonomous stress signal will be essential for a comprehensive understanding of how an organism copes with mitochondrial protein homeostasis.
Mitochondrial ETC dysfunction induces complex transcriptional networks
Although we have discussed the activities of several transcription factors separately, it is likely that at least some of these factors coordinate and act in a transcriptional network to respond to mitochondrial ETC dysfunction. For example, HIF-1 can inhibit DNA damage-induced apoptosis and CEP-1 activity in the germline [82]. Whether HIF-1, CEP-1, CEH-23, the factors that mitigate the UPRmt, and others collaborate in response to mitochondrial dysfunction awaits further investigation.
Consistent with a complex transcriptional network, the link between mitochondrial dysfunction and longevity is highly dependent on temporal and spatial regulation. Partial inhibition of mitochondrial ETC function either by RNAi knockdown or pharmacological intervention must occur prior to the fourth larval stage of development to confer a longer lifespan in worms [6,17]. And while ETC inhibition in adulthood effectively reduced respiration and ATP production, it had no effect on longevity [6]. Furthermore, using a genetic trick to limit the RNAi-mediated inhibition of the ETC only during development, but not after reaching adulthood, also extended lifespan robustly [6]. Collectively, these data suggest that inactivation of ETC components during development is crucial for longevity determination, and the signal initiated by the ETC stress maintains its effect later in life even if the original stressor no longer exists. It is important to note that the ETC inhibition timing requirement coincides with massive mitochondrial biosynthesis that accompanies germline development, suggesting that ETC dysfunction at this stage might exert the most potent effect. Looking forward, it will be important to determine whether and how the transcription factors and pathways we have discussed here respond to mitochondrial dysfunction in a temporal-specific manner.
Tissue-specific mitochondrial function is also important for longevity determination. Neuronal and intestinal tissues are key for the lifespan extension in ETC mutants [72]. For example, inducing mitochondrial ETC dysfunction only in neurons or the intestine was sufficient to promote the lifespan of the entire organism, whereas the same ETC defect in the body wall muscle did not significantly alter lifespan. Similar findings also hold true in D. melanogaster [83]. In C. elegans, some of the transcription factors that we have discussed exhibit distinct expression patterns. For example, CEH-23 is expressed in the neurons and intestine [68], CEP-1 is expressed in pharyngeal cells and the germline [84], and HIF-1 is ubiquitously expressed in all somatic cells [85]. To clearly delineate the cell-autonomous and non-cell-autonomous compensatory responses of mitochondrial ETC dysfunction, the cell types/tissues where the various factors we have discussed, as well as others, act in to modulate longevity must be investigated in the future. Given that ETC dysfunction can trigger both cell-autonomous and non-cell-autonomous compensatory responses with relatively strict temporal and spatial requirements [72], the existence of a complex and collaborative transcriptional network is likely.
While we have focused our review on the transcription factors HIF-1, CEP-1, CEH-23 and the UPRmt inducers, emerging research suggests that many more transcriptional regulators might respond to mitochondrial dysfunction and affect lifespan. Indeed, two independent RNAi screens identified a total of nine additional transcription factors that likely participate in longevity outcomes in response to ETC perturbation [68,86]. Therefore, further investigation of how these promising new candidates act singly or cooperatively with known transcription factors will likely lead to fruitful insights into the link between mitochondrial dysfunction and longevity in C. elegans.
Dietary restriction likely modulates lifespan through a mechanism distinct from that in ETC mutants
Dietary restriction (DR) has been shown to extend the lifespan of various organisms [87]. Despite the conflicting findings of its effects on the longevity of rhesus monkeys, the health benefits of DR have consistently been observed [88–90]. In mammals, the effects of DR on mitochondrial function have been studied extensively. DR has been shown to attenuate mitochondrial ROS emission from ETC complex I [91–94], a poignant finding given that increased ROS levels are one of the hallmarks of aging. Although there have been conflicting data regarding how DR impacts mitochondrial proton leak [95–98], recent evidence suggest that DR might in fact promote the efficiency of mitochondrial respiration [99–102, also reviewed in 103]. Lastly, DR has been shown to reduce the sensitivity of mitochondria to apoptosis stress, where less apoptosis is observed in DR animals [104,105] Together, these mammalian studies suggest that the longevity effects of DR might depend on altering mitochondrial functions. Consistent with this notion, the mitochondrial protein deacetylase SIRT3 can be activated by an increased NAD+/NADH ratio under DR conditions in mammals [106–108]. Furthermore, deacetylation of several factors has been demonstrated to mediate the beneficial effects of DR. For example, deacetylation of SOD2 is thought to reduce overall ROS in DR animals [109], and deacetylation of the mitochondrial protein cyclophilinD in DR animals lowers their sensitivity to apoptosis by delaying the opening of the mitochondrial permeability transition pore (mPTP), which is a key step towards inducing apoptosis [105].
In C. elegans, dietary restriction can be implemented using various regimens, including dilution of bacterial food on agar plates or in liquid culture, or complete bacterial deprivation post reproduction, all of which extend the organismal lifespan robustly but are thought to act through overlapping yet distinct pathways [110]. Similar to the mammalian models, several DR interventions in C. elegans have been shown to increase mitochondrial respiration [111–113], suggesting a conserved role for DR in mitochondrial function. Contrary to the effects of DR on mitochondria, the ETC mutants described in this review exhibit moderately reduced respiration and mitochondrial function. Several genetic studies suggest that although both DR and moderate ETC dysfunction prolong lifespan, the longevity phenotypes are likely mediated through distinct molecular players. For example, the eat-2 mutant is commonly used to study DR in C. elegans. eat-2 encodes a subunit of a ligand-gated channel of the pharyngeal muscle, thus eat-2 mutants exhibit a slower pharyngeal pumping rate that reduces food intake [114]. Consistent with a role for DR in longevity, eat-2 mutants live longer. Notably, the eat-2 mutant lifespan is further increased with the addition of the ETC mutation nuo-6 (qm200), suggesting that eat-2-mediated DR can act additively with mitochondrial dysfunction to prolong lifespan [13]. Moreover, the extended lifespan of eat-2 mutants requires the transcription factor pha-4, but the long-lived phenotype of isp-1 mutants does not [115]. However, the relationship between mitochondrial dysfunction and DR in mediating lifespan is complicated by the epistatic relationship between clk-1 and eat-2 mutations. Unlike the isp-1 and nuo-6 mutants, the clk-1 mutation seems to activate a similar pathway to the eat-2 mutation, as the clk-1 (e2519) mutation does not further lengthen the lifespan of eat-2 mutants [114]. As discussed in the previous section, clk-1 mutants have phenotypes that are quite distinct from the other ETC mutants. The epistatic relationship between clk-1 and eat-2 further supports the idea that clk-1 mutations trigger a longevity mechanism that might be distinct from other ETC mutations.
While the mitochondrial ETC longevity and DR longevity pathways appear to be distinct, they both promote the activity of the transcription factor skn-1 in C. elegans. SKN-1 is required for the extended lifespan of DR worms when they are cultured in liquid media with diluted bacteria as the food source [111]. Moreover, one of the skn-1 targets identified by Park et al., nlp-7, is required for the prolonged lifespan of the eat-2 mutant [53], which reinforces the pivotal role of skn-1 in DR-mediated longevity. As previously discussed, while the roles of SKN-1 in the longevity of ETC mutants are still controversial, the activity of SKN-1 is likely to be induced by mitochondrial ROS in the ASI neurons under ETC stress [51]. Interestingly, under conditions of glucose deprivation, increased mitochondrial respiration yields increases in ROS production, which is essential for the extended lifespan associated with this type of glucose deprivation-mediated DR [113]. Notably, SKN-1 activation and its requirement for prolonged longevity is only observed in some DR regimens but not all, suggesting that food availability plays a more complex role in lifespan than originally anticipated. How skn-1 affects longevity in response to ETC dysfunction is equally complicated as discussed in the previous section. A comparison of the activities of skn-1 under both ETC stress and DR stress may provide a more thorough look at the contribution of skn-1 in modulating longevity in response to these perturbations.
Mammalian mitochondrial dysfunction and aging
Mitochondrial dysfunction in mammals has largely yielded detrimental effects. In humans, inherited mitochondrial respiratory chain disorders encompass a large spectrum of clinical symptoms, including muscle weakness, neurological disorders, and lactic acidosis [116]. These disorders occur approximately 1 in 5,000 live births [117]. Furthermore, mitochondrial DNA itself accumulates mutations over time, and this has been proposed to be a cause and not an effect of aging using mouse models deficient for mtDNA polymerase proof reading activity. These mice not only lived shorter lives but also displayed premature aging-related pathologies in multiple tissues [118]. Mitochondrial dysfunction has also been observed in a variety of age-related human diseases, including neurodegeneration, type II diabetes, and cancer, where defects in nuclear-encoded proteins are suspected to contribute to mitochondrial dysfunction and thus some of the observed disease symptoms [119]. Given all these, how, if at all, could findings in C. elegans inform adaptive responses upon mitochondrial dysfunction in mammals?
Since, as discussed earlier, the age-dependent decline of mitochondrial ETC function is evolutionarily conserved, the presence of mitochondrial dysfunction-related aging pathologies in mammals is consistent with what we see in C. elegans. Furthermore, several mouse models of ETC dysfunction support the idea that mitochondrial ETC function also modulates lifespan in mammals. Similar to what has been observed in C. elegans, reduced function of the ubiquinone biosynthesis enzyme CLK-1/MCLK1 in mice causes reduced mitochondrial ETC function and extends lifespan [8,24,120]. While heterozygous loss of MCLK1 induces long life, homozygous loss of MCLK1 is lethal, reminiscent of the threshold model observed in C. elegans ETC mutants. In another model, Hughes et al. constructed RISP (+/P224S) mice to mimic the C. elegans isp-1 (qm150) mutation [121]. Similar to the MCLK1 model, the homozygous RISP mutant mice are not viable. Decreased function of RISP in mice reduces mitochondrial respiration in a substrate-dependent manner, where only the electron transfer from complex II to III to IV is affected. Interestingly, RISP (+/P224S) mice have a gender-specific longevity phenotype: only RISP (+/P224S) males are short-lived. Even more curious, RISP (+/P224S) females that survive past the median wild-type lifespan live slightly longer than wild-type mice. This suggests that RISP heterozygosity is mildly protective for female mice later in life. The beneficial effect of RISP (+/P224S) is reminiscent of the pro-longevity effect of the isp-1 mutation in C. elegans. Lifespan data for isp-1 mutants, and for most lifespan experiments, were collected using hermaphrodites, so a possible gender-specific effect would likely be overlooked in C. elegans.
In addition to MCLK1 and RISP mutant mice, mice with dysfunctional SURF1 are also used to study aging. One type of SURF1 mouse model harbors a prematurely truncated and highly unstable SURF1 protein, SURF1loxP [5]. As SURF1 encodes an assembly protein that is important for cytochrome c oxidase (complex IV) formation, SURF1loxP mice have reduced cytochrome oxidase activity and a long lifespan. In humans, SURF1 has been implicated in Leigh syndrome, which is typically caused by mutations that disrupt mitochondrial function. Moreover, patients with a cytochrome c oxidase deficiency usually have mutations in SURF1. While SURF1loxP mice exhibit lifespan extension in response to a mild reduction in ETC function, another SURF1 knockout mouse model exhibits the opposite longevity phenotype: SURF1neo mice, which carry an allele of SURF1 with a neomycin-resistant cassette [122], are actually short-lived. Curiously, these mice exhibit reduced cytochrome c oxidase activity similar to the SURF1loxP mice. Besides the short-lived phenotype, SURF1neo mice also exhibit high embryonic lethality, which is not observed in SURF1loxp mice. These inconsistencies may be due to an artefact of using a NEO cassette, as explained in Dell’Agnello et al., who pointed out that the SURF1loxP-NEO-loxP mice also have high embryonic lethality [5]. Taken together, several mouse models with ETC dysfunction described here demonstrate that reduced mitochondrial ETC function can also lead to lifespan extension in mammals, suggesting a possible conserved underlying mechanism between worms and mice.
In addition to the effects on lifespan, the broad spectrum of pathological manifestation, from relatively asymptomatic to severely debilitating, of mitochondrial respiratory chain disorders echoes the pleiotropy of C. elegans mitochondrial ETC mutants. In humans, mitochondrial DNA heteroplasmy, or a mixture of mutated and normal DNA within one cell, is one phenomenon that can account for these differences [123]. In these cases, penetrance depends on whether the presence of mutated mtDNA reaches a certain threshold in one or more tissues to manifest in a disease phenotype. Such a threshold phenomenon is reminiscent to the observations in C. elegans with dysfunctional mitochondria, where some ETC mutants live shorter than normal, whereas others live longer than normal. Furthermore, it has been observed that dietary intake and genetic background of individuals influence final phenotypic outcomes of human patients and mammalian models of mitochondrial dysfunction. Findings from C. elegans therefore could illuminate the cellular and organismal compensatory responses that determine the phenotypic manifestations [26,44,79].
The emerging data reviewed here indicate that cells and organisms have a large capacity to respond to mitochondrial stress, and different degrees of mitochondrial dysfunction likely induce compensatory responses that lead to divergent phenotypic outcomes. While C. elegans and mammals have very different physiologies, they likely share common cellular and organismal adaptive signaling responses to dysfunctional mitochondria. Insights gained from further analysis of the mechanistic basis of these responses in C. elegans and other model systems could be harnessed to provide therapeutic opportunities aimed at improving diverse mitochondrial disorders and possibly age-related diseases.
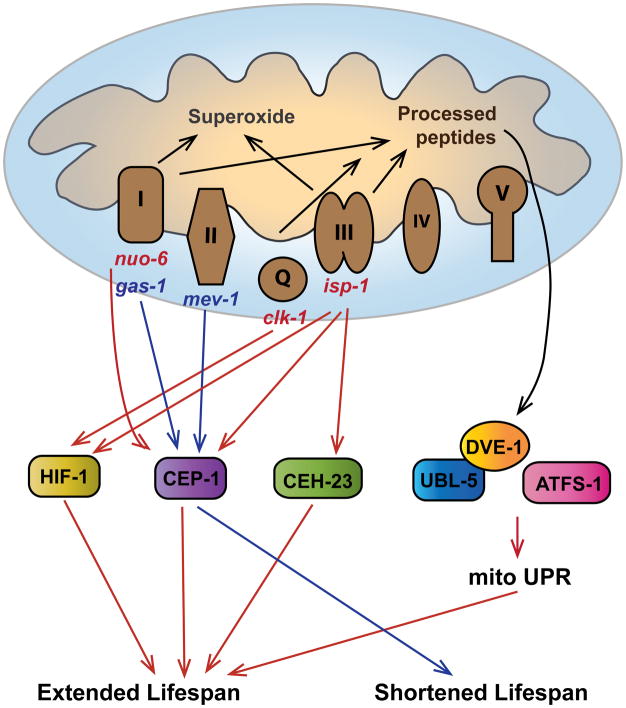
Figure 1. Mitochondrial ETC dysfunction affects lifespan in C. elegans by inducing a transcriptional network.
Mitochondrial mutations trigger differential transcriptional responses by activating distinct transcription factors. Red arrows indicate transcriptional pathways that are activated in long-lived ETC mutants and are important for their lifespan extension. Blue arrows indicate the transcriptional responses that are activated in the short-lived ETC mutants and are important for limiting the lifespan of these animals.
Highlights.
The mitochondrial electron transport chain (ETC) can mediate organismal lifespan.
Communications between mitochondria and the nucleus are vital for this regulation.
Transcription factors (TFs) can regulate the lifespan of C. elegans ETC mutants.
Key TFs participate in this regulation are highlighted.
Mammalian ETC dysfunction models of aging are likely conserved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, type setting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pulliam Da, Bhattacharya A, Van Remmen H. Mitochondrial Dysfunction in Aging and Longevity: A Causal or Protective Role? Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4950. 00. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 3.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dell’agnello C, Leo S, Agostino A, Szabadkai G, Tiveron C, Zulian A, Prelle A, Roubertoux P, Rizzuto R, Zeviani M. Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet. 2007;16:431–444. doi: 10.1093/hmg/ddl477. [DOI] [PubMed] [Google Scholar]
- 6.Dillin A, Hsu A-L, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 7.Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong A, Boutis P, Hekimi S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1994;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland JM, Cho J, Lo T, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol Elsevier Ltd. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Kirchman Pa, Kim S, Lai CY, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Bussière F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–447. doi: 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Pan KZ, Palter JE, Kapahi P. Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell. 2007;6:525–533. doi: 10.1111/j.1474-9726.2007.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman PS, Ishii N, Kayser EB, Morgan PG, Sedensky MM. Mitochondrial mutations differentially affect aging, mutability and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev. 2001;122:1187–1201. doi: 10.1016/s0047-6374(01)00259-7. [DOI] [PubMed] [Google Scholar]
- 17.Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekito T, Thornton J, Butow Ra. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol Biol Cell. 2000;11:2103–2115. doi: 10.1091/mbc.11.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jazwinski SM, Kriete A. The yeast retrograde response as a model of intracellular signaling of mitochondrial dysfunction. Front Physiol. 2012;3139 doi: 10.3389/fphys.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5e1000450 doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munkácsy E, Rea SL. The paradox of mitochondrial dysfunction and extended longevity. Exp Gerontol Elsevier Inc. 2014;56:221–233. doi: 10.1016/j.exger.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dancy BM, Sedensky MM, Morgan PG. Effects of the mitochondrial respiratory chain on longevity in C. elegans. Exp Gerontol Elsevier Inc. 2014;56:245–255. doi: 10.1016/j.exger.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Felkai S, Ewbank JJ, Lemieux J, Labbé JC, Brown GG, Hekimi S. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. EMBO J. 1999;18:1783–1792. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapointe J, Stepanyan Z, Bigras E, Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/− mice. J Biol Chem. 2009;284:20364–20374. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braeckman BP, Houthoofd K, De Vreese a, Vanfleteren JR. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Curr Biol. 1999;9:493–496. doi: 10.1016/s0960-9822(99)80216-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8e1000556 doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J Biol Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- 28.Kayser E-B, Sedensky MM, Morgan PG. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev. 2004;125:455–464. doi: 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Kondo M, Senoo-Matsuda N, Yanase S, Ishii T, Hartman PS, Ishii N. Effect of oxidative stress on translocation of DAF-16 in oxygen-sensitive mutants, mev-1 and gas-1 of Caenorhabditis elegans. Mech Ageing Dev. 2005;126:637–641. doi: 10.1016/j.mad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Ishii N, Senoo-Matsuda N, Miyake K, Yasuda K, Ishii T, Hartman PS, Furukawa S. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev. 2004;125:41–46. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Dingley S, Polyak E, Lightfoot R, Ostrovsky J, Rao M, Greco T, Ischiropoulos H, Falk MJ. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion Mitochondria Research Society. 2010;10:125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pujol C, Bratic-Hench I, Sumakovic M, Hench J, Mourier A, Baumann L, Pavlenko V, Trifunovic A. Succinate dehydrogenase upregulation destabilize complex I and limits the lifespan of gas-1 mutant. PLoS One. 2013;8e59493 doi: 10.1371/journal.pone.0059493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senoo-Matsuda N, Hartman PS, Akatsuka A, Yoshimura S, Ishii N. A complex II defect affects mitochondrial structure, leading to ced-3- and ced-4-dependent apoptosis and aging. J Biol Chem. 2003;278:22031–22036. doi: 10.1074/jbc.M211377200. [DOI] [PubMed] [Google Scholar]
- 34.Baruah A, Chang H-W, Hall M, Yuan J, Gordon S, Johnson E, Shtessel LL, Yee C, Hekimi S, Derry WB, Lee SS. CEP-1, the Caenorhabditis elegans p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants. In: Kim SK, editor. PLoS Genet. Vol. 10. e1004097. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk MJ, Zhang Z, Rosenjack JR, Nissim I, Daikhin E, Sedensky MM, Yudkoff M, Morgan PG. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans. Mol Genet Metab. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yee C, Yang W, Hekimi S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell Elsevier Inc. 2014;157:897–909. doi: 10.1016/j.cell.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honda S, Ishii N, Suzuki K, Matsuo M. Oxygen-dependent perturbation of life span and aging rate in the nematode. J Gerontol. 1993;48:B57–61. doi: 10.1093/geronj/48.2.b57. [DOI] [PubMed] [Google Scholar]
- 38.Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011;10:318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta R, Steinkraus Ka, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler J, Ventura N, Johnson TE, Rea SL. Long-lived mitochondrial (Mit) mutants of Caenorhabditis elegans utilize a novel metabolism. FASEB J. 2010;24:4977–4988. doi: 10.1096/fj.10-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S-J, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol Elsevier Ltd. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waypa GB. Mitochondrial Reactive Oxygen Species Trigger Calcium Increases During Hypoxia in Pulmonary Arterial Myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 43.Vanden Hoek TL. Reactive Oxygen Species Released from Mitochondria during Brief Hypoxia Induce Preconditioning in Cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 44.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker P. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerald D, Berra E, Frapart YM, Chan Da, Giaccia AJ, Mansuy D, Pouysségur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Hwang AB, Ryu E-A, Artan M, Chang H-W, Kabir MH, Nam H-J, Lee D, Yang J-S, Kim S, Mair WB, Lee C, Lee SS, Lee S-J. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci. 2014;1411199111 doi: 10.1073/pnas.1411199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- 48.Hwang AB, Ryu E, Artan M, Chang H, Humayun M, Nam H. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmeisser S, Schmeisser K, Weimer S, Groth M, Priebe S, Fazius E, Kuhlow D, Pick D, Einax JW, Guthke R, Platzer M, Zarse K, Ristow M. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell. 2013;12:508–517. doi: 10.1111/acel.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmeisser S, Priebe S, Groth M, Monajembashi S, Hemmerich P, Guthke R, Platzer M, Ristow M. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol Metab Elsevier. 2013;2:92–102. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tullet JMa, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S-K, Link CD, Johnson TE. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J. 2010;24:383–392. doi: 10.1096/fj.09-142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maduro MF, Broitman-Maduro G, Mengarelli I, Rothman JH. Maternal deployment of the embryonic SKN-1-->MED-1,2 cell specification pathway in C. elegans. Dev Biol. 2007;301:590–601. doi: 10.1016/j.ydbio.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 55.Paek J, Lo JY, Narasimhan SD, Nguyen TN, Glover-Cutter K, Robida-Stubbs S, Suzuki T, Yamamoto M, Blackwell TK, Curran SP. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab Elsevier. 2012;16:526–537. doi: 10.1016/j.cmet.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. 2005:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okuyama T, Inoue H, Ookuma S, Satoh T, Kano K, Honjoh S, Hisamoto N, Matsumoto K, Nishida E. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J Biol Chem. 2010;285:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celniker SE, Dillon LAL, Gerstein MB, Gunsalus KC, Henikoff S, Karpen GH, Kellis M, Lai EC, Lieb JD, Macalpine DM, Micklem G, Piano F, Snyder M, Stein L, White KP, Waterston RH. FEATURE Unlocking the secrets of the genome. 2009;459:927–930. doi: 10.1038/459927a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira RP, Porter Abate J, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arum O, Johnson TE. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J Gerontol A Biol Sci Med Sci. 2007;62:951–959. doi: 10.1093/gerona/62.9.951. [DOI] [PubMed] [Google Scholar]
- 62.McGee MD, Day N, Graham J, Melov S. cep-1/p53-dependent dysplastic pathology of the aging C. elegans gonad. Aging (Albany NY) 2012;4:256–269. doi: 10.18632/aging.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ventura N, Rea SL, Schiavi A, Torgovnick A, Testi R, Johnson TE. p53/CEP-1 Increases or Decreases Lifespan, Depending on Level of Mitochondrial Bioenergetic Stress. Aging Cell. 2010;8:380–393. doi: 10.1111/j.1474-9726.2009.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valentini S, Cabreiro F, Ackerman D, Alam MM, Kunze MBa, Kay CWM, Gems D. Manipulation of in vivo iron levels can alter resistance to oxidative stress without affecting ageing in the nematode C. elegans. Mech Ageing Dev Elsevier Ireland Ltd. 2012;133:282–290. doi: 10.1016/j.mad.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ventura N, Rea S, Samuel T, Condo I, Johnson TE, Testi R. Reduced expression of frataxin extends the lifespan of Caenorhabditis elegans. 2005;389:109–112. doi: 10.1111/j.1474-9726.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- 66.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huyen Y, Jeffrey PD, Derry WB, Rothman JH, Pavletich NP, Stavridi ES, Halazonetis TD. Structural differences in the DNA binding domains of human p53 and its C. elegans ortholog Cep-1. Structure. 2004;12:1237–1243. doi: 10.1016/j.str.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Walter L, Baruah A, Chang H-W, Pace HM, Lee SS. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol. 2011;9:e1001084. doi: 10.1371/journal.pbio.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkins NC, McGhee JD. Homeobox containing genes in the nematode Caenorhabditis elegans. Nucleic Acids Res. 1990;18:6101–6106. doi: 10.1093/nar/18.20.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- 71.Shen L, Hu Y, Cai T, Lin X, Wang D. Regulation of longevity by genes required for the functions of AIY interneuron in nematode Caenorhabditis elegans. Mech Ageing Dev Elsevier Ireland Ltd. 2010;131:732–738. doi: 10.1016/j.mad.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell Elsevier Ltd. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117:4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 74.Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. supp. Science. 2012;337:587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzenberger E, Boll M, Wenzel U. Impairment of the proteasome is crucial for glucose-induced lifespan reduction in the mev-1 mutant of Caenorhabditis elegans. Biochim Biophys Acta Elsevier BV. 2013;1832:565–573. doi: 10.1016/j.bbadis.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature Nature Publishing Group. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Runkel ED, Liu S, Baumeister R, Schulze E. Surveillance-activated defenses block the ROS-induced mitochondrial unfolded protein response. PLoS Genet. 2013;9e1003346 doi: 10.1371/journal.pgen.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haynes CM, Yang Y, Blais SP, Neubert Ta, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell Elsevier Ltd. 2010;37:529–540. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 2012;8:e1002760. doi: 10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature Nature Publishing Group. 2010;465:577–583. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell Elsevier Inc. 2013;155:699–712. doi: 10.1016/j.cell.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaramillo-Lambert A, Harigaya Y, Vitt J, Villeneuve A, Engebrecht J. Meiotic errors activate checkpoints that improve gamete quality without triggering apoptosis in male germ cells. Curr Biol Elsevier Ltd. 2010;20:2078–2089. doi: 10.1016/j.cub.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang H, Guo R, Powell-Coffman Ja. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci U S A. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan MH, Ligon M, Hussey LR, Hufnal B, Farber R, Munkácsy E, Rodriguez A, Dillow A, Kahlig E, Rea SL. TAF-4 is required for the life extension of isp-1, clk-1 and tpk-1 Mit mutants. Aging (Albany NY) 2013;5:1–18. doi: 10.18632/aging.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and Morbidity in Laboratory-maintained Rhesus Monkeys and Effects of Long-term Dietary Restriction. Journals Gerontol Ser A Biol Sci Med Sci. 2003;58:B212–B219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 89.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature Nature Publishing Group, a division of Macmillan Publishers Limited All Rights Reserved. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.López-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. 2002;32:882–889. doi: 10.1016/s0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- 92.Hagopian K, Chen Y, Simmons Domer K, Soo Hoo R, Bentley T, McDonald RB, Ramsey JJ. Caloric restriction influences hydrogen peroxide generation in mitochondrial sub-populations from mouse liver. J Bioenerg Biomembr. 2011;43:227–236. doi: 10.1007/s10863-011-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hagopian K, Harper M-E, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–84. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- 94.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 95.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper M-E. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286:E852–61. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- 96.Lal SB, Ramsey JJ, Monemdjou S, Weindruch R, Harper ME. Effects of caloric restriction on skeletal muscle mitochondrial proton leak in aging rats. J Gerontol A Biol Sci Med Sci. 2001;56:B116–22. doi: 10.1093/gerona/56.3.b116. [DOI] [PubMed] [Google Scholar]
- 97.Asami DK, McDonald RB, Hagopian K, Horwitz BA, Warman D, Hsiao A, Warden C, Ramsey JJ. Effect of aging, caloric restriction, and uncoupling protein 3 (UCP3) on mitochondrial proton leak in mice. Exp Gerontol. 2008;43:1069–1076. doi: 10.1016/j.exger.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagopian K, Harper M-E, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–84. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- 99.Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J. 2005;19:1320–1322. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- 100.Desai VG, Weindruch R, Hart RW, Feuers RJ. Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch Biochem Biophys. 1996;333:145–151. doi: 10.1006/abbi.1996.0375. [DOI] [PubMed] [Google Scholar]
- 101.Hempenstall S, Page MM, Wallen KR, Selman C. Dietary restriction increases skeletal muscle mitochondrial respiration but not mitochondrial content in C57BL/6 mice. Mech Ageing Dev. 2012;133:37–45. doi: 10.1016/j.mad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z, Nair KS. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gouspillou G, Hepple RT. Facts and controversies in our understanding of how caloric restriction impacts the mitochondrion. Exp Gerontol Elsevier Inc. 2013;48:1075–1084. doi: 10.1016/j.exger.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hafner AV, Dai J, Gomes AP, Xiao C-Y, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang Y, Cimen H, Han M-J, Shi T, Deng J-H, Koc H, Palacios OM, Montier L, Bai Y, Tong Q, Koc EC. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. J Biol Chem. 2010;285:7417–7429. doi: 10.1074/jbc.M109.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 108.Sauve AA. Sirtuin chemical mechanisms. Biochim Biophys Acta. 2010;1804:1591–1603. doi: 10.1016/j.bbapap.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 110.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bishop Na, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 112.Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. No reduction of metabolic rate in food restricted Caenorhabditis elegans. Exp Gerontol. 2002;37:1359–1369. doi: 10.1016/s0531-5565(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 113.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 114.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 116.McInnes J. Mitochondrial-associated metabolic disorders: foundations, pathologies and recent progress. Nutr Metab (Lond) Nutrition & Metabolism. 2013;10:63. doi: 10.1186/1743-7075-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menezes MJ, Riley LG, Christodoulou J. Mitochondrial respiratory chain disorders in childhood: insights into diagnosis and management in the new era of genomic medicine. Biochim Biophys Acta Elsevier BV. 2014;1840:1368–1379. doi: 10.1016/j.bbagen.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 118.Trifunovic A, Wredenberg A, Falkenberg M. Premature ageing in mice expressing defective mitochondrial DNA polymerase. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 119.Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J Neurochem. 2006;97:1659–1675. doi: 10.1111/j.1471-4159.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- 120.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hughes BG, Hekimi S. A mild impairment of mitochondrial electron transport has sex-specific effects on lifespan and aging in mice. PLoS One. 2011;6:e26116. doi: 10.1371/journal.pone.0026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agostino Alessandro, Invernizzi Federica, Tiveron Cecilia, Fagiolari Gigliola, Zeviani M. Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum Mol Genet. 2003;12:399–413. doi: 10.1093/hmg/ddg038. [DOI] [PubMed] [Google Scholar]
- 123.Lagouge M, Larsson N-G. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]