Abstract
Objective
Aminopeptidase N (CD13, EC 3.4.11.2) is a metalloproteinase expressed by fibroblast like synoviocytes (FLS). It has been suggested that CD13 can act chemotactically for T cells in rheumatoid arthritis (RA). The goals of this study were to measure CD13 in vivo and in vitro-in RA samples, and to determine whether CD13 could play a role in homing of T cells to the RA joint.
Methods
IL-17 treated FLS were used to immunize mice, from which a novel anti-human CD13 monoclonal antibody (591.1D7.34) was developed. 1D7 and a second anti-CD13 monoclonal, WM15, were used to develop a novel ELISA for CD13, and CD13 enzymatic activity was measured in parallel. Chemotaxis of cytokine activated T cells (Tck) was measured by an under-agarose assay.
Result
We detected substantial amounts of CD13 in synovial fluids, sera, FLS lysates, and culture supernatants by ELISA, with a significant increase in CD13 in RA synovial fluids when compared to osteoarthritis (OA). CD13 accounted for most but not all of the CD13-like enzymatic activity in synovial fluid. Recombinant human CD13 was chemotactic for Tck through a G-protein-coupled-receptor and contributed to the chemotactic properties of synovial fluid independently of enzymatic activity.
Conclusion
CD13 is released from FLS into culture supernatants and is found in synovial fluid. CD13 induces chemotaxis of Tck, a T cell population similar to that found in RA synovium. This data suggest that CD13 could play an important role as a T cell chemoattractant, in a positive feedback loop that contributes to RA synovitis.
Aminopeptidase N/CD13 (EC 3.4.11.2) is a metalloproteinase of the M1 family (1-4). It can be found on the membrane of many cell types (including cells of monocytic lineage and fibroblasts) and also as a truncated soluble protein (2-6). CD13 is a Zn+2 dependent ectoenzyme that cleaves the N-terminal peptide off substrates preferentially after a neutral amino acid (2-4). Substrates of CD13 include enkephalins, angiotensins, neuokinins, chemokines, and cytokines, and CD13 may degrade various extracellular matrix (ECM) components, aiding in cellular migration (2-4,7-8). CD13 has also been linked to immune mediated conditions including rheumatoid arthritis (RA), scleroderma, psoriasis, and chronic graft-versus-host disease (2-4,9-12).
RA is an autoimmune disease characterized by synovial inflammation and ultimately destruction of bone and cartilage. Infiltration of lymphocytes is one essential component of RA synovitis (13). In synovial tissue T cells can interact with other cells (including fibroblast like synoviocytes [FLS], macrophages, B cells, and dendritic cells) leading to cell activation and cytokine secretion (13-15). Various chemokines have been proposed to attract T cells to the RA joint including thymus and activation regulated chemokine (TARC/CCL17), IL-8/CXCL8, stromal cell-derived factor 1 (SDF-1/CXCL12), and macrophage inflammatory protein 1α (MIP-1α/CCL3) (15-17). CD13 involvement has been previously suggested in RA, and phenomena that can be functionally linked to CD13 are seen in RA (angiogenesis, cellular migration, monocyte activation) (2-4,10). However, a definitive role for CD13 in RA has not been shown, and the protein has not been directly measured. Enzymatic assays of CD13 activity and chemical inhibition of its enzymatic activity have been employed, but neither the assays nor the inhibitors of CD13 are specific for CD13 versus other aminopeptidases (18). Our goals in this study were to measure CD13 protein in RA and test the hypothesis that CD13 in synovial fluid is chemotactic for T cells. The results identify soluble CD13 (sCD13) as a protein that is preferentially produced in the RA joint and has chemotactic effects on a T cell population that is similar to RA synovial T cells, at concentrations found in synovial fluid in vivo.
Materials and Methods
Development of 591.1D7.34, a novel anti-CD13 monoclonal antibody
The investigations summarized herein commenced as an approach to defining novel inflammatory pathways in RA involving FLS, triggered preferentially by interleukin-17 (IL-17) rather than tumor necrosis factor α (TNFα). BALB/C mice were serially immunized with 106 FLS treated with IL-17, 10ng/ml, for 48 hours prior to immunization. A spleen from the most reactive mouse (assessed by flow cytometry of FLS using mouse sera) was fused to a non-secreting myeloma cell line. The resulting hybridoma clones were screened on resting FLS and IL-17 treated FLS, and then subcloned. An osteoarthritis (OA) FLS cell line was biotinylated and lysed. Immunopreciptiation was used to isolate the protein recognized by monoclonal antibody (mAb) 591.1D7.34 (1D7). Controls included MsIgG (isotype control) and anti-CD98. The eluted proteins were electrophoresed under reducing or non-reducing conditions on a 4-20% Tris-glycine polyacrylamide gel. The gel was transferred to an immobilon-P membrane and incubated with streptavidin-horseradish peroxidase then ECL substrate (Pierce). U937 myeloid cells were also lysed and 1D7 was used to immunoprecipitate a protein which was resolved by electrophoresis on two identical polyacrylamide gels. One was stained with Coomassie blue, and the other was excised at a spot corresponding to the primary band in the stained gel. The protein was subjected to trypsin digestion in-gel and the resulting peptide mixture was analyzed by LC-MS/MS on a Qtof premier instrument (Waters Inc). Protein Lynx Global Server and Mascot search engines were used to search the Uniprot and NCBI databases. The protein identified matched Aminopeptidase N/CD13.
Cell Culture
Procedures involving specimens obtained from human subjects were performed under a protocol approved by the University of Michigan Institutional Review Board. Histologic evaluation of synovial tissues was performed as previously described (19). FLS were cultured from human synovial tissue obtained at arthroplasty or synovectomy from RA or OA joints by digestion with 1% collagenase and separation through a 70μM cell strainer. The diagnosis of RA required at least four 1987 American College of Rheumatology criteria (20). The diagnosis of OA was based upon characteristic clinical and radiographic features. U937 cells were cultured in RPMI 1640 (10% FBS, 2% L-Glutamine, 1% HEPES, 1% Sodium Pyruvate, 0.5% glucose). FLS were maintained in CMRL (20% FBS, 2mM L-glutamine, 1% penicillin/streptomycin) and were used between passages 4 and 10. Cultures were moved to serum free media DMEM/F-12 with Peprogrow serum replacement (Peprotech) two days before harvesting. T cells were isolated from peripheral blood of healthy subjects using RosetteSep Human T cell enrichment cocktail (Stemcell) and Histopaque (Sigma-Aldrich). Cytokine activated T cells were prepared by culture of T cells in IL-6 (100ng/ml), IL-2 (25ng/ml), and TNFα (25ng/ml) for 7-10 days in 10% FBS RPMI medium (21,22). The COS-1 cell line (ATCC) was grown in 10% FBS DMEM medium.
Sample Preparation
Synovial fluid samples were treated with 0.05% Hyaluronidase (bovine testis, Sigma-Aldrich) one drop per 1 mL fluid for 5 min. Cells were lysed in cell lysis buffer (10% NP-40, 10% PMSF, 1% Iodoacetamide, and 0.1% E-64 in TSA) for one hour on ice. FLS culture supernatants were concentrated by centrifugation through an Amicon Ultracel 30K filter (Milliepore).
Flow cytometry
Fibroblasts were removed from flasks by 3mM EDTA in PBS. Cells were stained with MsIgG (negative control) or anti-CD13 (1D7, SJ1D1 [Abcam], or WM15 [Biolegend]), then goat anti-mouse IgG-Alexa fluor 488 (Molecular Probes). Cytometry was performed on a BD Biosciences FACSCalibur.
Aminopeptidase enzymatic activity
Aminopeptidase activity was measured by cleavage of L-Leucine-7-amido-4-methyl coumarin (L-leu-AMC, Sigma-Aldrich) to release the fluorescent molecule AMC. A standard curve was constructed using AMC (Sigma-Aldrich). The assay was run in 0.1 M Tris-HCl buffer (pH 8.0). Samples were incubated with the substrate at 37°C for one hour then read using a fluorescent plate reader at emission 450, excitation 365. Results were calculated as μM/hr of substrate cleaved.
CD13 Enzyme-Linked Immunosorbent Assay (ELISA)
High binding ELISA plates were coated with the anti-CD13 monoclonal antibody WM15 in 0.1M carbonate buffer pH9.5 overnight, and blocked with 1x Animal Free Block (Vector Laboratories) overnight. Samples were then applied to the plates either whole or diluted in block with 10mM EDTA. The standard curve was prepared using recombinant human CD13 (R&D Systems) in block with 10mM EDTA. 1D7 was biotinylated (Biotin-XX Microscale Protein Labeling Kit, Molecular Probes) and applied overnight. Streptavidin-HRP (Biolegend) was then added. Between steps plates were washed with PBS plus 0.05% Tween. The plates were visualized with TMB substrate (BD Biosciences), stopped with 2M H2SO4, and analyzed on a colorimetric plate reader.
Immunoprecipitation (IP)
Immunoprecipitation was performed using the Pierce Direct IP Kit (Thermo Scientific). In brief antibodies were bound to AminoLink Plus beads using cyanoborohydride, either anti-CD13 (1D7), anti-CD98 (7F8), or isotype control (MsIgG). Samples were diluted 1:1 with IP lysis/wash buffer and incubated overnight with the beads. The depleted portion was removed by centrifugation. Protein was eluted off the beads by low pH (2.8) which was neutralized with 1M Tris (pH 9.5).
Chemotaxis Assay
The chemotaxis assay was a modified under agarose chemotaxis system (23, 24). Some plates were coated with fibronectin (10μg/ml) overnight at 4°C then washed 2x with PBS. A 2.4% agarose (UltraPure, Invitrogen) solution in 1x HBSS was boiled and combined with 2 parts 1% BSA in RPMI and 1 part 1x HBSS. 3mls of this solution was added to each well of a 6-well plate and allowed to cool to room temperature. A 3-hole punch (University of Michigan Instrument Shop) was used to create three 4 mm wells, with edges 3 mm apart, and the plates were equilibrated to 37°C. Chemoattractants and T cells were adjusted to appropriate concentrations in chemotaxis medium (0.1% BSA, 2% L-glutamine, 1% pen/strep, in RPMI without phenol red). Some Tck samples were treated with 100ng/ml of pertussis toxin for 2hr at 37°C in 10% RPMI, then washed 1x with 0.1% BSA RPMI (viability ≥95%). 2.5×105 T cells were added to the center well. A medium only control was added to an outside well while the chemoattractant solution was added to the opposite outside well. Plates were incubated at 37°C, 5% CO2 overnight (uncoated) or for 5 hours (fibronectin). Pictures were taken of the area directly between the outside and center wells using an EVOS inverted microscope at 100x magnification. Cell counts were Ln transformed and data is expressed as a chemotactic index (CI= Ln cells migrated to stimulus – Ln cells migrated to medium alone).
CD13 enzymatic mutation
A CD13 clone (MGC Human ANPEP Sequence-Verified cDNA, BC058928) was obtained from Open Biosystems (Thermo Scientific). An E355Q enzymatic inactive mutant was created. The plasmid (1μg DNA) was linearized by double digestion with SgrAI (2U) and SbfI (10U) (New England Biolabs). The 7.3kb fragment was purified from a 1% agarose gel using the QIAquick Gel Extraction Kit (Qiagen). The plasmid fragment and two gene blocks, (IDT): 5’-TGGCCTGCCAGACTTCAACGCCGGCGCCATGCAGAACTGGGGACTGGTGACCTACCGGGAGAACTCCCTGCTGTTCGACCCCCTGTCCTCCTCCAGCAGCAACAAGGAGCGGGTGGTCACTGTGATTGCTCATGAGCTGGCCCACCAGTGGTTCGGGAACCTGGTGACCATAGAGTGGTGGAATGACCTGTGGCTGAACGAGGGCTTCGCCTCCTACGTGGAGTACCTGGGTGCTGACTATGCGGAGCCCACCTGGAACTTGAAAGACCTCATGGTGCTGAATGA-3’ and 5’-GACCTCATGGTGCTGAATGATGTGTACCGCGTGATGGCAGTGGATGCACTGGCCTCC TCCCACCCGCTGTCCACACCCGCCTCGGAGATCAACACGCCGGCCCAGATCAGTGAGCTGTTTGACGCCATCTCCTACAGCAAGGGCGCCTCAGTCCTCAGGATGCTCTCCAGCTTCCTGTCCGAGGACGTATTCAAGCAGGGCCTGGCGTCCTACCTCCACACCTTTGCCTACCAGAACACCATCTACCTGAACCTGTGGGACCACCTGCAGGAGGCTGTGAACAACCGGT-3’, were assembled and transformed into NEB 5-alpha E. Coli using the Gibson Assembly Cloning Kit (New England Biolabs). Clones were selected by Ampicillin resistance and several colonies were sequenced (University of Michigan Sequencing Core) to identify correct clones. Plasmids for wild-type CD13 or enzymatic inactive CD13 were grown up and isolated using an EndoFree Plasmid Maxi Kit (Qiagen). Plasmids were transfected into COS-1 cells (ATCC) using Lipofectamine LTX with Plus Reagent (Life Technologies). Mock transfection with Lipofectamine only was used as a control. Cultures were switched to serum free SFM4CHO media (Thermo Scientific) 48 hours after transfection and cultures were harvested 24 hours after.
Statistics
Concentrations, percent activity, and chemotactic index are expressed as mean±SEM. Enzymatic activity is expressed as mean±SD. Statistically significant chemotaxis was assessed by paired student T-test between the Ln stimulus count to Ln medium alone count; a paired T-test was used between groups. Otherwise significance was determined by unpaired student T-test, unless otherwise noted.
Results
Identification of CD13 as an IL-17-induced protein on FLS
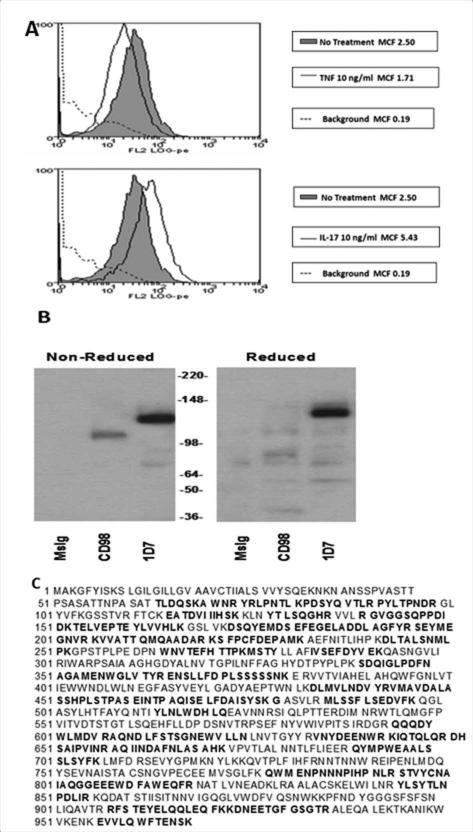
To identify a surface structure on FLS that was regulated by IL-17 independently of TNFα, IL-17 treated FLS were used to immunize BALB/c mice and hybridoma clones were created. The clones were screened on resting FLS, IL-17 treated FLS, and TNFα treated FLS. A hybridoma was selected that produced an antibody (591.1D7.34) that recognized an FLS surface protein upregulated by IL-17 but not TNFα at 48 hours (Figure 1A). Of the cell types tested, the monocytic cell line, U937, showed the strongest expression of the molecule identified by 1D7. We therefore used 1D7 to immunoprecipitate a protein from U937 that migrated on SDS-PAGE as a single band of 150kDA, and that was identified as CD13/Aminopeptidase N by sequencing of multiple peptide fragments (Figure 1B and C).
Figure 1. Identification of the protein recognized by mAb 591.1D7.34 and upregulated by IL-17 as CD13.
A, FLS were treated with medium alone, TNF-α (10ng/ml), or IL-17 (10ng/ml) for 48 hours before staining with 1D7 and anti-mouse FITC. B, An OA FLS cell line was biotinylated and lysed. Immunopreciptiation was used to isolate protein recognized by 1D7, MsIg (isotype control), or anti-CD98. The proteins from the 1D7, MsIg, and anti-CD98 beads were resolved by SDS-PAGE under reducing or non-reducing conditions. Protein from the same number of cells was loaded in each lane. C, Myeloid line U937 was lysed and 1D7 was used to immunoprecipitate the recognized protein, which was run on two gels. One was stained with Coomassie blue. The second gel was excised at a spot corresponding to the primary band in the stained gel. The protein was extracted and multiple peptide fragments sequenced, each of which matched portions of Aminopeptidase N/CD13 (bolded sequences).
CD13 in RA in vivo
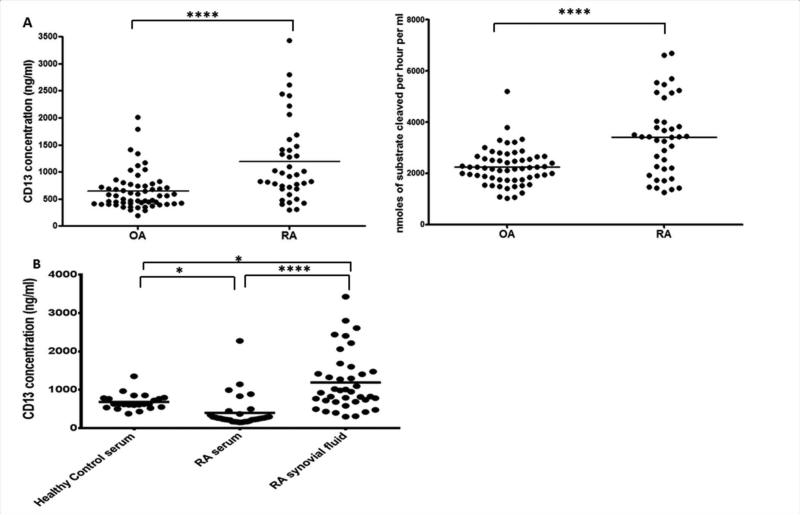
Having identified FLS CD13 as a potential part of an IL-17 induced pathway we next sought to determine whether the concentration of a putative soluble form of CD13 is elevated in the RA joint. This was previously suggested by determination of levels of aminopeptidase activity in synovial fluid (SF) samples, but without direct measurement of CD13 protein (9,10). We developed a novel ELISA to measure CD13 in healthy control (HC) serum (n=23), RA serum (n=33), and both RA and OA SFs (n=98). RA SFs (n=39) had an average CD13 concentration of 1191.09±121.58 ng/ml with an activity of 3402.45±239.58 μM/hr, and OA SFs (n=59) had an average CD13 concentration of 646.11±45.64 ng/ml and an activity of 2250.28±93.18 μM/hr (Figure 2A). The RA SFs were significantly higher in both amount (p<0.00001) and activity (p<0.00001). RA-SF CD13 concentration was also significantly higher than in RA sera, 397.95±72.18 (n=33, p<0.00001), or HC sera, 683.34±42.41 (n=23, p=0.0023), (Figure 2B). CD13 was significantly higher in HC sera than in RA sera, p=0.0040. The results are consistent with the observation that IL-17 upregulates CD13 expression by FLS and leads to the hypothesis that generation of sCD13 in the RA joint may play a role in a pro-inflammatory pathway.
Figure 2. CD13 is found in vivo and is higher in RA than in OA synovial fluid.
A, CD13 was measured by enzyme-linked immunosorbent assay (ELISA) and enzymatic activity in RA (n=39) and OA (n=59) synovial fluids. B, CD13 was measured by ELISA in sera from healthy controls (n=23), sera from RA patients (n=33) and in synovial fluids from RA patients (n=39, not matched to serum). * p≤0.05, ****p≤0.00001, by One-way ANOVA
CD13 on FLS from RA and OA
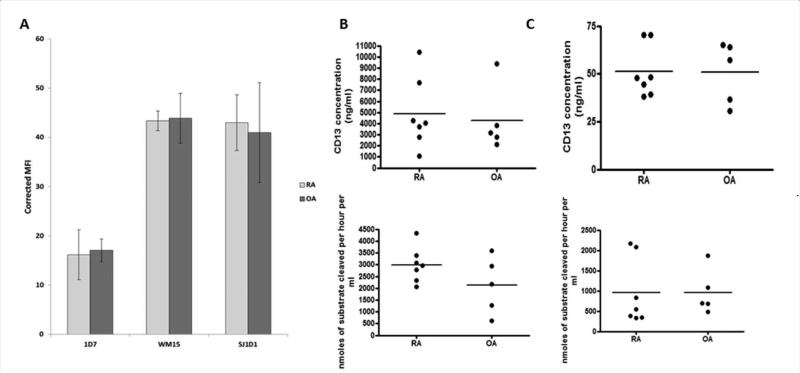
Having identified CD13 on FLS and sCD13 in synovial fluid, we asked whether the difference between RA and OA SFs corresponded to an increase in CD13 expression by RA FLS. We used antibodies WM15 and SJ1D1 in addition to 1D7 to measure CD13 by flow cytometry (Figure 3A). Each antibody detected CD13 on FLS, but with no significant difference in surface CD13 between resting RA (n=7) and OA (n=5) FLS. Moreover, measurement of total cell CD13 in lysates of RA and OA FLS by ELISA (p=0.74) and enzymatic activity assay (p=0.15) showed no significant differences (Figure 3B). We found 4867.39±1196.81ng/ml of CD13 in RA FLS with an activity of 2993.65±743.40 μM/hr and 4256.74±1306.71ng/ml of CD13 with 2123.05±1203.81 μM/hr of enzymatic activity for OA FLS. To determine whether FLS could release sCD13, we measured CD13 in FLS culture supernatants. FLS were cultured in serum free media to eliminate interference from bovine CD13. sCD13 was detected by ELISA in the supernatants and was enzymatically active, with almost identical results from RA (51.28±5.15 ng/ml, 957.69±819.02 μM/hr) and OA (50.78±7.16ng/ml, 962.69±552.26 μM/hr) FLS (Figure 3C).
Figure 3. Equivalent expression of CD13 by RA and OA FLS.
A, FLS were immunostained for CD13 using three antibodies which recognize different epitopes 1D7, WM15, SJ1D1. All three showed high expression. Mean fluorescent intensity (MFI) was adjusted for the isotype control as follows MFI CD13 – MFI MsIg = corrected MFI. B, RA (n=7) and OA (n=5) FLS were grown to confluence in 20% CMRL and switched to serum free media for 48 hours prior to harvesting. Cells were lysed and CD13 was measured by ELISA and enzymatic activity assay. C, The 48 hour culture supernatants were concentrated from 25mls to 1ml through at 30KDa centrifugal filter and measured by ELISA and enzymatic activity assay.
CD13 and Aminopeptidase Activity in the Joint
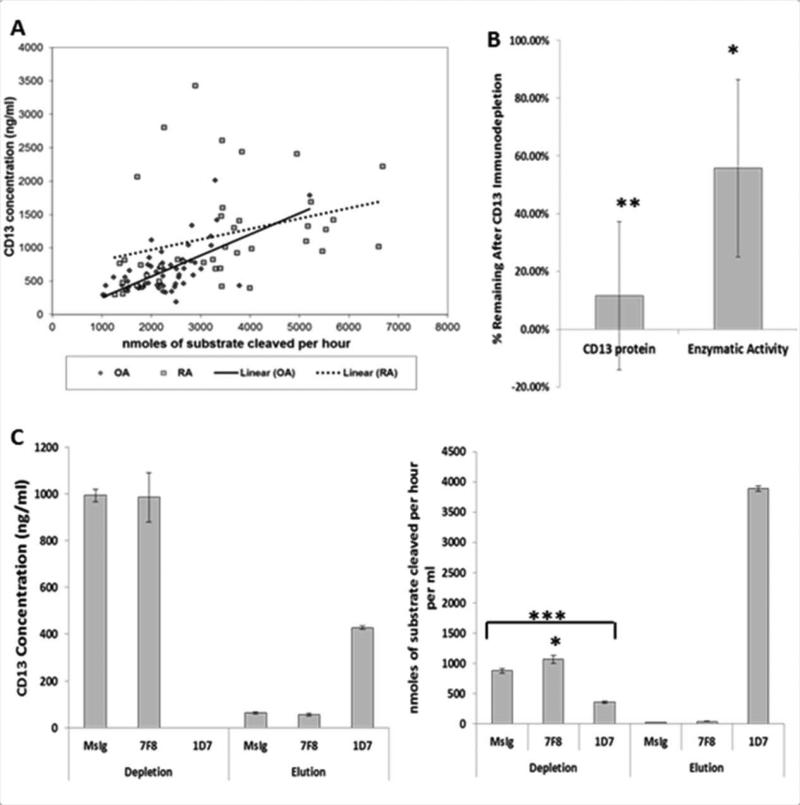
Although we found elevated levels of CD13 protein and enzymatic activity in RA in vivo, we did not find any differences in vitro between RA and OA FLS. We next sought to confirm that the N-aminopeptidase activity attributed to CD13 in SF samples was really due to CD13. The correlation between the concentration of CD13 and the aminopeptidase activity was R2=0.1365, p=4.24×10−9 by ANOVA in SF samples. Considering the OA samples separately, this correlation remained highly significant, R2=0.418, p=3.17×10−8. However, in the RA samples the correlation was non-significant, R2=0.0947, ANOVA p=0.057 (Figure 4A). Several outliers could indicate the variable presence of other proteins with aminopeptidase activity. To further assess the issue, we immunodepleted RA (n=6) and OA (n=3) SFs using 1D7 and assayed the depleted fractions for CD13 and aminopeptidase activity. Successful immunodepletion of CD13 (partial or complete) was verified by ELISA. We showed significant depletion of both the CD13 protein amount (11.50%±0.26% remaining, p=0.00055) and enzymatic activity (55.74%±0.31% remaining, p=0.0027) (Figure 4B). In most SFs the remaining percentage of aminopeptidase activity was higher than the remaining percentage of CD13 protein. Moreover, 100% depletion of CD13 protein failed to remove all of the aminopeptidaseactivity. An example in Figure 4C shows one RA SF with complete depletion of CD13 as measured by ELISA in which the enzymatic activity in the sample went from 876.61±36.06 μM/hr in the control depleted down to 356.00±17.47 μM/hr (p=2.3×10−5) in the CD13 depleted sample. Much of the enzymatic activity in this sample appeared in the eluate from the 1D7 immunopreciptation (3888.00±39.97 μM/hr). However, this only accounted for approximately 60% of the starting enzymatic activity.
Figure 4. CD13 accounts for most but not all of the aminopeptidase activity in synovial fluid.
A, ELISA and enzymatic activity assay (2B) results for RA and OA synovial fluids were correlated separately and together. Correlations were analyzed by ANOVA, all points together R2=0.1365, ANOVA p=4.24×10−9, OA alone R2=0.418, ANOVA p=3.17×10−8, RA alone R2=0.0947, ANOVA p=0.057. B, Synovial fluids (n=9) were immunodepleted with anti-CD13 (1D7), Ig isotype control (MsIgG1), or an isotype matched non-relevant antibody (7F8[CD98]). The removed protein was eluted off the beads by low pH. C, Example of complete depletion of an RA synovial fluid as measured by enzyme-linked immunosorbent assay (ELISA) and the corresponding enzymatic activity (1 synovial fluid 3 replicates). Expressed as mean±SD * p≤0.05, **p≤0.001, ***p≤0.0001 unpaired two tail T-test
Recombinant Human CD13 Aids in Migration of Cytokine Activated T Cells
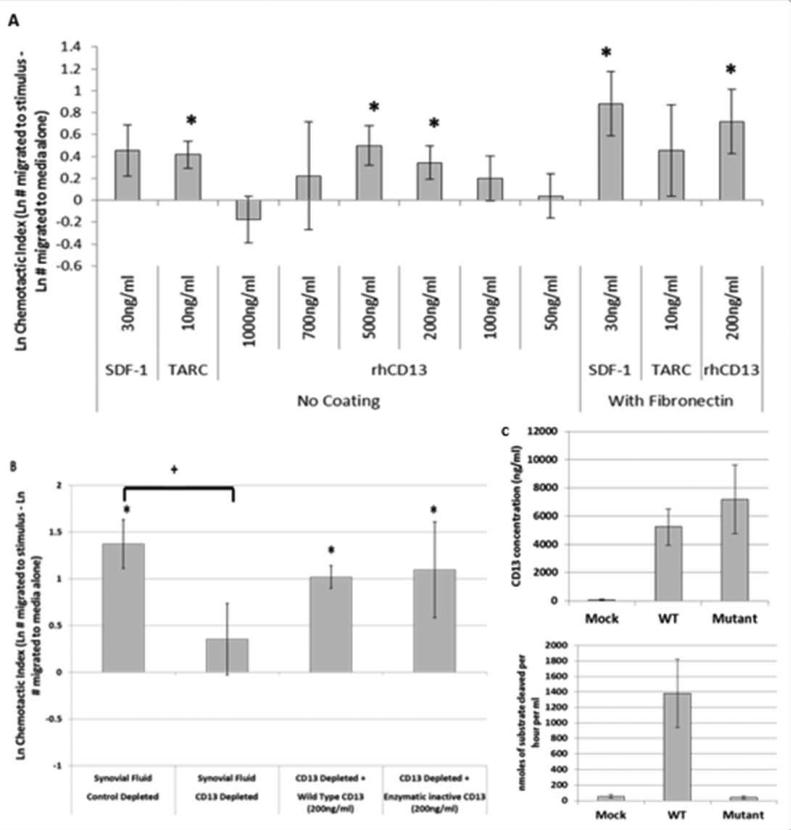
Having determined that CD13 is present in high amounts in SF and is enzymatically active we next considered a possible function for CD13 in RA. Two previous publications have suggested that CD13 is chemotactic for T cells (10,25). We sought to verify this observation and to determine whether CD13 could be chemotactic for cytokine activated T cells (Tck), an in vitro generated cell population that phenotypically and functionally resembles T cells found in RA synovium (21,22). We used a modified under agarose chemotaxis system with SDF-1/CXCL12 and TARC/CCL17 as positive controls. SDF-1 and TARC, had chemotactic indices (CI) of 0.45±0.23 (p=0.068) and 0.42±0.12 (p=0.0012) respectively without fibronectin and 0.88±0.29 (p=0.0046) and 0.46±0.42 (p=0.28) with fibronectin (Figure 5A). Recombinant human CD13 (rhCD13) was used over a range of concentrations, from 1000ng/ml to 50ng/ml, and was chemotactic for Tcks between 700ng/ml and 50ng/ml. Peak chemotaxis for CD13 was from 200ng/ml (0.34±0.29) to 500ng/ml (0.50±0.18) and was significant over medium alone (medium alone CI=0) at both concentrations, p=0.029 and 0.0079 respectively. RhCD13 was also significant at 200ng/ml with fibronectin coating (0.72±0.29, p=0.018) (Figure 5A). Representative images of the chemotaxis assays are shown in supplementary figure 1.
Figure 5. CD13 is chemotactic for cytokine activated T cells, independent of its enzymatic activity.
Tcks were placed in the center well of the under agarose chemotaxis system with a medium only control in one of the side wells and a chemotactic agent in the opposite well. Some plates were coated with fibronectin. A, Positive wells were loaded with chemotactic controls SDF-1/CXCL12 (30ng/ml) or TARC/CCL17 (10ng/ml) or rhCD13 (1000 to 50ng/ml) (n≥12). B, Positive wells were loaded with synovial fluid (3 different random donors), MsIgG control depleted or CD13 depleted (1D7), at a 1:10 dilution, or with CD13 depleted synovial fluid supplemented with supernatant from transfected COS-1 cells containing wild type or enzymatic inactive mutant CD13 (n≥19). C, The CD13 amount in the COS-1 supernatants was determined by ELISA and was used at 200ng/ml in assays shown in panel B. Enzymatic activity was measured by N-aminopeptidase activity assay. Data in panels A and B is expressed as a chemotactic index (CI= Ln # of cells migrated to stimulus – Ln # of cells migrated to media alone)±SEM. *p≤0.05 paired two tailed T-test between media alone and test wells; + p≤0.05 un-paired two tailed T-test between two groups
CD13 contributes to the chemotactic activity of SF independent of its enzymatic activity
We next asked whether CD13 contributes to the chemotactic potential of RA SF. To test for a potential role of CD13 in Tck chemotaxis, we immunodepleted SFs with anti-CD13 (1D7) or a mock (MsIg) depleted control (n=3). The mock depleted SFs had a CI of 1.37±0.44 which was significantly chemotactic, p=0.0065. Immunodepleting CD13 from the SFs resulted in a significant decrease in chemotaxis p=0.041, CI of 0.35±0.21 (Figure 5B).
We next examined whether CD13's chemotactic ability required its enzymatic activity. COS-1 cells were used to express plasmids containing either wild type (WT) CD13 or an enzymatically inactive mutant CD13 (E355Q) (26). The CD13 ELISA was used to measure CD13 concentration, and loss of enzymatic activity in the mutant was confirmed by the N-aminopeptidase assay (Figure 5C). We supplemented CD13 depleted synovial fluids with 200ng/ml of either WT or mutant CD13 from the COS-1 supernatants, both of which partially restored the chemotactic activity of the depleted SFs to a similar level. WT CD13 brought the CI from 0.35±0.21 to 1.02±0.31 and the mutant brought the CI to 1.10±0.30, each representing significant chemotaxis above medium alone, p=0.0031 and p=0.0018 respectively (Figure 5B).
CD13 initiates chemotaxis of T cells through a G-protein coupled receptor
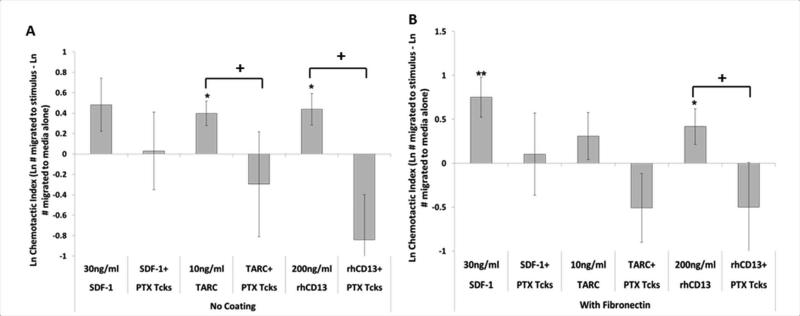
Most known chemokines function through a G-protein coupled receptor (GPCR) (27). We used Pertussis toxin (PTX) to determine whether the mechanism for CD13-induced chemotaxis functions similarly. PTX treatment of Tcks significantly reduced chemotaxis toward 200ng/ml rhCD13 either with a fibronectin coating (0.42±0.20 to −0.50±0.50, p=0.047) or without (0.44±0.15 to −0.84±0.44, p=0.00096), and also inhibited migration toward SDF-1 and TARC positive controls (significant for TARC, uncoated, at p=0.05) (Figure 6).
Figure 6. CD13 chemotaxis functions through a pertussis toxin sensitive G-protein coupled receptor.
Tcks were placed in the center well of the under agarose chemotaxis system with a media only negative control in one of the side wells and a chemotactic agent (SDF-1, TARC or rhCD13) at indicated concentration in the opposite well. Plates were (A) left untreated or (B) fibronectin (10μg/ml) coated overnight. Some Tcks were treated with 100ng/ml of pertussis toxin for 2hr at 37°C. (n≥20) Cell counts were Ln transformed and data is expressed as a chemotactic index (CI= Ln # of cells migrated to stimulus – Ln # of cells migrated to media alone) with error expressed as SEM. *p≤0.05 paired two tailed T-test between media alone and test wells, paired within each replicate; + p≤0.05 un-paired two tailed T-test between two groups of chemotactic indices
Discussion
It has become clear that RA is not mediated by a single cell type but rather by interactions between the various cells in the RA joint: including FLS and T cells. RA FLS express matrix metalloproteinases (MMPs), secrete pro-inflammatory mediators, and produce chemotactic agents to attract inflammatory cells to the joint. T cells are prime producers of multiple pro-inflammatory cytokines (such as IL-17, TNFα, interferon-γ [IFNγ]). We explored the potential role of CD13, which is highly expressed on FLS as one possible factor in the FLS/T cell interactions.
Although CD13 has been identified in synovial fluids by Western blot and through N-aminopeptidase enzymatic activity assays, it has never been directly measured. We developed a novel CD13 ELISA and showed that CD13 is significantly higher in RASFs than in OASFs, HC sera, or RA sera. We also noted a significantly higher amount of CD13 in HC sera when compared to RA sera (Figure 2). This could represent an influx of CD13 expressing cells (such as monocytes) into the joint leaving fewer circulating CD13-shedding cells. We observed higher co-staining of CD13 and a monocytic linage marker (CD11c) in RA synovial tissues than in OA or normal (Supplementary Figure 2). We were specifically interested in whether FLS could contribute to the sCD13 we found in the SF. FLS highly express surface CD13 and are therefore a logical choice for shedding of CD13. We were able to show that sCD13 does come from FLS (Figure 3C, Supplementary Figure 2), and can thereby infer that FLS contribute to the sCD13 in SF. We expected that CD13 would be elevated on RA FLS when compared to OA FLS, as previously published (10). However, our data show no noticeable difference (Figure 3). We noted a large variation in the intensity of CD13 expression on our cell lines and in SF and serum samples. We believe that this variation may account for the differences between our results and the previous publication (10).
There are at least four possible explanations for the higher amount of sCD13 in RA versus OASFs. First, the RA joint environment includes cytokines and growth factors that could upregulate CD13 expression or release from the FLS in vivo in a manner that might not be maintained in vitro (13). This is supported by our finding that IL-17 increases surface expression of CD13 (Figure 1A). Second, synovial tissue undergoes hyperplasia in RA resulting in much higher numbers of FLS that could release CD13. Third, the increase could be due to other cell types. Endothelial cells and cells of the monocytic cell lineage express surface CD13 (2-4). These cells are often found in higher numbers in the RA joint and so could account for the increased sCD13. Lastly, there is increased expression of proteases in the RA synovium which could increase shedding of CD13 from the FLS (28). The most likely explanation for the higher sCD13 in RA synovial fluid combines all four possibilities. The complex milieu present in the RA joint contributes to the higher number of FLS and other cells as well as regulation and shedding of CD13 from those cells, although monocytic shedding of CD13 has only been observed previously in apoptotic cells (29).
We wanted to ensure that the aminopeptidase activity we were measuring could be attributed to CD13. 1D7 was used for the immunodepletion since it does not block enzymatic activity (data not shown). We found that CD13 contributes the majority of the N-aminopeptidase activity to the synovial fluids; however, it appears that at least one other protein present has similar activity (Figure 4B, C) (5). The correlation analysis (Figure 4A) showed a few synovial fluid samples that also appear to have a high concentration of CD13 but lower enzymatic activity. This could indicate the presence of natural inhibitors and/or intrinsic differences in the CD13 protein from post-translational modifications resulting in differing degrees of enzymatic activity.
It has been previously suggested that CD13 may act as a chemokine for T cells (10, 25). We wanted to further explore this function as one possible aspect of the interaction between FLS and T cells in the RA joint. We tested for chemotaxis with SDF-1, TARC, or rhCD13 using not only Tcks, but also resting T cells, anti-CD3/CD28 activated, Jurkat, HUT78, and mitogen activated T cells (data not shown). We chose Tck because we achieved the most consistent and significant chemotaxis with both positive control chemokines and rhCD13 with this cell type. This is of particular interest as Tck phenotypically resemble T cells found in the RA joint (19). Chemotaxis assays with rhCD13 were performed both over a fibronectin coated and uncoated plate, to explore both a basic chemotaxis (uncoated) and a matrix invasion type function (fibronectin coating). Fibronectin improved adhesion of the cells to the plate, magnifying both specific and non-specific movement. Even though rhCD13 and synovial fluid were significantly chemotactic in both systems, the two known T cell chemokines (TARC and SDF-1) were each significantly chemotactic in only one of the systems. Nevertheless the chemotactic index is similar in both assays and the results of these assays are subject to the effects of high non-specific background movement. It is important to note that the optimal levels of CD13 in the chemotaxis assays correspond to sCD13 levels found in vivo indicating this process is likely biologically relevant (Figure 5A). Although the average CD13 concentration in RA synovial fluid is higher than the peak chemotactic range, it is possible that this serves to create a gradient between the joint and the serum with the high concentration in the joint aiding in T cell retention. Consistent with this notion the average concentration of CD13 in OA SF is similar to normal serum concentrations (Figure 2).
It is known that there are multiple chemokines that contribute to the migration of T cells to the RA joint (30,31). Notwithstanding the effects of other chemokines, CD13 appears to significantly contribute to the chemotactic activity of SF as Tck chemotaxis decreases when CD13 is depleted from such samples (Figure 5B). CD13 has been shown to be able to regulate other chemokines, but in vitro studies with rhCD13 indicate that CD13 can initiate chemotaxis directly (32-35). To determine whether the CD13 dependent chemotaxis in SF was direct or through enzymatic effects on other molecules, we introduced rhCD13 into the depleted SFs using either WT or an enzymatically inactive mutant (E355Q). This residue is part of the highly conserved GAMEN motif that is involved in substrate binding (36,37). Both the WT and mutant CD13 proteins were able to partially restore chemotactic activity to a similar degree demonstrating that the mechanism of chemotaxis is independent of CD13's enzymatic activity (Figure 5B). This also indicates that CD13 likely binds directly to a receptor on the T cells. To confirm this we used PTX treated Tck. PTX is known to inhibit cellular responses mediated through Gi/o proteins including most chemokine receptors (27,38). Here we were able to show that CD13 also acts through a PTX-sensitive GPCR (Figure 6). We also saw similar results with a fibronectin coating indicating a similar mechanism for basic chemotaxis or migration involving ECM.
Although we have defined one mechanism for CD13 mediated T cell chemotaxis in RA, CD13 in the RA joint is also likely involved in cleaving chemokines, extracellular matrix proteins, and other molecules that regulate migration of T cells and interaction of those T cells with FLS. Future investigation of these aspects of CD13 function and definition of the T cell receptor for CD13 will yield additional insights into the roles of CD13 in RA.
Supplementary Material
Supplementary Figure 1: Representative illustrations of under agarose chemotaxis. Pictures are representative of images obtained for counting from under agaorse chemotaxis (n≥19). Positive pictures show cells migrating toward the indicated chemotactic solution. Negative wells show the migration toward the medium only control for the same well. Arrow shows one migrated cell as an example.
Supplementary Figure 2: CD13 staining is present in synovial tissue from normal, OA, and RA patients. CD13 staining more strongly co-localizes with FLS (cadherin-11) and monocytic lineage cells (CD11c) in RA tissue. Tissues were harvested form normal, OA, and RA patients during surgery. Tissue sections were frozen with liquid nitrogen and 7 μm frozen sections were made using a cryostat. The slides were fixed in Acetone at 4°C for 30 minutes, washed, and blocked in 20% Fetal Bovine Serum and 5% goat serum in PBS. Slides were incubated with the primary antibodies anti-human CD13 (1D7, 10μg/mL) and rabbit anti-human CD11c (Abcam, 1:100 dilution) or rabbit anti-human Cadherin 11 (Zymed 10μg/mL). Controls were rabbit and mouse IgG at equivalent concentrations. Secondary antibodies for both sets of slides were Alexa fluor goat anti-mouse 488 and goat anti-rabbit 555 (Life Technologies 10μg/mL), and DAPI (Invitrogen, 1:5000 in PBS) was used on all slides. Slides were mounted with Fluoromount-G (Southern Biotech). Picutres were taken on an Olympus BX microscope at 40x magnification. Arrows point to examples of co-localization.
Acknowledgments
Grant Support: This work was supported by a NIH R01 grant F018446-05, NIH Research Supplements to Promote Diversity in Health-Related Research grant 10-PAF00195, and NIH training grant in Experimental Immunology 2T32A1007413-16.
References
- 1.Look AT, Ashmun RA, Shapiro LH, Peiper SC. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. Clin Investig. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riemann D, Kehlen A, Langner J. CD13 – not just a marker in leukemia typing. Immunol Today. 1999;20:83–88. doi: 10.1016/S0167-5699(98)01398-X. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14:639–47. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- 4.Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Cell. 2008;14:361–71. doi: 10.1016/j.molmed.2008.06.003. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favaloro EJ, Browning T, Facey D. CD13 (GP150; aminopeptidase-N): predominant functional activity in blood is localized to plasma and is not cell-surface associated. Exp Hematol. 1993;21:1695–1701. [PubMed] [Google Scholar]
- 6.Watanabe Y, Ito K, Iwaki-Egawa S, Yamaguchi R, Fujimoto Y. Aminopeptidase N in sea of healthy subjects is a different n-terminal processed derivative from the one obtained from maternal serum. Mol Genet Metab. 1998;63:289–94. doi: 10.1006/mgme.1998.2676. [DOI] [PubMed] [Google Scholar]
- 7.Larsen SL, Pedersen LØ, Buus S, Stryhn A. T cell responses affected by aminopeptidase N (CD13)-mediated trimming of major histocompatibility complex class II-bound peptides. J Exp Med. 1996;184:183–9. doi: 10.1084/jem.184.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiki I, Fujii H, Yoneda J, Abe F, Nakajima M, Tsuruo T, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–43. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan H, Tani K, Hase K, Shimizu T, Tamiya H, Biraa Y, et al. CD13/aminopeptidase N in collagen vascular diseases. Rheumatol Int. 2003;23:271–6. doi: 10.1007/s00296-003-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimuizu T, Tani K, Hase K, Ogawa H, Huang L, Shinomiya F, et al. CD13/aminopeptidase N-induced lymphocyte involvement in inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:2330–8. doi: 10.1002/art.10517. [DOI] [PubMed] [Google Scholar]
- 11.Kitko CL, Levine JE, Storer BE, Chai X, Fox D, Braun TM, et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123:786–93. doi: 10.1182/blood-2013-08-520072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thielitz A, Ansorge S, Bank U, Tager M, Wrenger S, Gollnick H, et al. The ectopeptidases dipeptidyl peptidase IV (DP IV) and aminopeptidase N (APN) and their related enzymes as possible targets in the treatment of skin diseases. Front Biosci. 2008;13:2364–75. doi: 10.2741/2850. [DOI] [PubMed] [Google Scholar]
- 13.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis: T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AMC, Colin EM, Hazes JMW, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Cho ML, Kim HR, Ju JH, Park MK, Oh HJ, et al. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes. Arthritis Rheum. 2007;56:1076–86. doi: 10.1002/art.22439. [DOI] [PubMed] [Google Scholar]
- 16.Szekanec Z, Koch AE, Tak PP. Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. Neth J Med. 2011;69:356–66. [Review] [PubMed] [Google Scholar]
- 17.Ruth JH, Bottman JB, Katschke KJ, Qin S, Wu L, LaRosa G, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44:2750–60. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Scornik OA, Botbol V. Bestatin as an experimental tool in mammals. Curr Drug Metab. 2001;2:67–85. doi: 10.2174/1389200013338748. [DOI] [PubMed] [Google Scholar]
- 19.Isozaki T, Ruth JH, Amin MA, Campbell PL, Tsou PS, Ha CM, Haines GK, 3rd, Edhayan G, Koch AE. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res Ther. 2014;16:R28. doi: 10.1186/ar4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988. 31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, Feldmann M. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells. Arthritis Rheum. 2002;46:31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Bryant J, Ahern DJ, Brennan FM. CXCR4/SDF-1 and VLA-4/VCAM-1 are key chemokine/adhesion molecules in the migration of cytokine-activated T cells. Arthritis Rheum. 2012;64:2137–46. doi: 10.1002/art.34394. [DOI] [PubMed] [Google Scholar]
- 23.Nelson RD, Quie PG, Simmons RL. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975;115:1650–6. [PubMed] [Google Scholar]
- 24.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci STKE. 2003:pl5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 25.Tani K, Ogushi F, Huang L, Kawano T, Tada H, Hariguchi N, et al. CD13/aminopeptidase N, a novel chemoattractant for T lymphocytes in pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000;161:1636–42. doi: 10.1164/ajrccm.161.5.9902008. [DOI] [PubMed] [Google Scholar]
- 26.Luciani N, Marie-Claire C, Ruffet E, Beaumont A, Roques BP, Fournie-Zaluski MC. Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of Aminopeptidase N (EC 3.4.11.2): insights into its mechanism of action. Biochemistry. 1998;37:686–692. doi: 10.1021/bi971705p. [DOI] [PubMed] [Google Scholar]
- 27.Allen SJ, Crown SE, Handel TM. Chemokine: Receptor Structure, Interactions, and Antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 28.Mantle D, Falkous G, Walker D. Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers. Clin Chim Acta. 1999;284:45–58. doi: 10.1016/s0009-8981(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 29.Brown SB, Kluck RM, Ellem KA. Loss and shedding of surface markers from the leukemic myeloid monocytic line THP-1 induced to undergo apoptosis. J Cell Biochem. 1996;60:246–59. doi: 10.1002/(sici)1097-4644(19960201)60:2<246::aid-jcb9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Miossec P, Dinarello CA, Ziff M. Interleukin-1 lymphocyte chemotactic activity in Rheumatoid Arthritis synovial fluid. Arthritis Rheum. 1986;29:461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mughales J, Blyth TH, Hunter JA, Wilkinson PC. The chemoattractant activity of rheumatoid synovial fluid for human lymphocytes is due to multiple cytokines. Clin Exp Immunol. 1996;106:230–236. doi: 10.1046/j.1365-2249.1996.d01-836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanayama N, Kajiwara Y, Goto J, Maradny EEL, Maehara K Andou K, et al. Inactivation of interleukin-8 by aminopeptidase N (CD13). J Leukoc Biol. 1995;57:129–134. doi: 10.1002/jlb.57.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wulfaenger J, Niedling S, Riemann D, Seliger B. Aminopeptidase N (APN)/CD13-dependent CXCR4 downregulation is associated with diminished cell migration, proliferation, and invasion. Mol Membr Biol. 2008;25:72–82. doi: 10.1080/09687680701551855. [DOI] [PubMed] [Google Scholar]
- 34.Chomarat P, Rissoan MC, Pin JJ, Banchereau J, Miossec P. Contribution of IL-1, CD14, and CD13 in the increased IL-6 production induced by in vitro monocyte-synoviocyte interactions. J Immunol. 1995;155:3645–52. [PubMed] [Google Scholar]
- 35.Proost P, Mortier A, Loos T, Vandercappellen J, Gouwy M, Ronsse I, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110:37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- 36.Wong AH, Zhou D, Rini JM. The X-ray crystal structure of human aminopeptidase N reveals a novel dimer and the basis for peptide processing. J Biol Chem. 2012;287:36804–13. doi: 10.1074/jbc.M112.398842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashmun RA, Shapiro LH, Look AT. Deletion of the zinc-binding motif of CD13/aminopeptidase N molecules results in loss of epitopes that mediate binding of inhibitory antibodies. Bood. 1992;79:3344–49. [PubMed] [Google Scholar]
- 38.Mangmool S, Kurose H. G(i/o) protein-dependent and –independent actions of Pertussis Toxin (PTX). Toxins. 2011;3:884–99. doi: 10.3390/toxins3070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Representative illustrations of under agarose chemotaxis. Pictures are representative of images obtained for counting from under agaorse chemotaxis (n≥19). Positive pictures show cells migrating toward the indicated chemotactic solution. Negative wells show the migration toward the medium only control for the same well. Arrow shows one migrated cell as an example.
Supplementary Figure 2: CD13 staining is present in synovial tissue from normal, OA, and RA patients. CD13 staining more strongly co-localizes with FLS (cadherin-11) and monocytic lineage cells (CD11c) in RA tissue. Tissues were harvested form normal, OA, and RA patients during surgery. Tissue sections were frozen with liquid nitrogen and 7 μm frozen sections were made using a cryostat. The slides were fixed in Acetone at 4°C for 30 minutes, washed, and blocked in 20% Fetal Bovine Serum and 5% goat serum in PBS. Slides were incubated with the primary antibodies anti-human CD13 (1D7, 10μg/mL) and rabbit anti-human CD11c (Abcam, 1:100 dilution) or rabbit anti-human Cadherin 11 (Zymed 10μg/mL). Controls were rabbit and mouse IgG at equivalent concentrations. Secondary antibodies for both sets of slides were Alexa fluor goat anti-mouse 488 and goat anti-rabbit 555 (Life Technologies 10μg/mL), and DAPI (Invitrogen, 1:5000 in PBS) was used on all slides. Slides were mounted with Fluoromount-G (Southern Biotech). Picutres were taken on an Olympus BX microscope at 40x magnification. Arrows point to examples of co-localization.