Abstract
Recent findings indicate an isoform-specific role for apolipoprotein E (apoE) in the elimination of beta-amyloid (Aβ) from the brain. ApoE is closely associated with various lipoprotein receptors, which contribute to Aβ brain removal via metabolic clearance or transit across the blood-brain barrier (BBB). These receptors are subject to ectodomain shedding at the cell surface, which alters endocytic transport and mitigates Aβ elimination. To further understand the manner in which apoE influences Aβ brain clearance, these studies investigated the effect of apoE on lipoprotein receptor shedding. Consistent with prior reports, we observed an increased shedding of the low density lipoprotein receptor (LDLR) and the LDLR-related protein 1 (LRP1) following Aβ exposure in human brain endothelial cells. When Aβ was co-treated with each apoE isoform, there was a reduction in Aβ-induced shedding with apoE2 and apoE3, while lipoprotein receptor shedding in the presence of apoE4 remained elevated. Likewise, intracranial administration of Aβ to apoE targeted replacement mice (expressing the human apoE isoforms) resulted in an isoform-dependent effect on lipoprotein receptor shedding in the brain (apoE4>apoE3>apoE2). Moreover, these results show a strong inverse correlation with our prior work in apoE transgenic mice in which apoE4 animals showed reduced Aβ clearance across the BBB compared to apoE3 animals. Based on these results, apoE4 appears less efficient than other apoE isoforms in regulating lipoprotein receptor shedding, which may explain the differential effects of these isoforms in removing Aβ from the brain.
Keywords: apolipoprotein E, lipoprotein receptor, blood-brain barrier, beta-amyloid
Introduction
Alzheimer’s disease (AD) is an age-related condition which affects approximately 36 million people worldwide (Gilbert 2013). This neurodegenerative process is characterized by a progressive deterioration in memory, executive function, and behavior (Reitz 2012) accompanied by selective neuronal degeneration and synaptic loss in the hippocampus, amygdala and temporal neocortex (Serrano-Pozo et al. 2011). The key pathological hallmarks of AD include the formation of neurofibrillary tangles and the deposition of beta-amyloid proteins (Aβ) in the brain and cerebrovasculature (Citron 2010). While the exact pathogenesis is unknown, the major toxic agent in AD is thought to be Aβ (Gilbert 2013), which accumulates in the brain and leads to neuronal cell death and ultimately dementia (Armstrong 2009; Reitz 2012). Mounting evidence now suggests the excessive accumulation of Aβ in AD is the result of impaired Aβ clearance from the brain (Castellano et al. 2011; Mawuenyega et al. 2010). Furthermore, studies in mouse models of AD have indicated that lowering Aβ levels in the brain can minimize neurodegeneration and slow cognitive decline (Boche et al. 2005). Thus, targeting clearance-related pathways may prove most effective in attenuating Aβ accumulation in the AD brain.
One explanation for the attenuated clearance in AD is dysfunctional Aβ transport at the blood-brain barrier (BBB). The low density lipoprotein receptor (LDLR) and the LDLR-related protein 1 (LRP1) are two BBB receptors that contribute to the brain-to-blood elimination of Aβ (Castellano et al. 2012; Deane et al. 2009). In addition to the transmembrane protein that transports molecules across the brain endothelium, these lipoprotein receptors also exist in a soluble form (Rebeck et al. 2006). The soluble receptor is generated via proteolytic cleavage at an extracellular region close to the cell surface, a process called ectodomain shedding (Begg et al. 2004; Etique et al. 2013; Selvais et al. 2010). When the soluble receptor is released from the cellular membrane, it retains the ability to bind ligands in the extracellular space (Grimsley et al. 1998; Quinn et al. 1997), but loses its functional capacity to internalize or transcytose ligands intracellularly (Rebeck et al. 2006; Selvais et al. 2010). It is believed the soluble receptor operates in a dominant negative fashion by attenuating the interaction between ligands and the membrane-associated receptor, thereby modulating endocytic activity and cell signaling (Etique et al. 2013; Rebeck et al. 2006).
While lipoprotein receptors interact with an array of ligands, one of the more closely associated is apolipoprotein E (apoE), which exists as three isoforms in humans (apoE2, apoE3, and apoE4). Numerous studies have acknowledged that possession of the apoE4 allele represents the strongest genetic risk factor for late-onset AD (Kim et al. 2009; Zhong and Weisgraber 2009). Our prior studies (Bachmeier et al. 2013) and the work of others indicate that when apoE is bound to Aβ, the BBB transport of Aβ is dramatically attenuated (Bell et al. 2007; Deane et al. 2008; Martel et al. 1997). However, when apoE is not bound to Aβ, apoE appears to have a supportive role in Aβ BBB clearance that is isoform-specific (Bachmeier et al. 2013). Along these lines, recent findings have suggested that apoE3 may promote Aβ clearance across the blood-cerebrospinal fluid barrier via the choroid plexus (Ruzali et al. 2012). As lipoprotein receptor shedding in the brain (and the BBB in particular) can be a major determinant in Aβ elimination, these studies investigated the influence of apoE on lipoprotein receptor shedding to further elucidate the role of apoE in Aβ removal from the brain.
Methods
Materials
Primary human brain microvascular endothelial cells (HBMEC) and associated culture reagents were purchased from Sciencell Research Laboratories (Carlsbad, CA, USA). Fibronectin, dextran (64,000–76,000 mol wt), and Hanks’ balanced salt solution (HBSS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DMEM/F-12 (Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12) and unlabeled human Aβ(1–42) were purchased from Invitrogen Corp. (Carlsbad, CA, USA). The enzyme-linked immunosorbent assay (ELISA) kits for LRP1 and LDLR were purchased from Cedarlane Labs (Burlington, NC, USA). The ELISA for human apoE was purchased from MBL International (Woburn, MA, USA).
Animals
All animals (male mice 4–6 months of age) were purchased from Taconic Farms (Germantown, NY, USA) and allowed to adapt to the vivarium for 2 weeks prior to any experimental procedures. Mice were singly housed in a temperature and humidity controlled room on a 12 hour light/dark cycle with free access to food and water. The apoE-targeted replacement (apoE-TR) mice were created by gene targeting, and carry one of the three human alleles (APOE2, APOE3, or APOE4) in place of the endogenous murine apoE gene (Sullivan et al. 1997). These mice retain the endogenous regulatory sequences required for apoE production and express the human apoE protein at physiological levels. The apoE knockout (apoE KO) mice were generated through disruption of the murine apoE gene, which results in a complete absence of the endogenous mouse apoE protein (Piedrahita et al. 1992). The wild-type mice were of the same background (C57BL/6) as the transgenic apoE animals described above. All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of the Roskamp Institute, Inc.
Aβ peptides
Using a standard process to limit aggregation, the Aβ peptides used in each of the studies were solubilized in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to acquire a monomeric/dimeric sample and minimize the formation of β-sheet structures as we previously described (Bachmeier et al. 2010).
ApoE isoforms
Dr. Mary Jo LaDu (University of Illinois at Chicago) kindly provided the mixed glial cultures. Cortical glial cultures were prepared from apoE-TR mice (apoE2-TR, apoE3-TR, or apoE4-TR) mice as previously described (Manelli et al. 2007). Briefly, dissected cortices from 1–2 day-old neonatal apoE-TR pups were dissociated by trypsinization and filtered sequentially through 100µm and 40µm cell strainers. Cells were plated in 150cm2 flasks (∼1½ brains per flask) and the medium (DMEM/F12 containing 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin/streptomycin) was changed every 3–5 days (Fan et al. 2011; Manelli et al. 2007). On day 10, confluent cultures were trypsinized and passaged into 75cm2 flasks (1 × 150cm2 flask into 4 × 75cm2 flasks). Upon confluency, cells were washed with serum-free media and incubated with serum-free media for 72 hours (Fan et al. 2011). Glial-conditioned media (GCM) was collected and centrifuged at 1,000g for 3 min to remove any residual cells. The GCM was concentrated (10x) using the Vivaspin 15 centrifugal concentrator with a molecular weight cutoff of 10,000 Da (Sartorius Mechatronics Corp., Bohemia, NY, USA). The resulting concentrate was analyzed for apoE content using a human apoE ELISA as per the manufacturer’s instructions and stored at −20°C until further use.
Antibodies
Polyclonal rabbit LRP1 antibody recognizing the 85 kDa C-terminal subunit (LRP-85) and polyclonal rabbit laminin (Sigma Chemical Co., St. Louis, MO, USA), monoclonal rabbit synaptophysin (Cell Signaling Technology, Inc., Danvers, MA, USA), and mouse monoclonal anti-actin, clone C4 (EMD Millipore Corp., Billerica, MA, USA).
Human brain endothelial cell culture
HBMEC were seeded at 50,000 cells per cm2 onto fibronectin-coated 6-well plates as previously described (Bachmeier et al. 2010). At approximately 80% confluency, the cells were treated with various concentrations (0.1, 0.2, 1, 2, and 10µM) of Aβ(1–42) and incubated for 48 hours at 37°C. Similarly, for the apoE studies, HBMEC cells were treated with each apoE isoform (25ng/ml) in the presence or absence of 2µM Aβ(1–42) and incubated for 48 hours at 37°C. It was recently determined that the average concentration of apoE found in the brain interstitial fluid (ISF) of the same apoE transgenic animals used in the current study is 25ng/ml (Ulrich et al. 2013). While the concentration of apoE is reported to be much greater in cerebrospinal fluid (5–10µg/ml) (Bekris et al. 2008; Wahrle et al. 2007; Yamauchi et al. 1999), these studies used the concentration found in the ISF, as this is most relevant in studying the brain microvasculature. Following each treatment period, the extracellular media was collected and the cell monolayer washed with ice-cold PBS. Cell lysates were collected using lysis buffer consisting of M-PER reagent (Pierce Biotechnology, Rockford, IL, USA) supplemented with phenylmethanesulfonyl fluoride (1mM) and Halt protease and phosphatase inhibitor cocktail (Thermo Scientific, Waltham, MA, USA). Cellular toxicity in the HBMEC was assessed via the extracellular media using a lactate dehydrogenase (LDH) detection assay (Roche Applied Science, Indianapolis, IN, USA).
Intracerebral Aβ(1–42) injections
Stereotaxic intracranial injections of Aβ were performed as previously described (Paris et al. 2011). Briefly, male mice (4–6 months of age) were anesthetized via inhalation using a 4% isoflurane / oxygen mix. While under anesthesia, the mice were injected bilaterally with 3µl of vehicle (DMSO) or 1mM human Aβ(1–42) into the caudate putamen of each hemisphere of the brain (0.5mm anterior to the bregma, 2 mm lateral to the midline, and 3 mm below the surface of the skull). Ten minutes after the second intracerebral injection, the mice were euthanatized. In addition, to determine the effect, if any, of the vehicle or the intracranial injection itself on lipoprotein receptor levels, we examined a group of age-matched naïve mice (i.e., no intracerebral injection). Upon sacrifice, all mouse brains were collected (minus the cerebellum) and the outer vessels and meninges were removed using a dry cotton swab (Coisne et al. 2005). All tissue samples were immediately snap frozen in liquid nitrogen and stored at −80°C.
Isolation of brain fractions
The cerebrovasculature and parenchyma from mouse brain tissue was isolated using a modified protocol (Triguero et al. 1990). As above, fresh mouse brains were collected (minus the cerebellum) and the outer vessels and meninges were removed using a dry cotton swab (Coisne et al. 2005). The mouse brains were pooled and minced with a blade prior to being ground with 6–8 passes of a Teflon pestle in a glass Dounce homogenizer (Erickson et al. 2012). Brain material was homogenized in fivefold excess of ice-cold HBSS containing 10 mM HEPES (Coisne et al. 2005). A sample of the brain homogenate was collected as a representation of the whole brain (Mitchell et al. 2011). An equal volume of 40% dextran solution was added to the brain homogenate for a final concentration of 20% dextran (Erickson et al. 2012) and immediately centrifuged at 6000g for 15 min at 4°C (Fryer et al. 2003). This procedure results in a pellet at the bottom of the container (cerebrovasculature) and a compact mass at the top of the solution (parenchyma) separated by a clear dextran interface (soluble fraction). The cerebrovascular pellet was washed with ice-cold HBSS and resuspended in lysis buffer. The parenchyma was collected in HBSS, centrifuged at 6000g for 10 min at 4°C, and the resulting pellet resuspended in lysis buffer. Finally, the dextran supernatant was added to an equal volume of HBSS, and centrifuged at 6000g for 5 min at 4°C to pellet any remaining cellular material. The supernatant was collected and all samples were stored at −80°C until analysis.
Lipoprotein receptor analysis
For the in vitro studies, extracellular media samples and cell lysates were analyzed by ELISA for human LRP1 and human LDLR as per the manufacturer’s instructions. Protein expression levels in the extracellular media were expressed as ng of LRP1 or LDLR per ml of media. For the in vivo samples, the cerebrovasculature, parenchyma, and soluble brain fraction were analyzed by ELISA for mouse LRP1 and mouse LDLR as per the manufacturer’s instructions and normalized to total protein content using the bicinchoninic acid (BCA) protein assay (Thermo Scientific, Waltham, MA, USA). Protein expression levels were expressed as ng of LRP1 or LDLR per mg protein for brain tissue and ng / ml for the soluble brain fraction.
Immunoblotting
The efficiency of the cerebrovascular isolation was assessed by light microscopy and immunoblotting using LRP-85 (marker for the membrane-bound subunit of LRP1), laminin (brain blood vessel marker), and synaptophysin (neuronal marker). Samples were examined for total protein content using the BCA protein assay. Brain supernatants were denatured by boiling in Laemmli Buffer (Bio-Rad, Hercules, CA, USA) and loaded (100µg of total protein) onto a Criterion 4–20% Tris-HCl gradient gel (Bio-Rad, Hercules, CA, USA). Migration transpired in 10x Tris/Glycine/SDS (Bio-Rad, Hercules, CA, USA) electrophoresis buffer diluted in deionized water using 50–130 V over a 2 hour period. Following migration, electrotransfer of 10x Tris/Glycine (Bio-Rad, Hercules, CA, USA) electrophoresis buffer and 20% HPLC grade methanol in deionized water to an Immun-Blot PVDF (polyvinylidene fluoride) membrane occurred overnight at 4°C and 90 mA. The membrane was blocked in 5% Blotting-Grade Blocker (nonfat dry milk) for 1 hour (Bio-Rad, Hercules, CA, USA) and then immunoprobed with antibodies for LRP-85 (1:500), laminin (1:800), synaptophysin (1:2000), and the housekeeping protein actin (1:1000) in 5% Blotting-Grade Blocker overnight. The membrane was washed with deionized water and exposed to HRP-linked secondary (1:1000) antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 hour. Following a 30 minute wash in deionized water, the membrane was revealed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Waltham, MA, USA) and exposed with a Bio-Rad ChemiDoc XRS molecular imager (Bio-Rad, Hercules, CA, USA).
Statistics
Statistical analyses were performed using an ANOVA and Bonferonni post-hoc test.
Results
Lipoprotein receptor shedding in human brain endothelial cells
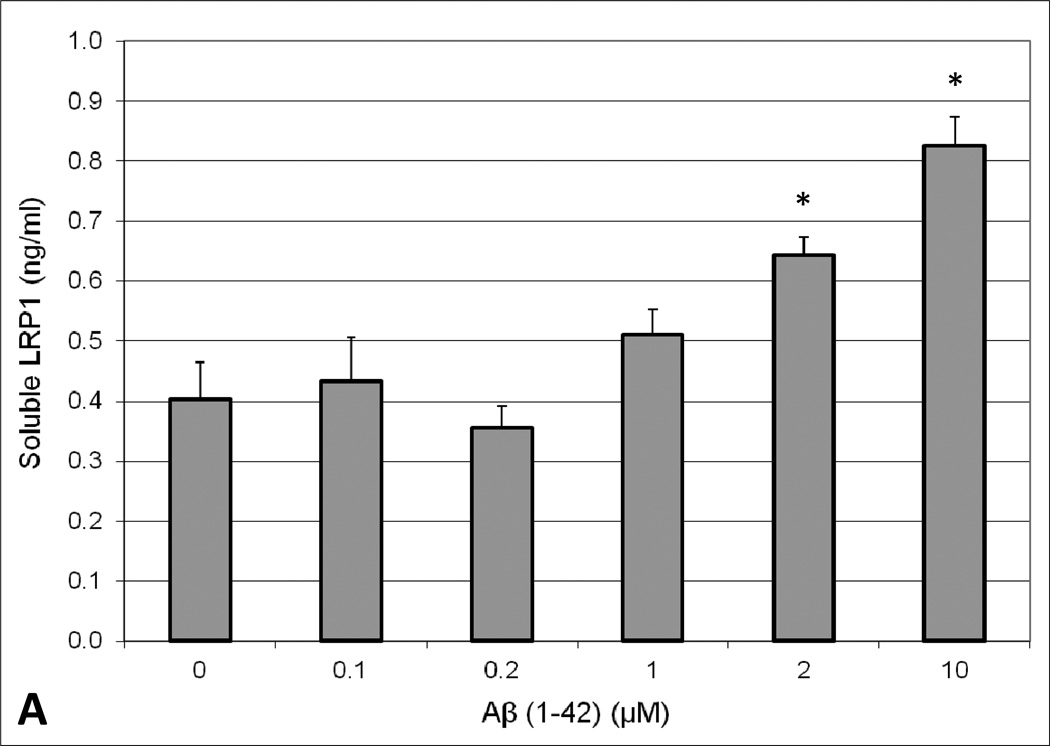
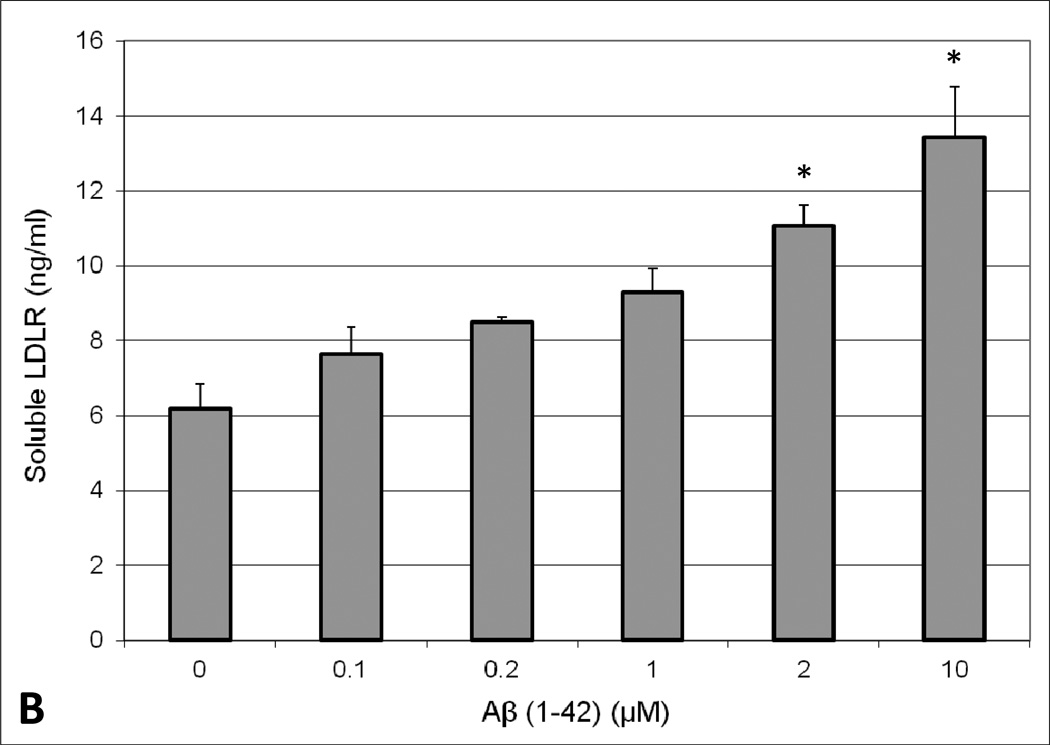
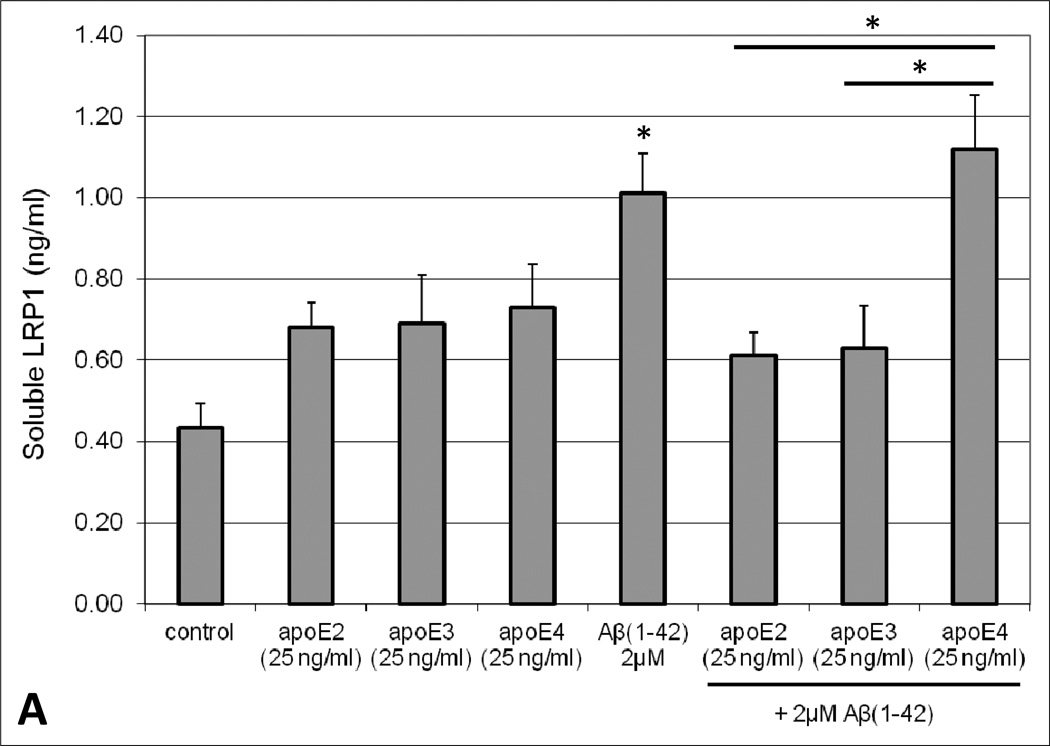
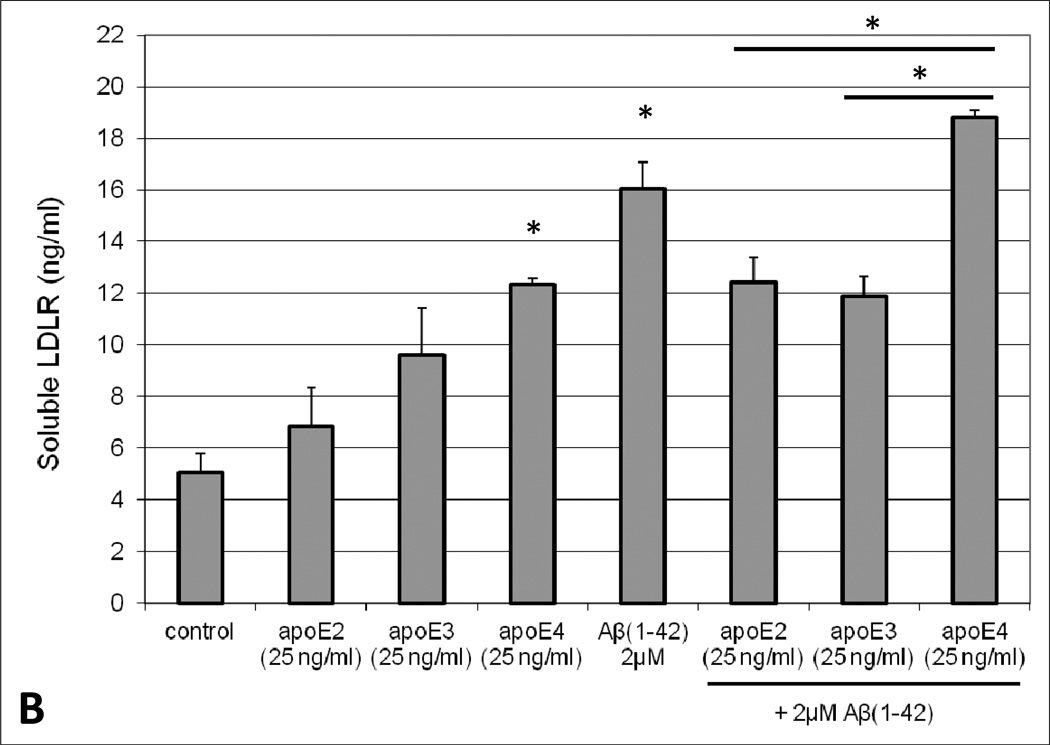
To determine the effect of Aβ exposure on lipoprotein receptor shedding in the BBB, human brain endothelial cells were treated with 2µM Aβ(1–42) for 48 hours and the extracellular media subsequently probed for soluble LRP1 and LDLR. A concentration-dependent increase in the appearance of LRP1 and LDLR in the media was observed upon exposure to Aβ(1–42). Moreover, Aβ concentrations ≥ 2µM resulted in a statistically significant increase (approximately 2-fold at 2µM) in lipoprotein receptor shedding compared to control conditions (Figure 1). Additionally, cellular toxicity was monitored using a LDH detection assay and there was no difference in LDH levels between control and Aβ-treated conditions (data not shown). The influence of apoE on lipoprotein receptor shedding was also examined in brain endothelial cells. For the most part, treatment with each apoE isofom alone demonstrated a modest increase in extracellular lipoprotein receptor levels compared to control conditions, though these values did not reach statistical significance and this effect was only around half of that produced with Aβ alone (Figure 2). For LDLR specifically, the differences between apoE isoforms were obvious, with a rank order of apoE2 < apoE3 < apoE4 (Figure 2B). Of these, only apoE4 treatment significantly altered LDLR levels (2.5-fold) from those observed under control conditions (Figure 2B). For the combination studies, co-treatment of apoE2 or apoE3 with Aβ mitigated the effect of Aβ on lipoprotein receptor shedding, reducing the levels in the media (at least for LRP1) to the baseline observed with each isoform alone (Figure 2A). In contrast, apoE4 treatment did not alter Aβ-induced lipoprotein receptor shedding as soluble LRP1 (Figure 2A) and soluble LDLR (Figure 2B) levels in the extracellular media were the same as those seen with Aβ insult alone. Importantly, for both lipoprotein receptors, the shedding levels in the media were significantly lower when apoE2 or apoE3 was administered with Aβ in comparison to apoE4 with Aβ (approximately a 1.6-fold difference). In addition, lipoprotein receptor expression was examined in the cell lysates and no statistically significant difference was observed between any of the treatment groups (data not shown).
Fig. 1.
Appearance of extracellular soluble (A) LRP1 or (B) LDLR in human brain endothelial cells (HBMEC) upon treatment with Aβ (1–42). HBMEC were exposed to various concentrations (0.1, 0.2, 1, 2, and 10 µM) of human Aβ(1–42) for 48 hours at 37°C. Following the treatment period, the extracellular media was collected and analyzed for LRP1 or LDLR content by ELISA. Values represent mean ± SEM (n = 3) and are expressed as ng of LRP1 or LDLR per ml of media. *P < 0.05 compared to control as determined by ANOVA and Bonferroni post-hoc test.
Fig. 2.
Appearance of extracellular soluble (A) LRP1 or (B) LDLR in human brain endothelial cells (HBMEC) in the presence of Aβ(1–42), apoE isoforms, or combinations thereof. HBMEC were treated with human Aβ(1–42) (2µM) and/or each apoE isoform (25ng/ml) for 48 hours at 37°C. Following the treatment period, the extracellular media was collected and analyzed for LRP1 or LDLR content by ELISA. Values represent mean ± SEM (n = 3) and are expressed as ng of LRP1 or LDLR per ml of media. *P < 0.05 compared to control. *P < 0.05 comparing apoE4 in the presence of Aβ versus apoE2 or apoE3 in the presence of Aβ, as indicated on the graph. Statistics determined by ANOVA and Bonferroni post-hoc test.
Lipoprotein receptor levels in vivo
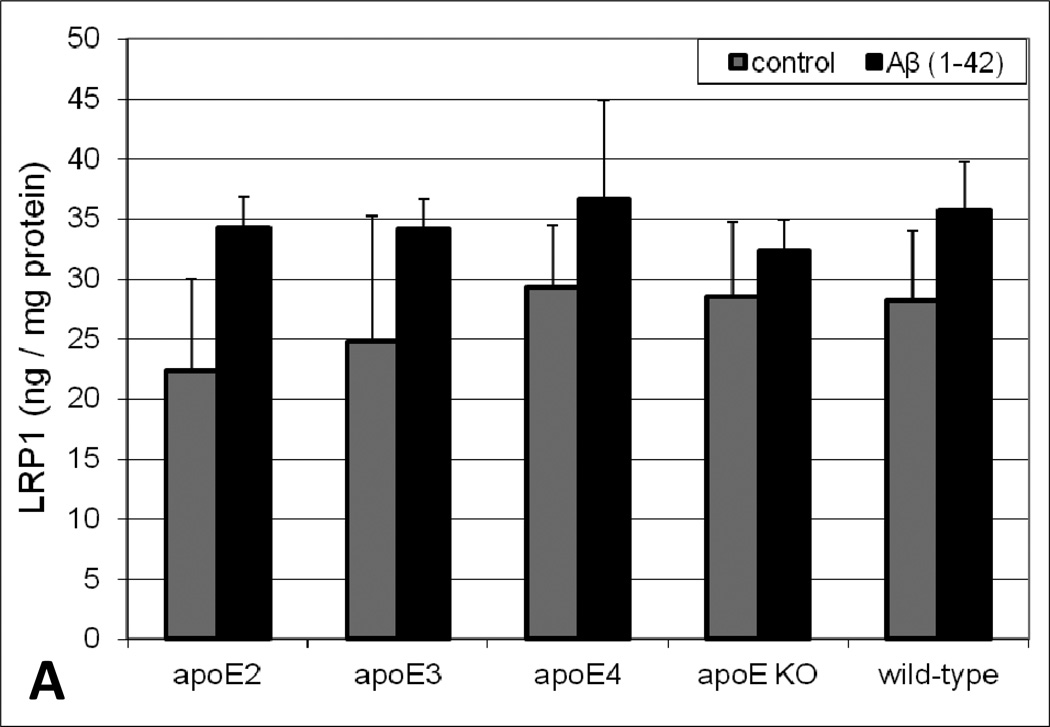
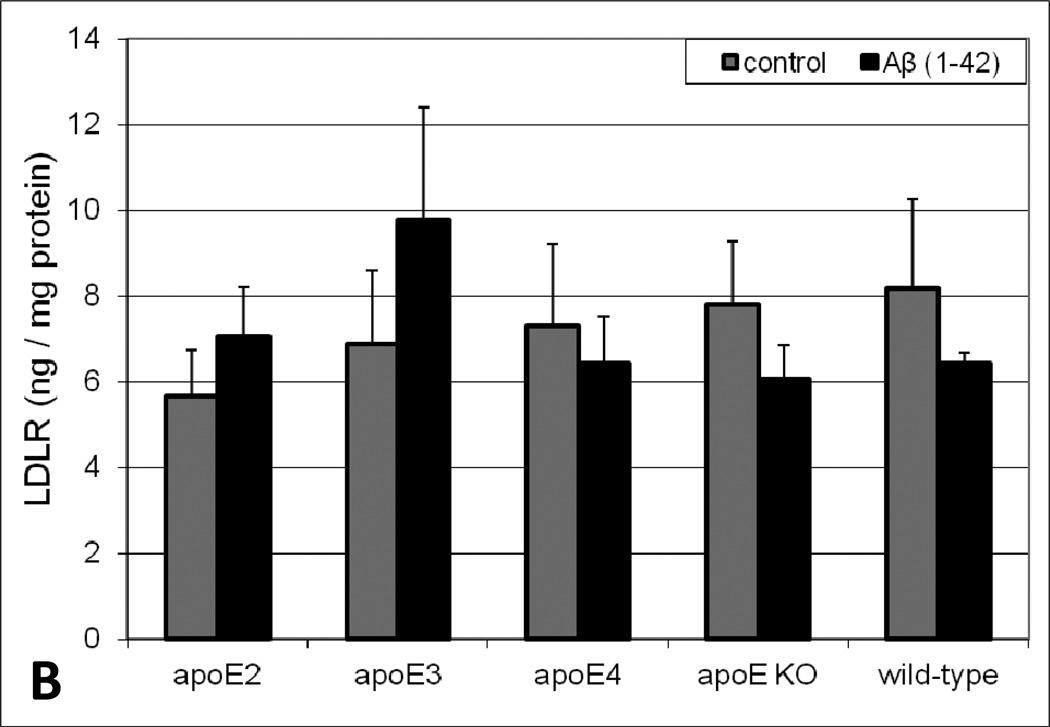
As a complement to the in vitro studies, brain lipoprotein receptor levels were evaluated in various brain fractions of apoE transgenic animals following acute intracerebral Aβ insult. While subtle variations in soluble LRP1 and LDLR levels in the parenchyma (data not shown) and cerebrovasculature (Figure 3) between apoE genotypes were apparent, there was no obvious trend and none of the values in these brain fractions were statistically different. While we did not observe demonstrable differences in lipoprotein receptor expression levels in the parenchyma and cerebrovasculature, the soluble brain fraction revealed both Aβ treatment- and apoE genotype-dependent differences in lipoprotein receptor levels. Under control conditions, the soluble brain levels of both LRP1 (Figure 4A) and LDLR (Figure 4B) varied across apoE genotype with a rank order of apoE2 < apoE3 < apoE4. Specifically, soluble lipoprotein receptor levels in apoE4 brains were significantly greater (2-fold) than that observed in apoE2 or apoE3 animals. Moreover, in every genotype, soluble brain lipoprotein receptor levels increased upon Aβ insult (especially for LRP1) compared to vehicle, with a rank order of apoE2 < apoE3 < apoE4 < apoE KO = wild-type (Figure 4). The most dramatic change was observed in the wild-type mice where soluble brain lipoprotein receptor levels were approximately 6-fold higher in the Aβ-treated animals compared to vehicle (Figure 4). Additionally, soluble brain lipoprotein receptor levels were examined in naïve mice (i.e., no intracerebral injection) and no significant differences were observed in these animals compared to the intracerebral vehicle-injected group (data not shown), indicating exposure to the vehicle or the intracranial injection itself does not appreciably impact the soluble levels of these proteins.
Fig. 3.
Expression of (A) LRP1 and (B) LDLR in cerebrovasculature isolated from apoE transgenic mice. Human Aβ(1–42) or vehicle was intracranially administered to male mice (4–6 months old). Ten minutes after the intracerebral injection, the brains were collected and various brain fractions were isolated. LRP1 or LDLR levels in the cerebrovasculature were determined using an ELISA and normalized to total protein content using the BCA protein assay. Values represent mean ± SEM (n = 6 animals) and are expressed as ng of LRP1 or LDLR per mg protein. No comparisons reached statistical significance as determined by ANOVA and Bonferroni post-hoc test.
Fig. 4.
Levels of (A) LRP1 and (B) LDLR in the soluble brain fraction of apoE transgenic mice. Human Aβ(1–42) or vehicle was intracranially administered to male mice (4–6 months old). Ten minutes after the intracerebral injection, the brains were collected and various brain fractions were isolated. LRP1 or LDLR levels in the soluble brain fraction were determined using an ELISA. Values represent mean ± SEM (n = 6 animals) and are expressed as ng of LRP1 or LDLR per ml of soluble brain material. *P < 0.05 comparing apoE4 to apoE2 or apoE3 for the respective vehicle and Aβ(1–42) groups as determined by ANOVA and Bonferroni post-hoc test.
Brain fraction isolation
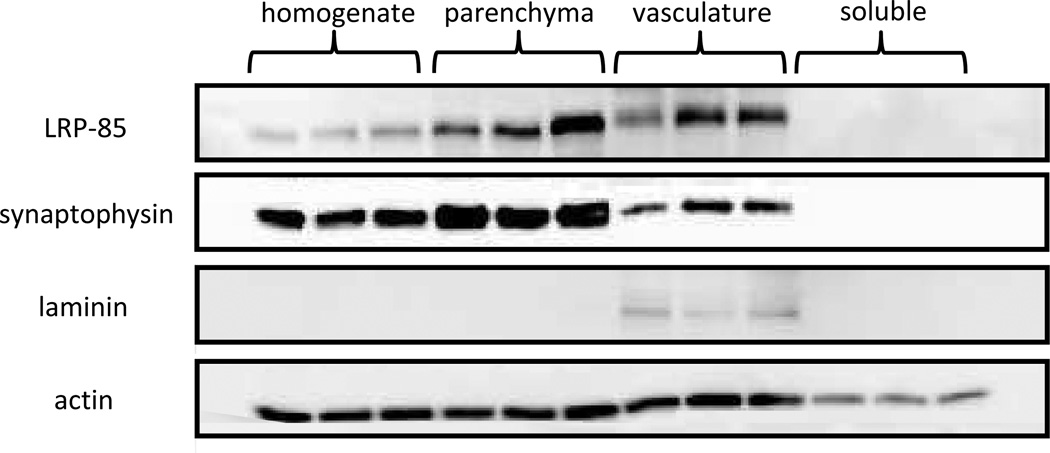
To assess the efficiency of our method to isolate various fractions of the brain, each of the resulting brain fractions were probed with specific protein markers (Figure 5). Since lipoprotein receptors are the focus of these studies, the presence of the C-terminal subunit of LRP1 (LRP-85) was examined in each brain fraction. LRP-85 contains the transmembrane and cytoplasmic domains of LRP1 and is associated with the cellular membrane prior to and following ectodomain shedding of the soluble receptor. The membranous LRP-85 was present in each of the cellular brain fractions (homogenate, parenchyma, and vasculature), which is expected as LRP1 is expressed in neurons (Bu et al. 1994) and cerebrovascular cells such as pericytes (Wilhelmus et al. 2007) and brain endothelia (Shibata et al. 2000). Furthermore, LRP-85 was not detected in the soluble protein fraction, indicating the soluble layer is devoid of cell-associated material. A neuronal marker (synaptophysin) and a blood vessel marker (laminin) were also examined in each brain fraction. Synaptophysin was present in each of the cellular fractions and not in the soluble preparation. While there were detectable levels of synaptopysin in the vasculature, these levels were considerably lower than those found in the parenchymal fraction. Laminin, on the other hand, was only detected in the vascular preparation, indicating all of the blood vessel components of the brain were confined to this fraction during the isolation process. Lastly, we employed a prototypical housekeeping protein (actin) and found similar levels in each fraction with the exception of the soluble layer, which is expected as this fraction consists only of soluble (non cell-associated) protein and would thus contain lower actin levels than the cell-based preparations (i.e., homogenate, parenchyma, and vasculature).
Fig. 5.
Western blot analysis of brain fractions isolated from wild-type mice. Brain fractions from wild-type male mice (4–6 months old) were examined for the presence of LRP-85 (marker for the membrane-bound subunit of LRP1), laminin (brain blood vessel marker), synaptophysin (neuronal marker) and the housekeeping protein actin. Samples were collected from the brain fractions of three naïve (i.e., no intracerebral injection) wild-type mice and loaded into separate lanes of the gel.
Discussion
Prior reporting has indicated Aβ clearance from the brain is differentially regulated by the type of apoE isoform expressed (Castellano et al. 2011). Multiple studies, including our own (Bachmeier et al. 2013), have demonstrated an isoform-specific disruption of Aβ transit across the BBB when Aβ is complexed with apoE (Bell et al. 2007; Deane et al. 2008; Martel et al. 1997). In addition, it has been proposed that soluble apoE (i.e., not bound to Aβ) can support Aβ clearance across the BBB in an isoform-dependent manner (Bachmeier et al. 2013). In line with this, recent findings suggest apoE3 may promote Aβ clearance across the blood-cerebrospinal fluid barrier in the choroid plexus as well (Ruzali et al. 2012). Despite the close association of apoE with lipoprotein receptors (Bu 2009; Zaiou et al. 2000), the manner in which apoE isoforms influence lipoprotein receptors and impact Aβ clearance from the brain is not entirely understood. To determine if the effects of apoE on brain Aβ removal are due to lipoprotein receptor quantity, we examined the expression of these receptors in both the parenchyma and cerebrovasculature isolated from the brains of apoE transgenic animals. While several studies have investigated the correlation between apoE and LRP1 expression in the brain (Akram et al. 2012; Arelin et al. 2002; Qiu et al. 2001; Shinohara et al. 2013), few have done so in relation to apoE genotype or isolated brain vasculature. Of those that have, LRP1 mRNA levels in whole brain homogenate were found to be different between genotypes in apoE transgenic animals (Kajiwara et al. 2010). At the protein level, in control and AD human brains, apoE genotype was not associated with significant variations in LRP1 in the frontal and occipital cortices or the meningeal blood vessels (Ruzali et al. 2012). Likewise, in our examination of lipoprotein receptor levels in the parenchyma and isolated brain vasculature of apoE transgenic mice, we did not observe substantial differences between apoE genotypes in LRP1 or LDLR protein expression. Thus, at least at the protein level in these apoE transgenic animals, it does not appear that alterations in lipoprotein expression are driving the isoform-specific effects of apoE on Aβ elimination from the brain.
Having observed a lack of variation in the lipoprotein expression amongst the apoE genotypes, we investigated the role of apoE in lipoprotein receptor processing, i.e., ectodomain shedding. When the soluble receptor is released from the cell following proteolysis, it is no longer involved in endocytic cellular transport (Rebeck et al. 2006; Selvais et al. 2010), which impairs Aβ clearance across the BBB. Lipoprotein receptor shedding has been shown to be induced by inflammation (Begg et al. 2004; Gorovoy et al. 2010), acute respiratory distress (Wygrecka et al. 2011), and exposure to Aβ(1–42) (Liu et al. 2009). In the current studies, treatment with Aβ(1–42) resulted in a dose dependent increase of lipoprotein receptor shedding in brain endothelial cells. In these same cells, we also examined the effect of each apoE isoform on lipoprotein receptor shedding and observed a modest increase upon apoE treatment. However, only apoE4 treatment resulted in shedding levels that were significantly greater than control (at least for LDLR) and the extent of shedding in the presence of apoE, regardless of isoform, was lower than that observed for Aβ. Importantly, there were no differences in lipoprotein receptor levels in the cell lysates between treatment groups indicating lipoprotein receptor expression changes are not driving the observed effects of Aβ and apoE in vitro, which is consistent with the in vivo findings discussed above. Our observation of increased lipoprotein receptor shedding in the presence of apoE is similar to prior work in which apoE binding to apoE receptors caused an increase in the release of the extracellular domain (Hoe and Rebeck 2005). Also, proteolysis of apoE receptors has been shown to be promoted by other ligands such as α2-macroglobulin and reelin (Hoe and Rebeck 2005), indicating some degree of receptor shedding is common with many ligands upon lipoprotein receptor binding, though the degree to which this occurs may vary between ligands. In the current studies, when the apoE isoforms were co-treated with Aβ in brain endothelial cells, Aβ-induced shedding in the presence of apoE2 and apoE3 was significantly lower than with apoE4. While apoE appears to play a role in the lipoprotein receptor shedding process (one that is isoform-specific), it is unclear whether apoE is simply less able to induce receptor shedding than other ligands (e.g., Aβ), or if apoE (apoE2 and apoE3 in particular) is meant to provide some protection to the lipoprotein receptor under certain conditions. Of note, a general protective role for apoE has been reported using a variety of experimental paradigms, and like our observations, these studies found apoE4 to be less adept than apoE3 in exerting a protective function (Buttini et al. 1999; Hayashi et al. 2007; Sen et al. 2012).
To complement our in vitro findings, we investigated lipoprotein receptor shedding in vivo by examining the soluble brain fraction in apoE transgenic animals. As prior reporting (Liu et al. 2009) and our in vitro studies demonstrate lipoprotein receptor shedding is induced by Aβ exposure, these in vivo studies also included an Aβ paradigm by administering Aβ via stereotaxic intracranial injection. Examination of the soluble fraction of the brain revealed substantial differences in lipoprotein receptor levels across apoE genotypes after Aβ insult. For mice administered vehicle intracerebrally, we observed differences in lipoprotein receptor shedding between apoE isoforms, similar to that found in vitro for apoE treatment alone (i.e., no Aβ exposure). Thus, even in the absence of Aβ insult, the degree of receptor shedding at baseline appears higher for apoE4 versus apoE2 or apoE3. In comparing the wild-type mice and the apoE KO animals, murine apoE does not appear to have a role in LRP1 shedding at baseline or in response to Aβ, while at the same time suppressing basal LDLR shedding levels. Consistent with the above in vitro studies, intracranial exposure to Aβ exacerbated receptor shedding in all genotypes with a rank order of wt = apoE KO > apoE4 > apoE3 > apoE2. Not only were the baseline shedding levels for apoE2 and apoE3 significantly lower than apoE4, but these two isoforms appear to offer more protection against Aβ insult as the degree of receptor shedding following intracerebral Aβ exposure was not nearly as extensive as that observed in the other genotypes (apoE4, apoE KO, and wt), especially for LDLR.
As mentioned above, the greatest degree of shedding was observed in the wild-type and apoE knockout animals, suggesting the presence of apoE (in particular human apoE) provides some protection to lipoprotein receptors from Aβ-induced shedding. This apparent protection provides rationale for reports that an absence of apoE altogether leads to reduced Aβ brain clearance (Shibata et al. 2000) and increased Aβ levels in the brain (DeMattos et al. 2004; Dodart et al. 2002). In the in vitro studies, apoE4 did not attenuate Aβ-induced lipoprotein receptor shedding as it did in the in vivo studies. This may be due to differences in cell type. The in vitro studies investigated lipoprotein receptor shedding exclusively in brain endothelial cells, while the in vivo studies examined lipoprotein receptor shedding in the soluble fraction of the entire brain. Thus, any lipoprotein receptor-expressing cell in the brain could have contributed to the soluble receptor levels we observed. This would include cells involved in metabolic Aβ clearance such as neurons (Kanekiyo et al. 2013), pericytes (Wilhelmus et al. 2007), smooth muscle cells (Kanekiyo et al. 2012), astrocytes (Basak et al. 2012; Koistinaho et al. 2004), and microglia (Lee and Landreth 2010). The dynamics of apoE4 in these cells may differ from brain endothelial cells, which might explain the discrepancy between the in vitro and in vivo studies. Moreover, it is important to note that altered lipoprotein receptor shedding by apoE in any of these cell types would likely contribute to Aβ accumulation in the AD brain. Overall, it is clear from both the in vitro and in vivo studies that an apoE isoform-specific effect exists such that lipoprotein receptor shedding is more prevalent in the presence of apoE4 than with apoE2 or apoE3.
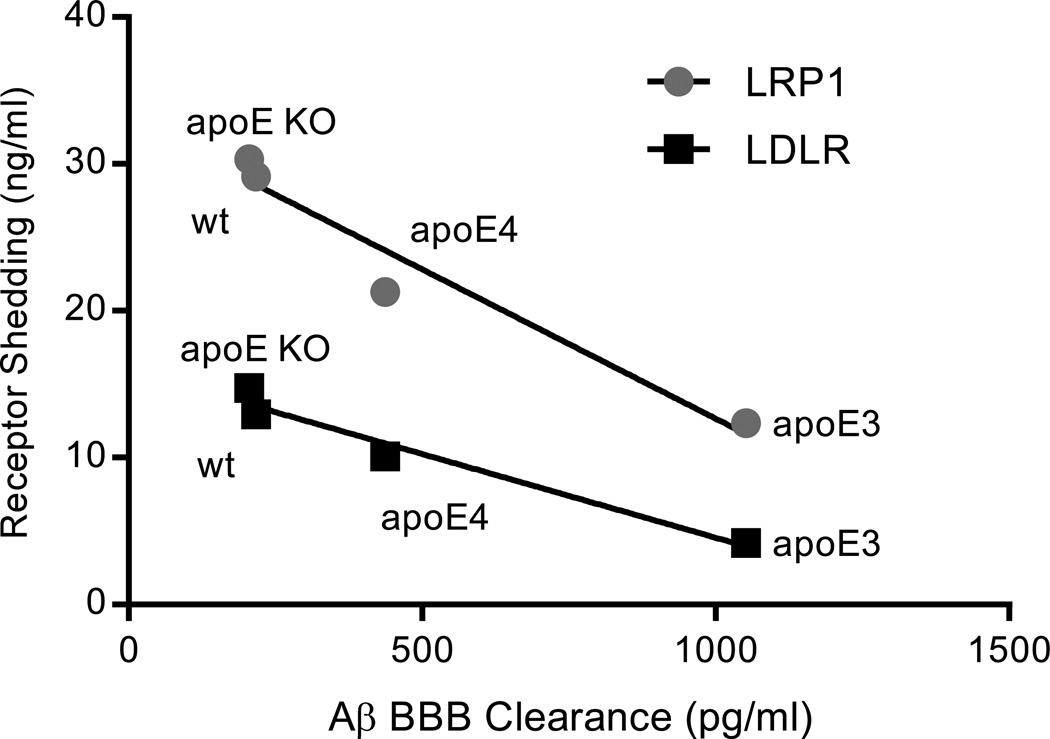
In correlating lipoprotein receptor shedding (in the current studies) with BBB-mediated Aβ clearance (from our prior work) (Bachmeier et al. 2013), there is a strong inverse relationship (p < 0.05, Pearson correlation) for both lipoprotein receptors (LRP1 and LDLR) that is apoE-genotype specific (Figure 6). Based on these data, Aβ clearance across the BBB appears to be at least partially mediated by lipoprotein receptor shedding, a process that is differentially regulated by the apoE isoforms. Receptor shedding not only depletes the population of endocytic transporters available for BBB clearance, but increases the concentration of soluble receptors in the extracellular space, which can bind Aβ (amongst other ligands) and extend its half-life in the brain. At this stage it is uncertain how apoE can alter Aβ-induced shedding. One explanation is that apoE directly binds to Aβ, preventing Aβ from accessing the liprorotein receptors. However, a recent study determined apoE does not readily bind soluble Aβ in physiological fluids, but instead acts through a shared receptor (e.g., LRP1) to influence Aβ brain removal (Verghese et al. 2013), a revelation that coincides with the findings of the current study and our prior work (Bachmeier et al. 2013). Alternatively, upon interacting with the lipoprotein receptor, apoE may promote Aβ endocytosis through cooperative binding or by inducing a conformational change in the receptor. Further exploration is necessary to understand the nature and consequence of these interactions.
Fig. 6.
Correlation between brain lipoprotein receptor shedding and Aβ clearance across the BBB in apoE transgenic mice. LRP1 and LDLR levels in the soluble fraction of the brain were plotted versus the appearance of Aβ in the plasma (i.e., Aβ BBB clearance) following intracerebral Aβ administration (LRP1, R2 = 0.94; LDLR, R2 = 0.96). The Aβ BBB clearance data was derived from our previously published work (Bachmeier et al. 2013). Values represent the mean of 6 animals for each genotype. p < 0.05 for both LRP1 and LDLR as determined by Pearson correlation.
Conclusion
Our previous findings and the work of others indicate an isoform-specific role for apoE in the elimination of Aβ from the brain (Bachmeier et al. 2013; Castellano et al. 2011). The present studies indicate apoE influences lipoprotein receptor shedding, a process which may explain the impact of apoE on Aβ brain BBB clearance, as increased shedding is associated with a loss of endocytic transport function (Rebeck et al. 2006; Selvais et al. 2010). Furthermore, the observed effect on shedding is apoE isoform-specific as both our in vitro and in vivo studies showed increased lipoprotein receptor shedding in the presence of apoE4, compared with apoE2 or apoE3, under basal conditions and following Aβ insult. Thus, apoE4 appears less efficient than other apoE isoforms in regulating lipoprotein receptor shedding, which culminates in reduced Aβ elimination from the brain. These studies further our understanding of the relationship between apoE and lipoprotein receptors and provide rationale for the elevated Aβ brain burden in apoE4 transgenic animals (Bales et al. 2009; Holtzman et al. 2000) and AD patients carrying the apoE4 allele (Bogdanovic et al. 2002; Schmechel et al. 1993). Moving forward, as our group (Kennelly et al. 2012) and others (Risner et al. 2006; Salloway et al. 2009) have observed that apoE4 carriers are often less responsive to therapeutic intervention than apoE4 non-carriers, new AD treatment modalities targeting this process could be particularly beneficial to individuals with this genotype.
Acknowledgements
We would like to thank the laboratory of Dr. Mary Jo LaDu (University of Illinois at Chicago) including Dr. Leon Tai and Susan Wohlgenant for isolating and preparing the mixed glial cultures. Research was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG041971. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of the Roskamp Institute, Inc.
The manuscript does not contain clinical studies or patient data.
References
- Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V. Association of ApoE and LRP mRNA levels with dementia and AD neuropathology. Neurobiol Aging. 2012;33(3):e1–e14. doi: 10.1016/j.neurobiolaging.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, et al. LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104(1):38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer's disease. Folia Neuropathol. 2009;47(4):289–299. [PubMed] [Google Scholar]
- Bachmeier C, Mullan M, Paris D. Characterization and use of human brain microvascular endothelial cells to examine beta-amyloid exchange in the blood-brain barrier. Cytotechnology. 2010;62(6):519–529. doi: 10.1007/s10616-010-9313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier C, Paris D, Beaulieu-Abdelahad D, Mouzon B, Mullan M, Crawford F. A multifaceted role for apoE in the clearance of beta-amyloid across the blood-brain barrier. Neurodegener Dis. 2013;11(1):13–21. doi: 10.1159/000337231. [DOI] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29(21):6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Abeta uptake and degradation by astrocytes. J Biol Chem. 2012;287(17):13959–13971. doi: 10.1074/jbc.M111.288746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg MJ, Sturrock ED, van der Westhuyzen DR. Soluble LDL-R are formed by cell surface cleavage in response to phorbol esters. Eur J Biochem. 2004;271(3):524–533. doi: 10.1046/j.1432-1033.2003.03953.x. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, Li G, et al. Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers Dis. 2008;13(3):255–266. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, et al. Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27(5):909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Nicoll JA, Weller RO. Immunotherapy for Alzheimer's disease and other dementias. Curr Opin Neurol. 2005;18(6):720–725. doi: 10.1097/01.wco.0000191513.60368.a7. [DOI] [PubMed] [Google Scholar]
- Bogdanovic N, Corder E, Lannfelt L, Winblad B. APOE polymorphism and clinical duration determine regional neuropathology in Swedish APP(670, 671) mutation carriers: implications for late-onset Alzheimer's disease. J Cell Mol Med. 2002;6(2):199–214. doi: 10.1111/j.1582-4934.2002.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J Biol Chem. 1994;269(28):18521–18528. [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19(12):4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Deane R, Gottesdiener AJ, Verghese PB, Stewart FR, West T, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc Natl Acad Sci U S A. 2012;109(38):15502–15507. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):57–67. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Coisne C, Dehouck L, Faveeuw C, Delplace Y, Miller F, Landry C, et al. Mouse syngenic in vitro blood-brain barrier model: a new tool to examine inflammatory events in cerebral endothelium. Lab Invest. 2005;85(6):734–746. doi: 10.1038/labinvest.3700281. [DOI] [PubMed] [Google Scholar]
- Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8(1):16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O'Dell MA, Taylor JW, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41(2):193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Johnstone EM, Little SP, Paul SM. Apolipoprotein E alters the processing of the beta-amyloid precursor protein in APP(V717F) transgenic mice. Brain Res. 2002;955(1–2):191–199. doi: 10.1016/s0006-8993(02)03437-6. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation. 2012;9:150–164. doi: 10.1186/1742-2094-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etique N, Verzeaux L, Dedieu S, Emonard H. LRP-1: a checkpoint for the extracellular matrix proteolysis. Biomed Res Int. 2013;2013:1–7. doi: 10.1155/2013/152163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Stukas S, Wong C, Chan J, May S, DeValle N, et al. An ABCA1-independent pathway for recycling a poorly lipidated 8.1 nm apolipoprotein E particle from glia. J Lipid Res. 2011;52(9):1605–1616. doi: 10.1194/jlr.M014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, et al. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23(21):7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BJ. The role of amyloid beta in the pathogenesis of Alzheimer's disease. J Clin Pathol. 2013;66(5):362–366. doi: 10.1136/jclinpath-2013-201515. [DOI] [PubMed] [Google Scholar]
- Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 2010;88(4):769–778. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley PG, Quinn KA, Owensby DA. Soluble low-density lipoprotein receptor-related protein. Trends Cardiovasc Med. 1998;8(8):363–368. doi: 10.1016/s1050-1738(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27(8):1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Regulation of ApoE receptor proteolysis by ligand binding. Brain Res Mol Brain Res. 2005;137(1–2):31–39. doi: 10.1016/j.molbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara Y, Franciosi S, Takahashi N, Krug L, Schmeidler J, Taddei K, et al. Extensive proteomic screening identifies the obesity-related NYGGF4 protein as a novel LRP1-interactor, showing reduced expression in early Alzheimer's disease. Mol Neurodegener. 2010;5(1):1–11. doi: 10.1186/1750-1326-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Cirrito JR, Liu CC, Shinohara M, Li J, Schuler DR, et al. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J Neurosci. 2013;33(49):19276–19283. doi: 10.1523/JNEUROSCI.3487-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer's amyloid-beta. J Neurosci. 2012;32(46):16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly S, Abdullah L, Kenny RA, Mathura V, Luis CA, Mouzon B, et al. Apolipoprotein E genotype-specific short-term cognitive benefits of treatment with the antihypertensive nilvadipine in Alzheimer's patients--an open-label trial. Int J Geriatr Psychiatry. 2012;27(4):415–422. doi: 10.1002/gps.2735. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117(8):949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Tran H, Verbeek MM, Reiss K, Estus S, et al. LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol Neurodegener. 2009;4:17–23. doi: 10.1186/1750-1326-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation. Neurobiol Aging. 2007;28(8):1139–1147. doi: 10.1016/j.neurobiolaging.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, et al. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer's amyloid beta. J Neurochem. 1997;69(5):1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117(4):735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- Paris D, Bachmeier C, Patel N, Quadros A, Volmar CH, Laporte V, et al. Selective Antihypertensive Dihydropyridines Lower Abeta Accumulation by Targeting both the Production and the Clearance of Abeta across the Blood-Brain Barrier. Mol Med. 2011;17(3–4):149–162. doi: 10.2119/molmed.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Elevation of LDL receptor-related protein levels via ligand interactions in Alzheimer disease and in vitro. J Neuropathol Exp Neurol. 2001;60(5):430–440. doi: 10.1093/jnen/60.5.430. [DOI] [PubMed] [Google Scholar]
- Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272(38):23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, LaDu MJ, Estus S, Bu G, Weeber EJ. The generation and function of soluble apoE receptors in the CNS. Mol Neurodegener. 2006;1:15–27. doi: 10.1186/1750-1326-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimers Dis. 2012;2012:1–11. doi: 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Ruzali WA, Kehoe PG, Love S. LRP1 expression in cerebral cortex, choroid plexus and meningeal blood vessels: relationship to cerebral amyloid angiopathy and APOE status. Neurosci Lett. 2012;525(2):123–128. doi: 10.1016/j.neulet.2012.07.065. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvais C, Dedieu S, Hornebeck W, Emonard H. Post-translational proteolytic events influence LRP-1 functions. Biomed Mater Eng. 2010;20(3):203–207. doi: 10.3233/BME-2010-0633. [DOI] [PubMed] [Google Scholar]
- Sen A, Alkon DL, Nelson TJ. Apolipoprotein E3 (ApoE3) but not ApoE4 protects against synaptic loss through increased expression of protein kinase C epsilon. J Biol Chem. 2012;287(19):15947–15958. doi: 10.1074/jbc.M111.312710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):1–23. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Petersen RC, Dickson DW, Bu G. Brain regional correlation of amyloid-beta with synapses and apolipoprotein E in non-demented individuals: potential mechanisms underlying regional vulnerability to amyloid-beta accumulation. Acta Neuropathol. 2013;125(4):535–547. doi: 10.1007/s00401-013-1086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54(6):1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, et al. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener. 2013;8(13):1–7. doi: 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A. 2013;110(19):E1807–1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Shah AR, Fagan AM, Smemo S, Kauwe JS, Grupe A, et al. Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol Neurodegener. 2007;2:1–9. doi: 10.1186/1750-1326-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Holler I, van Triel JJ, Veerhuis R, Maat-Schieman ML, Bu G, et al. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol. 2007;171(6):1989–1999. doi: 10.2353/ajpath.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wygrecka M, Wilhelm J, Jablonska E, Zakrzewicz D, Preissner KT, Seeger W, et al. Shedding of low-density lipoprotein receptor-related protein-1 in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;184(4):438–448. doi: 10.1164/rccm.201009-1422OC. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Tozuka M, Nakabayashi T, Sugano M, Hidaka H, Kondo Y, et al. Apolipoprotein E in cerebrospinal fluid: relation to phenotype and plasma apolipoprotein E concentrations. Clin Chem. 1999;45(4):497–504. [PubMed] [Google Scholar]
- Zaiou M, Arnold KS, Newhouse YM, Innerarity TL, Weisgraber KH, Segall ML, et al. Apolipoprotein E;-low density lipoprotein receptor interaction. Influences of basic residue and amphipathic alpha-helix organization in the ligand. J Lipid Res. 2000;41(7):1087–1095. [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the basis for the association of apoE4 with Alzheimer's disease: opening the door for therapeutic approaches. Curr Alzheimer Res. 2009;6(5):415–418. doi: 10.2174/156720509789207921. [DOI] [PMC free article] [PubMed] [Google Scholar]