Abstract
Background
Active surveillance (AS) is an important yet underutilized strategy to reduce prostate cancer (PCa) overtreatment.
Objective
To examine the 5-yr outcomes of AS in a population-based setting.
Design, setting, and participants
From the National Prostate Cancer Register of Sweden, we identified 11 726 men ≤70 yr diagnosed with very low-risk to intermediate-risk PCa from 2003 to 2007 who completed 5 yr of follow-up. Of these men, 1729 (15%) chose AS the primary management strategy.
Outcome measurements and statistical analysis
We calculated the probability of discontinuation of AS over time, and Cox proportional hazards models were used to determine factors associated with discontinuation. Reasons for discontinuation were assessed by data extraction from medical charts.
Results and limitations
By 5 yr, 64% of the men remained on AS. Predictors of discontinuation were younger age, fewer comorbidities, more education, higher prostate-specific antigen (PSA), and clinical stage T2 disease; marital status did not predict discontinuation. In a subset with data on the reason for discontinuation (86%), 20% of men discontinued because of patient preference, 52% because of PSA progression, 24% because of biopsy progression, and 3% for other reasons.
Conclusions
In a population-based setting, the majority of men remained on AS at 5 yr. However, one-fifth of the men who discontinued AS did so for nonbiologic reasons. Thus, there is a need for support and counseling for men to continue AS in the absence of signs of progression to improve adherence to AS and decrease overtreatment.
Patient summary
Active surveillance (AS) is an important option to delay or avoid treatment for men with favorable prostate cancer features. This study shows that at 5 yr, 64% of men across an entire population remained on AS. We concluded that AS is a durable option and that counseling may be useful to promote adherence for men without progression.
Keywords: Prostate cancer, Active surveillance, Adverse pathology, Predictors, Upgrading
1. Introduction
Active surveillance (AS) is an important management strategy to reduce prostate cancer (PCa) overtreatment [1]. While this strategy is supported in numerous guideline statements, adoption of AS demonstrates wide regional variability. AS is underutilized in the United States [2], whereas the majority of men with very low-risk PCa in Sweden currently select AS for their primary management strategy [3,4]. Little is known about long-term adherence to AS and the outcomes with this strategy. A more thorough understanding of these issues might help in encouraging broader adoption of AS.
A recent consensus statement from the National Institutes of Health emphasized the need for future research into the factors affecting adherence to AS [1]. A systematic review of seven major AS programs from around the world reported that one-third of patients received curative treatment after a median period of only 2.5 yr on AS [5]. However, these data may be difficult to generalize since they are based on a limited number of established AS programs, all with strict inclusion criteria and defined triggers for intervention. It is unclear to what extent the high rates of discontinuation are appropriate (ie, discontinuation for biologic reasons such as disease progression) or are secondary to patient fear, misinformation, or lack of emotional support. The latter reasons may present a potential target for interventions such as patient support and counseling.
Despite its great importance to the reduction of PCa overtreatment, relatively little is known about adherence to AS at the population level. Thus, the goal of our study was to examine real-world 5-yr adherence to AS using data from the entire nation of Sweden. We hypothesized that adherence rates would be lower across the entire Swedish population outside the confines of a predefined AS protocol. We obtained data on the reasons for AS discontinuation over time, which could have important implications for the successful implementation of this strategy around the world.
2. Patients and methods
The National Prostate Cancer Register (NPCR) of Sweden contains data on 98% of all PCa cases nationwide since 1998; in contrast, reporting to the Swedish Cancer Register is mandatory [4,6]. As previously described, clinical stage, prostate-specific antigen (PSA), biopsy Gleason score, ratio of positive biopsy cores, and primary therapy are recorded. Using the unique national identification number, cross-linkage was performed in Prostate Cancer Data Base (PCBaSe) between NPCR and several nationwide population-based health care registers and demographic databases, including the National Patient Register and the longitudinal integration database for health insurance and the labor market (LISA is its Swedish acronym), with data including marital status and educational level. The Charlson comorbidity index (CCI) was calculated based on discharge diagnoses in the patient register ≤10 yr prior to the date of PCa diagnosis and/or cancer diagnosis other than PCa in the NPCR [7].
In 2003, the NPCR began a follow-up study of all men ≤70 yr diagnosed with localized PCa (clinical stage T1/T2 with PSA <20 ng/ml). To examine 5-yr outcomes of AS, we identified 11 726 men from the follow-up study diagnosed with low- and intermediate-risk PCa from 2003 to 2007 with complete follow-up through December 31, 2013. As shown in Supplemental Figure 1, the primary treatment was radical prostatectomy for 57% of the men, radiation therapy in 18%, watchful waiting in 5%, hormonal therapy in 2%, and other forms of primary treatment in 2%. The remaining 1729 men (15%) were initially managed with AS and formed the current study population.
In these men, we examined adherence using Kaplan-Meier analysis to estimate the probability of discontinuation during follow-up. Multivariable Cox proportional hazards models were used to examine predictors of time to discontinuation of AS overall and for men in specific risk categories. In these models, the reference groups were year of diagnosis 2003, aged <60 yr, clinical stage T1c, Gleason score ≤6, very low-risk category, single, and low education; PSA and CCI were coded as continuous variables. A separate model was done with age as a continuous variable. Subset analysis was also performed in 1034 of the men on AS (60%) with data on the number of total and positive biopsy cores. To examine discontinuation by reason, we also calculated the cumulative incidence of discontinuation in a competing risks setting. The follow-up extraction form included the following options for reasons for discontinuing AS: PSA progression, biopsy progression (upgrading or a larger extent of cancer on biopsy), patient preference, and other reasons. Demographics and tumor features were then compared between men who discontinued AS because of biologic reasons (PSA or biopsy progression) versus because of preference. R v.3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. The study was approved by the research ethics board at Umeå University Hospital.
3. Results
Of 11 726 men aged ≤70 yr diagnosed with very low-risk to intermediate-risk PCa in Sweden nationwide from 2003 to 2007, 1729 men (15%) chose AS as initial management (Supplemental Fig. 1). Of these men, 644 (37%) were very low risk, 757 (44%) were low risk, and 328 (19%) were intermediate risk. Table 1 shows the demographics of the study population. During follow-up, 614 of 1729 men (36%) converted to active treatment.
Table 1.
Demographics and tumor characteristics of men in the 5-yr follow-up study in the National Prostate Cancer Register of Sweden who were placed on initial active surveillance
| Characteristic | Finding |
|---|---|
| Men, n (%) | 1729 (100) |
| Year of diagnosis, n (%) | |
| 2003 | 260 (15) |
| 2004 | 326 (19) |
| 2005 | 359 (21) |
| 2006 | 416 (24) |
| 2007 | 368 (21) |
| Age, yr, median (IQR) | 64.0 (60.0–67.0) |
| Clinical T stage, n (%) | |
| T1ab | 173 (10) |
| T1c | 1324 (77) |
| T2 | 232 (13) |
| Biopsy Gleason score, n (%) | |
| ≤6 | 1613 (93) |
| 7 | 116 (7) |
| Serum PSA, ng/ml, median (IQR) | 5.6 (4.1–8.0) |
| Biopsy cores, n (%) | |
| ≤6 | 514 (30) |
| 7–9 | 280 (16) |
| ≥10 | 240 (14) |
| Missing data | 695 (40) |
| Positive cores, n (%) | |
| ≤2 | 899 (52) |
| ≥3 | 135 (8) |
| Missing data | 695 (40) |
| Risk category, n (%)* | |
| Very low | 644 (37) |
| Low, not very low | 757 (44) |
| Intermediate | 328 (19) |
| Comorbidity, n (%) | |
| CCI 0 | 1425 (82) |
| CCI 1 | 165 (10) |
| CCI ≥2 | 139 (8) |
| Marital status, n (%) | |
| Single | 507 (29) |
| Married | 1222 (71) |
| Education, n (%)** | |
| Low | 569 (33) |
| Middle | 747 (43) |
| High | 403 (23) |
| Missing data | 10 (1) |
CCI = Charlson comorbidity index; IQR = interquartile range; PSA = prostate-specific antigen.
Risk categories modified from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: very low risk = T1c, Gleason ≤6, PSA <10 ng/ml, two or fewer positive cores; low, not very low risk = T1–2, Gleason ≤6, PSA <10 ng/ml; not very low, intermediate risk = T1–2, Gleason 7 and/or 10≤ PSA <20 mg/ml.
Educational level: low = compulsory school, ≤9 yr; middle = upper secondary school, 10–12 yr; high = college or university, ≥13 yr.
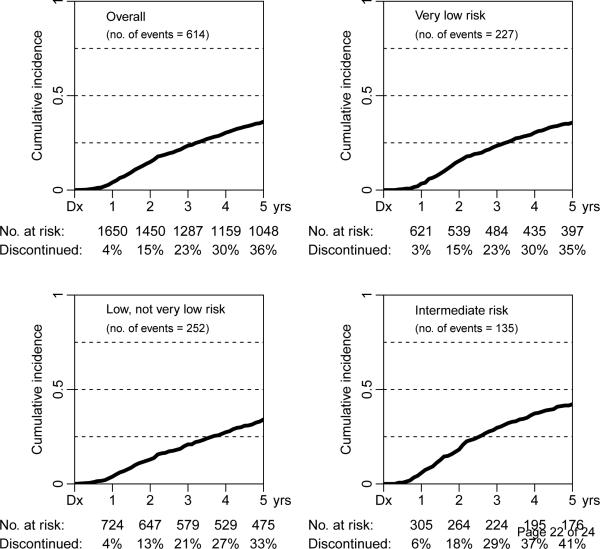
At 1 yr, 2 yr, 3 yr, 4 yr, and 5 yr, the probability of discontinuing AS was 4%, 15%, 23%, 30%, and 36%, respectively (Fig. 1). In the very low-risk group, the probability of discontinuing AS was 3%, 15%, 23%, 30%, and 35% at 1 yr, 2 yr, 3 yr, 4 yr, and 5 yr, respectively. In the low-risk group, the probability of discontinuing AS was 4%, 13%, 21%, 27%, and 33% at 1 yr, 2 yr, 3 yr, 4 yr, and 5 yr, respectively. In the intermediate-risk group, the probability of discontinuing AS was 6%, 18%, 29%, 37%, and 41% at 1 yr, 2 yr, 3 yr, 4 yr, and 5 yr, respectively. The median PSA at the time of discontinuing AS was 8.2 ng/ml, and the median absolute change from baseline to discontinuation was 3.0 (range: 0–32).
Fig. 1.
Time to discontinuation of active surveillance in the overall population and by clinical risk category in the National Prostate Cancer Register of Sweden. Dx = diagnosis.
On multivariable analysis (Table 2), clinical stage T2 (hazard ratio [HR]: 1.63; 95% confidence interval [CI], 1.32–2.02; p < 0.001 compared with nonpalpable disease), serum PSA (HR: 1.01; 95% CI, 1.00–1.01; p < 0.001), and high education (HR: 1.45; 95% CI, 1.17–1.80; p < 0.001) were significantly associated with greater risk of discontinuing AS. By contrast, men aged 65–70 yr (HR: 0.69; 95% CI, 0.55–0.85; p < 0.001) and men with high CCI (HR: 0.86; 95% CI, 0.75– 0.98; p = 0.02) had significantly lower risk of discontinuing AS. Men diagnosed in 2004 (HR: 0.61; 95% CI, 0.45–0.81; p < 0.001) and 2005 (HR: 0.69; 95% CI, 0.52–0.90; p = 0.007) also had a lower risk of discontinuation compared with men diagnosed in 2003. A separate multivariable model with age as a continuous variable found similar results, with a significantly lower risk of discontinuation with increasing age (HR: 0.97; 95% CI, 0.95–0.98; p < 0. 001). Another multivariable model in the subset with biopsy core data (n = 1034) showed that a greater number of positive biopsy cores was not a significant predictor of discontinuing AS (HR: 1.08; 95% CI, 0.98–1.19; p = 0.1).
Table 2.
Multivariable analysis for discontinuation of active surveillance
| HR | 95% CI | p value | |
|---|---|---|---|
| Year of diagnosis | |||
| 2003 | 1.00 | Ref. | |
| 2004 | 0.61 | 0.45–0.81 | <0.001 |
| 2005 | 0.69 | 0.52–0.90 | 0.007 |
| 2006 | 0.89 | 0.69–1.14 | 0.3 |
| 2007 | 0.96 | 0.75–1.25 | 0.8 |
| Age, yr | |||
| <60 | 1.00 | Ref. | |
| 60–64 | 0.94 | 0.76–1.16 | 0.5 |
| 65–70 | 0.69 | 0.55–0.85 | <0.001 |
| Clinical T stage | |||
| ≤T1c | 1.00 | Ref. | |
| T2 | 1.63 | 1.32–2.02 | <0.001 |
| Biopsy Gleason score | |||
| ≤6 | 1.00 | Ref. | |
| 7 | 1.27 | 0.94–1.73 | 0.1 |
| Serum PSA | 1.01 | 1.00–1.01 | <0.001 |
| Comorbidity | 0.86 | 0.75–0.98 | 0.02 |
| Marital status | |||
| Single | 1.00 | Ref. | |
| Married | 1.00 | 0.84–1.19 | 1 |
| Education | |||
| Low | 1.00 | Ref. | |
| Middle | 1.16 | 0.96–1.41 | 0.1 |
| High | 1.45 | 1.17–1.80 | <0.001 |
CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen; Ref. = reference.
The majority of men who discontinued AS (n = 420; 68%) received radical prostatectomy, while 32% underwent radiation therapy (Supplemental Fig. 1). Of the men who underwent radical prostatectomy, pathologic staging was available for 399. Pathologic stage T3 and N1 disease were present in 25% and 2% of these men, respectively.
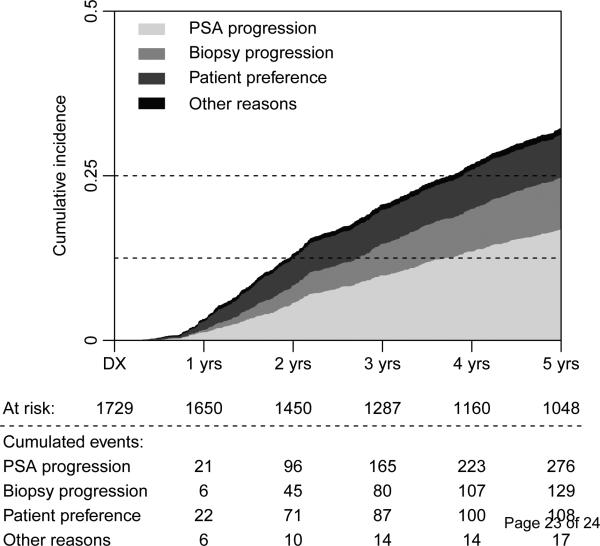
Data on the reason for discontinuation were available for 530 of 614 men who discontinued AS during follow-up (86%). In these men, the reason was patient preference in 108 men (20%), PSA progression in 276 men (52%), biopsy progression in 129 men (24%), and other reasons in 17 men (3%). Figure 2 shows the time to discontinuation according to reason for discontinuing AS. Among the men who discontinued AS because of PSA progression, the median (interquartile range) PSA at diagnosis and at discontinuation was 6.3 ng/ml (range: 4.7–8.9) and 9.8 ng/ml (range: 6.9–14.0), respectively.
Fig. 2.
Time to discontinuation of active surveillance by reason for discontinuation (biopsy/prostate-specific antigen progression, patient preference, or other reasons). Dx = diagnosis; PSA = prostate-specific antigen.
Table 3 shows separate multivariable models evaluating predictors of discontinuation for different reasons. Predictors of discontinuation because of patient preference were more recent diagnosis, clinical stage T2, biopsy Gleason 7, higher PSA, and high education; men aged 65–70 yr and men with increased comorbidities were less likely to discontinue because of patient preference. Predictors of discontinuation because of PSA or biopsy progression were clinical stage T2 disease and high education; men diagnosed in 2004–2005 and older men (65–70 yr) were less likely to discontinue AS because of biologic indications.
Table 3.
Multivariable analysis for predictors of discontinuing active surveillance because of patient preference or biopsy/prostate-specific antigen progression
| Patient preference | Biopsy/PSA progression | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Year of diagnosis | ||||||
| 2003 | 1.00 | Ref. | 1.00 | Ref. | ||
| 2004 | 0.67 | 0.46–0.99 | 0.05 | 0.55 | 0.29–1.01 | 0.06 |
| 2005 | 0.87 | 0.61–1.24 | 0.5 | 0.50 | 0.27–0.92 | 0.03 |
| 2006 | 1.13 | 0.81–1.58 | 0.5 | 0.64 | 0.37–1.12 | 0.1 |
| 2007 | 1.46 | 1.05–2.03 | 0.03 | 0.58 | 0.32–1.05 | 0.07 |
| Age, yr | ||||||
| <60 | 1.00 | Ref. | 1.00 | Ref. | ||
| 60–64 | 0.85 | 0.65–1.11 | 0.2 | 1.07 | 0.66–1.72 | 0.8 |
| 65–70 | 0.67 | 0.52–0.87 | 0.003 | 0.57 | 0.34–0.95 | 0.03 |
| Clinical T stage | ||||||
| ≤T1c | 1.00 | Ref. | 1.00 | Ref. | ||
| T2 | 1.69 | 1.31–2.19 | <0.001 | 1.80 | 1.10–2.95 | 0.02 |
| Biopsy Gleason score | ||||||
| ≤6 | 1.00 | Ref. | 1.00 | Ref. | ||
| 7 | 1.48 | 1.04–2.11 | 0.03 | 0.66 | 0.24–1.81 | 0.4 |
| Serum PSA | 1.01 | 1.00–1.01 | <0.001 | 1.00 | 1.00–1.01 | 0.6 |
| Comorbidity | 0.75 | 0.62–0.91 | 0.003 | 0.90 | 0.65–1.23 | 0.5 |
| Marital status | ||||||
| Single | 1.00 | Ref. | 1.00 | Ref. | ||
| Married | 0.99 | 0.80–1.24 | 1 | 1.11 | 0.72–1.70 | 0.6 |
| Education | ||||||
| Low | 1.00 | Ref. | 1.00 | Ref. | ||
| Middle | 1.16 | 0.91–1.47 | 0.2 | 1.55 | 0.95–2.52 | 0.08 |
| High | 1.45 | 1.11–1.89 | 0.007 | 1.78 | 1.04–3.05 | 0.04 |
CI = confidence interval; HR = hazard ratio; PSA = prostate-specific antigen; Ref. = reference.
4. Discussion
Using population-based data from the NPCR of Sweden, we found relatively high rates of adherence to AS, with a 64% probability of remaining on AS at 5 yr. These data suggest not only that AS is commonly accepted as the primary management strategy in Swedish men [3] but also that the majority of men continue AS during intermediate follow-up.
In the midst of ongoing debate about PCa screening, increasing use of AS is now widely recognized as an important strategy to preserve the ability of screening to identify men with high-risk PCa and simultaneously reduce downstream harms by limiting overtreatment [8]. Both in Sweden and in other countries around the world, an increasing proportion of men are initially choosing AS [3,9]. For example, in our previous paper based on data from 2007–2011 in the NPCR, 59% of very low-risk patients, 41% of low-risk patients, and 16% of intermediate-risk patients received AS for initial management [3], which continues to increase [4]. In men from the Gothenburg, Sweden, randomized PCa screening trial, nearly half (46%) were managed with AS, and 63% remained free from treatment at a median follow-up of 6 yr [10]. Similarly, a recent study from the British Association of Urologic Surgeons Cancer Registry reported that deferred treatment increased from 0% to 39% from 2000 to 2006 [11].
While these results are encouraging, for AS to truly fulfill the goal of delaying or avoiding unnecessary PCa treatment, long-term adherence is essential for men without evidence of disease progression. Unfortunately, adherence has been shown to be a challenge in some previous studies. In a study of US veterans by Lee et al, only 53% of the men followed through with the protocol-mandated repeat prostate biopsy at 1 yr [12]. Even in the very stringent Johns Hopkins AS protocol, only 59% and 41% of men remained free from intervention by 5 and 10 yr, respectively, suggesting that the majority did ultimately undergo curative treatment [13]. The current study is encouraging in that it demonstrates that a greater proportion of men (64%) did remain on AS at 5 yr across an entire population, outside the confines of a strict clinical protocol. From the public health perspective, it is critical to understand the underlying reasons for discontinuing AS. Among men in our population with a documented reason for AS discontinuation (n = 530), the majority sought curative treatment because of a rising PSA or biopsy progression, while approximately one-fifth discontinued AS because of patient preference. In our population, palpable disease and higher PSA were significantly associated with earlier AS discontinuation, suggesting appropriate instigation of treatment of men developing higher-risk tumor features. These results concur with a previous systematic review commissioned by the US Agency for Healthcare Research and Quality, in which higher clinical stage and PSA were associated with interruption of observational management strategies [14]. We also found that younger men and men with fewer comorbidities were more likely to discontinue AS.
Meanwhile, it is also important to understand the nonbiologic factors that influence AS adherence and therefore represent an important target for intervention. A recent study of men from the Prostate Cancer Research International Active Surveillance study in Italy suggested that the presence of a partner was associated with improved quality of life during AS [15]. Previous studies have reported that family members can dissuade men from pursuing conservative management [16], whereas other studies from the United States found that marital status did not affect the selection or discontinuation of observational management [17,18]. In our population, marital status did not predict discontinuation of AS, but higher education was a predictor of discontinuing AS. Creating an optimal form for support and counseling to continue AS in the absence of biologic progression is an important direction for further study.
Our study has several important strengths, including a 5-yr evaluation of adherence to AS across the entire nation of Sweden. This evaluation provides real-world data on the adherence to AS outside the confines of a specific protocol and on whether discontinuation was for biologic or nonbiologic reasons. In addition, the comprehensive cross-linkages of the NPCR to other national data sources allowed us to examine a greater number of predictors than many previous studies.
A limitation of our study is that we did not measure cancer-related anxiety, which previous studies have reported is a critical factor in adherence to AS [18]. Although there are data to suggest that consistent patient education and psychosocial interventions may promote adherence to AS [18,19], it was not possible to assess these interventions in our nationwide dataset. Other limitations of our study are that data from Swedish men may not be generalizable to other populations with different health care systems and cultural backgrounds. In addition, we do not have data on the follow-up protocols that were used, such as the number or interval of PSA tests, repeat biopsies, or imaging studies. While seemingly a limitation, this lack of data is actually a strength, demonstrating effectiveness in a real-world setting rather than merely effectiveness within the confines of a clinical trial. By contrast, data on the reason for discontinuation were available for 86% of men who discontinued AS, representing a unique insight into the factors involved in adherence.
5. Conclusions
In the nationwide Swedish registry, 64% of men remained on AS during 5 yr of follow-up. Younger men with fewer comorbidities, higher educational level, higher PSA, and higher-stage tumors were more likely to discontinue AS; other demographic and clinical factors were not significantly associated with discontinuation. One-fifth of men who discontinued AS did not have progression, suggesting the need for additional investigation into strategies of support and counseling to promote adherence. In a population-based setting outside clinical trials, we demonstrate that AS is a feasible and durable management strategy to reduce PCa overtreatment while at the same time maintaining the chances of detection of high-risk PCa.
Supplementary Material
Take-home message.
In Sweden, 64% of men remain on active surveillance for 5 yr. Age, comorbidities, education, prostate-specific antigen, and stage were predictors of discontinuation. One-fifth of men discontinuing active surveillance did not have progression, suggesting a role for support strategies to promote adherence.
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg, Ove Andrén, Anna Bill-Axelsson, Ann-Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm-Eriksson, Bodil Westman, Bill Pettersson, David Robinson, Mats Andén, Jan-Erik Damber, Jonas Hugosson, Ingela Frank-Lissbrant, Maria Nyberg, Göran Ahlgrén, Ola Bratt, René Blom, Rolf Lundgren, Lars Egevad, Calle Walller, Olof Akre, Per Fransson, Eva Johansson, Fredrik Sandin, Hans Garmo, Mats Lambe, Karin Hellström, Annette Wigertz, and Erik Holmberg.
Financial disclosures: Stacy Loeb certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stacy Loeb received an honorarium from Sanofi for speaking at the IGUCC conference. Danil V. Makarov has been a consultant for Castlight Health.
Funding/Support and role of the sponsor: The Swedish Research Council 825–2012–5047, Swedish Cancer Foundation 11 0471, Västerbotten County Council, and Lion's Cancer Research Foundation at Umeå University supported the collection and management of the data. Stacy Loeb is supported by the Louis Feil Charitable Lead Trust and the National Institutes of Health under award number K07CA178258. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Stacy Loeb had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Loeb, Bratt, Bill-Axelson, Stattin.
Acquisition of data: Bratt, Bill-Axelson, Stattin.
Analysis and interpretation of data: Loeb, Folkvaljon, Makarov, Bratt, Bill-Axelson, Stattin.
Drafting of the manuscript: Loeb, Folkvaljon, Stattin.
Critical revision of the manuscript for important intellectual content: Loeb, Folkvaljon, Makarov, Bratt, Bill-Axelson, Stattin.
Statistical analysis: Folkvaljon.
Obtaining funding: Loeb, Stattin.
Administrative, technical, or material support: Stattin.
Supervision: Loeb, Stattin.
Other (specify): None.
References
- 1.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Int Med. 2012;156:591–5. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–9. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 4. [May 28, 2014];National Prostate Cancer Register of Sweden. http://npcr.se/.
- 5.Dall'era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–83. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 6.Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer Data Base Sweden 2.0. Int J Epidemiol. 2012;42:956–67. doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 7.Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol. 2011;185:833–9. doi: 10.1016/j.juro.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 8.The Melbourne Consensus Statement on Prostate Cancer Testing [September 15, 2013];BJU International Web site. http://www.bjuinternational.com/bjui-blog/the-melbourne-consensus-statement-on-prostate-cancer-testing/. Updated August 7, 2013.
- 9.Evans SM, Millar JL, Davis ID, et al. Patterns of care for men diagnosed with prostate cancer in Victoria from 2008 to 2011. Med J Austr. 2013;198:540–5. doi: 10.5694/mja12.11241. [DOI] [PubMed] [Google Scholar]
- 10.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer: results from the Goteborg Randomised Population-based Prostate Cancer Screening Trial. Eur Urol. 2013;63:101–7. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 11.McVey GP, McPhail S, Fowler S, McIntosh G, Gillatt D, Parker CC. Initial management of low-risk localized prostate cancer in the UK: analysis of the British Association of Urological Surgeons Cancer Registry. BJU Int. 2010;106:1161–4. doi: 10.1111/j.1464-410X.2010.09288.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee EK, Baack J, Penn H, et al. Active surveillance for prostate cancer in a veteran population. Can J Urol. 2010;17:5429–35. [PubMed] [Google Scholar]
- 13.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 14.Dahabreh IJ, Chung M, Balk EM, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Int Med. 2012;156:582–90. doi: 10.7326/0003-4819-156-8-201204170-00397. [DOI] [PubMed] [Google Scholar]
- 15.Bellardita L, Rancati T, Alvisi MF, et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. Eur Urol. 2013;64:30–6. doi: 10.1016/j.eururo.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what's important. J Gen Int Med. 2000;15:694–701. doi: 10.1046/j.1525-1497.2000.90842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y, Carvalhal GF, Catalona WJ, Young JD. Primary treatment choices for men with clinically localized prostate carcinoma detected by screening. Cancer. 2000;88:1122–30. [PubMed] [Google Scholar]
- 18.Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment of patients with prostate cancer on surveillance. J Urol. 2007;178:826–31. doi: 10.1016/j.juro.2007.05.039. discussion 831–2. [DOI] [PubMed] [Google Scholar]
- 19.Goh AC, Kowalkowski MA, Bailey DE, Jr, Kazer MW, Knight SJ, Latini DM. Perception of cancer and inconsistency in medical information are associated with decisional conflict: a pilot study of men with prostate cancer who undergo active surveillance. BJU Int. 2012;110:E50–6. doi: 10.1111/j.1464-410X.2011.10791.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.