Abstract
Introduction
During exercise, the sympathetic nervous system is activated and blood pressure and heart rate increase. In heart failure (HF), the muscle metaboreceptor contribution to sympathetic outflow is attenuated and the mechanoreceptor contribution is accentuated. Previous studies suggest that lactic acid stimulates acid sensing channel subtype 3 (ASIC3), inducing a neurally mediated pressor response. Thus, we hypothesized that the pressor response to ASIC3 stimulation is smaller in HF rats due to attenuation in expression and function of ASIC3 in sensory nerves.
Methods
Lactic acid was injected into the arterial blood supply of the hindlimb to stimulate ASIC3 in muscle afferent nerves and evoke the muscle metaboreceptor response in control rats and HF rats. Also, western blot analysis was employed to examine expression of ASIC3 in dorsal root ganglion (DRG) and patch clamp to examine current response with ASIC3 activation.
Results
Lactic acid (4 µmol/kg) increased mean arterial pressure by 28±5 mmHg in controls (n=6) but only by 16±3 mmHg (P<0.05 vs. control) in HF (n=8). In addition, HF decreased the protein levels of ASIC3 in DRG (optical density: 1.03±0.02 in control vs. 0.79±0.03 in HF, P<0.05; n=6 in each group). The peak current amplitude of dorsal DRG neuron in response to ASIC3 stimulation is smaller in HF rats than that in control rats.
Conclusions
Compared with controls, cardiovascular responses to lactic acid administered into the hindlimb muscles are blunted in HF rats owing to attenuated ASIC3. This suggests that ASIC3 plays a role in engagement in the attenuated metaboreceptor component of the exercise pressor reflex in HF.
Keywords: blood pressure, afferent nerve, ASIC3, heart failure
INTRODUCTION
The sympathetic nervous system (SNS) is activated during exercise (34, 42). This leads to increases in blood pressure (BP), heart rate (HR), myocardial contractility, and vascular tone in inactive beds (15, 22, 26). A mechanism responsible for these exercise responses has been termed as the “exercise pressor reflex”, suggesting that afferents in contracting skeletal muscle are engaged and an autonomic reflex is initiated (10, 25). Group IV afferents are thought to be predominantly metabosensitive afferents, and group III afferents are thought to be predominantly mechanically sensitive (10). When these receptors are stimulated, thin-fiber muscle afferent nerves are engaged, cardiovascular nuclei in the brain stem are activated, sympathetic nerve activity (SNA) increases, and BP rises (25).
The accumulated data have demonstrated that lactic acid plays an important role in mediating the exercise pressor reflex (3, 23, 30, 34, 36, 40, 42). For example, static exercise increases muscle SNA via a reflex mechanism and the response is closely related to decreases in muscle pH in healthy subjects (42). Also, arterial injection of lactic acid into the hindlimb muscles reflexively increases BP and HR via its stimulation of metabosensitive muscle afferents in anesthetized cats (30). In addition, in healthy humans muscle SNA and BP responses to exercise are attenuated by muscle glycogen depletion or dichloroacetate to blunt contraction-induced lactic acidosis (3, 36). Moreover, acid sensing ion channels (ASICs), especially namely ASICs subtype 3 (ASIC3), have been identified to likely participate in the metabolic component component of the exercise pressor reflex (23, 40).
In heart failure (HF), during static handgrip the metaboreceptor contribution to muscle SNA is diminished (38), although the overall muscle sympathetic response is preserved (21). Subsequent studies in human subjects have suggested that activation of mechanosensitive afferents contributes to a greater degree in HF subjects than in controls (21, 24, 35). Moreover, data obtained a rat model of chronic HF demonstrated that BP response to stimulation of muscle metaboreceptors is blunted; whereas BP response to stimulation of mechanosensitive muscle afferents is amplified (13, 37).
Heightened SNA with mechanoreceptor stimulation in HF may contribute to the greater renal vasoconstriction seen early in exercise in HF(24, 28). This may allow HF subjects to partially compensate for reduced exercise muscle blood flow (7). However, the impaired metaboreceptor response may contribute to the impaired exercise tolerance seen with fatiguing exercise (32). Sympathetic tone at high workloads aids in the matching of blood flow and oxygen demand within the metabolically active muscle (41). Nevertheless, an important question as to what causes reduced responsiveness of metabosensitive muscle afferent nerves remains to be answered. Therefore, the purpose of this study was to examine the role played by ASIC3 in engagement in the attenuated metaboreceptor response in HF. Prior studies have demonstrated that in HF, exercise decreases intracellular pH of the exercising limb and increases venous level of lactic acid to a larger degree than in healthy individuals (31, 33). Accordingly, we postulated that the increased levels of lactic acid in the intersitium of active muscle could be one of mechanisms to blunt expression and function of ASIC3 in muscle afferent nerves and thereby lead to attenuated muscle metaboreceptor response with stimulation of lactic acid. Additionally, in chronic HF the levels of nerve growth factor (NGF) in sympathetic and afferent nerves are attenuated (6, 8) and prior studies further suggest that NGF is responsible for basal ASIC3 expression and its response (18, 20). Thus, we also postulated that NGF plays a role in attenuating ASIC3 expression and functional response with stimulation of muscle afferents.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of Penn State College of Medicine and complied with the NIH guidelines.
Coronary Artery Ligation
Male Sprague-Dawley rats (125 to 160 g) were anesthetized, intubated, and artificially ventilated. A left thoracotomy between the fourth and fifth ribs was performed, exposing the left ventricular wall. The left coronary artery was ligated (5, 13). Age and body weight-matched rats that underwent the same procedure as described except that a suture was placed below the coronary artery but was not tied served as controls. All experiments were performed six weeks after the surgery.
Transthoracic echocardiography was performed before the experiments. The rats were anesthetized by inhalation of an isoflurane-oxygen mixture. The transducer was positioned on the left anterior chest, and left ventricular dimensions were measured. In addition, a Millar catheter was inserted into the right carotid artery and was threaded into the left ventricle for measurement of left ventricular end-diastolic pressure (LVEDP) to further examine the rats’ cardiac function (5, 13). For the experiments of BP response, LVEDP was examined at the end of study. For the western blot and patch clamp experiments, LVEDP was examined before the dorsal root ganglion (DRG) tissues were taken.
Examination of the Reflex Cardiovascular Responses
Six control rats and eight HF rats were anesthetized by inhalation of an isoflurane-oxygen mixture. An endotracheal tube was inserted into the trachea and attached to a ventilator. Polyethylene catheters (PE-50) were inserted into the external jugular vein and common carotid artery for drug administration and measurement of arterial BP, respectively. The femoral arteries and arterial collaterals were isolated in both hindlimbs. The popliteal artery was cannulated with a PE-10 catheter for the injection of drugs into the arterial blood supply of the hindlimb muscles. The femoral and sciatic nerves of both legs were isolated so that they could be sectioned at the end of the study. Also, in this group of studies, decerebration was performed as previously described (5, 13, 37). Once the decerebration was completed, anesthesia was removed from the inhalant mixture. The animals were artificially ventilated, and respiratory parameters were monitored and maintained within normal ranges, as previously described (5, 13). Body temperature was maintained between 37.5°C and 38.5°C by a heating pad and heat lamps, and fluid balance was stabilized by a continuous infusion of saline.
Sixty minutes after surgery, lactic acid (4 µmol/kg) was injected into the arterial blood supply of the triceps surae muscle (17). The injection volume was 0.1 to 0.15 mL, and the duration of injection was 30 seconds. The same volume of saline was then injected to flush the arterial catheter. After waiting 20 minutes, the same dose of lactic acid was injected intra-arterially after sectioning of the femoral and sciatic nerves to determine that responses to lactic acid was via a reflex mechanism.
All measured variables were continuously recorded and stored on a computer that used the Power Lab system (AD Instruments, Castle Hill, Australia). Arterial BP was measured by connecting the carotid arterial catheter to a pressure transducer. Mean arterial pressure (MAP) was obtained by integrating the arterial signal with a time constant of 4 s. HR was determined from the arterial pressure pulse. The peak responses of MAP and HR to lactic acid were determined by the peak change from the control value.
Western Blot Analysis
Six control rats and six HF rats were anesthetized and decapitated. The DRG tissues were removed. The tissues were processed using a standard procedure to determine the protein levels of ASIC3 as reported previously (17). Briefly, total protein was extracted by homogenizing DRG sample in ice-cold radioimmunoprecipitation assay buffer and protein concentrations were measured. After being denatured, the samples containing 20 µg of protein were loaded onto gels and electrically transferred to a polyvinylidene fluoride membrane and incubated overnight with primary antibodies: rabbit anti-ASIC3 at 1:200 dilutions. Next, the membranes were washed and incubated with secondary antibodies: horse-radish peroxidase-linked anti-rabbit secondary antibody at 1:1,000 dilutions. The immunoreactive proteins were detected by enhanced chemiluminescence. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an x-ray film. Then, the film was scanned and the optical density of ASIC3 bands was analyzed using the NIH Scion Image software.
Electrophysiology
The eight control and six HF rats were anaesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). The skin was incised and pulled away from underlying muscle tissue, and the fluorescent retrograde tracer DiI (60 mg/ml) was injected into the white portion of the gastrocnemius muscle (44, 46). The injection volume of 1 µl was administered, and injection was repeated three times at different locations. The injection needle was left in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to permit the retrograde tracer to be transported to DRG neurons.
On the day to perform patch clamp experiments, the rats were anesthetized and decapitated. The L4–6 DRGs were quickly removed and transferred immediately into Dulbecco’s modified Eagle’s Medium (DMEM). The DRGs were minced, and the ganglion fragments were processed to obtain dissociated DRG neurons as described previously (44, 46). The cell suspension was centrifuged to remove the supernatant, and the cell pellet was resuspended in DMEM. The cells were then plated onto a 35-mm culture dish containing precoated coverslips.
Neurons were first visualized using a combination of epifluorescent illumination and differential interference contrast (DIC, 20–40X) optics on an inverted microscope (Nikon TE2000). Under DIC, images of Dil-positive neurons were displayed on a video monitor. Neurons were patched in the whole-cell configuration and recorded at a holding potential of −70 mV using a MultiClamp 700B amplifier (Axon Inc.). Seals (1–10 GΩ) between the glass electrode (2–5 MΩ resistance) and the cell were established in a modified Tyrode solution. After the whole-cell configuration was established, the cell membrane capacitance and series resistance were electronically compensated. All experiments were then conducted. Signals were acquired using the pClamp 9.0 software and experimental data were analyzed using the Clampfit software program. Neurons were considered proton-sensitive if acid solution elicited an inward current of > 50 pA in peak amplitude (44, 46).
Drugs stored in stock solutions were diluted in extracellular solution immediately before being used and were held in a series of independent syringes connected to corresponding fused silica columns (inner diameter 200 µm) (44, 46). The ends of the parallel columns were connected to a common silica column. The distance from the column mouth to the examined cell was 100 µm. Cells in the recording chamber were continuously bathed in Tyrode solution. The gravity-fed solutions containing each drug were delivered to the cells by controlling the corresponding valve switch (WP Instruments).
All DRG neurons used in this report were DiI-positive. A total of 41 responsive neurons obtained from eight control rats and six HF rats were tested. At the end of each experiment, the gastrocnemius muscle was dissected to confirm that DiI was located in the white portion of the gastrocnemius muscle.
Statistical Analysis
Experimental data were analyzed using one-way repeated measures analysis of variance (ANOVA). As appropriate, Tukey’s post hoc tests were used. All values were presented as mean ± SEM. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed using SPSS for Windows version 15.0.
RESULTS
General and Echocardiographic Measurements
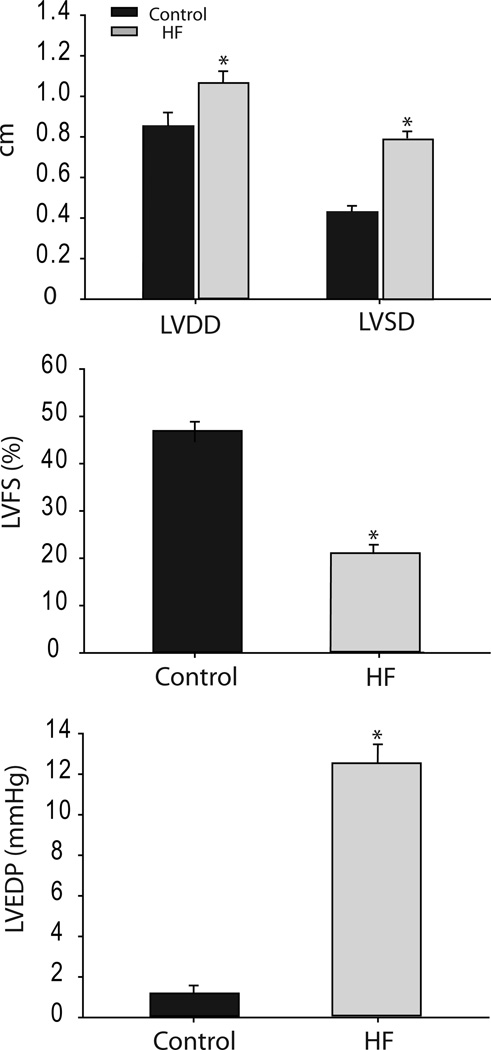
Left ventricular diastolic dimension (LVDD) and systolic dimension (LVSD) were determined by echocardiographic measurements. Then, the left ventricular fractional shortening (LVFS) was calculated as an index of the cardiac function. Rats with the left ventricular FS <30% showed increases in LVDD, LVSD and LVEDP. These rats were used as a HF group. In addition, LVFS is >40% in all control rats. Those measurements of the cardiac function are shown in Figure 1.
Figure 1.
Echocardiographic measurements and left ventricular end-diastolic pressure (LVEDP). *P <0.05, HF (n=20) vs. control (n = 20). LVDD: left ventricular diastolic dimension; LVSD: left ventricular systolic dimension; and LVFS: left ventricular fractional shortening.
Cardiovascular Responses Evoked by Lactic Acid
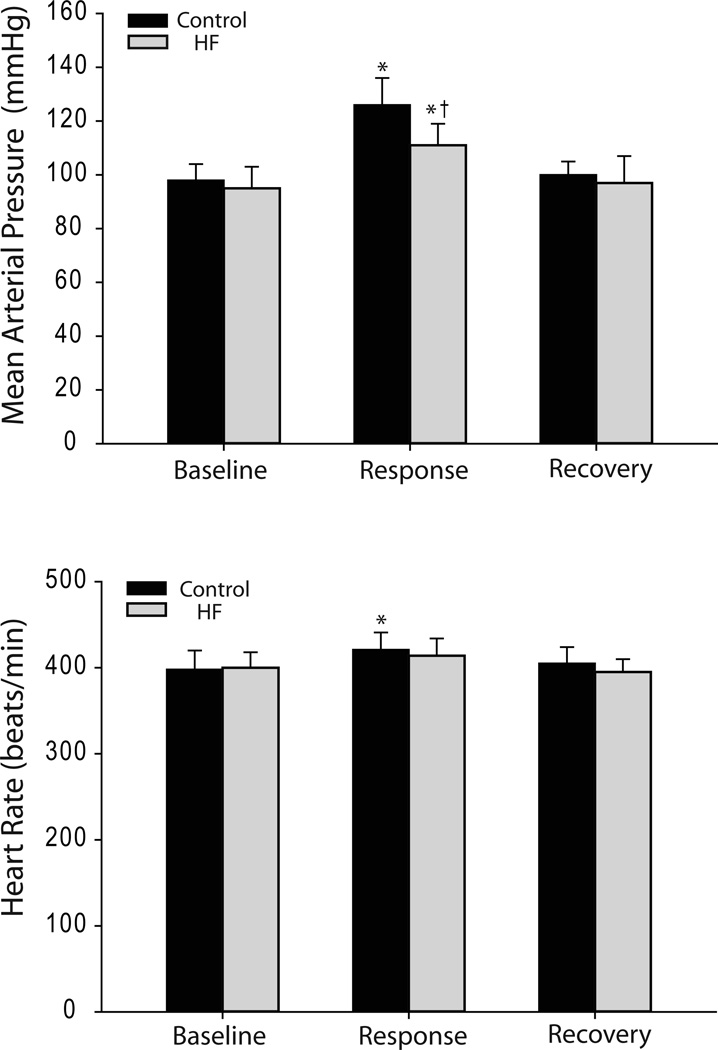
Baseline values for MAP and HR before arterial injections of lactic acid are presented in Figure 2. There were no significant differences in basal MAP and HR before drug injections in the control (n=6) and HF (n=8) groups. Lactic acid (4 µmol/kg) induced increases in BP and HR. BP was increased by 28 ± 5 mm Hg in control animals (baseline, 98 ± 6 mm Hg) but only by 16 ± 3 mm Hg (P<0.05 vs. control) in rats with chronic HF (baseline, 95 ± 8 mm Hg). Likewise, HR was elevated by 23 ± 3 bpm in control rats (P<0.05 vs. baseline) and 14 ± 3 bpm in HF rats (P>0.05 vs. baseline).
Figure 2.
Arterial administration of lactic acid (4 µmol/kg) into rat hindlimb muscle stimulated ASICs receptors and increased blood pressure. Increases in mean arterial pressure were attenuated in animals with HF (n=8) compared with control group (n=6). Data are mean ± SEM. *P < 0.05 vs. baseline and recovery in control group and HF group, respectively; †P < 0.05 vs. control. Note that the time between the lactic acid injection and the recovery measurements was ~2–3 min.
In addition, the pressor response evoked by lactic acid was attenuated by sectioning of the afferent nerves (30 ± 4 mm Hg before vs. 6 ±2 mm Hg after sectioning in control rats, P<0.05; and 20 ± 5 mm Hg before vs. 4 ± 1 mm Hg after sectioning in HF rats, P<0.05). This suggests that the acid-induced pressor response was mediated by engagement of a reflex pathway.
Expression of ASIC3 in DRG
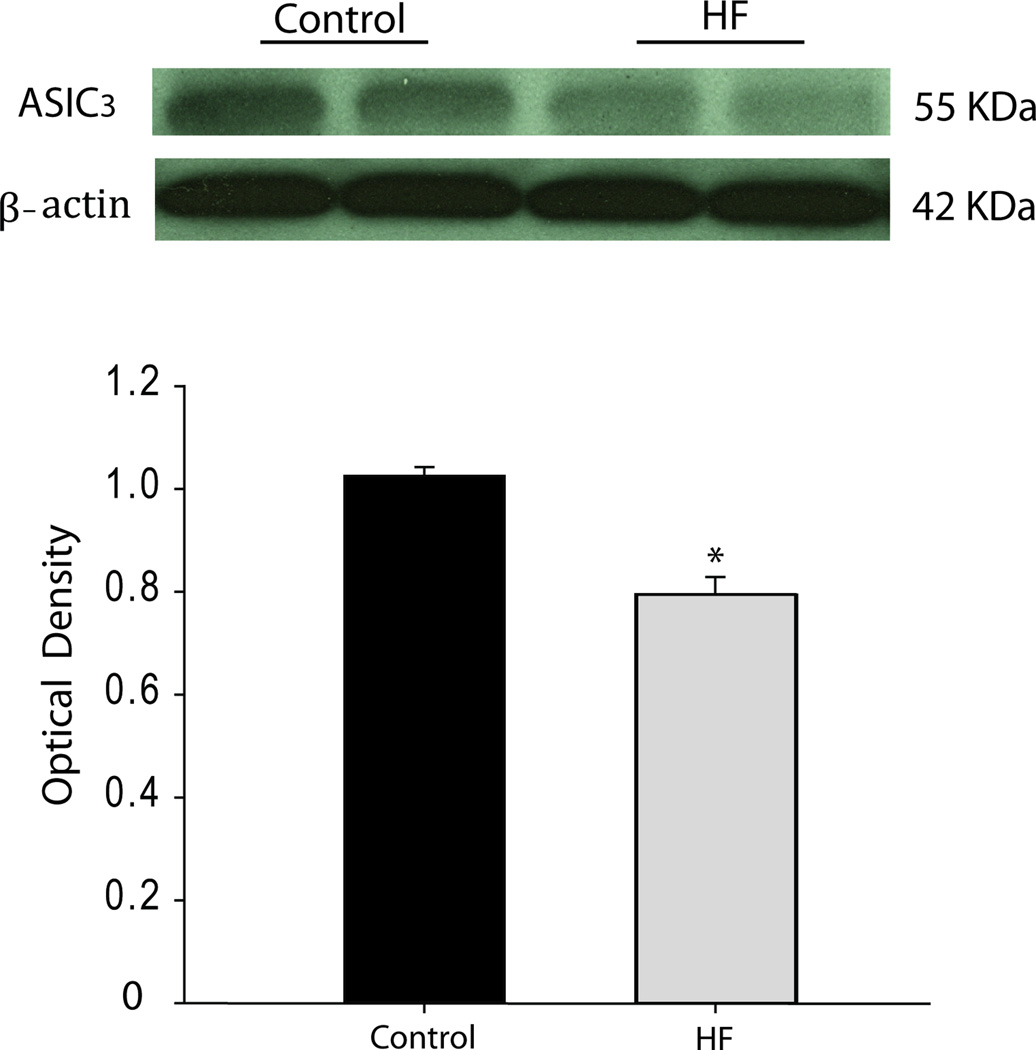
Figure 3 demonstrates typical blots and average data of ASIC3 expression in L4-L6 DRG tissues of control rats and HF rats. Chronic HF significantly decreased protein expression of ASIC3 in lumber DRG of HF rats. After six weeks of HF development, the intensity of ASIC3 signal in lumbar DRG tissues of HF was approximately 23% less than that in control (optical density: 1.03 ± 0.02 in control vs. 0.79 ± 0.03 in HF, P < 0.05; n=6 in each group).
Figure 3.
Expression of ASIC3 in dorsal root ganglion of control rats and HF rats. Top panels: Dual representative bands illustrate the protein levels of ASIC3 expression in control rats and HF rats. Bottom panels: Average data show that ASIC3 is significantly decreased in HF (n=6) compared with control (n= 6). Note that the same amount of protein was sampled to examine their individual expression. Beta-actin was used as control for equal loading of protein. *P <0.05 vs. control.
ASIC3-like Currents in DRG Neurons
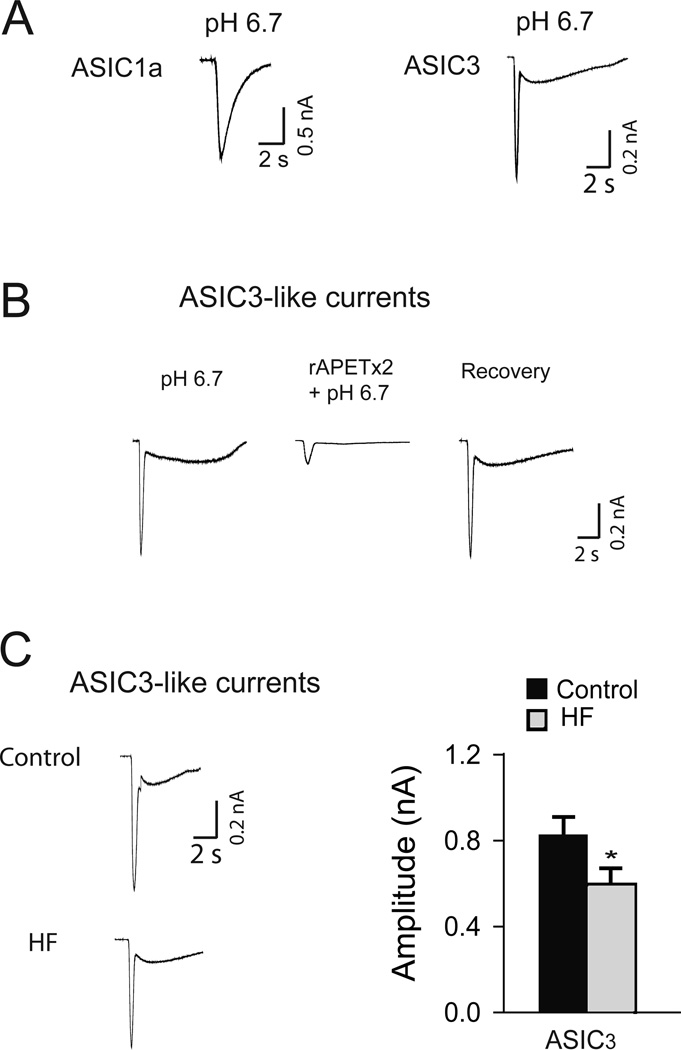
Because the prior studies have shown that ASIC3 is found predominantly on muscle afferent neurons and engaged in the reflex pressor response to muscle contraction (23, 40), in the current study we examined ASIC3-like current responses to pH 6.7 in DRG neurons of control rats and HF rats. As reported previously, two different types of ASIC currents were observed as pH 6.7 solution was applied onto DiI-labeled DRG neurons (44) (shown in Figure 4A). To distinguish ASIC currents, we examined their inactivation kinetics. Acid-induced currents with a slow inactivating rate are considered to be elicited by recombinant ASIC1a, and with rapid inactivation are typically observed as recombinant ASIC3 channels are activated. In this study, we also used a specific antagonist to ASIC3 channels rAPETx2 to determine ASIC3-like currents in DiI-labeled DRG neurons, as shown in Figure 4B. This figure further shows the inhibitory effects of rAPETx2 on ASIC3-like currents were reversible.
Figure 4.
ASIC3-like currents in DRG neurons innervating the hind limb muscles. (A), original traces of typical ASIC1a and ASIC3 current responses to pH 6.7. Their inactivation kinetics was examined to distinguish ASIC currents. (B), typical ASIC3-like current response to pH 6.7 with prior exposure to rAPETx2, a selective blocker of ASIC3. This was also used to determine ASIC3-like current in the current study. (C), original traces and averaged data showing that ASIC3-like current responses to pH 6.7 are attenuated in 16 responsive muscle DRG neurons of 6 HF rats (2–3 neurons/rat) as compared with 15 responsive muscle DRG neurons of 8 control rats (1–2 neurons/rat). *P < 0.05 vs .control.
Also, we examined the size distribution of DiI-labeled DRG neurons responding to pH 6.7 with ASIC3-like currents recorded in control and in HF groups. DRG neurons with ASIC3-like currents were distributed in small, medium and large size neurons as reported previously (44). The similar size distribution was observed in control and HF rats. However, DiI-labeled DRG neurons of HF rats exhibited a smaller peak inward ASIC3-like current when compared with that in the control. Figure 4C shows typical recordings from two neurons that express ASIC3-like current, which were obtained from a control rat and a HF rat, respectively. Averaged data in Figure 4C further demonstrates that the peak amplitude of induced current was 0.83 ± 0.1 nA in 15 neurons obtained from 8 control rats (1–2 neurons/rat), and 0.58± 0.1 nA in 16 neurons obtained from 6 rats with HF (2–3 neurons/rat, P < 0.05 vs. control). Moreover, chronic HF decreased the percentage of neurons expressing ASIC3-like current evoked by pH 6.7 from 47 ± 6% in control to 36 ± 5% in HF (P < 0.05 vs. control).
DISCUSSION
ASICs are members of a family of amiloride-sensitive sodium channels, and considered as molecular sensors in afferent neurons (9, 16, 27, 43, 47). They are almost ubiquitous in the mammalian nervous system and are activated as pH drops below 7.0. There are six different proteins of ASICs, ASIC1a, 1b, 2a, 2b, 3, and 4, encoded by four genes (ASIC1, 2, 3, and 4). Moreover, blocking ASICs receptors using amiloride and more selective antagonist A-317567 attenuated the pressor response evoked by static exercise and by arterial injection of lactic acid into the hindlimb muscles (23, 40). Thus, acid sensing has been considered as an important nature of sensory neurons with thin fiber afferents and contributes to the reflex cardiovascular responses to muscle contraction. The results of our previous studies also show the role played by ASICs in engagement of the pressor response to lactic acid (17). By recording discharges of the thin fiber muscle afferent nerves in cats, a recent study further suggests that ASICs participate in the metabolic component of the exercise pressor reflex (23).
The ASIC3 protein is mostly found in DRG where it forms functional channels that are opened by proton concentrations (43). It is well reasoned that ASIC3 is likely to play a role in engagement in the exercise pressor reflex. First, the ASIC3 receptors are activated with pH ranges that are seen in exercising muscles (19, 39). Second, lactate that is accumulated in active muscles tissues can enhance ASIC3 sensitivity to protons (9). Third, ASIC3 is a dominant ASIC subunit, preferentially localized in DRG neurons of thin fiber muscle afferent nerves (44). Also, a prior investigation supports the idea that ASIC3 are unlikely to contribute to mechanically activated currents in mammalian sensory neurons (1). Thus, ASIC3 is a suitable sensor for lactic acidosis as muscles undergo anaerobic metabolism.
Previous studies have suggested that the muscle metaboreceptor contribution to the SNA response to exercise is attenuated in HF (13, 37, 38); however, the underlying mechanisms responsible for these observations need to be defined. For the first time, the present study offers data suggesting that 1) BP response to stimulation of metabolically sensitive muscle afferents by lactic acid is blunted in HF rats and 2) ASIC3 expression and amplitude of current response with stimulation of ASIC3 in muscle sensory neurons of HF rats are reduced as compared with those expression and response in control rats. In HF, heightened SNA with mechanoreceptor stimulation and impaired metaboreceptor response are general notions to explain for decreased muscle blood flow during exercise and tolerance observed with fatiguing exercise (7, 24, 28, 32). Thus, results of the current study have addressed an important question as to what causes reduced responsiveness of metabosensitive muscle afferent nerves. Our data suggest that ASIC3 contributes to the attenuated metaboreceptor response in HF. Those results further indicate the role of sympathetic tone at high workloads in the matching of blood flow and oxygen demand within the metabolically active muscle (41)
In addition, abnormal responses of metabolically sensitive TRPV1 have been observed in rats with HF (13, 37). For instance, TRPV1 expression is found to be less in afferent nerves of HF rats compared with control animals (37), and the pressor response of TRPV1 activation is attenuated in HF (13, 37). Nevertheless, TRPV1 has been reported to play little role in mediating the cardiovascular responses to activation of muscle afferent in healthy cats, although capsaicin, a TRPV1 agonist injected into the arterial blood supply of the hindlimb muscles evokes increases in BP and HR (11). It is speculated that TRPV1-induced reflex responses require H+ (lower pH) in the muscle interstitium. It has been reported that TRPV1 and ASICs play a coordinated role in the processing of muscle sensory signals (4, 14). In situations without acidosis, the TRPV1 may not be effectively active. This idea is supported by another work showing that receptors mediating protons and capsaicin responses coexist in the DRG neurons innervating muscle because the responsiveness of acidosis and capsaicin is sensitized by each other (46).
Also, in HF the muscle mechanoreceptor contribution to sympathetic outflow is accentuated (13, 24, 38). Specifically, our studies have demonstrated that ATP accentuates muscle mechanoreceptor responses via P2X receptors and P2X-mediated muscle reflexes are amplified in rats with HF (13). Prostaglandin and bradykinin are also engaged in the exaggerated SNA response in HF (12, 29). Of note, heightened expression of P2X3 in sensory nerves and cyclo-oxygenase-2 within the hindlimb muscles contributes to augmented mechanoreceptor responses in HF (5, 29). It is speculated that muscle metabolites are accumulated to a greater degree in ischemic muscles of HF, which can sensitize mechanically sensitive muscle afferent nerves and enhance autonomic responses during activation of muscle mechanoreflex (35). However, how attenuated metaboreceptors (i.e. ASIC3 and TRPV1) in sensory muscle afferent nerves contribute to augmented muscle mechaoreflex or the overall muscle sympathetic response in HF remains unclear.
In this study, we have postulated that increased lactic acid levels in skeletal muscle interstitium of HF blunt expression and function of ASIC3 in muscle afferents. Nonetheless, this speculation seems inconsistent with the results observed in our previous work (17, 44), demonstrating that in rats with 24–72 hrs of femoral artery occlusion which possibly increased lactic acid levels in skeletal muscle interstitium causally upregulated ASIC3 and enhanced ASIC3 function. In HF with a chronic low flow state, the levels of NGF in afferent nerves are attenuated (6, 8), and the responses of NGF are closely related to impaired myocardial function (2). The reduced levels of NGF in HF are likely due to a systemic reduction of blood flow induced by damaged cardiac output. However, the femoral occlusion is an insufficient low flow rate. Hindlimb occlusion leads to localized muscle ischemia. Moreover, we have observed that the levels of NGF in DRG tissues are elevated 24–72 hrs after femoral ligation (45). Importantly, prior studies suggest that NGF is responsible for basal ASIC3 expression and functional response of muscle afferent nerves (18, 20). Thus, a distinction in ASIC3 expression and function between two ischemic diseases (HF and peripheral artery disease) is likely due to the levels of NGF and their different engagement in afferent nerves.
Study Limitations. ASIC3 is expressed in small- to large-diameter DRG cells (27). In this study, we examined the current response of muscle DRG neurons with stimulation of ASIC3. Nonetheless, it is unlikely to differentiate those neurons as C-fiber (group IV) or Aδ-fiber (group III). Thus, it should be noted that DRG cells analyzed in this study might not exclusively represent the cells that innervate C- fiber afferents.
CONCLUSIONS
Our data demonstrate that expression and function of ASIC3 in muscle afferent nerves are attenuated in chronic HF rats compared with control rats. Thus, the reflex pressor response is blunted in rats with HF when lactic acid is administered into the hindlimb to stimulate ASIC3 in muscle afferent nerves. Overall, alterations in ASIC3 influence the processing of sensory information in this disease and in turn, may alter the magnitude, timing, and distribution of SNA during exercise in HF.
ACKNOWLEDGEMENTS
This study was supported by NIH R01 HL090720 & AHA Established Investigator Award 0840130N.
Footnotes
The authors do not have any conflicts of interest and the results of this study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: Translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am. J. Physiol. Heart Circ. Physiol. 1991;261:H1653–H1658. doi: 10.1152/ajpheart.1991.261.5.H1653. [DOI] [PubMed] [Google Scholar]
- 4.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- 5.Gao Z, Xing J, Sinoway LI, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. American Journal of Physiology Heart & Circulatory Physiology. 2007;292:H939–H945. doi: 10.1152/ajpheart.00911.2006. [DOI] [PubMed] [Google Scholar]
- 6.Govonia S, Pascalea A, Amadioa M, Calvillo L, D’Elia E, Ceredad C, Fantuccie P, Ceronif M, Vanoli E. NGFand Heart: Is there a role in heart disease? Pharmacological Research. 2011;63:266–277. doi: 10.1016/j.phrs.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O’Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol. 2001;90:55–61. doi: 10.1152/jappl.2001.90.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Ieda M, Fukuda K. Cardiac innervation and sudden cardiac death. Curr Cardiol Rev. 2009;5:289–295. doi: 10.2174/157340309789317904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems. Chapter 10. New York: Oxford University Press; 1996. pp. 381–447. [Google Scholar]
- 11.Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1867–H1873. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- 12.Koba S, Xing J, Sinoway LI, Li J. Bradykinin receptor blockade reduces sympathetic nerve response to muscle contraction in rats with ischemic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1438–H1444. doi: 10.1152/ajpheart.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- 14.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lind A. Cardiovascular adjustments to isometric contractions: Static effort. In: Shepherd JT, Geiger SR, editors. Handbook of Physiology the Cardiovascular System: Peripheral Circulation and Organ Blood Flow. Bethesda, MD: American Physiological Society; 1983. pp. 947–966. [Google Scholar]
- 16.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Xing J, Li J. Role for ngf in augmented sympathetic nerve response to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J. Appl. Physiol. 2012;113:1311–1322. doi: 10.1152/japplphysiol.00617.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate and phosphate determined with msna during exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R563–R571. doi: 10.1152/ajpregu.2000.278.3.R563. [DOI] [PubMed] [Google Scholar]
- 20.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278:48907–48913. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- 21.McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. Journal of Clinical Investigation. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCord JL, Tsuchimochi H, Kaufman MP. Acid-sensing ion channels contribute to the metaboreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2009;297:H443–H449. doi: 10.1152/ajpheart.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J of Applied of Physiology. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JH, Reardon WC, McCloskey DI. Reflex effects on circulation and respiration from contracting skeletal muscle. American Journal of Physiology. 1977;233:H374–H378. doi: 10.1152/ajpheart.1977.233.3.H374. [DOI] [PubMed] [Google Scholar]
- 27.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momen A, Bower D, Boehmer J, Kunselman AR, Leuenberger UA, Sinoway LI. Renal blood flow in heart failure patients during exercise. American Journal of Physiology Heart & Circulatory Physiology. 2004;287:H2834–H2839. doi: 10.1152/ajpheart.00394.2004. [DOI] [PubMed] [Google Scholar]
- 29.Morales A, Gao W, Lu J, Xing J, Li J. Muscle cyclo-oxygenase-2 pathway contributes to the exaggerated muscle mechanoreflex in rats with congestive heart failure. Experimental Physiology. 2012;97:943–954. doi: 10.1113/expphysiol.2012.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol. 1989;67:256–263. doi: 10.1152/jappl.1989.67.1.256. [DOI] [PubMed] [Google Scholar]
- 31.Scott AC, Wensel R, Davos CH, Kemp M, Kaczmarek A, Hooper J, Coats AJ, Piepoli MF. Chemical mediators of the muscle ergoreflex in chronic heart failure: A putative role for prostaglandins in reflex ventilatory control. Circulation. 2002;106:214–220. doi: 10.1161/01.cir.0000021603.36744.5e. [DOI] [PubMed] [Google Scholar]
- 32.Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation. 1999;99:3002–3008. doi: 10.1161/01.cir.99.23.3002. [DOI] [PubMed] [Google Scholar]
- 33.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J. Appl. Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 34.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- 35.Sinoway LI, Li J. A perspective on the muscle reflex: Implications for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 36.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am. J. Physiol. Heart Circ. Physiol. 1992;263:H1499–H1505. doi: 10.1152/ajpheart.1992.263.5.H1499. [DOI] [PubMed] [Google Scholar]
- 37.Smith SA, Williams MA, Mitchell JH, Mammen PA, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- 38.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 39.Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J. Physiol. (London) 2001;537:993–998. doi: 10.1111/j.1469-7793.2001.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol. 2011;589:6173–6189. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victor RG, Bertocci L, Pryor S, Nunnally R. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J. Clin. Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 44.Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J. Physiol. 2012;590:1261–1272. doi: 10.1113/jphysiol.2011.221788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2009;296:H1380–H1387. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing J, Sinoway L, Li J. Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol. 2008;686:3245–3252. doi: 10.1113/jphysiol.2008.154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]