Abstract
The role of the novel costimulatory molecule TIM4 in anti-islet response is unknown. We explored TIM4 expression and targeting in Th1 (BALB/c islets into C57BL/6 mice) and Th2 (BALB/c islets into Tbet−/− C57BL/6 mice) models of anti-islet alloimmune response and in a model of anti-islet autoimmune response (diabetes onset in NOD mice). The targeting of TIM4, using the monoclonal antibody RMT4-53, promotes islet graft survival in a Th1 model, with 30% of the graft surviving in the long-term; islet graft protection appears mediated by a Th1 to Th2 skewing of the immune response. Differently, in the Th2 model, TIM4 targeting precipitates graft rejection by further enhancing the Th2 response. The effect of anti-TIM4 treatment in preventing autoimmune diabetes was marginal with only minor Th1 to Th2 skewing. B-cell depletion abolished the effect of TIM4 targeting. TIM4 is expressed on human B-cells and is upregulated in diabetic and islet-transplanted patients. Our data suggest a model in which TIM4 targeting promotes Th2 response over Th1 via B-cells. The targeting of TIM4 could become a component of an immunoregulatory protocol in clinical islet transplantation, aiming at redirecting the immune system toward a Th2 response.
Keywords: islet transplantation, autoimmune diabetes, costimulatory molecules, regulatory cells
Introduction
Islet transplantation has been shown to be a potentially effective cure for type 1 diabetes (T1D) and has been demonstrated to normalize glucose metabolism and halt the development of diabetic complications (9,38). Unfortunately, islet graft survival rates remain below those of other types of allografts (9,28). Considering the potential advantages of islet transplantation on diabetic complications (6,34,39) and the relatively low invasive nature of the procedure, much research has focused on increasing the success of islet cell transplantation (9,12,29). Although multiple factors have been deemed responsible for the failure of islet grafts, the development of the alloimmune response and the recurrence of autoimmune diabetes play primary roles. In the allo- and autoimmune responses, the reciprocal interaction between costimulatory molecules on T-cells and APCs (e.g. CD40L/CD40 or CD28/B7.1-2) determines, together with the cytokine environment, the differentiation of naïve Th0 cells into Th1, Th2, Th17, and Tregs predominantly (13,18,43), thus resulting in acceptance or rejection of the graft and in the onset or avoidance of autoimmunity. While the role of Th1 and Th17 cells in promoting allo- and auto-immune responses and of Tregs in promoting graft and self tolerance has been clearly assessed (30,42,43), the role of Th2 cells remains controversial. Although Th1 to Th2 skewing is considered a pivotal marker of graft acceptance, the Th2 response per se has been shown to be capable of causing graft rejection (17,25,33,41), and the exact role of the Th2 response in islet graft rejection remains unclear. TIM4 is a novel costimulatory molecule and a member of the T-cell Immunoglobulin Mucin (TIM) family, and it was recently shown to contribute to the development of the Th1 and Th2 responses (16). The TIM family consists of eight members in mice (TIM1-TIM8) and three in humans (TIM1, TIM3 and TIM4) (16). TIM1 and TIM3 are primarily expressed on T-cells, while TIM4 is localized on APCs (20) and serves as a ligand for TIM1 (1,22). The TIM1-TIM4 axis appears to provide a positive activation signal (20,37), leading to T-cell differentiation and activation, at least in models of autoimmunity, allergy and asthma (19). In allotransplantation, the targeting of TIM1 using an anti-TIM1 mAb has been shown to prolong allograft survival in a murine model of cardiac allograft rejection by reducing the Th1 and enhancing the Th2 response (35). Furthermore, anti-TIM1 mAb treatment was able to abrogate the Th17 response and to prolong allograft survival in a model of Th2/Th17 rejection (46). A recent study suggests that the TIM1-TIM4 costimulatory pathway may promote tolerance by expanding a population of regulatory B-cells (5). However, the specific role of TIM4 in immune activation and in anti-islet allo and autoimmune response is not clearly defined (26,45), and TIM4 has been implicated as both an inhibitor (21) and enhancer (23) of the immune response. In our study, we investigate the role of TIM4 and its targeting during the allo- and autoimmune anti-islet immune responses, with the aim of developing therapeutic tools to prolong the lifespan of exogenous (in the context of islet transplantation) and endogenous (in the pathogenesis of autoimmune diabetes) islets.
Materials and Methods
Patients
10 islet-transplanted patients, 10 patients with T1D and 10 healthy controls were enrolled at the San Raffaele Scientific Institute with Institutional Review Board approval. Table 1. All subjects provided informed consent before study enrolment.
Table 1.
Characteristics of islet-transplanted patients and healthy volunteers. Data are expressed as mean±SD.
| Healthy volunteers (n=10) |
T1D Patients (n=10) |
Islet- transplanted patients (n=10) |
|
|---|---|---|---|
| Gender (M/F) | 7/3 | 4/6 | 7/3 |
| Age (years) | 40.3±7.7 | 43.7±6.0 | 45.3±3.9 |
| T1D (years) | - | 24.2±8 | 27.0±8.1 |
| C-peptide (ng/ml) | - | 0.01±0.3 | 1.7±0.5 |
| EIR (U/day) | - | 30±4.2 | 8.8±2 |
| HbA1c(%) | - | 8.7±1.1 | 6.8±1.0 |
| Time from last islet infusion (months) | - | - | 32.6±11.6 |
| Rapamycin+FK506 | - | - | 5/10 |
| FK506+Mycophenolate | - | - | 4/10 |
| Rapamycin+Azathioprine | - | - | 1/10 |
T1D (type 1 diabetes); EIR (exogenous insulin requirement); HbA1c (glycated hemoglobin).
Human islet transplantation and immunosuppression
Islets were isolated from pancreata obtained from multi-organ donors, using a modified automated method, and were then purified by centrifugation on a discontinuous gradient as previously described (14). Islets were then transplanted intra-hepatically according to ABO matching. Islet-transplanted patients received the standard triple immunosuppressive regimen: anti-thymoglobulin (Thymoglobulin, Genzyme, Framingham, MA) as induction, followed by treatment with FK506 ([Astellas, Deerfield, IL]; target blood levels between 6 and 8 ng/ml) and/or Cyclosporine ([Novartis, Basel, Switzerland]; target blood level 100 ng/ml) and/or Rapamycin ([Pfizer, New York, NY]; 8-15 ng/ml) and/or Micophenolate ([Roche, Basel, Switzerland]; 2g/die) and prednisone ([Bruno Farmaceutici, Italy] 5–10 mg/day); Cyclosporine drug level was assessed by immunocolorimetric assay (Siemens, Munich, Germany), FK506 by liquid chromatography–mass spectrometry (). Steroids were tapered and then withdrawn within 6 months post-transplant. C-peptide level was assessed by immunofluorimetric assay (Tosoh, Tokyo, Japan); Hba1c level was assessed by high-performance liquid chromatography (Biorad, Hercules, CA); EIR (exogenous insulin requirement) was collected through patient interview.
PBMC from human patients
Peripheral blood mononuclear cells (PBMC) fractions were isolated from 20 ml of whole blood by Ficoll (GE Healthcare, Piscataway, NJ) density gradient centrifugation.
Mice
C57BL/6J, BALB/cJ, NOD/ShiLtJ and Tbet−/− mice (on a C57BL/6 background) were obtained from Jackson Laboratory and maintained as a breeding colony in our animal facility. All mice were cared for and used in accordance with institutional guidelines. Protocols were approved by the Harvard Medical School Animal Care and Use Committee.
Islet isolation and transplantation
Pancreatic islets derived from BALB/c donor mice were isolated by collagenase digestion followed by density gradient purification, and were handpicked. Islets were transplanted under the renal capsule of chemically induced 8-week-old mice (streptozotocin, Sigma-Aldrich, St. Louis, MO, 250 mg/kg, administered i.p.). Rejection of islet allografts was defined by blood glucose measurements > 250 mg/dL on two consecutive days.
In vivo treatment protocols
Islet-transplanted mice were treated with the anti-TIM4 mAb RMT4-53 (rat Ig2a; Bio X Cell; West Lebanon, NH) i.p. at doses of 500 μg on day 0 and 250 μg on days 2, 4, 6, 8, 10. For B-cell depletion, C57BL/6 mice were treated with an anti-CD22 monoclonal antibody conjugated with calicheamicin (anti-CD22/cal; Wyeth, Madison, NJ) at dose of 160 μg i.p. at days 0 and 5 (3). In the diabetes prevention study, 10-week-old female NOD mice were treated with RMT4-53 at doses of 500 μg on day 0 and 250 μg on days 2, 4, 6, 8, 10, or were left untreated. Treated mice were followed for diabetes onset, as defined by two consecutive days of blood glucose measurements > 250 mg/dL (BD Logic glucose meter, BD Biosciences, San Jose, CA) .
Islet pathology
Transplanted mice were sacrificed at various time points to obtain histology specimens. Kidney sections were stained with hematoxylin (Sigma-Aldrich) and eosin (Sigma-Aldrich) (3).
ELISPOT and stimulation assays
In vitro and ex vivo IFN-γ and IL-4 production measurements using ELISPOT assays were performed to monitor the alloimmune response (BD Biosciences). C57BL/6 splenocytes (1×106) were challenged in the presence of 1×106 BALB/c irradiated splenocytes or 0.5 μg/ml anti-CD3 Ig/anti-CD28 Ig (BD Biosciences) and were cultured for 24h (IFN-γ) or 48h (IL-4) as previously described (41). Spots were counted on an Immunospot analyzer (Cellular Technology Ltd., Cleveland, OH). In the autoimmune setting, splenocytes extracted from NOD mice were re-challenged with 150 μg/ml of the CD4-restricted peptide BDC2.5 (Chi Scientific, Maynard, MA) and 50 μg/ml of the CD8-restricted peptide IGRP (Abcam, Cambridge, MA). Bone Marrow derived DCs were generated as previously described (7,36). LPS (10 μg/ml) and PMA (50 ng/ml) used in the in vitro culture were obtained from Sigma-Aldrich.
Flow cytometry and intracellular cytokine staining
Anti-human CD19 (BD Bioscience) and anti-mouse B220 (BD Bioscience), CD11c (eBioscience, San Diego, CA), TIM4 (Biolegend, San Diego, CA), CD4 (BD Bioscience), CD25 (eBioscience), CD44 (BD Bioscience), CD40 (BD Bioscience), CD69 (BD Bioscience), CD80 (BD Bioscience), CD86 (BD Bioscience), CD62L (BD Bioscience), Annexin V (BD Bioscience), 7-AAD (BD Bioscience), IL-17 (eBioscience), and FoxP3 (eBioscience) were used according to the manufacturer’s recommendations (8). Anti-human TIM4 was obtained from Novus Biologicals (Littleton, CO): rabbit polyclonal conjugated to FITC for flow application (NBP1-76702F). Flow acquisition was performed on a FACSCalibur™ analyzer (Becton Dickinson, San Jose, CA), and data were analyzed using FlowJo software version 6.3.2 (Tree Star, Ashland, OR).
Th2 differentiation assay
Naive sorted ABM TCR-Tg CD4+CD25− T cells (Th0) were isolated (CD4+CD25− isolation kit, Milteny Biotec, Auburn, CA), and activated for 5 days with 1 μg/ml of plate-bound anti-CD3 and anti-CD28 antibodies (BD Biosciences) in presence of the same number of isolated (CD19+ isolation kit, Milteny Biotec) BM12 CD19+ B-cells (which are specifically recognized by the ABM TCR-Tg T-cells). Cultures were supplemented with 5 ng/ml mIL-4 (R&D System, Minneapolis, MN) and 1 μg/ml anti-IFN-γ-Ig (BD Bioscience) for Th2 differentiation. T-cells were the harvested and assessed by RT-PCR (40) for GATA3 expression (Th2 specific marker) to quantify level of differentiation (43).
Statistical Analyses
Data are expressed as mean±standard error for parametric data and median ± 95% confidence interval for non-parametric data. Kaplan-Meier analysis was used for survival analysis. When groups were compared cross-sectionally, the two-sided unpaired Student t-test (for parametric data) or the Mann-Whitney test (for non-parametric data) was used according to value distribution. In the comparison of 3 or more groups, after assessing normal distribution, ANOVA test was performed followed by post hoc T-test with Bonferroni correction. A P value of less than 0.05 (by two-tailed testing) was considered an indicator of statistical significance. Data were analyzed with a SPSS statistical package for Windows (SPSS Inc., Chicago, IL). Graphs were generated using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
Results
TIM4 expression in vitro: the percentage of TIM4+ B-cells decreases during T-cell stimulation in the allo- and autoimmune settings
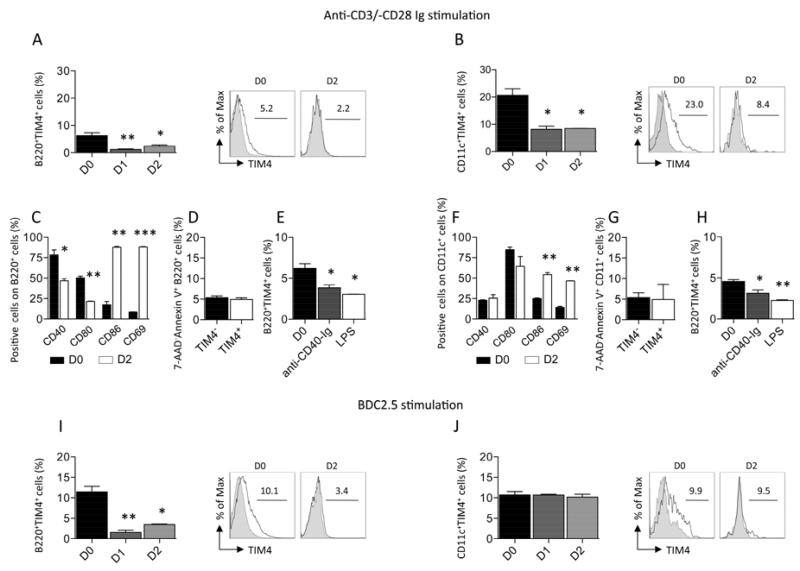
We first analyzed TIM4 expression on APCs [B-cells and dendritic cells (DCs)] during polyclonal T-cell stimulation in both a non-autoimmune setting to mimic the alloimmune response and during islet peptide-mediated T-cell stimulation to mimic the anti-islet autoimmune response. During polyclonal T-cell stimulation, splenocytes from C57BL/6 mice were challenged with 0.5 μg/ml anti-CD3 Ig and anti-CD28 Ig for 1 or 2 days, and FACS analysis was performed; a reduced percentage of B-cells positive for TIM4 (Figure 1A) and of DCs positive for TIM4 (Figure 1B) was observed. The expression level of the other costimulatory molecules assessed was also modified on B-cells and DCs by anti-CD3-Ig and anti-CD28-Ig-mediated stimulation, confirming active modulation of these cells. In particular, a reduced percentage of B-cells positive for CD40 or CD80 was observed after stimulation (Figure 1C), while the percentage of B-cells positive for CD86 or CD69 was increased (Figure 1C). To assess if the reduced percentage of TIM4+ B-cells was due to a relatively higher level of apoptosis in TIM4+ cells, we compared the level of apoptosis in TIM4+ and TIM4− cells after the stimulation. No difference was observed (Figure 1D). We thus hypothesize that the reduced percentage of TIM4+ cells after stimulation was due to the downregulation of TIM4 following T-cells-mediated transactivation. To assess that, we purified B-cells from splenocytes and we cultured them in presence of anti-CD40 for 1 day to mimic an interaction with activated CD40L+ T-cells. A reduction in TIM4+ B-cells was observed (Figure 1E). To assess if TIM4 dowregulation is a common feature following B-cells activation, we cultured B-cells in presence of LPS. A consistent reduction in TIM4 expression was observed (Figure 1E). PMA-mediated stimulation of B-cells was also associated to TIM4 downregulation (data not shown).
Figure 1.
During anti-CD3/-CD28 Ig stimulation of splenocytes extracted from C57BL/6 mice, the percentage of B-cells positive for TIM4 (B220+TIM4+) (n=3, *p<0.05 and **p<0.01 vs. D0; A) and of DCs positive for TIM4 (CD11c+TIM4+) decreases over time (n=3, *p<0.05 vs. D0; B). A reduction in B-cells positive for CD40 and CD80 and an increase in B-cell positive for CD86 and CD69 was also observed at day 2 after stimulation (n=3, *p<0.05, **p<0.01, and ***p<0.001 vs. D0; C). No difference in term of apoptosis was observed in TIM4+ and TIM4− B-cells at day 2 after stimulation (D). A reduction in the percentage of TIM4+ B-cells was observed after anti-CD40-Ig or LPS stimulation of isolated cells (n=3, *p<0.05 vs. D0; E). An increase in DCs positive for CD86 and CD69 was also observed at day 2 after stimulation (n=3, **p<0.01 vs. D0; F). No difference in term of apoptosis was observed in TIM4+ and TIM4− DCs at day 2 after stimulation (G). A reduction in the percentage of TIM4+ DCs was observed after anti-CD40-Ig or LPS stimulation of bone marrow derived DCs (n=3, *p<0.05 and **p<0.01 vs. D0; H). The percentage of B-cells positive for TIM4 decreases over time in splenocytes extracted from NOD mice and stimulated with the islet peptides BDC2.5 (n=3, *p<0.05 and **p<0.01 vs. D0; I). The percentage of DCs positive for TIM4 remained unchanged in splenocytes extracted from NOD mice stimulated with BDC2.5 (J).
No variation in the percentage of CD40 and CD80 positive DCs cells was observed by anti-CD3-Ig and anti-CD28-Ig-mediated stimulation, while CD86 and CD69 were found increased (Figure 1F). No difference in DCs apoptosis was observed (Figure 1G). A reduction in the expression of TIM4 in in vitro generated bone marrow derived DCs cultured in presence of LPS or anti-CD40-Ig (Figures 1H). For islet peptide-mediated T-cell stimulation, splenocytes extracted from normoglycemic NOD mice were stimulated with 150 μg/ml BDC2.5 (CD4-restricted) or 50 μg/ml IGRP (CD8-restricted) peptides (4,15). A decrease in the percentage of B-cells positive for TIM4 was evident during BDC2.5 stimulation (Figure 1I) and IGRP stimulation (data not shown). The percentage of CD40 and CD80 positive B-cells was also reduced, while stable levels of CD86 and CD69 were observed (data not shown). The percentage of DCs positive for TIM4 in the BDC2.5- and IGRP-stimulated settings remained stable (Figures 1J and data not shown, respectively). Stable levels of CD86 and CD80 were observed, while CD40 and CD69 were increased confirming that DCs were activated by peptide stimulus (data not shown). The activation of NOD B-cells with anti-CD40-Ig or LPS caused the dowregulation of TIM4 expression, while, differently then in C57BL/6 mice (data not shown), no reduction in TIM4 expression in DCs was observed. Thus, during both polyclonal antibody and islet peptide stimulation, a decrease in the percentage of TIM4+ B-cells is evident. The reduction in TIM4+ B-cells looks to be related to dowregulation of the receptor following transactivation of B-cells mediated by activated T-cells. TIM4 looks also dowregulated in C57BL/6-derived DCs, while is more stable in NOD-derived DCs.
TIM4 expression in vivo: the percentage of TIM4+ B-cells and DCs decreases in the anti-islet allo- and autoimmune responses
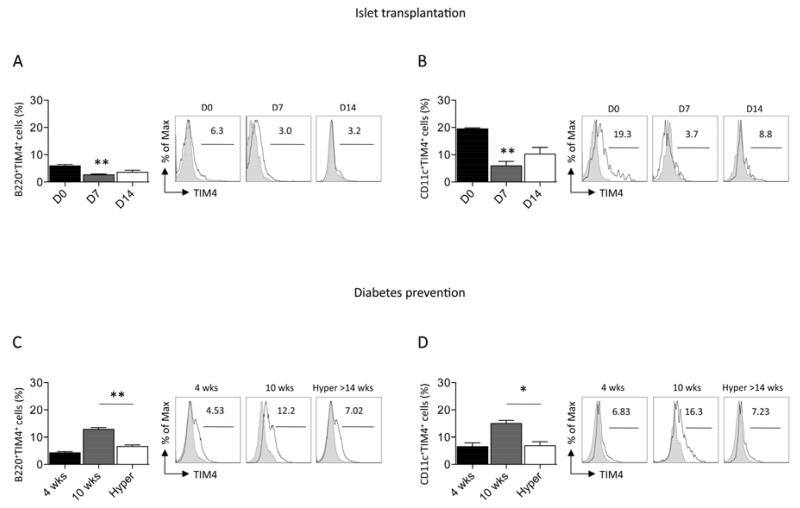
We then analyzed TIM4 expression in vivo during the alloimmune or autoimmune anti-islet response. To evaluate TIM4 expression on APCs during the alloimmune anti-islet response, we transplanted BALB/c mice islets into streptozotocin-treated C57BL/6 mice (fully mismatched), and recipient splenocytes were then harvested at 7 and 14 days after transplantation and assessed by flow cytometric analysis. A reduced percentage of B-cells positive for TIM4 (Figure 2A), and DCs positive for TIM4 (Figure 2B) was observed during the alloimmune response. To evaluate TIM4 expression on APCs during the autoimmune anti-islet response, we extracted splenocytes from NOD mice at different stages of disease (10-week-old prediabetic mice and >14-week-old hyperglycemic mice), and we then analyzed TIM4 expression. The percentage of B-cells positive for TIM4 was found reduced in hyperglycemic NOD mice compared to 10-week-old pre-diabetic mice (Figure 2C); an increase in the percentage of B-cells positive for TIM4 was observed between 4 and 10-week-old mice. A similar patter was observed For DCs (Figure 2D). A reduction in TIM4 expression on B-cells and DCs occurs during the anti-islet alloimmune and, after a temporary increase, autoimmune responses.
Figure 2.
TIM4 expression on APCs was evaluated by flow cytometry during the allo- (A, B) and autoimmune (C, D) anti-islet response in vivo. A decrease in the percentage of B-cells positive for TIM4 (n=3, **p<0.01 vs. D0; A) and DCs positive for TIM4 (n=3, **p<0.01 vs. D0; B) was evident after fully-mismatched islet transplantation of BALB/c islets into C57BL/6 recipients. In diabetes prevention studies, a reduction in the percentage of B-cells (n=3, **p<0.01 vs. 10wks; C) and DCs (n=3, *p<0.05 vs. 10wks; D) positive for TIM4 was observed in hyperglycemic mice.
Targeting TIM4 in vitro: RMT4-53 reduces IFN-γ production in the allo- but not in the autoimmune setting
To investigate the effect of TIM4 targeting in islet graft rejection and in the onset of autoimmune diabetes, we firstly tested the effect of RMT4-53 (an anti-TIM4 mAb) during polyclonal or islet peptide-specific stimulation. Splenocytes obtained from C57BL/6 mice were stimulated with anti-CD3/-CD28 Ig in the presence of RMT4-53 (1, 10, or 100 μg/ml) for 24 hours. ELISPOT analysis revealed that 100 μg/ml RMT4-53 reduced the number of IFN-γ-producing cells (Th1) (number of IFN-γ-producing spots: RMT4-53-treated=111±15, untreated=349±16; n=3; p=0.009; data not shown) and skews the immune response toward a Th2 profile (IL-4/IFN-γ ratio: RMT4-53-treated=0.7±0.2, untreated=0.2±0.03; n=3; p=0.02; data not shown). In autoimmune-relevant assays (BDC2.5 and IGRP stimulation for 24 hours), the use of RMT4-53 did not significantly affect the number of IFN-γ- or IL-4-producing cells nor did it change the IL-4/IFN-γ ratio. TIM4 targeting in vitro with RMT4-53 therefore reduces the Th1 and favors the Th2 response in vitro during polyclonal but not islet peptide-mediated T-cell stimulation.
Targeting TIM4 in vivo in a model of the Th1-mediated alloimmune anti-islet response: RMT4-53 promotes a Th2 over a Th1 response and prolongs islet graft survival
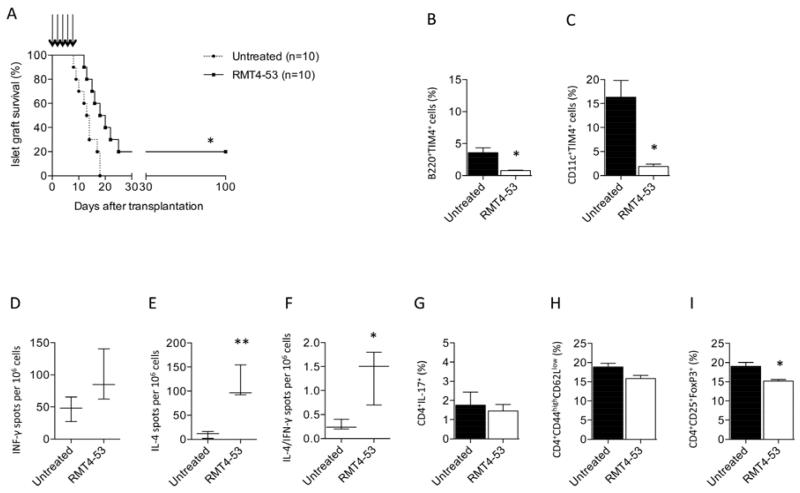
We tested the effect of TIM4 targeting in vivo in a model of the Th1-mediated alloimmune anti-islet response. BALB/c islets were transplanted into hyperglycemic C57BL/6 mice, and graft survival was evaluated in untreated and RMT4-53-treated mice (500 μg i.p. day 0 and 250 μg i.p. at days 2, 4, 6, 8, 10). A significant prolongation of graft survival was observed in treated mice compared to untreated mice (mean survival time [MST], days: RMT4-53-treated=19, untreated=13; n=10; p=0.01; Figure 3A). Flow cytometric analysis of splenocytes extracted from treated and untreated mice at 14 days after transplantation revealed a reduced percentage of B-cells positive for TIM4 (Figure 3B) and DCs positive for TIM4 (Figure 3C) in RMT4-53-treated mice The reduction in TIM4+ B-cells and DCs was not evident when transplanted mice were treated with anti-TIM1 antibodies. We hypothesize that RMT4-53 may have a depleting effect on TIM4+ cells during transplantation. We then phenotypically and functionally analyzed the immune system profile of treated and untreated mice. ELISPOT analysis of recipient splenocytes challenged with donor antigens revealed no significant differences in terms of number of IFN-γ-producing cells between RMT4-53-treated and untreated mice (Figure 3D), while on the contrary an increase in IL-4-producing cells was observed in RMT4-53-treated mice (Figure 3E). The Th2/Th1 ratio (IL-4-/IFN-γ-producing cells) increased significantly in RMT4-53-treated mice compared to controls (Figure 3F). We did not observe any significant differences in the percentages of CD4+IL-17+ T-cells and CD4 T effector cells (CD4+CD44highCD62Llow) between RMT4-53-treated and untreated mice (Figures 3G and 3H, respectively), while a slight reduction in Tregs (CD4+CD25+FoxP3+) was observed in the RMT4-53-treated group (Figure 3I). TIM4 targeting in vivo, in a model of the Th1-mediated anti-islet response, promotes islet graft survival and is associated with a Th1 to Th2 skewing of the immune response.
Figure 3.
The effect of TIM4 targeting with RMT4-53 was evaluated during the alloimmune anti-islet response; RMT4-53 treatment induced a prolongation of islet graft survival compared to untreated recipients (n=10, *p<0.05 vs. untreated; A). A decrease in the percentage of B-cells (n=3, *p<0.0 vs. untreated; B) and DCs (n=3, *p<0.05 vs. untreated; C) positive for TIM4 was observed 14 days after transplantation in the splenocytes obtained from RMT4-53-treated compared to untreated mice. In ELISPOT assays, an increase in the number of anti-donor specific IL-4- (n=3, **p<0.01 vs. untreated; E), but not IFN-γ-producing cells (D), was observed in splenocytes obtained from RMT4-53-treated compared to untreated recipients. The Th2/Th1 ratio shows a significant Th2 deviation in RMT4-53-treated mice (n=3, *p<0.05 vs. untreated; F). The percentage of Th17 (G) and CD4+ T effector cells (H) was similar in RMT4-53-treated and untreated mice, while a reduction in the percentage of Tregs was observed in RMT4-53-treated compared to untreated mice (n=3, *p<0.05 vs. untreated; I).
Targeting TIM4 in vivo in a model of the Th2-mediated alloimmune anti-islet response: RMT4-53 exacerbates the Th2 response and precipitates islet graft rejection
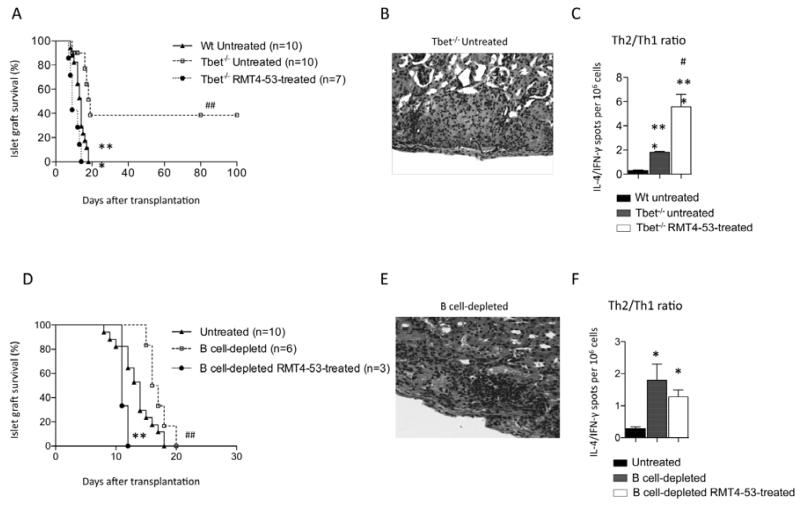
We then analyzed the effect of targeting TIM4 with RMT4-53 in a model of the Th2-mediated alloimmune anti-islet response: BALB/c islets were transplanted into streptozotocin-treated Tbet−/− C57BL/6 mice, which are characterized by high Th2 (and Th17) and virtually absent Th1 responses (31). Delayed islet graft rejection was observed in Tbet−/− mice compared to wild-type (Wt) recipients, with 40% of mice displaying long-term islet graft function (MST: Tbet−/− untreated=19 days; n=10; p=0.008 vs. Wt untreated; Figure 4A) and preserved islet morphology (Figure 4B). Splenocytes from Tbet−/− or wild-type mice were extracted at 14 days after transplantation and were stimulated with donor derived-antigens in an ELISPOT assay; an augmented Th2/Th1 ratio was observed in Tbet−/− compared to wild-type mice (Figure 4C). We then analyzed the effect of RMT4-53 treatment in Tbet−/− recipients. RMT4-53 treatment accelerated islet rejection compared to untreated Tbet−/− recipients (MST: Tbet−/− RMT4-53-treated=9 days, n=7; p=0.0002 vs. Tbet−/− untreated; Figure 4A). In our ELISPOT assay, a further increase in the Th2 anti-islet response was observed (Figure 4C). TIM4 targeting in a model of the Th2-mediated alloimmune anti-islet response thus precipitates graft rejection and further enhances the Th2 response.
Figure 4.
The effect of TIM4 targeting with RMT4-53 was tested in a model of the Th2 (and Th17) immune response (BALB/c islets into Tbet−/− C57BL/6 mice). A prolongation of islet allograft survival was observed in Tbet−/− C57BL/6 compared to wild-type recipients (##p<0.01 vs. Wt untreated; A). RMT4-53 treatment in Tbet−/− mice induced an acceleration of islet allograft rejection (Tbet−/− RMT4-53-treated ***p<0.001 vs. Tbet−/− untreated; A). Pathology of the graft revealed that islets were still preserved at 100 days after transplantation in normoglycemic untreated Tbet−/− C57BL/6 mice (B) with only a mild infiltrate within the islet graft. An increased Th2/Th1 ratio was demonstrated in Tbet−/− recipients challenged with donor-derived splenocytes compared to wild-type recipients (n=3, ***p<0.001 vs. Wt untreated; C); a further increase was observed in RMT4-53-treated Tbet−/− C57BL/6 compared to untreated Tbet−/− C57BL/6 (n=3, #p<0.05 vs. Tbet−/− untreated; C). When BALB/c islets were transplanted into B-cell-depleted C57BL/6 mice, islet graft survival was prolonged compared to untreated mice (##p<0.01 vs. untreated; D); RMT4-53 treatment in B-cell-depleted recipients promoted islet rejection (**p<0.01 vs. B-cell-depleted; D). In B-cell-depleted mice 14 days after transplantation, graft histology still displayed partially preserved islet morphology (E). An increased Th2/Th1 ratio was observed in B-cell-depleted recipients compared to undepleted recipients, while no further Th2/Th1 increase was observed after RMT4-53 treatment (n=3, *p<0.05 vs. untreated; F).
Targeting TIM4 in vivo does not promote the Th2-mediated anti-islet response in the absence of B-cells
We have previously demonstrated that the presence or absence of B-cells during allogeneic islet transplantation respectively favors a Th1 or Th2 anti-islet response (3,10). We thus evaluated whether the Th2 shift observed with RMT4-53 treatment was dependent upon B-cells. Depletion of B-cell was achieved with an anti-CD22/cal antibody, and graft survival and immune profile were analyzed (3). B-cell-depleted mice experienced a slight delay in islet graft rejection (MST: B-cell-depleted=17 days, n=6, p=0.004 vs. untreated; Figures 4D) with islet grafts that remained mildly infiltrated at 14 days after transplantation (Figure 4E); B-cell-depleted mice also displayed an increased Th2/Th1 ratio (Figure 4F). When B-cell-depleted mice were treated with RMT4-53, no further enhancement of the Th2/Th1 ratio was obtained by RMT4-53 treatment (Figure 4F) nor was any prolongation of graft rejection observed (on the contrary, an acceleration was evident) (MST: B-cell-depleted RMT4-53-treated=11 days, n=3; p=0.002 vs. B-cell-depleted; Figure 4D). These data demonstrate that the effect of anti-TIM4 treatment on Th2/Th1 ratio and islet graft protection is dependent upon the presence of B-cells.
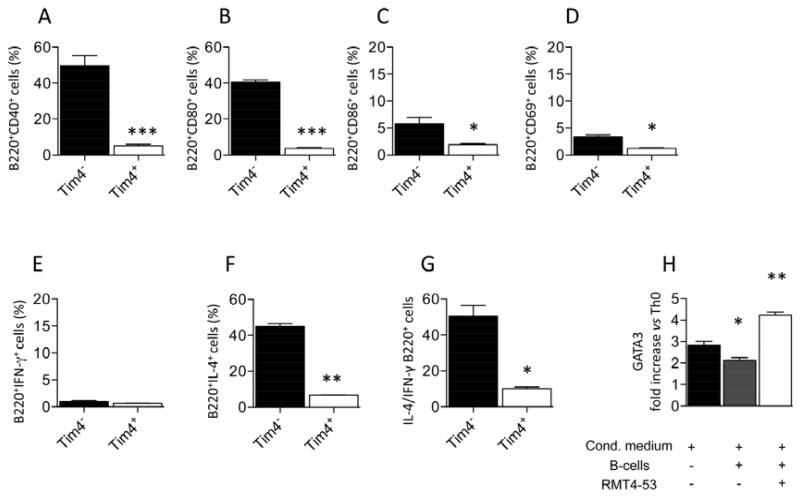
TIM4+ B-cells are characterized by a low costimulatory molecule expression profile and reduced IL-4 production
We investigated if the redirection towards a Th2 response obtained by the blockade of TIM4+ B-cells may be justified by the specific differences between TIM4+ and TIM4− B-cells. We first analyzed costimulatory molecule profile in TIM4+ and TIM4− B-cells extracted from C57BL/6 splenocytes. Lower expression in CD40 (Figure 5A), CD80 (Figure 5B), CD86 (Figure 5C) and CD69 (Figure 5D) was observed in TIM4+ B-cells compared to TIM4− B-cells. Cytokine profile is also different in TIM4+ and TIM4− B-cells. The percentage of IL-4+ B-cells is severely reduced in TIM4+ B-cells compared to TIM4− B-cells (Figure 5F), while no difference in IFN-γ production was observed (Figure 5E). IL-4/IFN-γ ratio is thus consequently higher in the TIM4− B-cells population than in TIM4+ (Figure 5G). These results indicate that TIM4− B-cells may be more functional than TIM4+ B-cells for Th2 differentiation than TIM4+ B-cells. To further investigate that we differentiated naïve Th0 cells towards Th2 in an appropriate cytokine milieu (43) in presence of B-cells and presence or absence of RMT4-53. T-cells were then harvested and assessed for level of differentiation by the analysis of the Th2-specific marker GATA3. RT-PCR analysis showed a higher GATA3 expression levels when RMT4-53 is added to the differentiation media (Figure 5H). This data indicate a better Th2 differentiation potential for TIM4− over TIM4+ B-cells.
Figure 5.
The different phenotype of TIM4+ and TIM4- B-cells was evaluated by flow cytometry. Reduced CD40 (A), CD80 (B), CD86 (C), CD69 (D) expression was observed in TIM4+ B-cells. (n=3, *<0.05 and ***p<0.001). No difference in term of IFN-γ positivity was observed (E), while IL-4 and IL-4/IFN-γ ratio is highly reduced in TIM4+ B-cells (n=3, *p<0.05; **<0.01; F, G). In an in vitro assay, the Th2 generation, assessed by RT-PCR as increase in GATA3 expression, was more efficient when Th0 were cultured in presence of B-cells and RMT4-53 (n=3, *<0.05 and **p<0.01 vs. Th0, H).
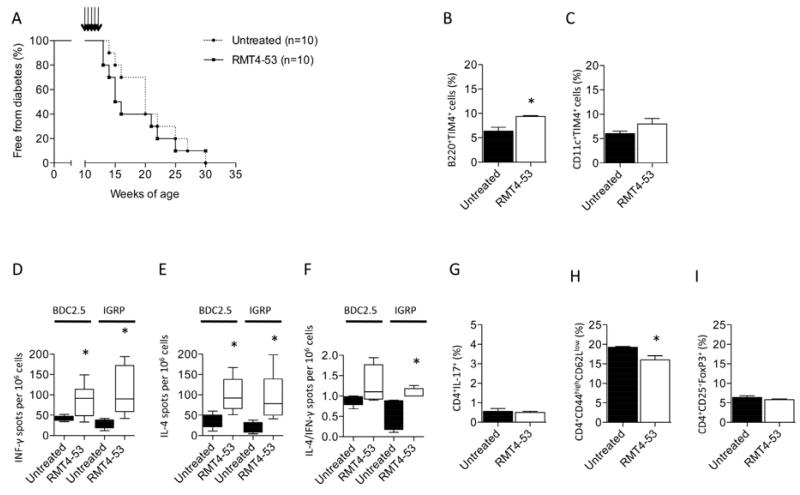
Targeting TIM4 in vivo in a model of the autoimmune anti-islet response: RMT4-53 does not promote the Th2 response or delay diabetes onset
The efficacy of TIM4 targeting was then tested in the prevention of diabetes in NOD mice. RMT4-53 treatment (500 μg i.p. at day 0 and 250 μg i.p. at days 2, 4, 6, 8, 10 beginning at 10 weeks of age) was ineffective in delaying diabetes onset (Figure 6A). An increased percentage of TIM4-expressing B-cells (Figure 6B), but not of TIM4-expressing DCs (Figure 6C) was observed in the spleens of RMT4-53-treated mice compared to untreated mice at 14 weeks of age. Analysis using an ELISPOT assay revealed a higher number of both IFN-γ- and IL-4-producing cells in splenocytes obtained from RMT4-53-treated mice challenged with BDC2.5 peptide (Figures 6D and 6E) and IGRP peptide (Figures 6D and 6E). An increased Th2/Th1 ratio in RMT4-53-treated mice was observed in response to IGRP but not to BDC2.5 peptide (Figure 6F). No significant differences were noted with regard to CD4+IL17+ T-cells or Tregs between RMT4-53-treated and untreated mice (Figures 6G and 6I), while a slight reduction in CD4 T effector cells was observed in RMT4-53-treated mice (Figure 6H). In the autoimmune diabetes model, targeting TIM4 did not appear to prevent or delay diabetes onset or to induce a clear Th2 skewing of the immune response.
Figure 6.
RMT4-53 treatment was evaluated in diabetes prevention in NOD mice. No effect was observed on the onset of diabetes after RMT4-53 treatment (A). An increase in the percentage of B-cells (but not of DCs) positive for TIM4 cells was observed in RMT4-53-treated mice compared to untreated mice (n=3, *p<0.05; B, C). An increase in the number of IFN-γ-producing cells was observed in RMT4-53-treated mice during BDC2.5 (n=5, *p<0.05 vs. untreated; D) and IGRP challenge (n=5, *p<0.05 vs. untreated; D) compared to untreated mice. An increase in the number of IL-4-producing cells was observed as well (BDC2.5 peptide: n=5, *p<0.05 vs. untreated; IGRP: n=5, *p<0.05 vs. untreated; E). The ratio of Th2/Th1 shows a significant skewing toward a Th2 response only with IGRP peptide challenge (n=5, *p<0.05 vs. untreated; F). No effect was observed on the percentage of Th17 cells (G), while a slight decrease in the percentage of T effector cells in RMT4-53-treated compared to untreated mice was observed (n=3, *p<0.05 vs. untreated; H). The percentage of Tregs was unchanged (I).
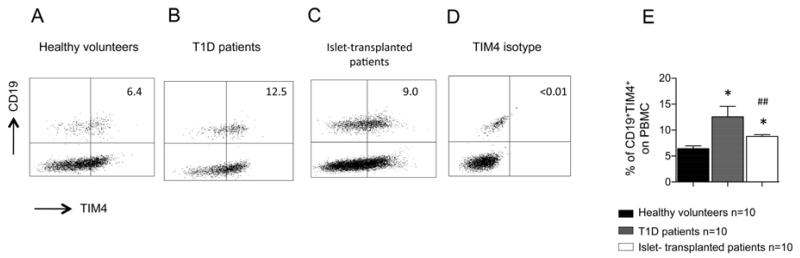
The TIM4+ B-cell population is increased in T1D and islet-transplanted patients
We then sought to examine the TIM4 expression profile by flow cytometry in B-cells obtained from islet-transplanted patients and from patients with T1D to determine the relevance of TIM4 in humans (Table 1). In healthy controls, TIM4+CD19+ B-cells represented approximately 6% of PBMC (Figures 7A and 7E) and 54% of all B-cells. An increase in the percentage of TIM4+ B-cells was seen in T1D patients (Figures 7B and 7E); a less pronounced increase was also observed in islet-transplanted patients (Figures 7C and 7E). The percentage of TIM4+ cells was gated according to isotype control staining (Figure 7D).
Figure 7.
TIM4 expression in B-cells obtained from T1D patients and islet-transplanted patients was evaluated using flow cytometry. An increase of TIM4+ B-cells was observed in T1D patients (n=6; *p<0.05 vs. healthy volunteers; B, E) and in islet-transplanted patients (n=10; *p<0.05 vs. healthy volunteers; ##p<0.01 vs. T1D; C, E) compared to healthy controls (A). TIM4-positive staining was determined according to isotype control staining (D).
Discussion
The finely tuned interactions between costimulatory molecules on APCs and T-cells determine the activation or the suppression of allo- and autoimmune responses (11,24). The targeting of costimulatory pathways represents an emerging therapeutic opportunity for the prevention of graft rejection and development of autoimmunity (18). The most well-studied and well-characterized pathways thus far are the CD28/B7.1-2 and the CD40L/CD40 costimulatory pathways, and the targeting of these has been shown to be efficient in preventing both graft rejection and autoimmunity in several animal models (41) and clinical settings (27,44). More recently, additional costimulatory pathways have been described (e.g. OX40/OX40L and the ICOS/B7h) (2). In our work we evaluated the possible efficacy of TIM1-TIM4 pathway targeting, using the monoclonal anti-TIM4-Ig RMT4-53 antibody, in preventing anti-islet allo- and autoimmunity.
TIM4 is expressed on B-cells and DCs and appears downregulated following alloimmune and autoimmune anti-islet response. The downregulation of TIM4 seems to be a common pattern after cell B-cell activation and was also evident in cell cultured with LPS or anti-CD40-Ig. The use of RMT4-53 (a mAb targeting TIM4) alleviates the anti-islet alloimmune response in C57BL/6 mice and delayed islet graft rejection; the effect seems to be associated with a skewing toward a Th2 immunoprofile. On the contrary, treatment with RMT4-53 was not shown to prevent diabetes onset in the NOD autoimmune model.
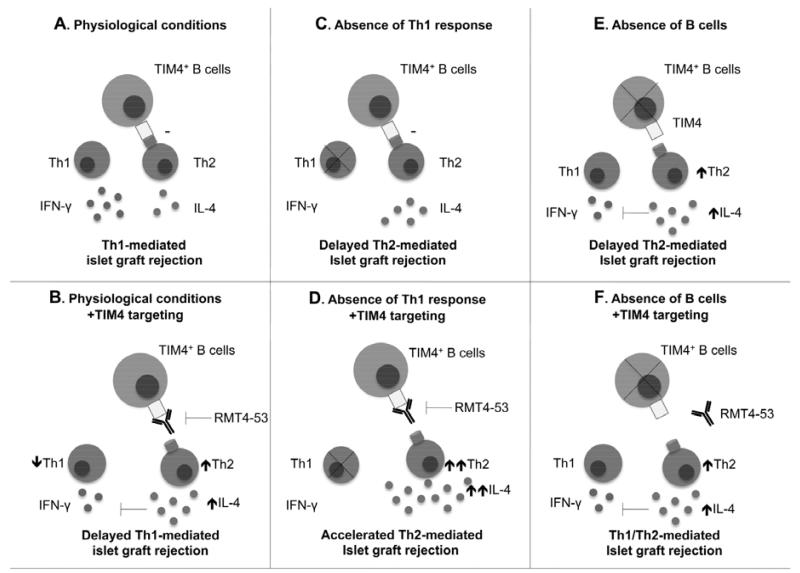
The redirection of the immune system toward a Th2 immunoprofile appears to be mediated by B-cells, as no increase in the Th2 response was observed after B-cell depletion in RMT4-53-treated or untreated recipients. We have previously demonstrated that the presence of B-cells is fundamental for directing the anti-islet alloimmune response toward a Th1 profile (3). Our data here demonstrate: (i) B-cell depletion causes Th2 deviation of the anti-islet response, (ii) RMT4-53 treatment causes a similar deviation of the anti-islet response, (iii) no synergistic or additive effect is observed on the Th2 response when combining the two treatments; these observations are compatible with an operative model in which the Th2 anti-islet alloimmune response is kept under control by a population of TIM4+ B-cells, and the function of these cells is suppressed by either anti-TIM4-Ig or B-cell depletion (Figure 8). Compared to TIM4− B-cells in fact TIM4+ B-cells appear less efficient in priming Th2 response and particular because a highly reduced expression of IL-4 which is fundamental for Th2 response development. Consistently with this model, the reduction of TIM4 expression on B-cells after anti-TIM4 treatment in the alloimmune but not the autoimmune setting can also contribute to the more evident redirection toward a Th2 profile observed in the alloimmune setting. The relative contribution in this model of the blockade of the phagocytosis of apoptotic bodies that could be obtained by RMT4-53 needs to be further investigated. However it is likely that TIM4 has a role in the redirection of the immune system, which is separate and independent by its role as a phagocytic receptor. In fact, we do not observe a Th2 redirection of the immune response with RMT4-53 after the depletion of B-cells, which have little function in the scavenging of apoptotic bodies. Our data also clarify the role of the Th2 response in anti-islet rejection. The switching from a Th1 to Th2 response in a model of the Th1-mediated anti-islet response functions in the delay of islet graft rejection, possibly due to a reduced rejecting capacity of the Th2 response. Interestingly, the rejection kinetics observed after TIM4 treatment in the Th1 model is similar to that which is observed in the genetic model of Th2 islet rejection, confirming the mechanism of action of RMT4-53. Conversely, in a model in which the Th2 response is already prominent, further stimulation of the Th2 response by RMT4-53 treatment precipitates graft rejection, confirming the potential capacity of Th2 cells in mediating islet rejection.
Figure 8.
Model of the role of TIM4+ B-cells in the alloimmune response in islet transplantation. TIM4 B-cells in normal conditions inhibits the Th2 immune response (A). TIM4 targeting with RMT4-53 enhances the Th2 response (B). In the absence of a Th1 response (as in Tbet−/− mice), TIM4 still exerts a Th2 inhibitory effect (C), and TIM4 targeting with RMT4-53 enhances the Th2 response (D). In the absence of B-cells, an increased Th2 response is evident due to the lack of TIM4 inhibition (E), and TIM4 targeting with RMT4-53 does not further increase Th2 response (F).
The increase in TIM4+ B-cells observed in islet-transplanted patients and T1D patients compared to healthy controls could contribute, according to our model, to the decreased Th2/Th1 ratio demonstrated in these patients (32). Consistently with the murine model of islet transplantation a relatively reduction in TIM4 expression on B-cells is observed in T1D patients after transplantation.
In conclusion, our work demonstrates a significant role for TIM4 and TIM4 targeting in the anti-islet alloimmune response, while the role of TIM4 or its blockade in the autoimmune response appears to be marginal. TIM4 inhibition may favor the Th2 over the Th1 response, and, although in the context of islet transplantation a high Th2 response can still be deleterious for the graft, given its reduced anti-graft rejecting capacity, we hypothesize that anti-TIM4 treatment could serve as a component of a combination therapy to promote islet graft survival.
ACKNOWLEDGMENTS
Paolo Fiorina is the recipient of a JDRF Career Development Award, an ASN Career Development Award, and an ADA Mentor-based Fellowship grant. P.F. is also supported by a Translational Research Program (TRP) grant from Boston Children’s Hospital; Harvard Stem Cell Institute grant (“Diabetes Program” DP-0123-12-00); Italian Ministry of Health grant RF- 2010-2303119. P. F. and Andrea Vergani are supported of an Italian Ministry of Health grant: (“Staminali”RF-FSR-2008-1213704). A.V. has been supported by an NIH-Research Training grant to Boston Children’s Hospital in Pediatric Nephrology (T32DK007726-28). A.V. is supported by the “AMD-SID Pasquale di Coste Scolarship”. Francesca D’Addio is a recipient of Italian Scientists and Scholars of North America Foundation (ISSNAF)-Fondazione Marche Fellowship. Roberto Bassi is supported by an ADA Mentor-based Fellowship grant to P.F and by an AST Genentech Clinical Science Fellowship grant. A.V. conducted this study as partial fulfillment of his PhD in Molecular Medicine, San Raffaele University, Milan, Italy.
Footnotes
Authors declare no conflicts of interest.
References
- 1.Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, Kobayashi N, Baumgarth N, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J. Immunol. 2010;185(11):6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari MJ, Fiorina P, Dada S, Guleria I, Ueno T, Yuan X, Trikudanathan S, Smith RN, Freeman G, Sayegh MH. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin. Immunol. 2008;126(2):140–147. doi: 10.1016/j.clim.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Carvello M, Petrelli A, Vergani A, Lee KM, Tezza S, Chin M, Orsenigo E, Staudacher C, Secchi A, Dunussi-Joannopoulos K, Sayegh MH, Markmann JF, Fiorina P. Inotuzumab ozogamicin murine analog-mediated B-cell depletion reduces anti-islet allo- and autoimmune responses. Diabetes. 2012;61(1):155–165. doi: 10.2337/db11-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai YD, Jensen KP, Lehuen A, Masteller EL, Bluestone JA, Wilson DB, Sercarz EE. A peptide of glutamic acid decarboxylase 65 can recruit and expand a diabetogenic T cell clone, BDC2.5, in the pancreas. J. Immunol. 2005;175(6):3621–3627. doi: 10.4049/jimmunol.175.6.3621. [DOI] [PubMed] [Google Scholar]
- 5.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 2011;121(9):3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorina P, Gremizzi C, Maffi P, Caldara R, Tavano D, Monti L, Socci C, Folli F, Fazio F, Astorri E, Del Maschio A, Secchi A. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care. 2005;28(6):1358–1365. doi: 10.2337/diacare.28.6.1358. [DOI] [PubMed] [Google Scholar]
- 7.Fiorina P, Jurewicz M, Vergani A, Augello A, Paez J, Ricchiuti V, Tchipachvili V, Sayegh MH, Abdi R. Phenotypic and functional differences between wild-type and CCR2−/− dendritic cells: implications for islet transplantation. Transplantation. 2008;85(7):1030–1038. doi: 10.1097/TP.0b013e31816843a0. [DOI] [PubMed] [Google Scholar]
- 8.Fiorina P, Jurewicz M, Vergani A, Petrelli A, Carvello M, D’Addio F, Godwin JG, Law K, Wu E, Tian Z, Thoma G, Kovarik J, La Rosa S, Capella C, Rodig S, Zerwes HG, Sayegh MH, Abdi R. Targeting the CXCR4-CXCL12 axis mobilizes autologous hematopoietic stem cells and prolongs islet allograft survival via programmed death ligand 1. J. Immunol. 2011;186(1):121–131. doi: 10.4049/jimmunol.1000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am. J. Transplant. 2008;8(10):1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 10.Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, Wu E, Tian Z, Abdi R, Guleria I, Rodig S, Dunussi-Joannopoulos K, Bluestone J, Sayegh MH. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57(11):3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorina P, Vergani A, Petrelli A, D’Addio F, Monti L, Abdi R, Bosi E, Maffi P, Secchi A. Metabolic and immunological features of the failing islet-transplanted patient. Diabetes Care. 2008;31(3):436–438. doi: 10.2337/dc07-1831. [DOI] [PubMed] [Google Scholar]
- 12.Gremizzi C, Vergani A, Paloschi V, Secchi A. Impact of pancreas transplantation on type 1 diabetes-related complications. Curr. Opin. Organ. Transplant. 2010;15(1):119–123. doi: 10.1097/MOT.0b013e32833552bc. [DOI] [PubMed] [Google Scholar]
- 13.He W, Fang Z, Wang F, Wu K, Xu Y, Zhou H, Du D, Gao Y, Zhang WN, Niki T, Hirashima M, Yuan J, Chen ZK. Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation. 2009;88(6):782–790. doi: 10.1097/TP.0b013e3181b47f25. [DOI] [PubMed] [Google Scholar]
- 14.Ichii H, Ricordi C. Current status of islet cell transplantation. J. Hepatobiliary Pancreat. Surg. 2009;16(2):101–112. doi: 10.1007/s00534-008-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, Santamaria P, Thomas HE, Kay TW. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J. Immunol. 2008;180(7):4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 16.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 2003;3(6):454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 17.Lenschow DJ, Zeng Y, Thistlethwaite R, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of Xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 18.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol. Rev. 2009;229(1):271–293. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 19.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2001;2(12):1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 20.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 2005;6(5):455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 21.Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 2005;11(8):362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 23.Mizui M, Shikina T, Arase H, Suzuki K, Yasui T, Rennert PD, Kumanogoh A, Kikutani H. Bimodal regulation of T cell-mediated immune responses by TIM-4. Int. Immunol. 2008;20(5):695–708. doi: 10.1093/intimm/dxn029. [DOI] [PubMed] [Google Scholar]
- 24.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J. Clin. Invest. 2008;118(5):1806–1814. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najafian N, Sayegh MH. CTLA4-Ig: a novel immunosuppressive agent. Expert opinion on investigational drugs. 2000;9(9):2147–2157. doi: 10.1517/13543784.9.9.2147. [DOI] [PubMed] [Google Scholar]
- 26.Nurtanio N, Yang PC. Role of TIM-4 in innate or adaptive immune response. N. Am. J. Med. Sci. 2011;3(5):217–221. doi: 10.4297/najms.2011.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59(6):1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr. Diab. Rep. 2011;11(5):345–354. doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 30.Shi Q, Lees JR, Scott DW, Farber DL, Bartlett ST. Endogenous expansion of regulatory T cells leads to long-term islet graft survival in diabetic NOD mice. Am. J. Transplant. 2012;12(5):1124–1132. doi: 10.1111/j.1600-6143.2011.03943.x. [DOI] [PubMed] [Google Scholar]
- 31.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 32.Szebeni A, Schloot N, Kecskemeti V, Hosszufalusi N, Panczel P, Prohaszka Z, Fust G, Uray K, Hudecz F, Meierhoff G. Th1 and Th2 cell responses of type 1 diabetes patients and healthy controls to human heat-shock protein 60 peptides AA437-460 and AA394-408. Inflamm. Res. 2005;54(10):415–419. doi: 10.1007/s00011-005-1362-9. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J. Immunol. 2007;179(8):5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, Begg I, Al Mehthel M, Kondi J, Harris C, Fensom B, Kozak SE, Tong SO, Trinh M, Warnock GL. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 35.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, Popoola J, Wang Y, Yagita H, Akiba H, Ansari MJ, Yang J, Turka LA, Rothstein DM, Padera RF, Najafian N, Sayegh MH. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J. Clin. Invest. 2008;118(2):742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno T, Tanaka K, Jurewicz M, Murayama T, Guleria I, Fiorina P, Paez JC, Augello A, Vergani A, Wong M, Smith RN, Abdi R. Divergent role of donor dendritic cells in rejection versus tolerance of allografts. J. Am. Soc. Nephrol. 2009;20(3):535–544. doi: 10.1681/ASN.2008040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005;6(5):447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 38.Vantyghem MC, Marcelli-Tourvieille S, Fermon C, Duhamel A, Raverdy V, Arnalsteen L, Kerr-Conte J, Noel C, Fontaine P, Pattou F. Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation. 2009;87(1):66–71. doi: 10.1097/TP.0b013e31818bbdab. [DOI] [PubMed] [Google Scholar]
- 39.Venturini M, Fiorina P, Maffi P, Losio C, Vergani A, Secchi A, Del Maschio A. Early increase of retinal arterial and venous blood flow velocities at color Doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation. 2006;81(9):1274–1277. doi: 10.1097/01.tp.0000208631.63235.6a. [DOI] [PubMed] [Google Scholar]
- 40.Vergani A, Clissi B, Sanvito F, Doglioni C, Fiorina P, Pardi R. Laser capture microdissection as a new tool to assess graft-infiltrating lymphocytes gene profile in islet transplantation. Cell Transplant. 2009;18(8):827–832. doi: 10.3727/096368909X472278. [DOI] [PubMed] [Google Scholar]
- 41.Vergani A, D’Addio F, Jurewicz M, Petrelli A, Watanabe T, Liu K, Law K, Schuetz C, Carvello M, Orsenigo E, Deng S, Rodig SJ, Ansari JM, Staudacher C, Abdi R, Williams J, Markmann J, Atkinson M, Sayegh MH, Fiorina P. A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes. 2010;59(9):2253–2264. doi: 10.2337/db09-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vergani A, Fotino C, D’Addio F, Tezza S, Podetta M, Gatti F, Chin M, Bassi R, Molano RD, Corradi D, Gatti R, Ferrero ME, Secchi A, Grassi F, Ricordi C, Sayegh MH, Maffi P, Pileggi A, Fiorina P. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes. 2013;62(5):1665–1675. doi: 10.2337/db12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013;127(4):463–475. doi: 10.1161/CIRCULATIONAHA.112.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 2005;353(8):770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 45.Yeung MY, McGrath M, Najafian N. The emerging role of the TIM molecules in transplantation. Am. J. Transplant. 2011;11(10):2012–2019. doi: 10.1111/j.1600-6143.2011.03727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan X, Ansari MJ, D’Addio F, Paez-Cortez J, Schmitt I, Donnarumma M, Boenisch O, Zhao X, Popoola J, Clarkson MR, Yagita H, Akiba H, Freeman GJ, Iacomini J, Turka LA, Glimcher LH, Sayegh MH. Targeting Tim-1 to overcome resistance to transplantation tolerance mediated by CD8 T17 cells. Proc. Natl. Acad. Sci. U S A. 2009;106(26):10734–10739. doi: 10.1073/pnas.0812538106. [DOI] [PMC free article] [PubMed] [Google Scholar]