Abstract
Background
There is increasing use of high intensity, interval type exercise training in the management of many lifestyle-related diseases.
Purpose
To test the hypothesis that vigorous-intensity, interval exercise is as effective as traditional, moderate-intensity aerobic exercise training on nonalcoholic fatty liver disease (NAFLD) outcomes in obese, Otsuka Long-Evans Tokushima Fatty (OLETF) rats.
Methods
OLETF rats (age 20 wks; n= 8–10/group) were assigned to sedentary (O-SED), moderate-intensity exercise training (O-MOD EX; 20 meters/min, 15% incline, 60 min/d, 5 d/wk treadmill running), or vigorous-intensity interval exercise training (O-VIG EX; 40 meters/min, 15% incline, 6×2.5 min bouts/d, 5 d/wk treadmill running) groups for 12 weeks.
Results
Both MOD EX and VIG EX effectively lowered hepatic triglycerides (TGs), serum ALTs, perivenular fibrosis, and hepatic collagen 1α1 mRNA expression (vs. O-SED, p<0.05). In addition, both interventions increased hepatic mitochondrial markers (citrate synthase activity and fatty acid oxidation) and suppressed markers of de novo lipogenesis (FAS, ACC, Elovl6, and SCD-1); whereas, only MOD EX increased hepatic mitochondrial β-HAD activity and hepatic TG export marker apoB100 and lowered fatty acid transporter CD36 compared with O-SED. Moreover, while total hepatic macrophage population markers (CD68 and F4/80 mRNA) did not differ among groups, MOD EX and VIG EX lowered M1 macrophage polarization markers (CD11c, IL-1β, and TNFα mRNA) and MOD EX increased M2 macrophage marker, CD206 mRNA, compared with O-SED.
Conclusions
The accumulation of 15 min/day of VIG EX for 12 weeks had similar effectiveness as 60 min/day of MOD EX in the management of NAFLD in OLETF rats. These findings may have important health outcome implications as we work to design better exercise training programs for NAFLD patients.
Keywords: nonalcoholic fatty liver disease, mitochondria, de novo lipogenesis, macrophage polarization, physical exercise
INTRODUCTION
Poor dietary habits and sedentary lifestyles have led to an obesity epidemic in Westernized societies that is accompanied by increasing clinical complications, including nonalcoholic fatty liver disease (NAFLD). NAFLD is a progressive liver disease ranging from simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis (38). It is characterized by elevated hepatic triglyceride (TG) storage (≥ 5% by weight) in the absence of excess alcohol consumption (<20 g/d) and is considered to be the hepatic manifestation of the metabolic syndrome (reviewed in (38)). NAFLD affects ~30% the adult population in the United States, and is present in 75–100% of obese or morbidly obese individuals (3, 5).
Lifestyle modifications that alter net energy status (increased physical activity, reduced energy intake) are the most commonly recommended therapies for individuals with diagnosed NAFLD (8). This is supported by cross-sectional reports in which more habitual physical activity is associated with reduced incidence and severity of NAFLD (19, 32). Currently, the American College of Sports Medicine recommends 150 min/week of moderate physical activity (i.e. aerobic exercise) in order to promote health (16), and clinical research suggests that similar moderate exercise training can reduce hepatic steatosis in adults (17, 45). In fact, four weeks of moderate intensity exercise (70% of VO2peak, 30–45 min/d, 3 d/wk) reduced serum free-fatty acids (FFAs), visceral adiposity, and hepatic TG content in obese men and women with clinical hepatic steatosis (17). However, higher intensity exercise may be needed for disease management in NAFLD patients in order to prevent the advancement of NAFLD to NASH (19).
Vigorous intensity interval training is becoming a popular means of exercise training due to its lower volume and shorter time commitments and emerging evidence suggests that it has many potential health benefits (33, 41, 47). Yet, it remains unclear if this type of exercise training may be as efficacious as moderate intensity aerobic exercise for the treatment of NAFLD. Here we utilized the Otsuka Long-Evans Tokushima Fatty (OLETF) rat model to test the hypothesis that vigorous intensity interval exercise training is as efficacious as moderate intensity aerobic exercise for the treatment of NAFLD. The OLETF rat, which is selectively bred for null expression of the cholecystokinin-1 receptor exhibits a within meal feedback defect for satiety, leading to the development of obesity, insulin resistance, type 2 diabetes, and NAFLD, with significant hepatic TG accumulation occurring as early as 8 weeks of age (37). By 40 weeks of age, these animals experience a progression of NAFLD that results in hepatocyte ballooning and increased fibrosis and collagen deposition in a progressive pattern similar to the human disease (35–37). We have previously shown that voluntary wheel running effectively prevents and treats NAFLD in the OLETF rat (4, 35, 36), and recently demonstrated that 12 weeks of moderate-intensity aerobic treadmill exercise training is effective in the treatment of NAFLD in these animals (23). However, the effects of lower volume, high-intensity exercise training for the treatment of NAFLD is unknown. Because of this, we hypothesized that vigorous intensity interval exercise training would effectively treat NAFLD outcomes. We sought to test this hypothesis by determining if a) vigorous intensity interval exercise training is equally effective as moderate intensity exercise training for the treatment of NAFLD in obese OLETF rats and b) gain mechanistic insight into these two types of exercise training in the management of NAFLD.
METHODS
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri and adhered to the animal care standards of the American College of Sports Medicine. Four-week old hyperphagic OLETF male rats (Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan) were individually housed in temperature and light controlled animal quarters (12h light-12h dark cycle at 21°C). The animals were given ad libitum access to standard rodent chow (Formulab 5008, Purina Mills, St. Louis, MO, USA) and remained sedentary until 20 weeks of age. At 20 weeks of age, OLETF rats were randomly assigned to one of the following treatment groups (n=8–10/group): sedentary (O-SED), moderate intensity aerobic exercise training (O-MOD EX), or vigorous intensity interval training (O-VIG EX) for 12 weeks. Non-hyperphagic, control Long-Evans Tokushima Otsuka rats remained in sedentary cage conditions (L-SED) throughout the study. Animals in the O-MOD EX treatment initially began treadmill running at a speed of 15 m/min on a 15% incline for 5 min/day. Duration and speed were gradually increased by 2–3 min/day and 1–2 m/min per wk such that by wk 4 the animals were running at a speed of 20 m/min on a 15% incline for 60 min/day, 5 days/wk. For O-VIG EX, the animals completed six bouts of treadmill running with 4.5 min of rest between bouts. The duration and intensity of the bouts were increased over 5 wk, such that animals completed six sprints at a speed of 40 m/min, 15% incline, 2.5min/bout, 5days/wk. This intensity was chosen based on previous reports from our group and others in which a similar speed and grade elicited near maximal oxygen consumption (≥90% of VO2max) in untrained, healthy rats (13, 20, 31, 43). Body mass and food intake were measured weekly throughout the study. At 32 weeks of age, rats were anesthetized with sodium pentobarbital (100 mg·kg−1) and then exsanguinated by removal of the heart. All animals were fasted for 12 hours and the last exercise bout for O-MOD EX and O-VIG EX was performed ~18h prior to sacrifice. These conditions were chosen because in a clinical setting, patients are typically not instructed to withdraw from physical activity prior to a fasting blood draw; however, residuals effects from the last bout of exercise may have contributed to some of the current findings.
Dual-energy x-ray absorptiometry
Whole-body fat composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats.
Blood and serum assays
Whole blood was collected in EDTA coated tubes for HbA1c analysis by boronate-affinity HPLC (Primus Diagnostics, Kansas City, MO) in the Diabetes Diagnostics Lab at the University of Missouri. Serum glucose, insulin, TG, and FFA assays were performed by Comparative Clinical Pathology Services (Columbia, MO) using commercially available assays. Serum leptin, monocyte chemoattractant protein-1, interleukin (IL)-1β, TNFα, and IL-10 were determined using a Milliplex immunoassay kit (EMD Millipore Corp, Billerica, MA, USA). All assays were completed according to manufacturers' guidelines.
Intraperitoneal glucose tolerance tests
At 30–31 weeks of age, animals underwent intraperitoneal glucose tolerance tests (IPGTT) to assess glucose tolerance following a 12h fast. Animals in exercise training groups completed their last bout of exercise ~18h prior to the test. Whole blood samples were collected from the tail vein prior to and 15, 30, 45, 60, and 120 min following the intraperitoneal dextrose injection (50% solution, 2g/kg body weight) as previously described (39). After centrifugation at 4°C at 3000 × g, serum samples were stored at −80°C until insulin was assessed by ELISA (EMD Millipore). The total area under the glucose and insulin curves (AUC) were calculated using the trapezoidal method (46). Additionally, the insulin-glucose index (IG index), an indicator of the effectiveness of insulin, was calculated (11).
Tissue collection and preparation procedure
Livers were quickly removed from anaesthetized rats and flash frozen in liquid nitrogen or placed in 10% formalin, RNAlater, or ice-cold isolation buffer (100mM KCl, 40mM Tris-HCl, 10 mM Tris-Base, 5mM MgCl2․6H2O, 1mM EDTA and 1mM ATP; pH 7.4). The heart and retroperitoneal, epididymal, and omental fat pads were excised from animals and weighed.
Fatty acid oxidation
As previously described (36), fatty acid oxidation assays were performed in fresh hepatic tissue preparations using radiolabeled [1-14C] palmitate (American Radiochemicals). The oxidation rate of 14C palmitate was measured by collecting and counting the 14CO2 produced (representing complete fatty acid oxidation) and 14C-labeled acid-soluble metabolites (representing incomplete fatty acid oxidation) that were collected within a trapping device and counted with a liquid scintillation counter as previously described (40).
Measures of mitochondrial content
Citrate synthase and β-HAD activities were determined using the methods of Srere (44) and Bass et al. (2), respectively, as previously described by our group (36).
Intrahepatic lipid content, liver morphology, and hepatic glycogen content
To examine liver morphology, formalin-fixed, paraffin-embedded livers were sectioned and stained with haematoxylin and eosin (H&E) or trichrome stain (for collagen deposition/fibrosis). Biochemical intrahepatic TG content and hepatic glycogen content was determined as previously described (36).
Quantitative RT- PCR
Hepatic mRNA expression was quantified using the ABI 7500 Fast Sequence Detection System and Software (Applied Biosystems, Carlsbad, CA) as previously described by our group (30). Primers were obtained from Sigma (St. Louis, MO, USA) for all analyses and analyses were conducted using Fast Sybr Green Master Mix kits (Applied Biosystems). β-actin (Forward: GCTCTCTTCCAGCCTTCCTT, Reverse: CTTCTGCATCCTGTCAGCAA) was used as the housekeeping gene and all gene expression data are represented relative to β-actin expression using the ΔΔCT method, in which the L-SED group served as the referent group. Gene expression was assessed for carnitine palmitoyltransferase 1a (CPT-1a, Forward: GCCTCTATGTGGTGTCCAAG, Reverse: TAGACAACCTCCATGGCTCA), Elolv6 (Forward: CGTAGCGACTCCGAAGATCA, Reverse: CCAAATATAAAGGCAGCGTA), CD68 (Forward: TCACAAAAAGGCTGCCACTCTT, Reverse: TCGTAGGGCTTCGTTGCTGTGCTT), F4/80 (Forward: GGAGGACCAATGTTCCAGGG, Reverse: TGGGCAAGAACAGCTGTAGG), CD11c (Forward: CAAAGCTGAGCTGGGAGACA, Reverse: TGGCTGCTGATGACAGTGTA), interleukin-1β (IL-1β; Forward: CCTATGTCTTGCCCGTGGAG, Reverse: CACACACTAGCAGGTCGTCA), tumor necrosis factor α (TNFα; Forward: GTCGTAGCAAACCACCAAGC, Reverse: GCAGCCTTGTCCCTTGAAGA), CD163 (Forward: TGTAGTTCATCATCTTCGGTCC, Reverse: CACCTACCAAGCGGAGTTGAC), CD206 (Forward: GAGGACTGCGTGGTGATGAA, Reverse: CAGCGAACGTTGAAAGGGTG), and collagen 1α1 (Col 1α1; Forward: GATGGACTCAACGGTCTCCC, Reverse: CGGCCACCATCTTGAGACTT).
Western blot analysis
Western blot analyses were used to determine hepatic protein expression of markers related to mitochondrial content as well as fatty acid uptake, TG synthesis, de novo lipogenesis, and TG secretion. The following proteins were assessed: peroxisome proliferator-activated receptor α (PPARα; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α; EMD Millipore Corp.), CPT-1a (Alpha Diagnostics International, San Antonio, TX, USA), total AMP-activated protein kinase α (AMPK; Cell Signaling, Beverly, MA, USA) AMPK Thr 172 phosphorylation-specific (pAMPK; Cell Signaling), glycogen synthase (GS; Cell Signaling), glycogen synthase Ser641 phosphorylation-specific (pGS; Cell Signaling), glycogen synthase kinase 3β (GSK3β; Cell Signaling), GSK3β Ser9 phosphorylation- specific (pGSK3β; Cell Signaling), CD36/FAT (Santa Cruz Biotechnology, Inc.), acetyl coenzyme A carboxylase (ACC; Cell Signaling), ACC Ser79 phosphorylation-specific (pACC; Cell Signaling), fatty acid synthase (FAS; Cell Signaling), steroyl CoA desaturase-1 (SCD-1; Alpha Diagnostics International), adipose triglyceride lipase (ATGL; Cell Signaling), microsomal triglyceride transfer protein (MTTP; Santa Cruz Biotechnology), apolipoprotein B (ApoB; Abcam, Cambridge, MA, USA), CD68 (Santa Cruz Biotechnology, Inc.), IL-1β (Abcam), and CD206 (Abcam). Content of phospho-proteins was calculated from the density of the band of the phospho-protein divided by the density (content) of the total protein using the appropriate antibodies (35, 36). Liver samples were homogenized using lysis buffer. Protein (20–40 µg) was loaded into a SDS-PAGE gel and probed with primary antibody. After washing, the membrane was incubated with horse-radish peroxidase-conjugated secondary antibodies. Protein bands were quantified using a densitometer (Bio-Rad). In order to control for equal protein loading and transfer, the membranes were stained with 0.1% amido-black (Sigma) and total protein staining was quantified (36).
Statistical analysis
Each outcome measure was examined in 8–10 animals per group. For each outcome measure, a one-way analysis of variance was performed (SPSS/20.0, IBM, Chicago, IL, USA), with significant interactions followed up using Fisher LSD post-hoc comparisons. Values are reported as mean ± standard error (SE) and statistical significance was determined as p<0.05.
RESULTS
Exercise training and body weight, adiposity, food intake, and heart weight
Both MOD EX and VIG EX reduced body weight by 10–15% compared to O-SED (p<0.001; Table 1), with no significant differences between MOD EX and VIG EX. These reductions in body weight were associated with improved body composition and lower fat pad weights, with MOD EX inducing greater reductions in both body fat percentage and fat pad mass (p<0.05, O-MOD EX vs. O-VIG EX; Table 1). Although animals in both the MOD EX and VIG EX groups consumed less food on average than O-SED, when food consumption was expressed relative to body weight, no differences were observed (Table 1). Both exercise training groups exhibited increases in the heart weight to body weight ratio, compared to O-SED (p<0.05; Table 1).
Table 1.
Animal characteristics.
| L-SED | O-SED | O-MOD EX | O-VIG EX | |
|---|---|---|---|---|
| Final body weight (g) | 467.33± 17.27a | 696.88± 12.38b | 580.75± 14.70c | 610.00± 10.66c |
| DEXA body composition (% fat) | 14.5± 0.8a | 36.8± 1.4b | 24.1± 1.8c | 29.7± 1.1d |
| Fat pad mass (g) | 14.65± 1.27a | 70.94± 4.20b | 40.30± 4.45c | 51.20± 3.57d |
| Average weekly food intake (g/wk) | 149.18± 3.79a | 220.06± 3.32b | 202.19± 2.51c | 200.23± 5.00c |
| Average relative weekly food weight (g food/g BW/wk) | 0.323± 0.003 | 0.339± 0.007 | 0.331± 0.007 | 0.319± 0.006 |
| Heart weight : body weight | 3.11± 0.09a | 2.73±0.07b | 3.26± 0.07a | 3.20±0.07a |
| Triglyceride (mg/dl) | 44.0± 3.7a | 343.4± 47.3b | 103.0± 9.8a,c | 114.0± 10.9c |
| Free fatty acids (mmol/L) | 0.28± 0.02a | 0.89± 0.07b | 0.52± 0.04c | 0.61± 0.04c |
| Leptin (pg/ml) | 18,144± 4,720a | 106,027± 10,531b | 39,285± 6,998a,c | 42,912± 6,784c |
| MCP-1 (pg/ml) | 19.74± 10.47a | 49.04± 6.98b | 14.98± 6.49a | 26.92± 10.76a,b |
| TNFα (pg/ml) | 3.16± 1.28 | 6.71± 3.10 | 4.55± 1.90 | 3.50± 1.21 |
| IL-1β (pg/ml) | 0.84± 0 | 3.19± 1.73 | 5.71± 2.83 | 3.77± 1.58 |
| IL-6 (pg/ml) | 3.41± 0.0a | 735.00± 182.14b | 227.43± 51.80a,c | 542.63± 84.91b,c |
| IL-10 (pg/ml) | 30.43± 7.94a | 426.25± 86.03a,b | 1,001.73± 236.61c | 598.00± 149.48b,c |
Values are mean ±SE; n=8-10 animals per group. L-SED, LETO-sedentary; O-SED, OLETF-SED; MOD EX, moderate intensity exercise training; VIG EX, vigorous intensity interval training; MCP-1, monocyte chemoattractant protein; TNFα, tumor necrosis factor α; IL, interleukin. Fat pad mass was determined by summing the weights of the omental, retroperitoneal, and epididymal fat pads. Values with different letter superscripts are significantly different (p<0.05).
Serum lipids, inflammatory markers, and measures of glycemic control
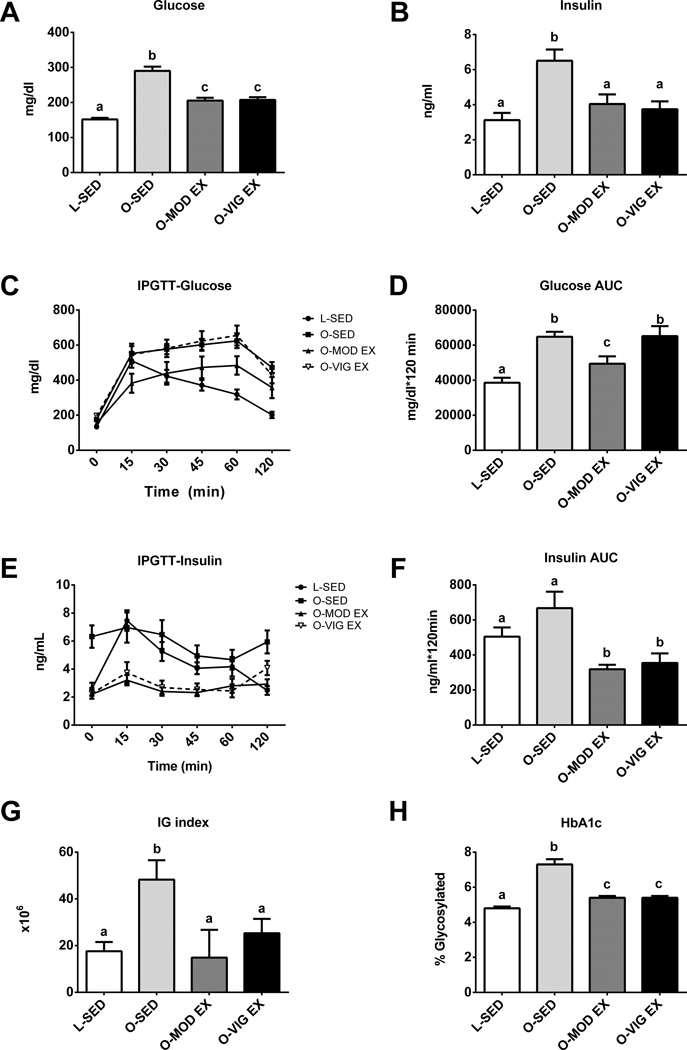
Both MOD EX and VIG EX effectively lowered fasting serum TG, FFAs, and leptin (p<0.001; Table 1). However, only MOD-EX altered the systemic inflammatory markers MCP-1, IL-6, and IL-10 compared to O-SED (p<0.05) while no differences were observed between groups for circulating TNFα or IL-1β (Table 1). Both exercise treatments reduced HbA1c (p<0.05; Figure 1H), a measure of long-term glycemic control, as well as fasting serum glucose and insulin (p<0.01; Figure 1A–B). It should be noted that fasting insulin values fell by 40% from 20 weeks of age to 32 weeks of age in O-SED rats (20 wk, insulin=11.0± 1.4 ng/ml) and, on average, O-SED rats demonstrated only a slight insulin response during the IPGTT (Figure 1E). Only MOD EX partially attenuated the post glucose challenge glucose area under the curve (AUC) (p<0.05 O-EndEx vs O-SED; Figure 1D); whereas, both MOD EX and VIG EX elicited lower insulin AUC (Figure 1F) and IG index (Figure 1G) compared to O-SED during the IPGTT (p<0.05).
Figure 1.
Moderate and vigorous exercise-induced alterations in glycemic control. Fasting glucose (A), fasting insulin (B), post-glucose challenge glucose curves (C), glucose area under the curve (D), post-glucose challenge insulin curves (E), insulin area under the curve (F), IG index (G), and hemoglobin A1c (HbA1c; H). Values (n=6–10 per group, means ± SE) with different letter superscripts are significantly different (p<0.05).
Treatment of NAFLD by MOD EX and VIG EX
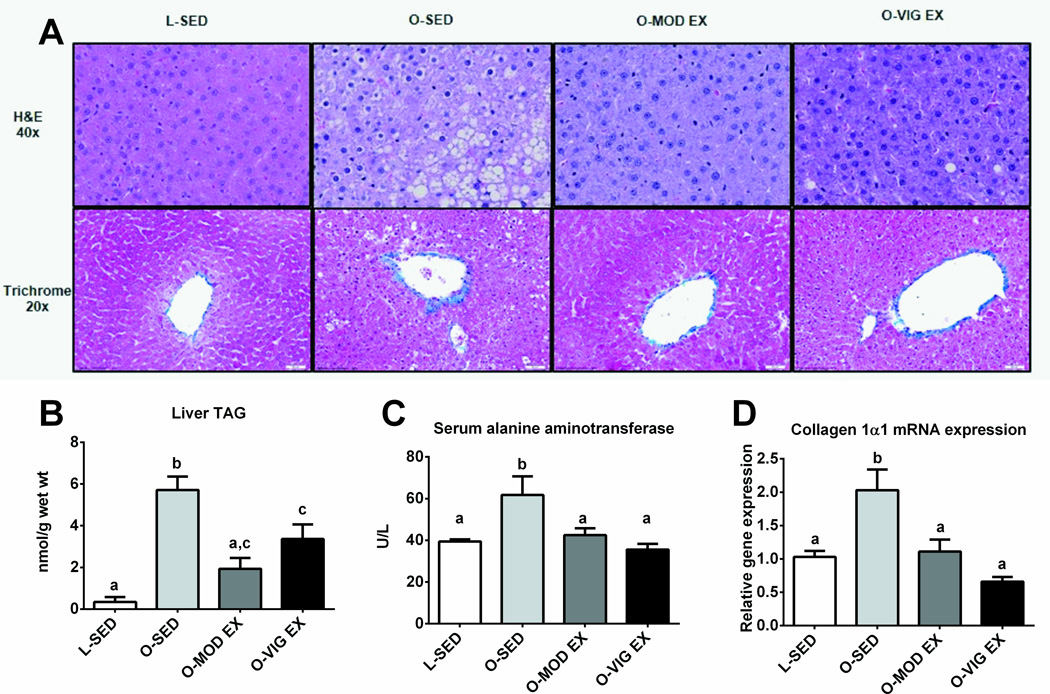
Both MOD EX and VIG EX reduced hepatic lipid accumulation and collagen formation (Figure 2A representative H&E and trichrome stained images, note less lipid vacuolization and less thickening of collagen surrounding vessels in treatment groups compared to O-SED). Biochemical TG analyses showed similar findings, with MOD EX restoring hepatic TG content to the level of the LETO (Figure 2B) and both treatments effectively lowered hepatic collagen 1α1 mRNA expression compared to O-SED (p<0.01; Figure 2C). Additionally, both MOD EX and VIG EX induced ~30–40% reductions in serum ALT concentrations (p<0.01 vs. O-SED), a marker used to indicate liver injury, resulting in ALT levels similar to the lean control animal (Figure 2D).
Figure 2.
Effects of MOD EX vs. VIG EX on measures of NAFLD. Representative images of hematoxylin and eosin and trichrome staining. Note the large lipid vacuoles, macro- and micro-vesicular steatosis, hepatocyte ballooning, nuclear displacement, and thickening of collagen in the O-SED animals compared with the other animal groups (A). Hepatic TGs, collagen 1α1 mRNA expression, and serum ALTs are shown in B, C, and D respectively. Values (n=8–10 per group, means ± SE) with different letter superscripts are significantly different (p<0.05).
Measures of hepatic mitochondrial content and function and glycolytic proteins
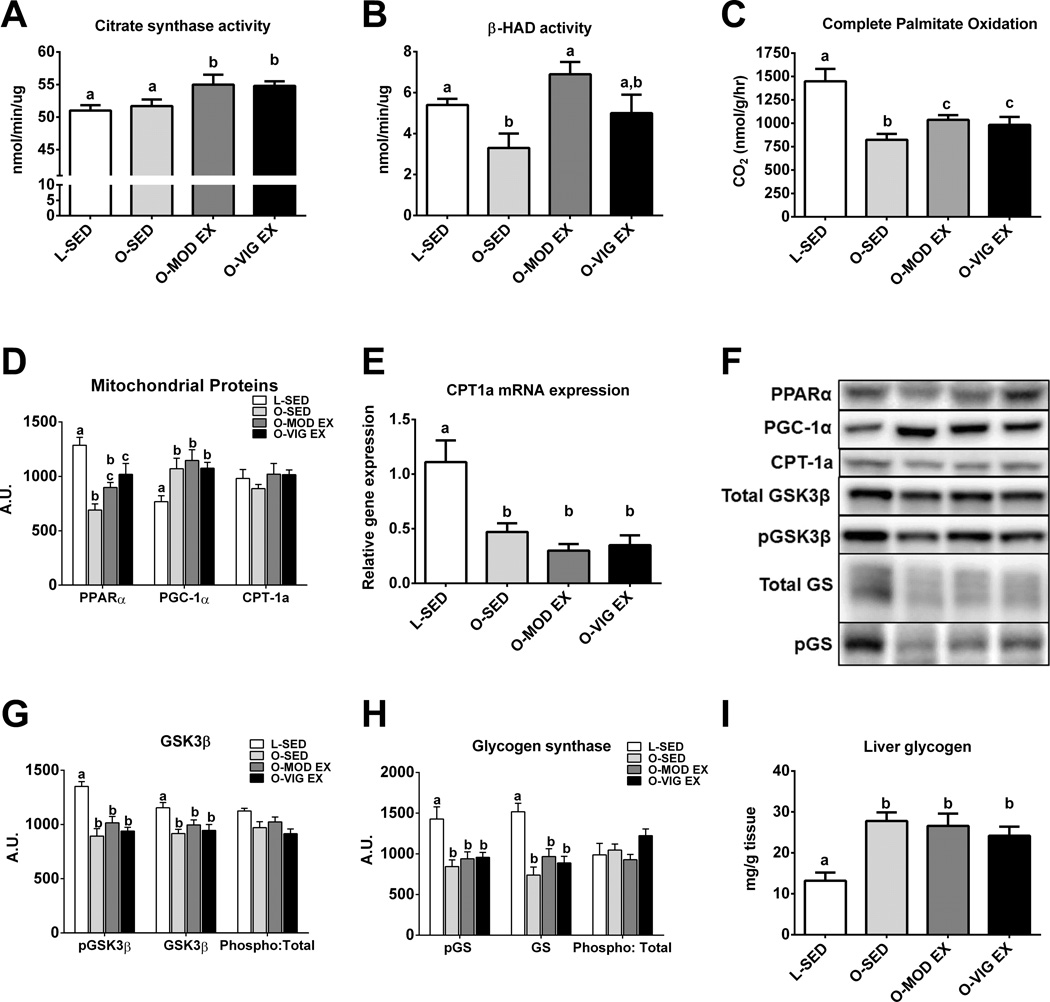
In order to better understand the potential mechanisms by which the lower volume VIG EX improved NAFLD outcomes, measures of mitochondrial content and function were assessed. Both MOD EX and VIG EX effectively improved mitochondrial content and function, as indicated by increases in citrate synthase activity (p<0.05 vs. O-SED; Figure 3A) and the complete oxidation of palmitate to CO2 (p<0.05 vs. O-SED; Figure 3C), with no observed differences between MOD EX and VIG EX. However, only MOD EX improved hepatic β-HAD activity (p<0.05; Figure 3B) when compared to O-SED, and only VIG EX increased the mitochondrial protein PPARα compared to O-SED (p<0.01; Figure 3D). Neither exercise treatment significantly altered hepatic PGC-1α protein content (Figure 3D), CPT-1a protein content (Figure 3D), or CPT-1a mRNA expression (Figure 3F) compared to O-SED. All hyperphagic OLETF groups had elevated hepatic glycogen content in the fasted state compared with non-hyperphagic LETO rats (Figure 3I). O-SED rats demonstrated reduced total hepatic GSK3β protein content (p<0.05; Figure 3G) and total hepatic GS protein content (p<0.05; Figure 3F) compared to L-SED, and exercise training did not affect basal levels of these proteins. The ratios of phospho-GSK3β to total GSK3β and phospho-GS to total GS did not differ among groups.
Figure 3.
Mitochondrial and glycolytic adaptations with MOD EX or VIG EX. Citrate synthase activity (A), β-HAD activity (B), complete palmitate oxidation (C), mitochondrial proteins (D), carnitine palmitoyltransferase-1a mRNA expression (CPT-1a; E), representative Western blots (F), glycogen synthase kinase 3β (G), glycogen synthase (H), and liver glycogen (I). Values (n=8–10 per group, means ± SE) with different letter superscripts are significantly different (p<0.05).
Hepatic markers of fatty acid uptake, de novo lipogenesis and TG secretion
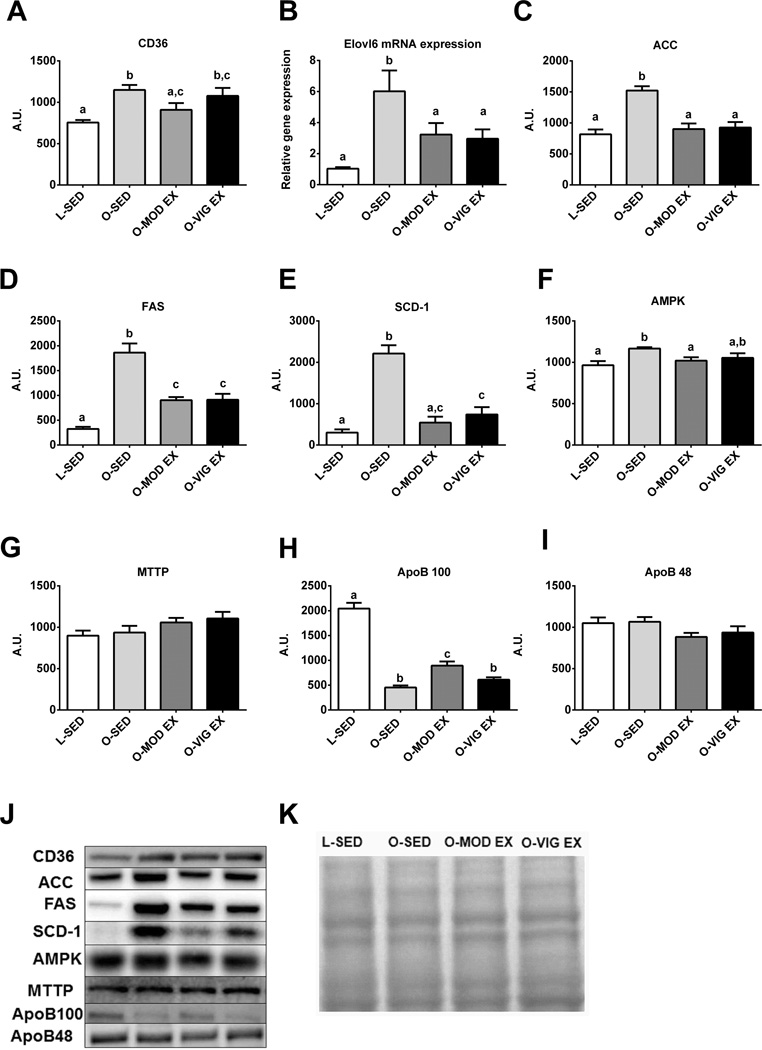
We also sought to assess other known contributing factors to TG accumulation associated with NAFLD. Only MOD EX lowered hepatic CD36/FAT, a protein important for the cellular uptake of fatty acids (p< 0.05 vs. O-SED; Figure 4A). However, both exercise training programs lowered markers of de novo lipogenesis, including the mRNA expression of Elovl6 (p<0.05; Figure 4B), and the protein content of ACC (p<0.001; Figure 4C), FAS (p<0.001; Figure 4D), and SCD-1 (p<0.001; Figure 4E), with MOD EX lowering SCD-1 to levels similar to the LETO controls. Total AMPK was higher in O-SED compared with L-SED and O-MOD EX animals (p<0.05, Figure 4F), but there were no observed differences among groups for phospho-AMPK or phospho-ACC (data not shown). Hepatic TG secretion protein MTTP (Figure 4G) was not altered with either exercise intervention; however, the dramatically suppressed hepatic expression of ApoB100 in the O-SED rats was partially rescued with MOD EX (p<0.001; Figure 4H). ApoB48 may also play an important role in hepatic TG secretion in rodents (42), but its protein content did not differ among groups in the present report (Figure 4I).
Figure 4.
Hepatic markers of fatty acid uptake, de novo lipogenesis, and triglyceride secretion. CD36/FAT protein expression (A), Elovl fatty acid elongase 6 mRNA expression (Elovl6; B), acetyl CoA carboxylase protein expression (ACC; C), fatty acid synthase protein expression (FAS; D), steroyl CoA desaturase-1 protein expression (SCD-1; E), AMPK (F), microsomal triglyceride transfer protein protein expression (MTTP; G), apolipoprotein B100 protein expression (ApoB;H), Apo B48 (I), representative Western blot images (J), and representative amido black stain (K).Values (n=8–10 per group, means ± SE) with different letter superscripts are significantly different (p<0.05).
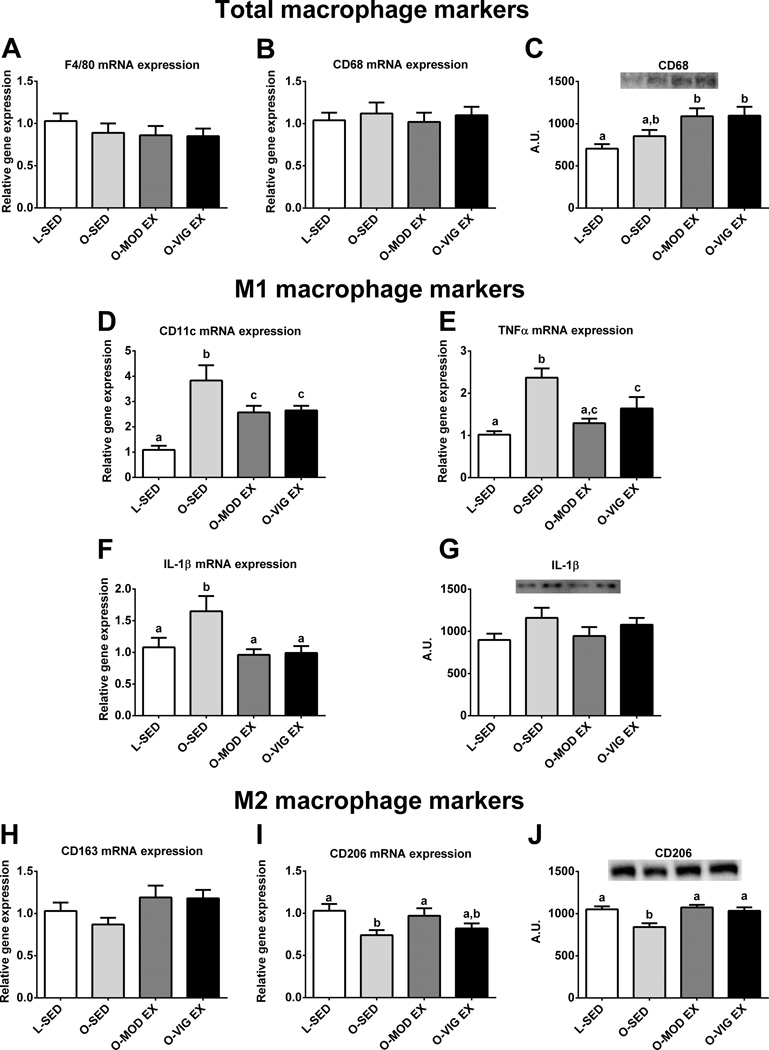
Hepatic macrophage markers
Because inflammation may play a role in the progression to advanced liver disease, markers of hepatic macrophage polarization were also assessed. Hepatic mRNA expression for total macrophage population markers F4/80 and CD68 were similar among all groups (p>0.05; Figure 5A and 5B, respectively); however CD68 protein content was increased in the OLETF rat compared to the LETO (Figure 5C), with exercise training having no effect on CD68 protein content. Interestingly, both MOD EX and VIG EX reduced mRNA expression of pro-inflammatory M1 macrophage markers CD11c (p<0.05; Figure 5D), TNFα (p<0.05; Figure 5E), and IL-1β (p<0.05; Figure 5F) by ~40% compared to SED. However, no differences were observed between groups for hepatic IL-1β protein content (Figure 5G). MOD EX also increased mRNA expression and both exercise treatments increased the protein content of the M2 macrophage marker CD206 compared to O-SED (p<0.05; Figure 5I and 5J, respectively); however, no differences were observed among groups for the M2 marker CD163 (p=0.164; Figure 5H).
Figure 5.
Altered hepatic macrophage markers in MOD and VIG EX. F4/80 mRNA expression (A), CD68 mRNA expression (B), CD68 protein content (C), CD11c mRNA expression(D), tumor necrosis factor α mRNA expression (TNFα; E), interleukin-1β mRNA expression (IL-1β; F), IL-1β protein content (G), CD163 mRNA expression (H), CD206 mRNA expression (I), and CD206 protein content (J). Values (n=8–10 per group, means ± SE) with different letter superscripts are significantly different (p<0.05).
DISCUSSION
Increasing physical activity aids in the prevention and treatment of NAFLD (reviewed in (34)), but the optimal duration and intensity of exercise needed to treat NAFLD remains unclear. In this report, sedentary OLETF rats displayed characteristics of the metabolic syndrome, including hyperglycemia, low grade inflammation, and NAFLD, which is considered the hepatic manifestation of the metabolic syndrome (reviewed in (38)). Importantly, both MOD EX and VIG EX effectively improved NAFLD outcomes, including hepatic TG, serum ALTs, and perivenular fibrosis. The observed improvements in NAFLD with VIG EX were associated with increased hepatic mitochondrial function and reduced hepatic markers of de novo lipogenesis. Additionally, this is one of the first reports to suggest that VIG EX can effectively lower hepatic M1 macrophage polarization markers and prevent advanced liver disease. When taken together, these novel findings suggest that the accumulation of 15 min/day of VIG EX 5 days/week for 12 weeks by in large was as effective as 60 min/day, 5 days/week of MOD EX in the management of NAFLD in the OLETF rat, and provides compelling evidence for further study of VIG EX as a potential therapy for NAFLD.
The incidence of lifestyle-related diseases such as NAFLD is rising at alarming rates and one of the first recommendations for treatment of this condition is exercise training. Currently it is recommended that adults complete at least 150 minutes of moderate physical activity a week in order to promote health (16) and similar recommendations have effectively treated NAFLD in animal models (23, 36, 40) and humans (17, 45). However, individuals often have difficulty adhering to moderate intensity exercise regimens due to the associated time commitment; therefore, it is important to determine whether alternative exercise training methods may aid in the treatment of NAFLD.
High intensity interval training is becoming more popular due to its reductions in exercise volume and time commitment. Clinical trials and animal studies have shown interval training (30s-4 min bouts) not only improves aerobic capacity (15, 33, 47) but also many health outcomes including blood pressure (15, 47), circulating TG concentrations (33), and insulin sensitivity and glycemic control (41, 47). Additionally, interval training can induce positive mitochondrial adaptations within skeletal muscle (7, 9, 21, 47). Moreover, 12 weeks of continuous high-intensity aerobic exercise training (85% of VO2peak, 4 d/wk, 30 min/d) reduced hepatic TG content by ~40% in sedentary, obese adolescents in the absence of weight loss (48). The current findings support previous work using vigorous intensity interval training and expand upon these findings by showing that the accumulation of 15 minutes of vigorous exercise (estimated here to be >80–90% of animals’ VO2max (43)) through interval training was as effective as MOD EX at lowering hepatic steatosis in the OLETF rat, which occurred without attenuating weight gain to the same extent as MOD EX.
Interval sprint training has been shown to improve skeletal muscle mitochondrial content and function (6, 9, 21, 47) as well as glycolytic capacity in skeletal muscle (26). Here we highlight novel data in which similar mitochondrial adaptations occurred in the liver in response to VIG EX, with increased hepatic PPARα protein content, citrate synthase activity, and palmitate oxidation compared to O-SED animals. These observed adaptations were similar to those seen with MOD EX, with exceptions being that MOD EX failed to increase hepatic PPARα but did increase hepatic β-HAD activity (not witnessed in the VIG EX rats). However, neither MOD EX nor VIG EX significantly altered the elevated fasting hepatic glycogen content witnessed in the O-SED animals or the protein regulators of glycogen synthesis (GS and GSK3β), despite similar reductions seen in fasting serum glucose by both exercise training groups. The exercise-induced increases in hepatic mitochondrial markers are important given that hepatic mitochondrial dysfunction precedes NAFLD development and is associated with NAFLD progression in OLETF rats (37). In addition, the significant increases in hepatic PPARα in the VIG EX rats perhaps suggest an upregulation in peroxisomal activation which may be playing a role in exercise-mediated alleviation of hepatic steatosis in this group. This possibility warrant future investigation.
Fatty acid uptake and hepatic de novo lipogenesis can also contribute significantly to NAFLD (reviewed in (34)). The fatty acid transporter CD36/FAT is elevated in NAFLD patients (28) and within the liver of the obese OLETF rat (24), potentially allowing for greater uptake of circulating fatty acids into hepatocytes and contributing to NAFLD. In the present report, hepatic CD36/FAT protein content was only reduced in the MOD EX group. Additionally, it is estimated that >25% of hepatic TG accumulation in NAFLD patients can be accounted for by de novo lipogenesis (12). We have previously shown a dramatic up-regulation in hepatic de novo lipogenesis in the OLETF rat, which is suppressed with chronic physical activity (24, 36, 40) and moderate intensity treadmill exercise training (23). Here, VIG EX was as effective as MOD EX at lowering ACC, FAS, and SCD-1 protein content and reducing the mRNA expression of Elov6, an elongase that has not only been associated with hepatic steatosis but also implicated in the progression of NAFLD to NASH by promoting inflammation (27).
Hepatic MTTP and ApoB also were assessed due to their role in hepatic VLDL TG secretion. Here we report no differences among OLETF and LETO groups for hepatic MTTP and ApoB48 protein content, which may play an important role for hepatic VLDL TG secretion in rodents (42). However, hepatic ApoB100 was dramatically reduced in the O-SED compared with L-SED, and these reductions were partially rescued with MOD EX but not VIG EX. The effects of MOD EX on ApoB100 levels may partially explain the exercise-induced improvements in hepatic steatosis witnessed in these animals, but future research is warranted in understanding its role in the modulation of hepatic TG export in this animal model.
Finally, inflammation is thought to contribute to the progression to advanced liver disease (22) and individuals with NAFLD have been shown to have elevations in systemic inflammation (14, 25), which was supported in the present report in the sedentary OLETF rats with elevations in leptin, MCP-1, and IL-6. Additionally, it is known that obesity affects the balance in macrophage polarization within white adipose tissue (1) and similar imbalances may promote inflammation and fibrosis through stellate cell activation in the liver (18, 29). M1 polarized macrophages promote the release of pro-inflammatory cytokines while alternatively activated, or M2 polarized macrophages, promote anti-inflammatory responses (10). Recently, M1 polarization markers have been shown to be elevated in animal models of NASH (18) and diet induced obesity (49). The current findings support these limited investigations, with observed elevations in hepatic mRNA expression of M1/inflammatory markers CD11c, IL-1β, and TNFα in the obese, O-SED rats. Additionally, our results of lower CD206 mRNA expression and protein content support research in which hepatic mRNA expression of M2 polarization markers were lowered in obese individuals with elevated hepatic steatosis (49). Interestingly, mRNA expression of the total macrophage markers CD68 or F4/80 did not differ among groups in the present study and CD68 protein content was actually elevated with both MOD EX and VIG EX. Interestingly, both MOD EX and VIG EX attenuated the mRNA expression of CD11c, IL-1β, and TNFα, supporting previous work in which treadmill exercise training lowered CD11c mRNA expression (18). Additionally, MOD EX increased mRNA of the M2 marker CD206 and both MOD and VIG EX increased CD206 protein content, suggesting an exercise-induced increase in M2 polarization of hepatic macrophages (18). These exercise-induced phenotypic shifts towards M2 macrophage polarization were associated with lowered collagen 1α1 mRNA expression and reduced perivenular fibrosis. These are novel, exciting findings that suggest a potential mechanism by which exercise training may prevent the advancement of liver disease through alterations in hepatic macrophage polarization status. Future work is needed to better understand the interactions between hepatic macrophage polarization and the progression of NAFLD to NASH.
In summary, these data highlight novel findings that the accumulation of 15 minutes per day (6 sessions of 2.5 minute duration), 5 days/week of vigorous-intensity interval exercise training for 12 weeks was by in large as effective as 60 minutes per day, 5 days/week of moderate-intensity exercise training in the management of NAFLD in the OLETF rat. The current findings highlight the importance of exercise training for the treatment of NAFLD and indicate that the effectiveness of exercise as a therapy appears to be mediated through improvements in hepatic mitochondrial function, lipid metabolism, and alterations in hepatic macrophage polarization status. These findings may have important health outcome implications as we work to design better exercise training programs for patients with nonalcoholic fatty liver disease.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the excellent technical assistance of Pam Thorne and Timothy Tan. We also thank Eric Gibson, Brittany Muller, Kelcie Tacchi, Matt Brielmaier, and Nicholas Fleming for all of their hard work as animal trainers. This work was partially supported by NIH T32 AR 048523-07(JAF), DK088940 (JPT), RO1HL036088 (MHL), HL73101-07 (JRS), HL107910-03 (JRS), VA-Merit System 0018 (JRS), and VHA-CDA2 1299 (RSR). This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Footnotes
Author Contributions: Involved in the study concept and design (FWB, MHL, JRS, JAI, JPT, RSR); acquisition of data (MAL, GMM, EMM, JAF, RSR); analysis and interpretation of data (MAL, GMM, FWB, MHL, EMM, JAI, JPT, RSR); drafting of the manuscript (MAL, RSR); critical revision of the manuscript for important intellectual content (MAL, EMM, JAF, GMM, FWB, MHL, JAI, JRS, JPT, RSR); statistical analysis (MAL, RSR).
The authors have no conflicts of interest to disclose.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human Adipose Tissue Macrophages: M1 and M2 Cell Surface Markers in Subcutaneous and Omental Depots and after Weight Loss. J Clin Endocrinol Metab. 2009;94(11):4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 2.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic Differentiation of Distinct Muscle Types at the Level of Enzymatic Organization. Eur J Biochem. 1969;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and Risk Factors for Hepatic Steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Borengasser SJ, Rector RS, Uptergrove GM, et al. Exercise and Omega-3 Polyunsaturated Fatty Acid Supplementation for the Treatment of Hepatic Steatosis in Hyperphagic Oletf Rats. J Nutr Metab. 2012;2012:268680. doi: 10.1155/2012/268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of Hepatic Steatosis in an Urban Population in the United States: Impact of Ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of Short-Term Sprint Interval Training on Human Skeletal Muscle Carbohydrate Metabolism During Exercise and Time-Trial Performance. J Appl Physiol. 2006;100(6):2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 7.Burgomaster KA, Howarth KR, Phillips SM, et al. Similar Metabolic Adaptations During Exercise after Low Volume Sprint Interval and Traditional Endurance Training in Humans. J Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell S, Lazo M. Is Exercise an Effective Treatment for Nash? Knowns and Unknowns. Ann Hepatol. 2009;8(Suppl 1):S60–S66. [PubMed] [Google Scholar]
- 9.Chilibeck PD, Bell GJ, Farrar RP, Martin TP. Higher Mitochondrial Fatty Acid Oxidation Following Intermittent Versus Continuous Endurance Exercise Training. Can J Physiol Pharmacol. 1998;76(9):891–894. doi: 10.1139/cjpp-76-9-891. [DOI] [PubMed] [Google Scholar]
- 10.Claria J, Gonzalez-Periz A, Lopez-Vicario C, Rius B, Titos E. New Insights into the Role of Macrophages in Adipose Tissue Inflammation and Fatty Liver Disease: Modulation by Endogenous Omega-3 Fatty Acid-Derived Lipid Mediators. Front Immunol. 2011;2:49. doi: 10.3389/fimmu.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez MY, Torgan CE, Brozinick JT, Jr, Ivy JL. Insulin Resistance of Obese Zucker Rats Exercise Trained at Two Different Intensities. Am J Physiol. 1991;261(5 Pt 1):E613–E619. doi: 10.1152/ajpendo.1991.261.5.E613. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of Fatty Acids Stored in Liver and Secreted Via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley GA, Abraham WM, Terjung RL. Influence of Exercise Intensity and Duration on Biochemical Adaptations in Skeletal Muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(4):844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 14.Genc H, Dogru T, Kara M, et al. Association of Plasma Visfatin with Hepatic and Systemic Inflammation in Nonalcoholic Fatty Liver Disease. Ann Hepatol. 2013;12(4):548–555. [PubMed] [Google Scholar]
- 15.Haram PM, Kemi OJ, Lee SJ, et al. Aerobic Interval Training Vs. Continuous Moderate Exercise in the Metabolic Syndrome of Rats Artificially Selected for Low Aerobic Capacity. Cardiovasc Res. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haskell WL, Lee IM, Pate RR, et al. Physical Activity and Public Health: Updated Recommendation for Adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 17.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic Exercise Training Reduces Hepatic and Visceral Lipids in Obese Individuals without Weight Loss. Hepatology. 2009;50(4):1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 18.Kawanishi N, Yano H, Mizokami T, Takahashi M, Oyanagi E, Suzuki K. Exercise Training Attenuates Hepatic Inflammation, Fibrosis and Macrophage Infiltration During Diet Induced-Obesity in Mice. Brain Behav Immun. 2012;26(6):931–941. doi: 10.1016/j.bbi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB. Physical Activity Recommendations, Exercise Intensity, and Histological Severity of Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2011;106(3):460–468. doi: 10.1038/ajg.2010.488. quiz 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laughlin MH, Korthuis RJ, Sexton WL, Armstrong RB. Regional Muscle Blood Flow Capacity and Exercise Hyperemia in High-Intensity Trained Rats. J Appl Physiol (1985) 1988;64(6):2420–2427. doi: 10.1152/jappl.1988.64.6.2420. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval Sprint Training Enhances Endothelial Function and Enos Content in Some Arteries That Perfuse White Gastrocnemius Muscle. J Appl Physiol (1985) 2004;96(1):233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Diehl AM. Innate Immunity in the Liver. Curr Opin Gastroenterol. 2003;19(6):565–571. doi: 10.1097/00001574-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Linden MA, Fletcher JA, Morris EM, et al. Combining Metformin and Aerobic Exercise Training in the Treatment of Type 2 Diabetes and Nafld in Oletf Rats. Am J Physiol Endocrinol Metab. 2014;306(3):E300–E310. doi: 10.1152/ajpendo.00427.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden MA, Meers GM, Ruebel ML, et al. Hepatic Steatosis Development with Four Weeks of Physical Inactivity in Previously Active, Hyperphagic Oletf Rats. Am J Physiol Regul Integr Comp Physiol. 2013;304(9):R763–R771. doi: 10.1152/ajpregu.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucero D, Zago V, Lopez GI, et al. Pro-Inflammatory and Atherogenic Circulating Factors in Non-Alcoholic Fatty Liver Disease Associated to Metabolic Syndrome. Clin Chim Acta. 2011;412(1–2):143–147. doi: 10.1016/j.cca.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 26.MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM. Muscle Performance and Enzymatic Adaptations to Sprint Interval Training. J Appl Physiol (1985) 1998;84(6):2138–2142. doi: 10.1152/jappl.1998.84.6.2138. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaka T, Atsumi A, Matsumori R, et al. Elovl6 Promotes Nonalcoholic Steatohepatitis. Hepatology. 2012;56(6):2199–2208. doi: 10.1002/hep.25932. [DOI] [PubMed] [Google Scholar]
- 28.Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, et al. Hepatic Fatty Acid Translocase Cd36 Upregulation Is Associated with Insulin Resistance, Hyperinsulinaemia and Increased Steatosis in Non-Alcoholic Steatohepatitis and Chronic Hepatitis C. Gut. 2011;60(10):1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 29.Miura K, Kodama Y, Inokuchi S, et al. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1beta in Mice. Gastroenterology. 2010;139(1):323–334. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris EM, Meers GM, Booth FW, et al. Pgc-1alpha Overexpression Results in Increased Hepatic Fatty Acid Oxidation with Reduced Triacylglycerol Accumulation and Secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303(8):G979–G9792. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patch LD, Brooks GA. Effects of Training on Vo2 Max and Vo2 During Two Running Intensities in Rats. Pflugers Arch. 1980;386(3):215–219. doi: 10.1007/BF00587471. [DOI] [PubMed] [Google Scholar]
- 32.Perseghin G, Lattuada G, De Cobelli F, et al. Habitual Physical Activity Is Associated with Intrahepatic Fat Content in Humans. Diabetes Care. 2007;30(3):683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 33.Racil G, Ben Ounis O, Hammouda O, et al. Effects of High Vs. Moderate Exercise Intensity During Interval Training on Lipids and Adiponectin Levels in Obese Young Females. Eur J Appl Physiol. 2013;113(10):2531–2540. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 34.Rector RS, Thyfault JP. Does Physical Inactivity Cause Nonalcoholic Fatty Liver Disease? J Appl Physiol. 2011;111(6):1828–1835. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- 35.Rector RS, Thyfault JP, Laye MJ, et al. Cessation of Daily Exercise Dramatically Alters Precursors of Hepatic Steatosis in Otsuka Long-Evans Tokushima Fatty (Oletf) Rats. J Physiol. 2008;586(Pt 17):4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rector RS, Thyfault JP, Morris RT, et al. Daily Exercise Increases Hepatic Fatty Acid Oxidation and Prevents Steatosis in Otsuka Long-Evans Tokushima Fatty Rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 37.Rector RS, Thyfault JP, Uptergrove GM, et al. Mitochondrial Dysfunction Precedes Insulin Resistance and Hepatic Steatosis and Contributes to the Natural History of Non-Alcoholic Fatty Liver Disease in an Obese Rodent Model. J Hepatol. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-Alcoholic Fatty Liver Disease and the Metabolic Syndrome: An Update. World J Gastroenterol. 2008;14(2):185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rector RS, Uptergrove GM, Borengasser SJ, et al. Changes in Skeletal Muscle Mitochondria in Response to the Development of Type 2 Diabetes or Prevention by Daily Wheel Running in Hyperphagic Oletf Rats. Am J Physiol Endocrinol Metab. 2010;298(6):E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rector RS, Uptergrove GM, Morris EM, et al. Daily Exercise Vs. Caloric Restriction for Prevention of Nonalcoholic Fatty Liver Disease in the Oletf Rat Model. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G874–G883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards JC, Johnson TK, Kuzma JN, et al. Short-Term Sprint Interval Training Increases Insulin Sensitivity in Healthy Adults but Does Not Affect the Thermogenic Response to Beta-Adrenergic Stimulation. J Physiol. 2010;588(Pt 15):2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salter AM, Wiggins D, Sessions VA, Gibbons GF. The Intracellular Triacylglycerol/Fatty Acid Cycle: A Comparison of Its Activity in Hepatocytes Which Secrete Exclusively Apolipoprotein (Apo) B100 Very-Low-Density Lipoprotein (Vldl) and in Those Which Secrete Predominantly Apob48 Vldl. Biochem J. 1998;332(Pt 3):667–672. doi: 10.1042/bj3320667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepherd RE, Gollnick PD. Oxygen Uptake of Rats at Different Work Intensities. Pflugers Arch. 1976;362(3):219–222. doi: 10.1007/BF00581173. [DOI] [PubMed] [Google Scholar]
- 44.Srere PA. Citrate Synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- 45.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized Trial of Exercise Effect on Intrahepatic Triglyceride Content and Lipid Kinetics in Nonalcoholic Fatty Liver Disease. Hepatology. 2012;55(6):1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai MM. A Mathematical Model for the Determination of Total Area under Glucose Tolerance and Other Metabolic Curves. Diabetes Care. 1994;17(2):152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 47.Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome: A Pilot Study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Heijden GJ, Wang ZJ, Chu ZD, et al. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity (Silver Spring) 2010;18(2):384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 49.Wan J, Benkdane M, Teixeira-Clerc F, et al. M2 Kupffer Cells Promote M1 Kupffer Cell Apoptosis: A Protective Mechanism against Alcoholic and Nonalcoholic Fatty Liver Disease. Hepatology. 2014;59(1):130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]