Abstract
Introduction
Minimally invasive parathyroidectomy (MIP) with intraoperative parathyroid hormone assay (IOPTH) has successfully replaced conventional neck exploration in most patients with primary hyperparathyroidism (pHPT) and preoperatively localized parathyroid adenoma.
Aim
To compare outcomes of video-assisted MIP (MIVAP) to open MIP (OMIP).
Material and methods
A retrospective case-controlled study of 455 patients with sporadic pHPT undergoing MIP with IOPTH at our institution in 2003–2012 was undertaken. The primary outcome measure was postoperative pain. Secondary outcome measures were: duration of surgery, recurrent laryngeal nerve (RLN) identification rate, conversion rate, length of hospital stay, cure rate, patients’ satisfaction with cosmetic outcome, morbidity, costs, and diagnostic accuracy of IOPTH.
Results
Of 455 patients with pHPT and a solitary parathyroid adenoma on preoperative imaging, 151 underwent MIVAP and 304 had OMIP. The following outcomes were favourable for MIVAP vs. OMIP: lower pain intensity during 24 h postoperatively (p < 0.001), lower analgesia request rate (p < 0.001), lower analgesics consumption (p < 0.001), higher recurrent laryngeal nerve identification rate (p < 0.001), shorter scar length (p < 0.001), and better cosmetic satisfaction at 1 month (p = 0.013) and at 6 months (p = 0.024) after surgery. However, MIVAP vs. OMIP had longer duration of surgery (p < 0.001), and was more expensive (p < 0.001). No differences were noted in the conversion rate, length of hospital stay, and morbidity.
Conclusions
Both MIVAP and OMIP approaches were equally safe and effective. However, the outcomes of MIVAP operations were superior to OMIP in terms of lesser postoperative pain, lower analgesics consumption, and better cosmetic satisfaction resulting from a smaller scar.
Keywords: minimally invasive parathyroidectomy, minimally invasive video-assisted parathyroidectomy, open minimally invasive parathyroidectomy, intraoperative parathyroid hormone assay
Introduction
Minimally invasive parathyroidectomy (MIP) has recently replaced the gold standard of bilateral neck exploration (BNE) in the surgical treatment of most patients with sporadic primary hyperparathyroidism (pHPT) [1–6]. Minimally invasive parathyroidectomy focuses on resecting an image-indexed solitary parathyroid adenoma through a short skin incision, without a need for intraoperative identification and assessment of the remaining glands; intraoperative intact parathyroid hormone (IOPTH) assay is often used instead to confirm cure of the hyperparathyroid state. Among fundamental advantages of MIP – besides better cosmetic effects and less pain – is a significant decrease in the percentage of postoperative transient hypoparathyroidism, which drops to approximately 5–10% as compared to some 20–25% following BNE, as well as complete elimination of the risk of permanent hypoparathyroidism. This results from preserving intact the blood supply of the remaining, normal parathyroid glands, which are not exposed during MIP. In turn, a lower transient hypocalcaemia percentage after MIP is associated with a significantly lower demand for calcium and vitamin D3 preparations and shorter hospitalisation, whereas the success rate of this technique in restoring normal calcium levels in expert hands of endocrine surgeons exceeds 98% [2, 5]. Among the presently employed methods of MIP, the most commonly selected techniques include minimally invasive video assisted parathyroidectomy (MIVAP) developed by Miccoli [7, 8], Udelsmann's open minimally invasive parathyroidectomy (OMIP) [9, 10], Gagner's endoscopic parathyroidectomy using the central approach [11, 12], the same procedure, but performed from the lateral approach, as described by Henry [1, 13], or else parathyroidectomy guided by a gamma-probe [14]. Which of these approaches is superior remains an open question [6, 15]. Many endocrine surgeons recommend that the treatment of choice for solitary parathyroid adenoma should be OMIP, due to advantages in operative duration, a shorter learning curve and improved cost-effectiveness [6]. Unfortunately, the few hitherto published randomized controlled trials comparing different minimally invasive surgical approaches for removal of a solitary parathyroid adenoma were explanatory rather than pragmatic trials due to the small number of patients involved [16, 17]. Thus, from the clinical viewpoint, it is important to validate outcomes of these explanatory trials in large cohort studies. To address this issue, we performed a retrospective case-controlled analysis comparing outcomes of the two MIP techniques most commonly utilized at our institution, MIVAP and OMIP, among a large series of patients operated on within the last decade.
Aim
The aim of this study was to compare outcomes of MIVAP to OMIP.
Material and methods
Study design and patient selection
This was a retrospective cohort study of patients who underwent MIP for sporadic pHPT at the Third Department of General Surgery, Jagiellonian University Medical College, Krakow, Poland. The prospectively collected database of parathyroid surgery was searched for eligible patients (treated in 2003–2012). All the patients provided written informed consent for the storage and use of their data. Of 610 patients meeting the inclusion criteria who were identified in the register, 460 patients were eligible for MIP. The inclusion criteria for MIP were: biochemically confirmed sporadic primary hyperparathyroidism, suspicion of a single parathyroid adenoma localized by at least one imaging examination (ultrasound of the neck and/or 99mTc-MIBI subtraction scintigraphy), adenoma size as confirmed by ultrasonography not exceeding 30 mm in the largest diameter and absence of any concomitant thyroid pathology that would require surgical treatment. The exclusion criteria from MIP were: past surgery involving the neck, history of cervical irradiation, including 131I therapy, multinodular goitre or suspected thyroid carcinoma, suspected multiglandular parathyroid disease, familial hyperparathyroidism and suspected MEN syndrome, as well as incomplete clinical data or follow-up information. Five of 460 eligible patients refused to undergo MIP and underwent conventional BNE. Finally, 455 patients (398 females and 57 males) underwent MIP and were included in the study. The study group comprised patients who underwent MIVAP. They were compared to patients who had OMIP. The type of initial parathyroid exploration (MIVAP vs. OMIP) was based in the majority of cases on a patient's choice (395/455 = 86.8%), following a detailed discussion with all patients undergoing initial parathyroid exploration for apparent sporadic pHPT. However, the initial series of patients (60/455 = 13.2%) reported in this cohort was randomly assigned to MIVAP or OMIP intervention at a 1: 1 ratio [16]. All the patients had preoperatively biochemically confirmed pHPT and laryngoscopy. Table I presents clinical and pathological characteristics of the study patients. All the patients qualified for MIP had parathyroid imaging with at least two modalities: neck ultrasound and subtraction parathyroid scintigraphy, or SPECT-CT with 99mTc-MIBI involving the neck and superior mediastinal region (Orbiter, Siemens, Erlangen, Germany), evaluated by a nuclear medicine specialist experienced in parathyroid imaging. Neck ultrasonography was performed using a 7.5–15 MHz linear-array transducer by a surgeon experienced in parathyroid imaging. The primary outcome measure was postoperative pain. Secondary outcome measures were: duration of surgery, recurrent laryngeal nerve identification rate, conversion rate, length of hospitalisation, cure rate, patients’ satisfaction with cosmetic outcome, morbidity and costs. In addition, diagnostic accuracy of IOPTH in prognostication of cure of hyperparathyroidism was evaluated in the entire cohort of patients. The protocol of this study was approved by the Institutional Review Board.
Table I.
Clinical and pathological characteristics of patients in the study
| Parameter | MIVAP (n = 151) | OMIP (n = 304) | Value of p |
|---|---|---|---|
| Gender, n (%): | 0.075 | ||
| Female | 138 (91.4) | 260 (85.5) | |
| Male | 13 (8.6) | 44 (14.5) | |
| Age, mean ± SD (range) [years] | 51.7 ±14.9 (18–75) | 57.7 ±16.3 (18–82) | < 0.001 |
| BMI, mean ± SD (range) [kg/m2] | 27.2 ±4.5 (19.2–35.0) | 27.3 ±4.8 (19.4–35.7) | 0.838 |
| Total serum calcium level, mean ± SD (range) [mmol/l] | 2.95 ±0.16 (2.69–3.21) | 3.02 ±0.19 (2.69–3.65) | < 0.001 |
| iPTH serum level, mean ± SD (range) [ng/l] | 257.5 ±97.2 (101.1–421.5) | 280.0 ±105.7 (110.2–860.4) | 0.028 |
| Creatinine, mean ± SD (range) [µmol/l] | 86.1 ±15.7 (65–116) | 87.9 ±15.0 (63–117) | 0.238 |
| Alkaline phosphatase, mean ± SD (range) [IU/l] | 130.5 ±27.0 (37–230) | 140.3 ±36.7 (39–239) | 0.231 |
| Pathology, n (%): | 0.709 | ||
| Single parathyroid adenoma | 147 (97.3) | 294 (96.7) | |
| Double parathyroid adenoma | 3 (2.0) | 9 (3.0) | |
| Four-gland parathyroid hyperplasia | 1 (0.7) | 1 (0.3) | |
| Parathyroid cancer | 0 (0.0) | 0 (0.0) | |
| Adenoma weight, mean ± SD (range) [g] | 1.97 ±1.46 (0.25–4.68) | 2.30 ±1.65 (0.48–5.69) | < 0.001 |
| Localization of the parathyroid adenoma, n (%): | 0.737 | ||
| Right superior | 35 (23.3) | 74 (24.4) | |
| Right inferior | 40 (26.7) | 82 (27.1) | |
| Left superior | 37 (24.7) | 74 (24.4) | |
| Left inferior | 41 (27.3) | 82 (27.1) | |
| Disease, n (%): | 0.089 | ||
| Symptomatic | 104 (68.9) | 232 (76.3) | |
| Asymptomatic | 47 (31.1) | 72 (23.7) |
BMI – body mass index, iPTH – intact parathyroid hormone. Reference ranges: total calcium (2.2–2.6 mmol/l), iPTH (12–65 ng/l), creatinine (60–120 µmol/l), total alkaline phosphatase (30–260 IU/l)
Surgical technique
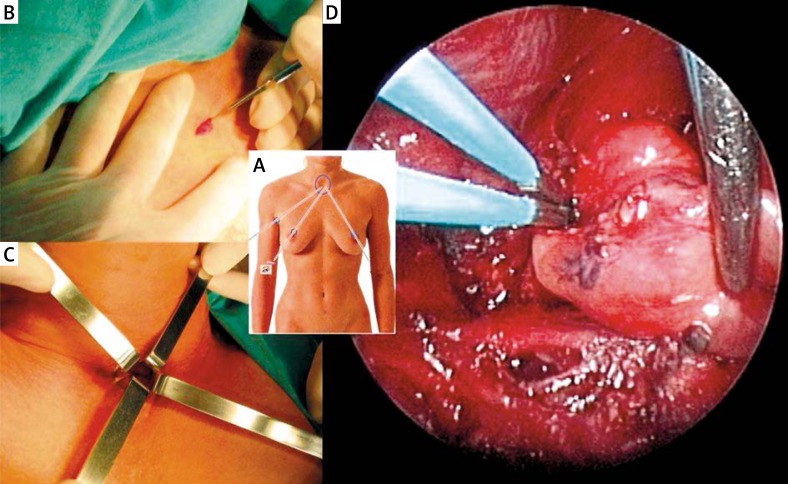
All operations were performed under general anaesthesia by one of two endocrine surgeons involved, with each performing a similar number of respective interventions (p = 0.678). The MIVAP procedures were performed employing the Miccoli technique (Photo 1) [7, 8]. The patients were placed in the same position as required in classic thyroid surgery, but hyperextension of the neck was avoided in order not to decrease the working space beneath the short muscles of the platysma. A 1.5 cm-long horizontal section was made approximately 2 cm above the sternal notch. The short muscles of the platysma were longitudinally dissected into layers in the midline and the working space was bluntly dissected manually between the thyroid gland and the muscles, avoiding pneumoperitoneum. A 5 mm 30-degree endoscope was introduced into the incision, and the working space was maintained using 2–4 conventional retractors to retract the thyroid lobe medially towards the trachea and the muscles laterally towards the cervical vessels, thus exposing the thyroid sulcus. The tissues were dissected using microsurgical instruments, mostly a suction spatula and a dissector. Videoscopic magnification significantly facilitated identification of the cervical anatomical structures, including the recurrent laryngeal nerve. Having exposed the adenoma of the parathyroid, the surgeon performed a blunt resection, exercising caution not to damage the capsule and thus risking implantation of disseminated parathyroid cells. Following the exposure of the vascular pedicle of the parathyroid gland, it was electrocauterized by bipolar coagulation. The adenoma was delivered through the main surgical incision, which was subsequently closed with loose single stitches, followed by closing the skin with intracutaneous absorbable sutures. No wound drainage was employed. The material was sent for histopathology. At the same time, the surgeon waited for the result of IOPTH determination.
Photo 1.
Minimally invasive video assisted parathyroidectomy. A, B – A 5 mm rigid endoscope and all working instruments are inserted into the neck through one small incision above the sternal notch. C – Working space is created by blunt dissection and maintained by retractors without gas insufflation. D – Intraoperative view of the superior left parathyroid adenoma, the vascular pedicle of which is ensured with bipolar coagulation
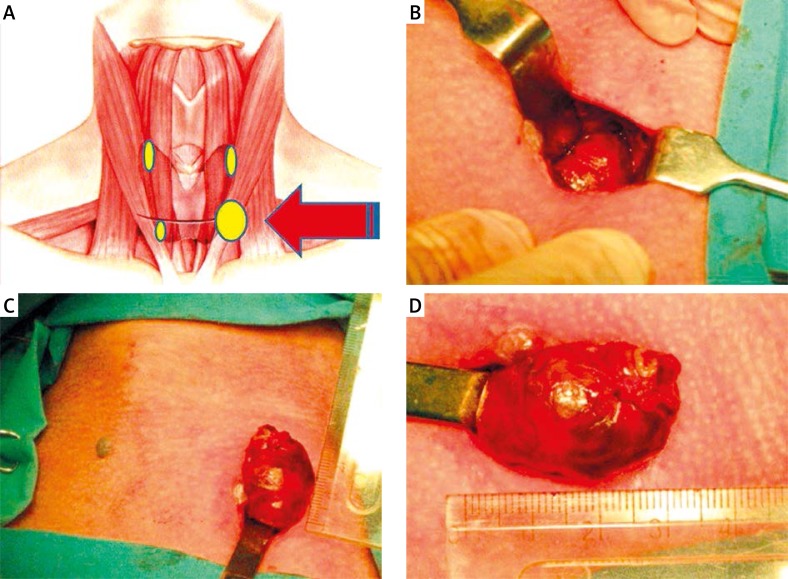
In the OMIP procedures, the surgical technique relied on the method developed by Irvin and modified by Udelsmann (Photo 2) [9, 10, 18]. A 2–3 cm-long skin incision (a short Kocher incision) was made in the majority of patients above the jugular notch. In adenomas of the superior parathyroids or situated outside the oesophagus, the skin incision was made somewhat more superiorly, along the anterior margin of the sternocleidomastoid muscle. Having dissected laterally into layers the short muscles of the platysma, the thyroid lobe was mobilized; the adenoma was identified, resected and sent for histopathology, with the surgeon waiting for the result of IOPTH determination. Routinely, the exposure of the other parathyroid gland situated on the ipsilateral (operated on) side of the neck was avoided. The wound was closed loosely and no wound drainage was employed.
Photo 2.
Open minimally invasive parathyroidectomy. A – Conventional approach for parathyroid adenoma during bilateral neck exploration through the Kocher incision. B, C – In the OMIP approach, a small skin incision is made directly over the preoperatively image-indexed parathyroid lesion. D – Intraoperative view of the superior left parathyroid adenoma delivered over the skin incision
Intraoperative iPTH assay
IOPTH determinations were routinely performed in all MIP operations using the STAT-Intraoperative-iPTH-Assay (Future Diagnostics, Wijchen, The Netherlands). Blood samples were collected from a Venflon catheter inserted into the basilic vein. Three ml of blood were collected in an EDTA vacuum test tube. The specimens were collected according to the following protocol: 1) preoperatively, after anaesthesia induction but before incising the skin; 2) after exposing and dissecting free the parathyroid adenoma, immediately before its resection; 3) 10 min following the adenoma resection. To assess the effectiveness of the procedure and to predict postoperative normocalcaemia, the modified Miami criterion was employed (a decrease in serum parathyroid hormone concentration by more than 50% at 10 min) following adenoma resection as compared to the higher of the two values describing the parathyroid hormone level prior to surgery or prior to adenoma resection [19]. In cases not meeting the Miami criterion, an additional determination was made 20 min following adenoma resection, and if no decrease in parathyroid hormone concentration was observed, the procedure was extended to unilateral and bilateral neck exploration [20]. All IOPTH determinations were performed within the operating suite complex. A single determination required 8 min. Intraoperative histopathology was used in selected patients.
Postoperative follow-up
Pain intensity was assessed by an independent clinical investigator using the visual analogue scale (VAS) at 4, 8, 12, and 24 h postoperatively. The patients were aware that the scale analysed the intensity of pain alone and was not a representation of their generalized postoperative discomfort. Nurse-controlled analgesia rate (NCA) on the patient's request, as well as analgesics consumption, was recorded for 24 h postoperatively. Ketoprofen (Sandoz, Poland) was used as a painkiller, the dose being 50 mg administered on demand (not more frequently than every 6 h). Duration of surgery was calculated from skin incision to skin closure. Conversion from MIP to unilateral neck exploration was used in cases not meeting the modified Miami criterion of IOPTH [19, 20]. In cases of intraoperative demonstration of a normally appearing second parathyroid gland on the operated side or of unsuccessful unilateral neck exploration in converted patients, BNE was performed. Total serum calcium concentration values at 24 and 48 h postoperatively were routinely measured. If serum calcium levels dropped below 2 mmol/l, oral supplementation with calcium preparations was administered, along with vitamin D metabolites. Cure of hyperparathyroidism was defined as postoperative normocalcaemia lasting for at least 6 months after surgery. Hypercalcaemia within 6 months postoperatively signified persistent disease, whereas hypercalcaemia after more than 6 months following surgery denoted recurrent disease. Hypocalcaemia was defined as total serum calcium level below 2.0 mmol/l irrespectively of iPTH level. A serum calcium level below 2.0 mmol/l with a subnormal serum iPTH level (< 10 ng/l) was defined as transient hypoparathyroidism if restored to normal within 12 months following withdrawal of oral calcium or calcium plus calcitriol therapy. Persistent hypocalcaemia with serum iPTH level below 10 ng/l for more than 12 months postoperatively, requiring substitution with calcium with or without calcitriol, was considered permanent hypoparathyroidism. Indirect laryngoscopy was used to identify and follow RLN injury (on postoperative day 1, and in cases of vocal fold paresis, every 2 months within 1 year after surgery, or until the vocal folds recovery). The nerve events incidence was calculated based on the number of nerves at risk. Patients’ satisfaction with the cosmetic result of the procedure (based on the Verbal Rating Scale (VRS); 0 – poor, 1 – weak, 2 – satisfactory, 3 – good, 4 – very good, 5 – excellent) including scar length and healing was assessed at 1, 6 and 12 months postoperatively during outpatient follow-up visits. Cost analysis was performed using an official in-hospital price list for medical procedures. The costs included (in EUR): 20 EUR for ultrasonography, 200 EUR for sestamibi scintigraphy, 100 EUR for IOPTH, 5 EUR/min for anaesthesia, 5 EUR/min for operating theatre use, 5 EUR/min for videoscopic equipment, and 80 EUR/day of hospitalisation.
Statistical analysis
For sample size calculation, an assumption was made that MIVAP should result in pain intensity lower by 30% as compared to OMIP. To detect this, it was calculated that 124 patients would be required in each treatment arm to give the study a power of 95%. Anticipating a 15% loss to follow-up, 150 patients per arm were required in the study.
Data are presented as mean with standard deviation (SD) or range, unless stated otherwise. The statistical significance of categorical variables was evaluated by the χ2 test, whereas Student's t test was used for analysis of continuous variables. Receiver operating characteristics (ROC) were used to evaluate the diagnostic accuracy of IOPTH in prognostication of cure of the hyperparathyroid state. All the data were collected prospectively and stored in a computer-based institutional register of parathyroid surgery and analysed retrospectively by a statistician. Statistical analyses were performed with MedCalc (version 13, MedCalc Software, Belgium). Value of p < 0.05 was considered to indicate a significant difference.
Results
Of 1107 patients referred for parathyroid surgery during the study interval, 690 had pHPT and were potential candidates for the study. Two-hundred and twenty-seven patients did not meet the inclusion criteria (negative or discordant preoperative imaging 65; concomitant goitre necessitating surgical removal 51; persistent pHPT 50; recurrent pHPT 22; MEN 1 syndrome 17; lithium treatment 12; lacking consent for MIP 5; MEN 2A syndrome 5), and 8 had incomplete histopathology or follow-up data, leaving 455 patients who were finally included in this study.
One-hundred and fifty-one patients underwent MIVAP, whereas 304 patients underwent OMIP.
There were 398 women and 57 men with a mean age at operation of 55.7 ±16.1 years (range: 18–82). Analysis of baseline characteristics identified that MIVAP patients were significantly younger (51.7 ±14.9 years vs. 57.7 ±16.3 years, respectively; p < 0.001), had lower total serum calcium levels (2.95 ±0.16 mmol/l vs. 3.02 ±0.19 mmol/l, respectively; p < 0.001), lower preoperative serum iPTH levels (257.5 ±97.2 ng/l vs. 280.0 ±105.7 ng/l, respectively; p = 0.028) and smaller adenomas (1.97 ±1.46 g vs. 2.30 ±1.65 g, respectively; p < 0.001) than OMIP patients. Despite concordant results of preoperative imaging in all the patients in this cohort, a solitary parathyroid adenoma was found in 441 (96.9%) patients, whereas 14 (3.1%) patients had multiglandular disease (double adenoma 12, four-gland hyperplasia 2). Table I shows clinical and pathological characteristics of patients analysed in this study.
Primary outcome
Pain assessed by VAS was significantly lower at 4, 8, 12, and 24 h postoperatively in MIVAP versus OMIP patients, respectively (p < 0.001). The difference in pain intensity varied between the groups, being 22.6% at 4 h, 7.3% at 8 h, 25.2% at 12 h, and 32.7% at 24 h after surgery. The analgesia request rate was significantly lower in MIVAP versus OMIP patients (65.6% vs. 91.4%, respectively; p < 0.001). Mean analgesics consumption was also lower among MIVAP compared with OMIP patients (48.0 ±41.6 mg vs. 89.1 ±48.9 mg of ketoprofen, respectively; p <0.001). Detailed data are shown in Table II.
Table II.
Primary and secondary outcomes
| Parameter | MIVAP (n = 151) | OMIP (n = 304) | Value of p |
|---|---|---|---|
| Postoperative pain (VAS), mean ± SD (range) [pts] | |||
| At 4 h | 22.3 ±4.6 (15–30) | 28.8 ±5.8 (19–38) | < 0.001 |
| At 8 h | 25.3 ±5.0 (17–34) | 27.3 ±5.7 (18–37) | < 0.001 |
| At 12 h | 16.6 ±4.1 (10–25) | 22.2 ±4.9 (14–30) | < 0.001 |
| At 24 h | 10.9 ±3.7 (5–18) | 16.2 ±3.8 (10–22) | < 0.001 |
| Analgesia request, n (%) | 99 (65.6) | 278 (91.4) | < 0.001 |
| Analgesics consumption, mean ± SD (range) [mg] | 48.0 ±41.6 (0–150) | 89.1 ±48.9 (0–200) | < 0.001 |
| Duration of surgery, mean ± SD (range) [min] | 42.5 ±10.1 (25–60) | 37.4 ±10.9 (20–55) | < 0.001 |
| RLN identification, n (%) | 140 (92.7) | 221 (72.7) | < 0.001 |
| Conversion, n (%): | 4 (2.7) | 10 (3.3) | 0.709 |
| UNE | 1 (0.7) | 4 (1.3) | |
| BNE | 3 (2.0) | 6 (2.0) | |
| Length of hospital stay, mean ± SD (range) [days]: | 1.14 ±0.48 (1–3) | 1.20 ±0.51 (1–3) | 0.216 |
| 1 day, n (%) | 138 (91.4) | 258 (84.9) | |
| 2 days, n (%) | 5 (3.3) | 31 (10.2) | |
| 3 days, n (%) | 8 (5.3) | 15 (4.9) | |
| Cure of hyperparathyroidism, n (%) | 150 (99.3) | 303 (99.7) | 0.613 |
| Length of scar, mean ± SD (range) [mm] | 17.8 ±1.9 (15–21) | 31.4 ±4.2 (25–38) | < 0.001 |
| Patients’ satisfaction with cosmetic outcome (VRS), mean ± SD (range) [pts]: | |||
| At 1 month | 3.77 ±0.62 (2–5) | 3.60 ±0.75 (1–5) | 0.013 |
| At 6 months | 4.10 ±0.71 (2–5) | 3.95 ±0.70 (2–5) | 0.024 |
| Morbidity, n (%): | 0.627 | ||
| Transient hypocalcaemia | 7 (4.6) | 16 (5.3) | |
| Permanent hypoparathyroidism | 0 (0.0) | 0 (0.0) | |
| Transient RLN injury | 2 (1.3) | 5 (1.6) | |
| Permanent RLN injury | 1 (0.7) | 3 (1.0) | |
| Costs, mean (range) [EUR] | 1048.7 (775.0–1460.0) | 841.0 (600.0–1110.0) | < 0.001 |
VRS – verbal rating scale (0 – poor, 1 – weak, 2 – acceptable, 3 – good, 4 – very good, 5 – excellent), SD – standard deviation, RLN – recurrent laryngeal nerve, UNE – unilateral neck exploration, BNE – bilateral neck exploration
Secondary outcomes
Mean operative time was significantly longer for MIVAP vs. OMIP operations (42.5 ±10.1 min vs. 37.4 ±10.9 min, respectively; p < 0.001). The RLN identification rate was significantly higher in the MIVAP vs. OMIP group (92.7% vs. 72.7%, respectively, p < 0.001). Scar length was significantly shorter in the MIVAP vs. OMIP group (17.8 ±1.9 mm vs. 31.4 ±4.2 mm, respectively; p < 0.001). Patients’ satisfaction with cosmetic outcome assessed by VRS was significantly higher after MIVAP vs. OMIP, both at 1 month (3.77 ±0.62 vs. 3.60 ±0.75 points, respectively; p = 0.013) and at 6 months (4.10 ±0.71 vs. 3.95 ±0.70 points, respectively; p = 0.024) postoperatively. Mean hospitalisation costs were higher in the MIVAP group compared with the OMIP group for total hospital charges, but the difference was related to the higher costs of endoscopic tools involvement and longer operating time, both being independently charged 5 EUR each per minute of operation, with the mean difference being 207.7 EUR. Thus, MIVAP was on average 25% more expensive than OMIP.
Non-significant differences were observed between both groups in cure rate, length of hospitalisation and morbidity. Details are shown in Table II. ROC analysis identified the following diagnostic accuracy of the modified Miami criterion employed for IOPTH interpretation in prognostication of cure of hyperparathyroidism: sensitivity 97.7%, specificity 96.4%, positive predictive value 99.8%, negative predictive value 73.0%, and overall accuracy 97.6%.
Discussion
This retrospective case-controlled study compared outcomes of video-assisted vs. open MIP and suggested a similar cure rate of the hyperparathyroid state, but lower pain intensity within 24 postoperative hours, lower analgesia request rate, lower analgesics consumption, improved RLN identification rate, shorter scar length and higher cosmetic satisfaction rate after video-assisted parathyroidectomy. However, all these benefits were achieved in the MIVAP group at higher costs than in the OMIP group, mainly due to the need for use of endoscopic tools. Similar outcomes were initially reported by our group in a small (n = 60) randomized study, and now have been confirmed in a large cohort (n = 455) of patients with sporadic pHPT treated in 2003–2012. However, somewhat conflicting outcomes were reported by Hessman et al., with 143 patients randomized to undergo video-assisted vs. open MIP. In this study, surgery duration was significantly shorter for open compared to video-assisted MIP (60 ±35 min vs. 84 ±47 min, respectively; p = 0.001), but both groups of patients had similar conversion rates and the same outcome, with comparable incision lengths, low scores for postoperative neck discomfort, high cosmetic satisfaction and low complication rates [17]. A possible explanation of the differing outcomes found in these two studies is the mixed type of intervention in the video-assisted arm in the Hessman et al. study, as patients underwent either MIVAP according to the Miccoli technique or video-assisted parathyroidectomy using a lateral approach (VAPLA) according to the Henry technique [17].
Minimally invasive parathyroidectomy has gained worldwide acceptance in the surgical treatment of sporadic pHPT, replacing the gold standard of BNE in patients with a presumed solitary parathyroid adenoma [1–6]. Recent advances in parathyroid imaging allow for highly accurate pre-selection of patients with pHPT for MIP, whereas IOPTH can be used to monitor intraoperatively the quality of surgery, minimizing the risk of overlooking multiglandular disease [21–23]. As expressed in a survey distributed among members of the International Association of Endocrine Surgeons (IAES), MIP is currently offered to patients by almost two-thirds of endocrine surgeons worldwide [24]. There are many different MIP techniques (open, video-assisted or endoscopic), but regardless of the technique, all these minimally invasive operations share the same philosophy of the image-guided selective removal of a single parathyroid adenoma [23]. Thus, it is not surprising that all these techniques have a similar success rate in correcting the hyperparathyroid state. On the other hand, many soft endpoints can be different and influence postoperative recovery.
The primary outcome of this study was postoperative pain assessed by VAS and it was found to be significantly lower in MIVAP versus OMIP patients at all evaluated time points within the initial 24 postoperative hours. The reason for this could not have been entirely related to the shorter skin incision in MIVAP versus OMIP patients (17.8 ±1.9 mm vs. 31.4 ±4.2 mm, respectively; p < 0.001) and less tissue damage. In fact, postoperative pain is also caused by neck hyperextension, which is avoided in the video-assisted technique [7, 8]. Mean differences in pain intensity varied from 22.6% at 4 h to 32.7% at 24 h postoperatively (p < 0.001). The benefit of lower postoperative pain in the MIVAP group was also confirmed by a lower analgesia request rate (p < 0.001) and reduced analgesics consumption (p < 0.001).
Both the MIVAP and OMIP techniques were safe, as the prevalence of RLN injury was not significantly different between the groups. However, in MIVAP patients, the RLN identification rate was significantly higher than in OMIP patients (92.7% vs. 72.7%, respectively; p < 0.001).
Theoretically, during OMIP, the tissues are subjected to a higher degree of traction, which may increase the risk of transient RLN injury. It can also be hypothesized that the video-assisted technique provides magnification of the dissected structures (up to 10 times), allowing for easier recognition of the nerve. But even in the most experienced hands, nerve events still occur in about 1% of patients, irrespectively of the intraoperative neural monitoring use.
In addition, MIVAP patients experienced significantly higher cosmetic satisfaction with their scars (the scars being significantly shorter than in OMIP patients). This was true for 1 and 6 months after surgery, and cosmetic satisfaction tended to increase with time in both groups. Similar observations were made among 240 patients undergoing minimally invasive video-assisted thyroidectomy (MIVAT) at our institution [25, 26]. No differences were observed in the mean length of hospitalisation. Although overnight hospitalisation was mandatory due to the reimbursement-associated insurer's regulations, both procedures could have been successfully performed in the majority of non-complicated cases as outpatient operations under local anaesthesia [10, 27]. Mean hospital stay costs were significantly higher in the MIVAP compared with the OMIP group, and the difference was related mostly to the charges for endoscopic tool involvement, and marginally to somewhat longer surgery duration, whereas all other components, including localization studies, were similar.
A clearly identifiable bias in this study is that type of initial parathyroid exploration (MIVAP vs. OMIP) was based in the majority of individuals on a patient's choice, following a detailed discussion of potential advantages and disadvantages of each technique. Surprisingly, only one-third of patients preferred MIVAP, whereas the remaining two-thirds were in favour of OMIP. Patients who gave their consent for MIVAP were younger (p < 0.001), had lower serum calcium levels (p < 0.001), lower serum iPTH levels (p = 0.028), and lower adenoma weight (p < 0.001) than their OMIP counterparts (details are shown in Table I). Despite these statistically significant differences in baseline characteristics of both groups, they can be considered marginal from a clinical viewpoint, allowing for a fair comparison of both techniques evaluated in this study. Melck et al. found similar differences when comparing 125 video-assisted MIP cases with 95 open MIP control subjects. In this study, patients undergoing OMIP had higher mean preoperative levels of calcium (p = 0.007) and parathyroid hormone (p = 0.008), greater mean adenoma weight (p < 0.001), and increased long-term mortality (4% OMIP vs. 0% MIVAP, p = 0.03) [28].
The main concerns voiced by adversaries of MIP address the risk of recurrent hyperparathyroidism becoming evident in the late postoperative period [29, 30]. For many years, the incidence of multiglandular disease in patients subjected to BNE was estimated at approximately 15–22% (chiefly on the basis of the macroscopic criterion, i.e. the size of the parathyroid glands), whereas after MIP, the corresponding rate is only 4–5% [31]. On the one hand, this phenomenon results from pre-selection of patients qualified for MIP based on imaging studies, but on the other, it is a consequence of the parathyroid size not always correlating with the function of the gland. The concern about a higher risk of recurrent hyperparathyroidism after MIP seems unwarranted, since in the most extensive investigations, the follow-up period is longer than 10 years. Westerdahl et al. observed only sporadic cases of hyperparathyroidism recurrence in patients operated on in their young age, who were later diagnosed with type I MEN syndrome [32]. Among our patients with the mean follow-up of 42.7 ±15.8 months (range: 12–112 months), we noted one case of recurrent hyperparathyroidism (the menin gene mutation) diagnosed 36 months postoperatively. Moreover, Schneider et al. retrospectively reviewed a series of 1368 parathyroid operations for primary hyperparathyroidism performed within the last decade. A total of 1006 were MIP whereas 380 were OP. There were no differences in recurrence between the MIP and OP groups (2.5% vs. 2.1%; p = 0.68), and the operative approach (MIP vs. OP) did not independently predict recurrent disease in the multivariable analysis [30].
Finally, it should be noted that the MIP procedure, in spite of its simplicity, might not be recommended as the method of choice performed in each and every surgical ward in view of the possible lack of one or several key elements of this operation. The most important component of MIP is the surgeon experienced in parathyroid surgery. The surgeon should not attempt employing the MIP method while operating on the parathyroids until he gains expertise in classic BNE procedures. Secondly, availability of high-level imaging techniques, such as subtraction scintigraphy or high-resolution ultrasonography, is necessary to properly preselect patients qualified for MIP. Thirdly, availability of IOPTH is needed to minimize the risk of missing multiglandular disease in patients with false positive results of imaging examinations [33–35].
Whether the differences in pain intensity, analgesics consumption and improved cosmetic satisfaction are sufficient to compensate for the additional costs of endoscopic tool involvement will depend on local circumstances – the healthcare system and the acceptance of improved cosmetic outcomes as the leading benefits of the video-assisted MIP approach [16]. However, in most healthcare centres, the treatment of choice can be OMIP, due to advantages in operative duration, a short learning curve and improved cost-effectiveness [6].
Conclusions
Both MIVAP and OMIP approaches were equally safe and effective in surgical treatment of patients with sporadic primary hyperparathyroidism. However, outcomes of MIVAP operations were superior to OMIP in terms of lesser postoperative pain, lower analgesics consumption, and better cosmetic satisfaction resulting from a smaller scar. Nevertheless, a video-assisted procedure is more expensive and requires experience not only in parathyroid surgery, but also in endoscopic surgery, as well as availability of appropriate endoscopic instruments.
Acknowledgments
The authors wish to express their gratitude to Aleksander Konturek, M.D., Ph.D., and Małgorzata Stopa, M.D., who assisted at many of the operations through the duration of this study.
References
- 1.Henry JF, Raffaelli M, Iacobone M, Volot F. Video-assisted parathyroidectomy via the lateral approach vs conventional surgery in the treatment of sporadic primary hyperparathyroidism: results of a case-control study. Surg Endosc. 2001;15:1116–9. doi: 10.1007/s00464-001-9013-x. [DOI] [PubMed] [Google Scholar]
- 2.Bergenfelz A, Kamigiesser V, Zielke A, et al. Conventional bilateral cervical exploration versus open minimally invasive parathyroidectomy under local anaesthesia for primary hyperparathyroidism. Br J Surg. 2005;92:190–7. doi: 10.1002/bjs.4814. [DOI] [PubMed] [Google Scholar]
- 3.Miccoli P, Materazzi G, Bonari G, et al. Minimally invasive video-assisted parathyroidectomy. Operative Techniques in Otolaryngology Head and Neck. 2008;19:22–5. [Google Scholar]
- 4.Miccoli P, Berti P, Materazzi G, et al. Endoscopic bilateral neck exploration versus quick intraoperative parathormone assay (qPTHa) during endoscopic parathyroidectomy: a prospective randomized trial. Surg Endosc. 2008;22:398–400. doi: 10.1007/s00464-007-9408-4. [DOI] [PubMed] [Google Scholar]
- 5.Slepavicius A, Beisa V, Janusonis V, Strupas K. Focused versus conventional parathyroidectomy for primary hyperparathyroidism: a prospective, randomized, blinded trial. Langenbecks Arch Surg. 2008;393:659–66. doi: 10.1007/s00423-008-0408-1. [DOI] [PubMed] [Google Scholar]
- 6.Gracie D, Hussain SS. Use of minimally invasive parathyroidectomy techniques in sporadic primary hyperparathyroidism: systematic review. J Laryngol Otol. 2012;126:221–7. doi: 10.1017/S0022215111002908. [DOI] [PubMed] [Google Scholar]
- 7.Miccoli P, Pinchera A, Cecchini G, et al. Minimally invasive, video-assisted parathyroid surgery for primary hyperparathyroidism. J Endocrinol Invest. 1997;20:429–30. doi: 10.1007/BF03347996. [DOI] [PubMed] [Google Scholar]
- 8.Miccoli P, Berti P, Materazzi G, et al. Results of video-assisted parathyroidectomy: single institution's six-year experience. World J Surg. 2004;28:1216–8. doi: 10.1007/s00268-004-7638-3. [DOI] [PubMed] [Google Scholar]
- 9.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–70. doi: 10.1097/00000658-200205000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585–91. doi: 10.1097/SLA.0b013e318208fed9. [DOI] [PubMed] [Google Scholar]
- 11.Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996;83:875. doi: 10.1002/bjs.1800830656. [DOI] [PubMed] [Google Scholar]
- 12.Naitoh T, Gagner M, Garcia-Ruiz A, Heniford BT. Endoscopic endocrine surgery in the neck. An initial report of endoscopic subtotal parathyroidectomy. Surg Endosc. 1998;12:202–5. doi: 10.1007/s004649900634. [DOI] [PubMed] [Google Scholar]
- 13.Henry JF, Iacobone M, Mirallie E, et al. Indications and results of video-assisted parathyroidectomy by a lateral approach in patients with primary hyperparathyroidism. Surgery. 2001;130:999–1004. doi: 10.1067/msy.2001.119112. [DOI] [PubMed] [Google Scholar]
- 14.Rubello D, Piotto A, Casara D, et al. Role of gamma probes in performing minimally invasive parathyroidectomy in patients with primary hyperparathyroidism: optimization of preoperative and intraoperative procedures. Eur J Endocrinol. 2003;149:7–15. doi: 10.1530/eje.0.1490007. [DOI] [PubMed] [Google Scholar]
- 15.Chen H. Surgery for primary hyperparathyroidism: what is the best approach? Ann Surg. 2002;236:552–3. doi: 10.1097/00000658-200211000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barczyński M, Cichoń S, Konturek A, Cichoń W. Minimally invasive video-assisted parathyroidectomy versus open minimally invasive parathyroidectomy for a solitary parathyroid adenoma: a prospective, randomized, blinded trial. World J Surg. 2006;30:721–31. doi: 10.1007/s00268-005-0312-6. [DOI] [PubMed] [Google Scholar]
- 17.Hessman O, Westerdahl J, Al-Suliman N, et al. Randomized clinical trial comparing open with video-assisted minimally invasive parathyroid surgery for primary hyperparathyroidism. Br J Surg. 2010;97:177–84. doi: 10.1002/bjs.6810. [DOI] [PubMed] [Google Scholar]
- 18.Irvin GL, 3rd, Carneiro DM, Solorzano CC. Progress in the operative management of sporadic primary hyperparathyroidism over 34 years. Ann Surg. 2004;239:704–11. doi: 10.1097/01.sla.0000124448.49794.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barczynski M, Konturek A, Hubalewska-Dydejczyk A, et al. Evaluation of Halle, Miami, Rome, and Vienna intraoperative iPTH assay criteria in guiding minimally invasive parathyroidectomy. Langenbecks Arch Surg. 2009;394:843–9. doi: 10.1007/s00423-009-0510-z. [DOI] [PubMed] [Google Scholar]
- 20.Barczyński M, Konturek A, Cichoń S, et al. Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol. 2007;66:878–85. doi: 10.1111/j.1365-2265.2007.02827.x. [DOI] [PubMed] [Google Scholar]
- 21.Westerdahl J, Bergenfelz A. Sestamibi scan-directed parathyroid surgery: potentially high failure rate without measurement of intraoperative parathyroid hormone. World J Surg. 2004;28:1132–8. doi: 10.1007/s00268-004-7484-3. [DOI] [PubMed] [Google Scholar]
- 22.Sugg SL, Krzywda EA, Demeure MJ, Wilson SD. Detection of multiple gland primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Surgery. 2004;136:1303–9. doi: 10.1016/j.surg.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 23.Mihai R, Barczynski M, Iacobone M, Sitges-Serra A. Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbecks Arch Surg. 2009;394:785–98. doi: 10.1007/s00423-009-0529-1. [DOI] [PubMed] [Google Scholar]
- 24.Sackett WR, Barraclough B, Reeve TS, Delbridge LW. Worldwide trends in the surgical treatment of primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Arch Surg. 2002;137:1055–9. doi: 10.1001/archsurg.137.9.1055. [DOI] [PubMed] [Google Scholar]
- 25.Barczyński M, Konturek A, Stopa M, et al. Minimally invasive video-assisted thyroidectomy: seven-year experience with 240 cases. Videosurgery Miniinv. 2012;7:175–80. doi: 10.5114/wiitm.2011.28871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konturek A, Barczyński M, Stopa M, Nowak W. Total thyroidectomy for non-toxic multinodular goiter with versus without the use of harmonic FOCUS dissecting shears – a prospective randomized study. Videosurgery Miniinv. 2012;7:268–74. doi: 10.5114/wiitm.2011.30675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunstman JW, Udelsman R. Superiority of minimally invasive parathyroidectomy. Adv Surg. 2012;46:171–89. doi: 10.1016/j.yasu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Melck AL, Armstrong MJ, Yip L, Carty SE. Case-controlled comparison of video-assisted and conventional minimally invasive parathyroidectomy. Am Surg. 2012;78:125–32. [PubMed] [Google Scholar]
- 29.Schneider DF, Mazeh H, Sippel RS, Chen H. Is minimally invasive parathyroidectomy associated with greater recurrence compared to bilateral exploration? Analysis of more than 1,000 cases. Surgery. 2012;152:1008–15. doi: 10.1016/j.surg.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider DF, Mazeh H, Chen H, Sippel RS. Predictors of recurrence in primary hyperparathyroidism: an analysis of 1386 cases. Ann Surg. 2014;259:563–8. doi: 10.1097/SLA.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genc H, Morita E, Perrier ND, et al. Differing histologic findings after bilateral and focused parathyroidectomy. J Am Coll Surg. 2003;196:535–40. doi: 10.1016/s1072-7515(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 32.Westerdahl J, Bergenfelz A. Parathyroid surgical failures with sufficient decline of intraoperative parathyroid hormone levels. Unobserved multiple endocrine neoplasia as an explanation. Arch Surg. 2006;141:589–94. doi: 10.1001/archsurg.141.6.589. [DOI] [PubMed] [Google Scholar]
- 33.Stalberg P, Sidhu S, Sywak M, et al. Intraoperative parathyroid hormone measurement during minimally invasive parathyroidectomy: does it ‘value-add’ to decision-making? J Am Coll Surg. 2006;203:1–6. doi: 10.1016/j.jamcollsurg.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Kandil E, Malazai AJ, Alrasheedi S, Tufano RP. Minimally invasive/focused parathyroidectomy in patients with negative sestamibi scan results. Arch Otolaryngol Head Neck Surg. 2012;138:223–5. doi: 10.1001/archoto.2011.1419. [DOI] [PubMed] [Google Scholar]
- 35.Wharry LI, Yip L, Armstrong MJ, et al. The final intraoperative parathyroid hormone level: how low should it go? World J Surg. 2014;38:558–63. doi: 10.1007/s00268-013-2329-6. [DOI] [PubMed] [Google Scholar]