Abstract
Minimally invasive gastrectomy has become the standard of care in many centers in Asia but remains unpopular in Europe. The aim of this article is to present the technique of laparoscopic robot-assisted total gastrectomy. The presented case involved a 66-year-old female patient with an advanced gastric cancer on the lesser curvature of the stomach. The laparoscopic part of the procedure involved opening the lesser sac, mobilization of the greater curvature and transection of the duodenum. A robot was used for the D2 lymphadenectomy and creation of the anastomosis. In summary, we have found that during a total gastrectomy for advanced gastric cancer a successful oncological resection can be achieved using a minimally invasive approach. We have also found that by combining conventional laparoscopy with robotic assistance we could overcome the technical difficulties with regards to lymph node dissection and anastomosis.
Keywords: robotic surgery, laparoscopic surgery, gastrectomy, gastric resection
Introduction
Gastric cancer (GC) is the fourth most common malignancy in the world, with about one million new cases diagnosed each year. Stomach cancer is the second leading cause of death in both sexes worldwide. The highest mortality rates are observed in Eastern Asia (28.1 per 100,000 in men, 13.0 per 100,000 in women) but also in Central and Eastern Europe [1]. In Poland, GC is the fifth most common cancer; with approximately 5500 new cases diagnosed every year and the third leading cause of death from malignancy [2].
Surgery remains the mainstay of treatment for GC, whereas currently chemo- and radiotherapy play a secondary role in the form of adjuvant and neo-adjuvant treatment as well as palliative care. The extent of a surgical resection for GC depends on the stage of disease and tumor location. In general, total or partial stomach resections with appropriate lymph node dissection are the most common surgical options.
Minimally invasive gastrectomy for cancer has become the standard of care in many centers in Korea and Japan [3–5]. Laparoscopic surgery of the upper gastrointestinal tract for benign indications is widespread in Europe, but gastric cancer surgery remains unpopular [6–8].
Aim
In this article we present the laparoscopic robot-assisted total gastrectomy method and discuss the potential advantages and disadvantages of the minimally invasive surgery (MIS) approach in the treatment of gastric cancer.
Case report
The patient was a 66-year-old woman with a suspected gastric cancer. She was admitted to her regional hospital with symptoms of upper gastrointestinal bleeding. An esophagogastroduodenoscopy revealed a 5 mm ulcer located on the lesser curvature of the stomach. The bleeding was controlled using epinephrine and proton pump inhibitors. Histopathology raised the suspicion of a stomach adenocarcinoma; however, an additional biopsy was required to confirm the initial diagnosis. The patient was in turn referred to our outpatient clinic for further treatment. Neither a repeated endoscopy nor an abdominal computed tomography (CT) scan showed any pathology. Due to the fact that an endoscopic ultrasound was not available at our institution, the patient was offered a diagnostic laparoscopy. Surgical options were thoroughly discussed with the patient and she consented to a partial or total stomach resection, depending on the intraoperative findings. From the beginning the patient insisted on a minimally invasive approach. The patient had body mass index (BMI) of 34 kg/m2 and no history of previous abdominal surgery.
Surgical technique
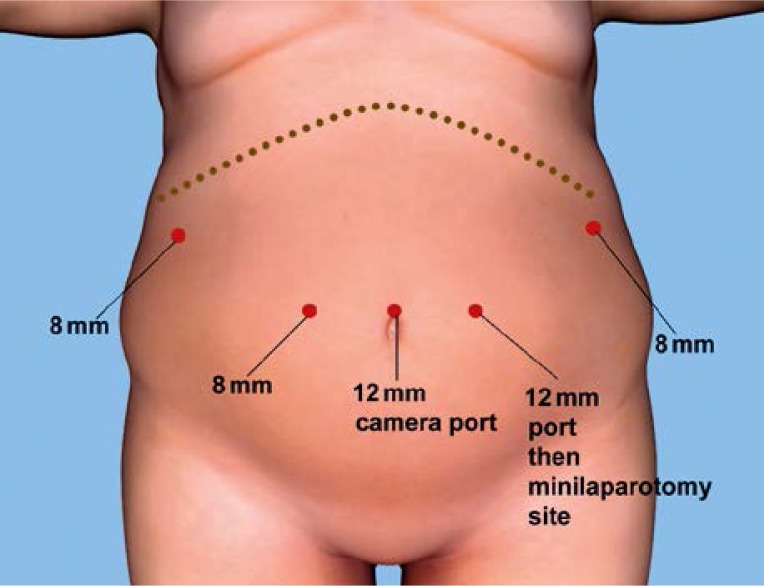
The procedure began with the patient placed in the reverse Trendelenburg position. As depicted in Figure 1, five trocars were used. The diagnostic laparoscopy revealed an approximately 4 cm × 3 cm tumor located on the upper part of the lesser curvature of the stomach. The extent and location of the tumor precluded a partial stomach resection. There was no evidence of lymphadenopathy or any other additional pathology within the abdominal cavity. The decision was made to proceed with a minimally invasive total gastrectomy. The first part of the procedure was performed using a laparoscopic technique, with the operator standing between the patient's legs using 2 instruments: a Harmonic ACE Shears (Ethicon, Inc., Cincinnati, OH, USA) and a bowel grasper. Two assistants, placed on the right and left side of the patient, used side ports and provided additional retraction. Initially the greater omentum was resected from the transverse colon. After the division of the right gastroepiploic vessels, dissection was performed toward the pylorus. Then, the right gastric vessels were identified and secured with Hem-o-Lok clips (Teleflex Medical, Research Triangle Park, NC, USA). At that point, the duodenum was transected 1–2 cm distal to the pylorus using a blue cartridge 45 mm Echelon Flex Endopath stapler (Ethicon, Inc., Cincinnati, OH, USA). Dissection continued towards the left. First the left gastroepiploic vessels, and afterwards the short gastric vessels were divided. Mobilization of the greater curvature was carried out until the full exposure of the left diaphragmatic crus. This was also the final step of the laparoscopic part of the procedure.
Figure 1.
Port placement
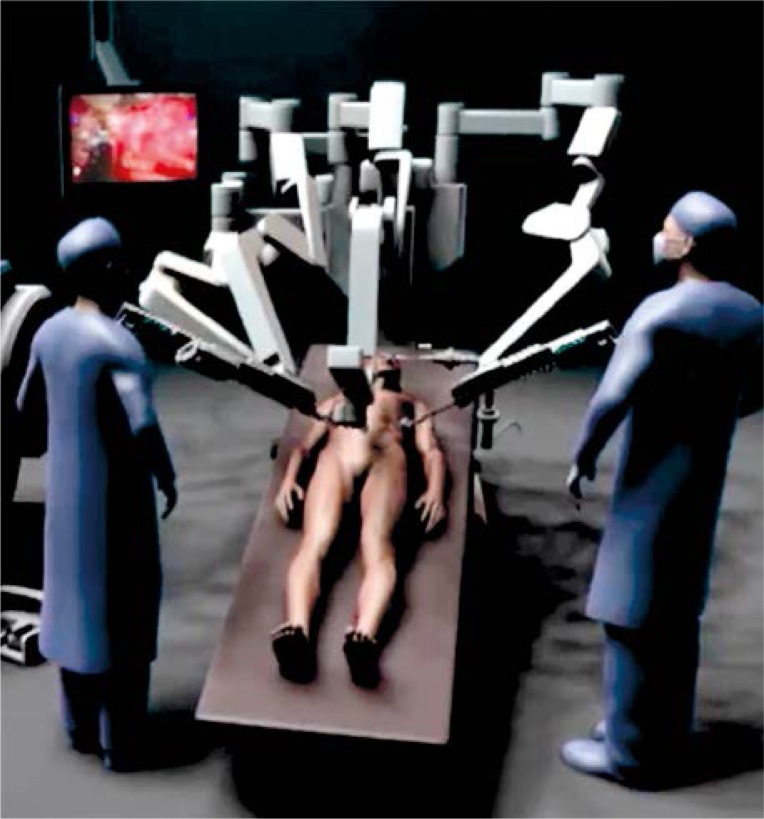
Next, the surgical robot (da Vinci Si surgical system, Intuitive Surgical, Sunnyvale, CA, USA) was brought into the operative field and docked into a position over the patient's head (Figure 2). We used three robotic working arms and a camera arm. In total, four robotic instruments were used. On robotic arm 1 we used a Permanent cautery hook which was replaced with a Large needle driver during the creation of the anastomosis. A robotic Fenestrated bipolar forceps and Cadiere forceps were used on robotic arms 2 and 3.
Figure 2.
Position of the robot
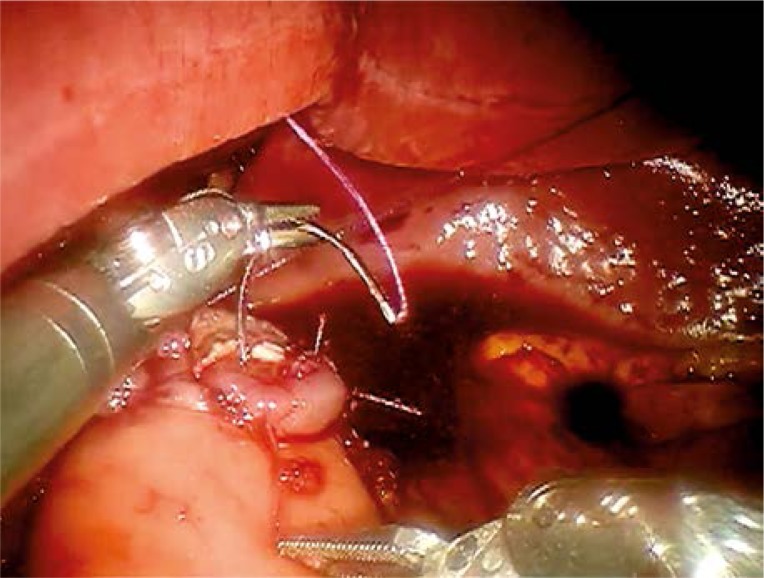
The robotic part of the procedure started with the identification of the left gastric vessels. The left gastric vein was secured with a vascular clip and divided. The left gastric artery was then dissected at its root and secured in a similar way but using three locking clips for safety. At this point, without division of the artery, dissection of the lymph nodes around the coeliac axis was performed. The lymph nodes along the common hepatic artery and splenic artery were harvested. For this we worked mostly with the robotic monopolar cautery hook and bipolar fenestrated grasper. The harmonic shears placed into the assistant's port were used when necessary. Once we finished the lymphadenectomy, the left gastric artery was divided. The dissection continued towards the right diaphragmatic crus and distal esophagus. The latter was mobilized from the surrounding tissue. After the division of vagus nerves the esophagus was transected with the monopolar cautery hook. Prior to this two stay sutures were placed between the esophagus and both diaphragmatic crura in order to avoid its retraction into the thoracic cavity. The incision of the 12 mm left epigastric port was enlarged into an approximately 5 cm long mini-laparotomy which was secured with an Alexis wound retractor (Applied Medical, Rancho Santa Margarita, CA, USA). The resected stomach was then extracted from the abdominal cavity. Then, the first loop of the jejunum was identified and brought out. The jejuno-jejuno anastomosis of the Roux-en-Y loop was created extracorporeally, then the Roux-en-Y loop was returned to the abdominal cavity and the pneumoperitoneum was re-established. The esophago-jejuno anastomosis was created robotically using a two-layered, interrupted suture technique (Photo 1).
Photo 1.
Robotic-sewn esophago-jejuno anastomosis
Results
The procedure took a total of 370 min and blood loss was estimated at 150 ml. The patient returned to the surgical oncology ward after the operation and was managed with a standardized postoperative algorithm. A nasogastric tube and suction drain were removed on postoperative day 3 and 5 respectively. The patient was given sips of water on postoperative day 4, a liquid diet on postoperative day 5, and a soft diet on postoperative day 6. She was discharged home on postoperative day 7.
The pathology report showed an undifferentiated adenocarcinoma G3, stage pT4aN3 with 20 out of 43 metastatic lymph nodes.
Discussion
Laparoscopy was first reported in the treatment of gastric cancer in 1994 by Kitano et al. [9]. In Korea and Japan the MIS approach has been rapidly adopted and has become the accepted standard of care [3–5]. This phenomenon is related to the fact that early gastric cancer accounts for 40–50% of all gastric malignancies in those countries [10]. This is possible because of well-established national screening programs as well as the lower BMI averages of East Asian countries, which favor the minimally invasive approach.
Reports from Eastern Asia were able to demonstrate oncologic safety of laparoscopic gastrectomy in early stage gastric cancer [3]. In addition, the MIS approach is believed to be associated with less postoperative pain and faster recovery than in open surgery [5]. However, evidence regarding the effectiveness of a laparoscopic procedure in the management of advanced gastric cancer (AGC) is limited [11–13]. Existing studies are retrospective in nature, involving a small number of patients while also mainly focusing on distal gastrectomies. Nevertheless, groups of experienced surgeons were able to show acceptable short-term outcomes and satisfactory oncologic results in the laparoscopic treatment of AGC [11–13]. These findings have sparked further discussion on the role of MIS in management of AGC. Recently, three large randomized clinical trials studies investigating the MIS approach in AGC have begun in Korea, Japan and China [14]. They aim to investigate the short- and long-term outcomes of MIS in management of cT2-T4a, cN0-N3 gastric cancer.
One of the main technical difficulties in the minimally invasive treatment of AGC is carrying out a D2 lymphadenectomy with oncologic adequacy [15, 16].
More recently the robotic system has been introduced as a solution. It is believed that it may overcome some of the shortcomings of a laparoscopy [17–20]. The surgical robot provides technical advantages such as endowristed instruments and a three-dimensional view of the operating field. The application of a robotic system for gastric cancer surgery has been studied extensively in Korea. Recently it has been shown that a robotic gastrectomy compared to laparoscopy reduces intraoperative blood loss and has similar morbidity and mortality rates [21, 22]. The value of robotic assistance during a gastrectomy is the subject of another multicenter observational study which has started the enrollment of patients in Korea [14].
The clinicopathological pattern of GC differs considerably between West and East [10]. In western countries gastric cancer tends to present at a more advanced stage, is located more proximally and more often has a diffuse histology. Additionally, western patients suffering from GC are on average 10 years older, have a higher body mass index and a higher prevalence of comorbidities. Not surprisingly, all of these factors negatively influence the outcome of the treatment.
As shown in a German multicenter observation trial, more than 70% of all patients with a GC resection had stage II–IV of the disease [23]. The majority of patients had a tumor located on the proximal or middle third of the stomach and 70% of them required a total or extended total gastrectomy. Any surgical team willing to start performing MIS gastric cancer surgery in Europe should take into account these variables. Even in a specialized center it is virtually impossible to select a reasonable number of early, distally localized tumors. One should therefore expect a total or subtotal gastrectomy with a D2 lymphadenectomy to be the procedure of choice.
In this article we present a hybrid laparoscopic-robotic approach for a total gastrectomy. Based on our experience with the hybrid concept for a low anterior resection, we applied a similar approach in gastrectomy [24]. We used laparoscopy when dealing with redundant tissue such as the greater omentum or greater curvature and applied the robot when accuracy and precision were paramount. As reported by others, also in our opinion robotic assistance indeed enhanced the surgeon's capability during a lymphadenectomy [25]. It brings enormous precision when working around celiac trunk vessels and allows the surgeon to simply replicate the technique use in an open procedure. Robotic dexterity was for us equally important during the creation of esophago-jejuno anastomosis. Anastomotic failure after gastrectomy is a potentially lethal complication [26]. The morbidity due to a leak may outweigh the benefits associated with MIS. As shown by authors from Yonsei University, even in experienced hands, both laparoscopic and robotic gastrectomies are associated with a higher risk of anastomotic failure [27]. Therefore maximum effort should be made to minimize the risk of this complication. Robotic assistance allows a surgeon to choose his preferred techniques for esophago-jejuno anastomosis. As shown in the presented case, even an intracorporeal robot-sewn method can be carried out without difficulty. Spurred on by the experience gained during the presented case and by earlier bariatric procedures, we plan to further investigate the application of robotics in gastric cancer surgery.
Conclusions
We have found that during a total gastrectomy for advanced gastric cancer a successful oncological resection can be achieved using a minimally invasive approach while maintaining surgical radicality. We have also found that by combining conventional laparoscopy with robotic assistance we could overcome the technical difficulties with regards to lymph node dissection and anastomosis.
Nevertheless, a laparoscopic-robotic total gastrectomy with D2 lymphadenectomy is a complex procedure and should be performed by surgeons well versed in stomach surgery.
Acknowledgments
This publication is part of the project “Wrovasc – Integrated Cardiovascular Centre”, co-financed by the European Regional Development Fund, within Innovative Economy Operational Program, 2007–2013 realized in Regional Specialist Hospital, Research and Development Centre in Wroclaw.
References
- 1. http://globocan.iarc.fr/factsheets/cancers/stomach.asp.
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Vinuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a metaanalysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–56. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 4.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report – a phase III multicenter, prospective, randomized trial (KLASS Trial) Ann Surg. 2010;251:417–20. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 5.Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 6.Stanowski E, Wylężoł M, Paśnik K. Laparoscopy in bariatric surgery in Poland – present status. Videosurgery Miniinv. 2007;2:18–23. [Google Scholar]
- 7.Migaczewski M, Pędziwiatr M, Matłok M, et al. Laparoscopic Nissen fundoplication in the treatment of Barrett's esophagus – 10 years of experience. Videosurgery Miniinv. 2013;8:139–45. doi: 10.5114/wiitm.2011.32941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piątkowski J, Jackowski M, Szeliga J. Laparoscopic surgery of esophageal hiatus hernia – single center experience. Videosurgery Miniinv. 2014;9:13–7. doi: 10.5114/wiitm.2014.40174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–8. [PubMed] [Google Scholar]
- 10.Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: east vs. west. J Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SI, Kim HO, Yoo CH, et al. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23:1252–8. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Ahn SH, Park J, et al. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg. 2012;36:2394–9. doi: 10.1007/s00268-012-1669-y. [DOI] [PubMed] [Google Scholar]
- 13.Park DJ, Han SU, Hyung WJ, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548–53. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 14.Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: current status and future perspectives. Gut Liver. 2014;8:229–33. doi: 10.5009/gnl.2014.8.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong O, Jung MR, Kim GY, et al. Comparison of short-term surgical outcomes between laparoscopic and open total gastrectomy for gastric carcinoma: case-control study using propensity score matching method. J Am Coll Surg. 2013;216:184–91. doi: 10.1016/j.jamcollsurg.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693–8. doi: 10.1002/jso.21400. [DOI] [PubMed] [Google Scholar]
- 17.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258:554–9. doi: 10.1097/SLA.0b013e3182a4e87c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marecik SJ, Zawadzki M, Desouza AL, et al. Robotic cylindrical abdominoperineal resection with transabdominal levator transection. Dis Colon Rectum. 2011;54:1320–5. doi: 10.1097/DCR.0b013e31822720a2. [DOI] [PubMed] [Google Scholar]
- 19.Witkiewicz W, Zawadzki M, Rząca M, et al. Robot-assisted right colectomy: surgical technique and review of the literature. Videosurgery Miniinv. 2013;8:253–7. doi: 10.5114/wiitm.2011.33761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkiewicz W. Robotic surgery – a new quality and breakthrough or an expansive gadget? J Oncol. 2013;63:423–9. [Google Scholar]
- 21.Marano A, Choi YY, Hyung WJ, et al. Robotic versus laparoscopic versus open gastrectomy: a meta-analysis. J Gastric Cancer. 2013;13:136–48. doi: 10.5230/jgc.2013.13.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–80. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zawadzki M, Velchuru VR, Albalawi SA, et al. Is hybrid robotic laparoscopic assistance the ideal approach for restorative rectal cancer dissection? Colorectal Dis. 2013;15:1026–32. doi: 10.1111/codi.12209. [DOI] [PubMed] [Google Scholar]
- 25.Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610–5. doi: 10.1007/s00464-009-0618-9. [DOI] [PubMed] [Google Scholar]
- 26.Lang H, Piso P, Stukenborg C, et al. Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol. 2000;26:168–71. doi: 10.1053/ejso.1999.0764. [DOI] [PubMed] [Google Scholar]
- 27.Kim KM, An JY, Kim HI, et al. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–7. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]