Abstract
Introduction
Currently, the predominant question is whether a laparoscopic approach is comparatively radical in comparison with an open access approach, especially in the circumferential resection margin and quality of the completeness of total mesorectal excision. These factors are important in determining the quality of surgical care as well as long-term results of the treatment.
Aim
This article focuses on the evaluation of circumferential resection margins and on the quality of mesorectal excision of middle and lower rectum tumors. In addition, laparoscopic and open techniques are compared.
Material and methods
Data were collected prospectively and stored in a rectal cancer registry over a 3-year period. The parameters studied were age, sex, body mass index, localization and topography of the tumor, clinical stage, neoadjuvant chemotherapy and its response, the type of surgery, character of the circumferential and distal margins, quality of the mesorectal excision, pT and pN.
Results
One hundred and twenty-five patients were chosen for our study. Laparoscopy was performed in 53 operations and a conventional approach was performed in 72 operations. Complete mesorectal excision was achieved in 54.7% of laparoscopic operations versus 44.4% in the conventional technique; partially complete excision was performed in 20.8 and 12.5%, respectively. Incomplete excisions were described in 24.5 and 43.1% (p = 0.085). Positive circumferential margin occurred during laparoscopic surgery in 11 (20.8%) patients, and in the case of conventional resection in 27 (37.5%) patients (p = 0.044).
Conclusions
Our study showed comparable results between laparoscopic and open access procedures during rectal resection. The results achieved, in particular in the quality of the mesorectal excision and negative circumferential resection margin, show that the laparoscopic approach is comparable to conventional surgical techniques, with an adequate surgical outcome, in the treatment of rectal cancer.
Keywords: colorectal cancer, total mesorectal excision, circumferential resection margin
Introduction
The circumferential resection margin (CRM) and the quality of the completeness of total mesorectal excision (TME) are important factors. Without these, radical resections for rectal carcinoma cannot be evaluated [1, 2]. It turns out that pathological circumferential margin (pCRM) can be used as a direct indicator of oncological radicality, which significantly affects the outcome of treatment [1, 3]. According to some, a positive pathological circumferential margin is a more significant independent predictive factor than the actual pT stage of the tumor [3].
The laparoscopic approach for resection of the rectum has been repeatedly analyzed for many years. Currently, the predominant question is whether a laparoscopic approach is comparatively radical in comparison with an open access approach, especially in the circumferential resection margin.
Our study is focused on the results of rectal resection of the aboral 10 cm. One such study published on this subject was unfortunately hindered by methodological shortcomings. For example, patients with cancer of the upper rectum were included [4, 5], while amputation procedures for cancer of the distal third of the rectum were excluded [6]. In addition, overall patient selection was questionable [7, 8].
Aim
This article focuses on the evaluation of circumferential resection margins and on the quality of mesorectal excision of middle and lower rectum tumors. In addition, laparoscopic and open techniques are compared.
Material and methods
Our study includes patients with carcinoma of the middle and lower rectum who underwent surgery at the Department of Surgery at the University Hospital in Hradec Kralove in the period from January the 1st 2010 to December the 31st, 2012. Rectal cancer diagnosis was performed by standard procedures (colonoscopy, biopsy). Preoperative TNM staging was determined by abdominal computed tomography (CT). Almost all patients underwent a pretreatment pelvic nuclear magnetic resonance (NMR), and in some cases endorectal endosonography was also provided. The height of the lower edge of the tumor in all patients was based on clinical examination, including digital rectal or endorectal sonography and MRI measurement.
Monitored data
All observed data were prospectively entered into the registry for rectal cancer – ProMED. The factors investigated in the laparoscopic group and in the classic procedures were age, sex, body mass index, tumor location and its location in the rectal circumference, clinical stage, type of preoperative therapy and response to it, the type of surgery, tumor distance from the distal resection margins, quality of the total mesorectal excision, pathological tumor invasion and pathological evaluation of lymph nodes.
Patients in whom the lower tumor margin was higher than 10 cm from the anal verge were excluded. Compliance with this condition guaranteed the possibility of performing a total mesorectal excision. In addition, patients who have not undergone resection but only palliative derivative stoma were also excluded. Patients in whom the laparoscopic operations were converted were also excluded from the study. The last exclusion criterion was a complete pathological response (ypT0) or if the tumor was not histologically found after non-radical endoscopic polypectomy. In these cases it was not possible to assess the circumferential margins.
The treatment strategy for each patient was determined by the decision of the oncosurgical multidisciplinary committee.
Preoperative oncological treatment was indicated in patients with locally advanced tumors (T2N+, T3N+, T4 regardless of N+). After the treatment was carried out, NMR re-staging took place and patients were operated on at least 8 weeks after completing the neoadjuvant cancer treatment.
Either a conventional or laparoscopic technique was employed for each operation. Abdominoperineal excision of the rectum and Hartmann's operation were also carried out. Since 2012, intersphincteric resection and extralevator abdominoperineal excision of the rectum has been performed at our institution. Adjuvant chemotherapy was indicated again on the basis of the conclusions of the oncosurgical committee, especially for patients with tumors of stage III and IV and high-risk patients in stage II.
Histopathological evaluation of rectal resection
A fresh specimen of the rectum was sent for histopathological examination immediately after terminating the resection phase of the operation. The histopathological evaluation was done by a knowledgeable pathologist in accordance with the “Histopathological Protocol for Rectal Cancer”, which has been a standard part of our institutional evaluation for surgical treatment of rectal cancer since 2008. The quality of the mesorectal excision was assessed by Quirke [1]. A positive circumferential resection margin is evaluated as a tumor or tumor-affected lymph nodes less than or equal to 1 mm. A distance greater than 1 mm is considered negative.
Statistical analysis
For statistical evaluation the software NCSS 8 was used. For comparing the quantitative data we used either the two-sided t-test or the nonparametric Mann-Whitney, or the Kolmogorov-Smirnov test. To evaluate the qualitative data we used the χ2 test of independence in a contingency table or Fisher's exact test. Values of p were obtained with the likelihood-ratio test and considered significance if < 0.05.
Results
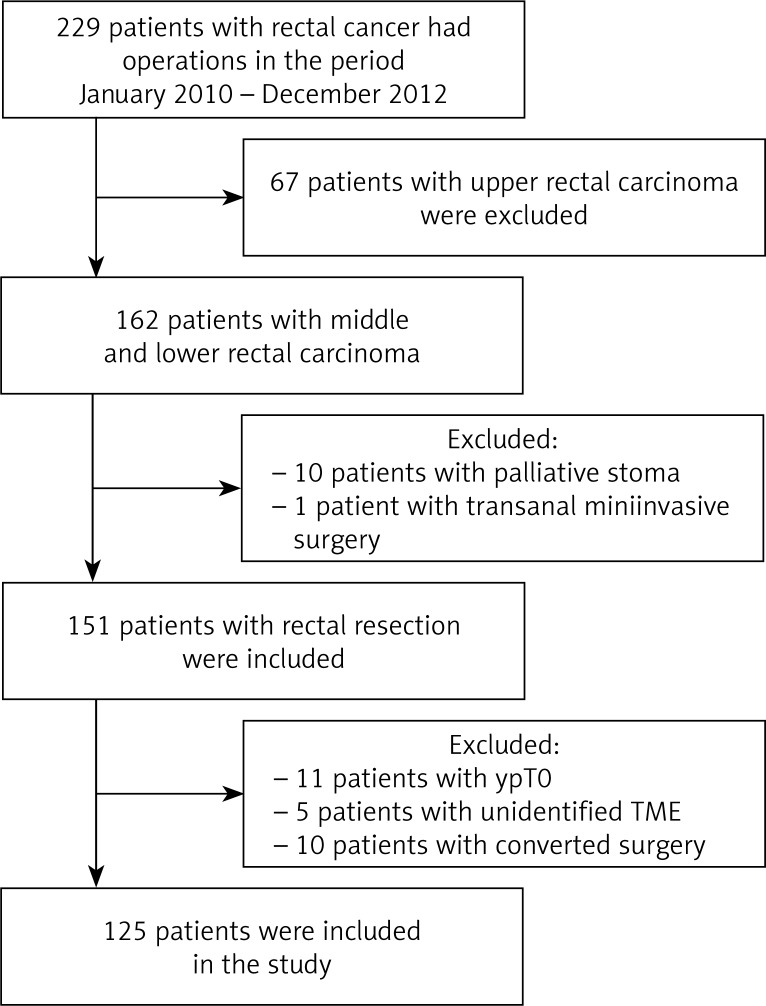
In the period from January the 1st 2010 to December 31st 2012 a total of 229 patients were operated on for rectal cancer at the Department of Surgery at the Faculty Hospital in Hradec Králové. Sixty-seven (29.3%) patients with carcinoma of the upper rectum were excluded based on the given exclusion criteria. From the remaining group of 162 patients with carcinoma of the middle and lower rectum, 11 further patients were excluded: 10 (6.2%) who underwent only palliative stoma and 1 patient on whom transanal local surgery was performed. In addition, from the remaining 151 patients, 11 (7.3%) patients with tumors of the middle and lower rectum were excluded (Figure 1). These patients had achieved a pathologically complete response following neoadjuvant chemoradiotherapy. Ten patients (6.2%) were excluded due to converted surgery. The main reason was to achieve homogeneous groups. Five (3.3%) other patients were not be intended quality of the mesorectal excision were also excluded. The total number of enrolled patients was 125: 76 men and 49 women, with an age range of 38–86 years.
Figure 1.
Study profile
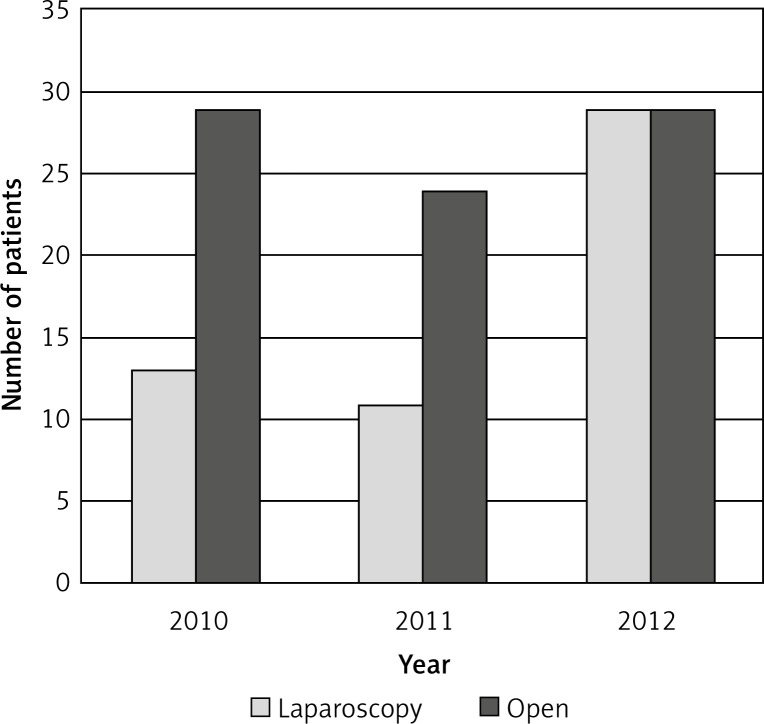
Laparoscopy was performed in 53 cases, while a conventional approach was used in 72 cases (Figure 2). A diverting ileostomy was performed in 49 (36.3%).
Figure 2.
Low and mid third rectal cancer. Number of resection procedures in successive years
These two groups, the laparoscopic and conventional groups, differed significantly in body mass index (BMI, median 26.2 kg/m2 vs. 27.9 kg/m2) (p = 0.021). In both groups there was a higher number of men than women (64.2% in the laparoscopic group and 58.3% in the conventional group). In the group of laparoscopic procedures, middle rectal carcinoma represented 52.8% of the procedures, tumors of the lower rectum 47.2%. A conventional operation was performed for middle rectal tumors in 47.2% of the cases and lower rectum in 52.8%. In the group of middle rectal tumors there were 14 patients (22.2%) with tumor localization within 9–10 cm from the anal verge.
Distribution based on clinical stage and type of surgery was not statistically significant (stage I – 43.4%. vs. 29.2%, stage II – 15.1% vs. 29.2%, stage III – 28.3% vs. 23.6% and stage IV – 13.2% vs. 18.1%) (Table I).
Table I.
Comparison of the laparoscopic and open group. Demographic and basic oncologic data
| Parameter | Laparoscopic | Open | Value of p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of patients | 53 | 72 | |||
| Age [years]: | 0.63 | ||||
| Mean | 65.4 | 66.4 | |||
| Median | 65 | 66 | |||
| Range | 40–85 | 38–86 | |||
| Gender: | 0.51 | ||||
| Male | 34 | 64.2 | 42 | 58.3 | |
| Female | 19 | 35.8 | 30 | 41.7 | |
| BMI [kg/m2]: | 0.021 | ||||
| Mean | 26.6 | 28.4 | |||
| Median | 26.2 | 27.9 | |||
| Range | 19.4–38.2 | 19.9–41.9 | |||
| Tumor location: | 0.54 | ||||
| Middle rectum (5–10 cm) | 28 | 52.8 | 34 | 47.2 | |
| Lower rectum (0–5 cm) | 25 | 47.2 | 38 | 52.8 | |
| Tumor topography: | 0.19 | ||||
| Circular | 11 | 20.8 | 24 | 34.2 | |
| Anterior | 10 | 18.9 | 19 | 27.1 | |
| Dorsal | 13 | 24.5 | 9 | 12.9 | |
| Lateral on the right | 9 | 17 | 9 | 12.9 | |
| Lateral on the left | 10 | 18.9 | 9 | 12.9 | |
| Clinical stage: | 0.17 | ||||
| I | 23 | 43.4 | 21 | 29.2 | |
| II | 8 | 15.1 | 21 | 29.2 | |
| III | 15 | 28.3 | 17 | 23.6 | |
| IV | 7 | 13.2 | 13 | 18.1 | |
Ninety-seven (77.6%) patients underwent total preoperative oncologic treatment, while 60.4% of the patients underwent chemoradiotherapy before the laparoscopic procedure, and 52.8% before conventional resection. Eight patients (2 vs. 6) were irradiated in long mode, and 19 patients (5 vs. 14) in short mode. Twenty-eight patients received no preoperative oncological treatment. No statistically significant difference was found in response to neoadjuvant therapy (p = 0.098), where a partial pathological response in the laparoscopic group occurred in 45.3% of the patients, in contrast to 30.6% in the group with open surgery.
Laparoscopy was performed in 43 patients (81.1%) by means of low anterior resection, including 4 intersphincteric resections. An abdominoperineal amputation was performed in 9 patients of this group (17%), along with 1 (1.9%) Hartmann's operation. In the group of open surgery 26 (36.1%) low anterior resections were performed, 39 (54.2%) abdominoperineal amputations and 7 (9.7%) Hartmann's resections. A statistically significant difference (p = 0.000001) was observed between these groups. The difference was mainly due to the high number of low anterior resections in the laparoscopic group and more than 50% share of amputation procedures in the open surgery group (Table II).
Table II.
Comparison of the laparoscopic and open group. Factors connected with treatment
| Parameter | Laparoscopic | Open | Value of p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Neoadjuvant therapy: | 0.28 | ||||
| Without | 14 | 26.4 | 14 | 19.4 | |
| Chemoradiotherapy | 32 | 60.4 | 38 | 52.8 | |
| Radiotherapy – long course | 2 | 3.8 | 6 | 8.4 | |
| Radiotherapy – short course | 5 | 9.4 | 14 | 19.4 | |
| Response to therapy: | 0.098 | ||||
| Partial pathological response | 24 | 45.3 | 22 | 30.6 | |
| Stable disease | 9 | 16.9 | 23 | 31.9 | |
| Progressive disease | 3 | 5.7 | 4 | 5.6 | |
| Not available | 17 | 32.1 | 23 | 31.9 | |
| Type of procedure: | 0.000001 | ||||
| Low anterior resection | 43 | 81.1 | 26 | 36.1 | |
| Abdominoperineal amputation | 9 | 17 | 39 | 54.2 | |
| Hartmann's procedure | 1 | 1.9 | 7 | 9.7 | |
Histopathological aspects
The first quality of TME, namely complete mesorectal excision, was achieved in 54.7% of patients in the laparoscopic group, compared with 44.4% in the group of patients operated on conventionally. Partially complete excision was performed in 20.8% and 12.5%, respectively. Incomplete excision was described in 24.5% and 43.1%, respectively. Differences in mesorectal excision in both surgical approaches were not statistically significant.
A positive circumferential margin was described in the laparoscopic procedure in 11 (20.8%) patients. In the case of an open resection, the number was 27 (37.5%). The results between groups were statistically significant (p = 0.044) (Table III).
Table III.
Comparison of the laparoscopic and open group. Results of the pathological examination
| Parameter | Laparoscopic | Open | Value of p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Character of distal resection margin: | 0.23 | ||||
| Positive | 3 | 5.7 | 10 | 13.9 | |
| Negative | 50 | 94.3 | 62 | 86.1 | |
| Distance from distal resection margin to tumor [mm]: | 0.45 | ||||
| Mean | 20.7 | 24.2 | |||
| Median | 15 | 20 | |||
| Range | 0–60 | 0–99.9 | |||
| Circumferential margin [mm]: | 0.20 | ||||
| Mean | 7.2 | 6.2 | |||
| Median | 5 | 2.8 | |||
| Range | 0–40 | 0–45 | |||
| Character of circumferential margin: | 0.044 | ||||
| ≤ 1 mm | 11 | 20.8 | 27 | 37.5 | |
| > 1 mm | 42 | 79.2 | 45 | 62.5 | |
| Total mesorectal excision quality: | 0.085 | ||||
| Complete | 29 | 54.7 | 32 | 44.4 | |
| Nearly complete | 11 | 20.8 | 9 | 12.5 | |
| Incomplete | 13 | 24.5 | 31 | 43.1 | |
| pT stage | |||||
| Middle rectum: | 0.074 | ||||
| pT1 | 2 | 7.1 | 1 | 2.9 | |
| pT2 | 17 | 60.8 | 12 | 35.3 | |
| pT3 | 9 | 32.1 | 19 | 55.9 | |
| pT4 | 0 | 0 | 2 | 5.9 | |
| Low rectum: | 0.068 | ||||
| pT1 | 5 | 20.0 | 3 | 7.9 | |
| pT2 | 10 | 40.0 | 9 | 23.7 | |
| pT3 | 9 | 36.0 | 17 | 44.7 | |
| pT4 | 1 | 4.0 | 9 | 23.7 | |
| Number of harvested lymphatic nodes: | 0.26 | ||||
| Mean | 17.2 | 19.0 | |||
| Median | 14 | 16 | |||
| Range | 2–55 | 6–55 | |||
| pN stage: | 0.85 | ||||
| N0 | 31 | 58.5 | 44 | 61.1 | |
| N+ | 22 | 41.5 | 28 | 38.9 | |
The ypCRM (after neoadjuvant oncologic treatment) was evaluated and compared in selected group of patients treated preoperatively with the laparoscopic or conventional technique (Table IV). The results were not statistically significant (Fisher p = 0.11). We suppose that a relatively small group of patients has been included in our study to demonstrate the difference between laparoscopic and conventional groups. The distance from the distal tumor resection margin was not statistically significant, but it was in favor of the open method (median 20.7 mm in laparoscopy, 24.2 mm in the conventional procedure) (p = 0.45).
Table IV.
ypT results in laparoscopic and open group with regard to CRM
| CRM | Laparoscopic | Open | Value of p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Negative | 32 | 82.1 | 39 | 67.2 | 0.11 |
| Positive | 7 | 17.9 | 19 | 32.8 | |
Discussion
The benefits of laparoscopic resection of the rectum, which include reduced postoperative pain and faster recovery, with both early resumption of bowel function and shortened hospitalization, are very well known and confirmed by prospective clinical studies [5, 7–11]. An important and strictly monitored parameter is the oncological aspects of laparoscopic procedures. When comparing the results of surgical treatment of rectal cancer, particular attention must be given to the varying anatomy and principles of surgical treatment, especially in tumors of the lower third. Unfortunately, many studies do not reflect the view of the divergent results of TME and CRM tumors above and below 10 cm from the anus. Incorporating upper rectum resections into procedures has led to an “improvement” in the results and thus distorted the results of many studies. It has been shown that while the prognosis of rectal cancer depends principally on the stage of disease at diagnosis, the incidence of local recurrence after resection of the rectum reflects the quality of the surgeon [8, 12–16].
Traction using laparoscopic instrumentation is difficult and can lead to tears in the mesorectum, which can also be the cause of the poor quality of the mesorectal excision. On the other hand, laparoscopy provides a detailed view of the pelvis and greater control over the management of the identification of the resection line, essential for the high quality of the oncologic outcome. Capnoperitoneum plays a role, which helps separate the avascular tissue layers [16, 17]. The open operation is facilitated by hand traction, which does not damage fat mesorectal tissue. It has been repeatedly demonstrated that total mesorectal excision is a significant milestone in reducing local recurrence [2].
Obese patients have larger mesorectal fat tissue, so handling and dissecting in tighter spaces makes identifying the avascular layer difficult. Identifying the layers also poses problems even in thin patients. The layer of perirectal fat is low, and dissection is closer to the intestinal wall and thus to the tumor, making it difficult to prevent tearing the mesorectum [4]. The BMI difference between these two groups was evaluated in our study and showed statistical significance, probably due to the fact that patients with a lower BMI were previously operated on laparoscopically. Some studies take into account the distribution of “internal” fat and pelvic proportions as factors to be considered in terms of surgical tactics [18]. Also sex of the patient plays an important role. A woman and a man carry the risk of injury of the mesorectum and pCRM disruption. Injury of the mesorectum can be caused by a thin fat layer, especially in the ventral part of the mesorectum in a woman. However, the combination of a narrow small pelvis with bulky mesorectal tissue in a man can be the cause of worse results of TME.
The quality of mesorectal excision and pCRM are related yet independent prognostic factors. The relationship between pCRM+ and the completeness of the mesorectal excision has been documented by Nagtegaal. In 44% of patients with pCRM+ a torn mesorectum was observed, while patients with an intact mesorectum exhibited pCRM positivity in 11% of cases [13].
The study COLOR II, which prospectively compared the laparoscopic and open technique for rectal resection, showed no statistically significant difference in the frequency of pCRM+ between these two groups (93% of laparoscopically operated patients had negative pCRM vs. 91% of patients in the open surgery group). Conversely, a difference was observed in the positivity of the pCRM in carcinoma of the lower rectum. A significantly lower number was found in the laparoscopic than in the open approach (the difference was 12.4%). The difference in pCRM positivity was even more pronounced in the case of abdominoperineal amputation. A positive pCRM was found in 25% of open procedures, but only in 8% during laparoscopic procedures [17].
Similar results have also been obtained by the prospective multicenter study COREAN, dealing with the results of the treatment of rectal cancer within 9 cm. Positive pCRM was exhibited in 2.9% of patients in the laparoscopic resection group, and in the open technique group the figure was 4.1%. Even from other studies comparing the influence of the chosen surgical techniques to pCRM it is obvious that the results in this category are identical [14, 16, 19, 20].
In a study by Laurent, which included more than 80% of patients with lesions of the middle and lower rectum, there were no differences in the number of positive CRM for both techniques (7% vs. 6%), and thus no differences in local recurrence in both groups (3.9% vs. 5.5%). These results were probably achieved due to the high number of R0 resections (92% vs. 95%) [21]. However, the results may be affected by including in the overall group patients (12%) with tumors of the upper rectum. The difference we found between ypCRM+ after laparoscopic and conventional resection of the rectum for cancer within 10 cm (12.5% and 27.8%, respectively) was not statistically significant. There is, however, the assumption that increasing the number of patients in the study resulted in a statistically significant difference in favor of laparoscopic resection. Partial selection of the patients who are candidates for laparoscopic resection (patients with a positive response in tumor size on preoperative treatment, no tumor infiltration of surrounding organs and patients in whom bulky tumors were not found) probably affects the outcome of this study.
Achieving complete mesorectal excision is very important in the case of obtaining a negative circumferential resection margin with all consequences of treatment for the patient [22].
Another factor to consider is the impact of positive lymph nodes and radial spread of tumor through the mesorectal tissue on positive pCRM. Patients with pN+ and in whom positive CRM was observed had a damaged mesorectum in 44% of cases. This is compared to patients with mesorectal excision quality of the third degree and pN–, where a positive circumferential margin was found in 24% of cases [13].
In the COREAN study positive CRM after conventional abdominoperineal amputation was described in 8.3% and 5.3% for the laparoscopic procedure [7]. In our study we observed positive CRM in the laparoscopically assisted APR in 33.3%, and in the open procedure in 47.6%. Such a high rate of non-radical performance can be attributed to the relatively small group of patients with amputation.
A higher frequency of positive pCRM has been found in patients with abdominoperineal amputation (10.2% to 13.9%) compared to low anterior resection (3.6% to 8.7%) [23, 24].
Positive CRM contributes to the increased incidence of local recurrence (36.5% vs. 22.3%) and a shorter overall survival rate (52.3% vs. 65.8%) [22, 24]. In our group similar results were confirmed and led to a change in surgical technique for AP amputation.
The distance of the tumor from the anal verge is closely related to the quality of the removed mesorectum. Low anterior resections for tumors of the distal rectum achieve complete excision only in 39% of cases, compared with 67% in cases where tumors are located at distances more than 10 cm from the anus. Similar results were observed in amputation procedures. The APR group had a complete mesorectum in only 34% of patients. Compare that with low anterior resections, where complete excision was achieved in 73% of patients. No difference was observed between the genders and age of the patients [13].
Problems during dissection in the perineal phase of the Miles operation can be tumor infiltration of the levator muscle, which can lead to nonradical surgery with pCRM+. Specimen perforation during resection is in even more aboral localisation due to poor orientation of the surgeon in the case of a bulky tumor with a close relationship to nearby organs – vagina, prostate, urethra or urinary bladder.
Subsequently, the result is low quality mesorectal excision together with more positive CRM. The solution of this problem may be cylindrical excision of the rectum. Laurent compared the long-term oncological outcome between laparoscopic and conventional resection of the rectum and found that, over a 5-year period, the incidence of local recurrence was not significantly different (3.9% vs. 5.5%). Furthermore, the long-term disease-free survival results are comparable for the two methods [21, 25].
Conclusions
Our study showed no differences between laparoscopic and open access with regard to oncological outcomes in rectal resections. Evaluating both pCRM and the quality of excision confirmed the legitimacy of the laparoscopic approach. To confirm these results, further studies on a larger cohort of patients is necessary.
Acknowledgments
This article was supported by MH CZ - DRO (UHHK, 00179906).
References
- 1.Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO 16randomised clinical trial. Lancet. 2009;373:821–8. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagtegaal ID, Quirke P. What is the role for circumferential margin in the modern treatment of rectal cancer. Br J Surg. 2009;96:982–9. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 3.Ströhlein MA, Grützner KU, Jauch KW, et al. Comparison of laparoscopic vs. open access surgery in patients with rectal cancer: a prospective analysis. Dis Colon Rectum. 2008;51:385–91. doi: 10.1007/s10350-007-9178-z. [DOI] [PubMed] [Google Scholar]
- 4.Leonard D, Penninckx F, Fieuws S, et al. Factors predicting the quality of total mesorectal excision for rectal cancer. Ann Surg. 2010;252:982–8. doi: 10.1097/SLA.0b013e3181efc142. [DOI] [PubMed] [Google Scholar]
- 5.Kellokumpu IH, Kairaluoma MI, Nuorva KP, et al. Short- and long-term outcome following laparoscopic versus open resection for carcinoma of the rectum in the multimodal setting. Dis Colon Rectum. 2012;55:854–63. doi: 10.1097/DCR.0b013e31825b9052. [DOI] [PubMed] [Google Scholar]
- 6.Fukunaga Y, Higashino M, Tanimura S, et al. Laparoscopic rectal surgery for middle and lower rectal cancer. Surg Endosc. 2010;24:449–57. doi: 10.1007/s00464-009-0551-y. [DOI] [PubMed] [Google Scholar]
- 7.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–45. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee SD, Park SC, Park JW, et al. Laparoscopic versus open surgery for stage I rectal cancer: long-term oncologic outcomes. World J Surg. 2013;37:646–51. doi: 10.1007/s00268-012-1846-z. [DOI] [PubMed] [Google Scholar]
- 9.D'Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–95. doi: 10.1007/s00464-012-2731-4. [DOI] [PubMed] [Google Scholar]
- 10.Örhalmi J, Klos D, Jackanin S, et al. Intersphincteric resection of the rectum. Rozhl Chir. 2012;91:101–4. [PubMed] [Google Scholar]
- 11.Skrovina M, Duda M, Srovnal J, et al. Evaluation of laparoscopic resection of colorectal carcinoma from the viewpoint of molecular biology. Videosurgery Miniinv. 2012;7:19–26. doi: 10.5114/wiitm.2011.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez QH, Rodriguez-Zentner HA, Moreno-Berber JM, et al. Laparoscopic versus open total mesorectal excision: a nonrandomized comparative prospective trial in a tertiary center in Mexico City. Am Surg. 2009;75:33–8. [PubMed] [Google Scholar]
- 13.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–34. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Hotta T, Yamaue H. Laparoscopic surgery for rectal cancer: review of published literature 2000-2009. Surg Today. 2011;41:1583–91. doi: 10.1007/s00595-010-4555-y. [DOI] [PubMed] [Google Scholar]
- 15.Herzog T, Belyaev O, Chromik AM, et al. TME quality in rectal cancer surgery. Eur J Med Res. 2010;15:292–6. doi: 10.1186/2047-783X-15-7-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krane MK, Fichera A. Laparoscopic rectal cancer surgery: where do we stand? World J Gastroenterol. 2012;18:6747–55. doi: 10.3748/wjg.v18.i46.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Pas MHGM, Haglind E, Vuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–8. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 18.Baik SH, Kim NK, Lee KY, et al. Factors influencing pathologic results after total mesorectal excision for rectal cancer: analysis of consecutive 100 cases. Ann Surg Oncol. 2008;15:721–8. doi: 10.1245/s10434-007-9706-z. [DOI] [PubMed] [Google Scholar]
- 19.Bosch SL, Nagtegaal ID. The importance of the pathologist's role in assessment of the quality of the mesorectum. Curr Colorectal Cancer Rep. 2012;8:90–8. doi: 10.1007/s11888-012-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang MJ, Liang JL, Wang H, et al. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomised controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis. 2011;26:415–21. doi: 10.1007/s00384-010-1091-6. [DOI] [PubMed] [Google Scholar]
- 21.Laurent C, Leblanc F, Wutrich P, et al. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250:54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]
- 22.Schneider PM, Vallbohmer D, Ploenes Y, et al. Evaluation of quality indicators following implementation of total mesorectal excision in primarily resected rectal cancer changed future management. Int J Colorectal Dis. 2011;26:903–9. doi: 10.1007/s00384-011-1155-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim JC, Yu CS, Lim SB, et al. Abdominoperineal resection and low anterior resection: comparison of long-term oncologic outcome in matched patients with lower rectal cancer. Int J Colorectal Dis. 2013;28:493–501. doi: 10.1007/s00384-012-1590-8. [DOI] [PubMed] [Google Scholar]
- 24.Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2013;20:1179–84. doi: 10.1245/s10434-012-2722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asoglu O, Balik E, Kunduz E, et al. Laparoscopic surgery for rectal cancer: outcomes in 513 patients. World J Surg. 2013;37:883–92. doi: 10.1007/s00268-013-1927-7. [DOI] [PubMed] [Google Scholar]