Abstract
Background
Recent studies show that endemic hepatitis E virus (HEV) infection occurs frequently in some developed countries. In the Netherlands in 2013, the routine screening of 35,220 plasma donations for HEV RNA showed 20 donors to be viremic (1:1761), which seems to contradict reports of declining HEV seroprevalence in the recent past.
Study Design and Methods
To asses HEV infection pressure changes over time, archived samples from Dutch blood donations collected in 1988 and 2000 were tested for anti-HEV immunoglobulin (Ig)G. The findings were compared to the HEV seroprevalence among donors in 2011.
Results
The age-adjusted prevalence of anti-HEV IgG for Dutch donors aged 18 to 64 declined from 46.6% in 1988 to 27.3% in 2000 and to 20.9% in 2011. The reduction of seroprevalence was apparent for all age groups between 1988 and 2000, and for donors older than 40 between 2000 and 2011, but the seroprevalence among donors aged 18 to 29 increased between 2000 and 2011. Recent changes in HEV infection pressure are more apparent in the youngest donors, who to a lesser extent reflect cumulative exposure to HEV in the past. Donors aged 18 to 21 showed decreasing HEV seroprevalence from 19.8% in 1988 to 7.0% in 1995 and to 4.3% in 2000, followed by an increase to 12.7% in 2011.
Conclusion
HEV antibody patterns in young and old Dutch donors, in 1988 to 2011, suggest that decades ago, HEV was ubiquitous and most persons acquired infection. Subsequently HEV incidence was low during a prolonged period, to increase again in recent years.
Recently it became clear that indigenous infection with hepatitis E virus (HEV) Genotype 3 is common in some industrialized countries.1,2 Although the transmission routes are not well understood, domesticated swine are a likely source of infection.3 In the Netherlands pig farming is very intensive, with 12,000,000 piglets being reared each year. Among Dutch blood donors, a low anti-HEV seroprevalence of 0.4% was reported in 1993, determined using experimental HEV antibody screening and confirmatory assays from Abbott Laboratories (Chicago, IL) and Diagnostic Biotechnology (DB, Singapore).4 A more recent study reported 1.9% of the Dutch population to be confirmed anti-HEV positive in 2007, using an enzyme immunoassay (EIA; MP Diagnostics, Santa Ana, CA).5 The assays used in these studies are not the most sensitive HEV IgG tests, in particular for detecting past infection with HEV Genotype 3.6 More sensitive, validated HEV antibody tests have become available.7 We reported a strikingly higher seroprevalence of 27% among Dutch blood donors in 2011, employing an anti-HEV IgG EIA (Wantai Biological Pharmacy Enterprise Co., Ltd, Beijing, China).8 The seroprevalence increased strongly with age, which could be indicative of an age cohort effect. An age cohort effect results in a higher seroprevalence in older persons, not (only) because of age-dependent cumulative exposure, but because infection pressure was higher in the past. Indeed an age cohort effect has been demonstrated in the United Kingdom, Denmark, and the United States.9-11 Our previous study suggested a high incidence of HEV infection of 1.1% per person-year between 2009 and 2011. In 2013, the routine screening of 3000 Dutch plasma donations each month, in pools of 96, showed 20 of 35,220 donors (1 in 1761) to be viremic (data not shown). Assuming that the higher anti-HEV seroprevalence in donors older than 40 is caused by an age cohort effect, the recent high incidence suggests strong fluctuations of HEV infection pressure over time. To assess the prevalence of HEV infection in the past we studied archived donor plasma samples collected in the years 1988 to 2004 and compared the results with recent findings among donors in 2011.
Materials and Methods
Repository samples
The repository sample archive consists of over more than 1.5 million plasma samples of blood donations collected between 1988 and 2004. Samples were stored in 96-vial plates and kept at less than –20°C. Tightly closed vials were used for archival samples; even the oldest samples did not show signs of volume loss through evaporation. The archive was started in 1988 and initially only included donations from the Amsterdam area. Later, when blood banks merged, samples from a larger area in the Northwest of the Netherlands (including Amsterdam) were archived. After 2004, prolonged storage of samples from new donations ceased. Limited donor information (age, sex, anonymous donor identification) is available for all samples, allowing the testing of longitudinal samples of a given donor.
Sample selection
Samples of donations from 1988 and from 2000 were retrieved for HEV antibody testing. For each year of birth at least 10 donors were sampled, for 1988 and for 2000. Because the retrieval of specific samples from 1.5 million frozen samples is very labor intensive, the following approach was adopted. First, all donations in a set of random archive plates, containing donations from 1988 and from 2000, were tested. Subsequently, to compensate for the distribution of donor age, additional donations were retrieved and tested, to obtain at least 10 tested donors for each year of birth. The final selection included 538 donations from 1988 of donors aged 18 to 64 and 621 donations from 2000 of donors aged 18 to 69. (Between 1988 and 2000 the maximum age at which people may donate was increased from 64 to 69 years.) Because the change in HEV antibody prevalence over time was most pronounced in young donors (see Results), the number of samples from donors aged 18 to 21 in 1988 and 2000 was increased to at least 100 by testing additional donations. In addition, 100 donations of donors aged 18 to 21 in 1995 were tested.
HEV antibody testing
Samples were tested using an anti-HEV IgG assay (Wantai Biological Pharmacy Enterprise Co., Ltd) according to the manufacturer's instructions. Recently this assay has been validated and compared to other anti-HEV assays.7 Borderline reactive samples (optical density/cutoff [OD/CO] > 0.9 and < 1.1) were considered negative. Results were compared with those from our previous study in which the anti-HEV IgG prevalence was determined in 5329 donor samples, collected on 2 days in March 2011 in all Dutch blood collection centers.8
Prolonged sample storage may cause increased nonspecific reactivity in antibody assays.12 To investigate this issue the distribution of the signal strength in samples of 1988, 2000, and 2011 was compared. The persistence of anti-HEV IgG levels over a 22-year period was investigated by testing serial samples of 23 donors who tested positive in 1988.
Statistical analysis
Statistical significance was calculated using the chi-square test. The Newcombe-Wilson method was applied to determine 95% confidence intervals (CIs).
Results
The anti-HEV IgG seroprevalence among Dutch blood donors in 1988 and 2000 was determined in archived samples and compared to the seroprevalence in 2011. Between 1988 and 2011 the age-adjusted seroprevalence for donors aged 18 to 64 declined from 46.6% in 1988 to 27.3% in 2000 and to 20.9% in 2011. At each time point, anti-HEV IgG prevalence strongly increased with age. The reduction of seroprevalence was apparent for all age groups between 1988 and 2000 (Fig. 1) and for donors older than 40 years between 2000 and 2011. The seroprevalence among donors aged 18 to 29 increased between 2000 and 2011. Recent changes in HEV infection pressure are more apparent in younger donors, who to a lesser extent reflect cumulative exposure to HEV in the past. Samples of donors aged 18 to 21 in 1988, 1995, 2000, and in 2011 showed decreasing anti-HEV seroprevalence from 19.8% in 1988 to 7.0% in 1995 (p = 0.007) and to 4.3% in 2000 (p = 0.39), followed by a significant increase to 12.7% in 2011 (p = 0.016; Fig. 2).
Figure 1.
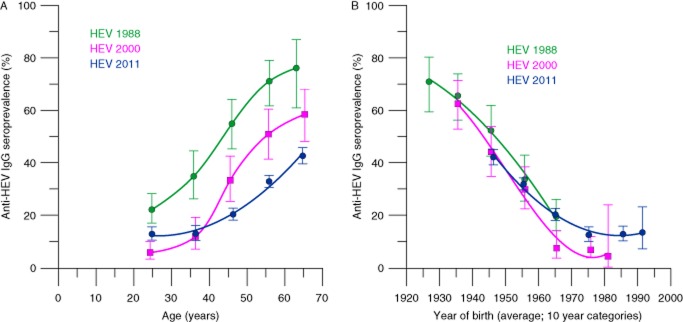
Anti-HEV IgG seroprevalence in 1988, 2000, and 2011 among blood donors: (A) by age and (B) by year of birth. The youngest age groups contain donors aged 18 to 29 instead of 20 to 29. Between 1988 and 2000 the maximum age for donors increased from 64 to 69, resulting in a shift of the average age of the oldest donors. Error bars indicate 95% CIs.
Figure 2.
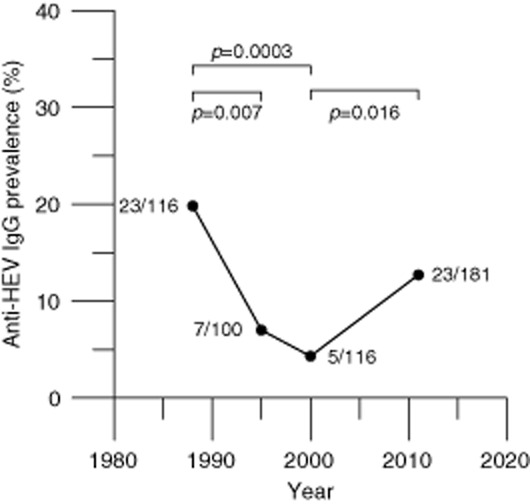
Anti-HEV IgG seroprevalence among donors aged 18 to 21 in 1988, 1995, 2000, and 2011. The total number of samples and the number of anti-HEV–positive samples are indicated for each time point. The numbers above the bars denote the two-sided p values calculated using the chi-square method.
The dynamics of anti-HEV IgG levels over time were investigated testing recent samples (obtained in 2011 and 2012) from 23 donors who tested positive in 1988, spanning 23.1 years on average per donor. It appears that anti-HEV IgG signals as measured with the Wantai EIA are stable over time: only five donors testing IgG positive in 1988 reverted to seronegativity 23 years later. These donors already showed a low-positive IgG signal (OD/CO < 5) in the early sample. The anti-HEV signal was reduced to less than 50% of the original value in eight donors (including four of the donors that reverted to seronegativity). In conclusion, the decline of anti-HEV signals was small and slow.
Because long-term storage of samples may cause nonspecific EIA reactivity, we compared the distribution of signals in samples stored in 1988 and 2011, from donors that could have donated in both years (i.e., donors aged 18-46 in 1988 and donors aged 41-69 in 2011). As shown in Fig. 3, the signal distribution of the 1988 samples was very similar to that of the 2011 samples. Similar results were obtained when samples of 2000 were compared with samples of 1988 and 2011 (data not shown). These findings indicate that the long-term storage of the samples did not cause nonspecific background reactivity.
Figure 3.
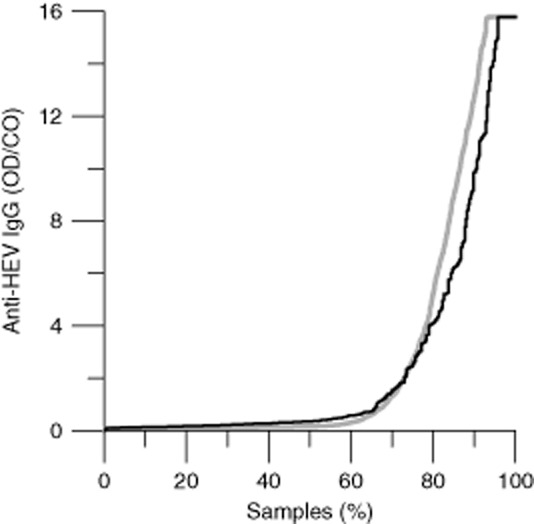
Distribution of the anti-HEV signals in archived samples. (Black) Sorted OD/CO ratios of 333 donors aged 18 to 46 and sampled in 1988; (gray) 4000 samples of donors aged 41 to 69 and sampled in 2011.
Discussion
The anti-HEV seroprevalence has been shown to differ in western countries. Part of the variation is due to the use of different HEV IgG assays.3 Recent studies, with sensitive assays, showed seroprevalences among blood donors of 16% in the United States and in southwest England, 17% in Germany, 21% in Denmark, 27% in the Netherlands, and 52% in the southwest of France.6,8,10,11,13,14 The anti-HEV seroprevalence invariably increases with age. In England, Denmark, and the United States the testing of archived samples showed that the seroprevalence declined in recent decades.9-11 In the Netherlands HEV seroprevalence also declined over time. Hence the age-related seroprevalence seems largely caused by decreased infection pressure, not by variability in acquiring infection in the course of life. Nevertheless, currently at least one acutely infected Dutch donor donates each day.8 Our findings provide an explanatory scenario. Decades ago, HEV infection pressure was very high and most people acquired infection. Subsequently a prolonged period of low incidence occurred, causing the age cohort effect. Recently the incidence of HEV infection increased again, as illustrated by the increasing seroprevalence after 2000 among blood donors aged 18 to 21 and the frequent finding of viremic donors.
Conclusions about the dynamics of HEV infection can only be drawn if the IgG anti-HEV response is detected reliably over time, in terms of both sensitivity and specificity. Our longitudinal data show that, employing the Wantai assay, anti-HEV IgG responses are detected consistently over time and seroprevalence seems a reliable indicator for cumulative HEV infection in the past. The use of a sensitive screening test is hampered by the unavailability of an equally sensitive, more specific confirmatory test. Probably some donors tested false positive. We showed that long-term storage did not cause nonspecific reactivity. The current study is limited to samples collected in the western part of the Netherlands. Previously we demonstrated that the regional variation of HEV seroprevalence in the Netherlands is neglectible.8
The source and transmission routes of current autochthonous HEV infection in Europe are unknown; domesticated swine probably play a role.15 In the Netherlands in 2005, 55% of pooled stool samples from pigs tested positive for HEV RNA,16 showing the same HEV-3 subtypes as found among Dutch blood donors.8 The cause of the decline and reemergence of HEV in recent decades is enigmatic. The source and the genotype of HEV strains that caused the high seroprevalence in the 1980s is unknown; it cannot be ruled out that the seroreactivity is the result of cross-reacting antibodies against an unknown HEV-like virus.
HEV transmission by blood transfusion has been reported in various countries.17-19 The recent high incidence of HEV infection may be less than before 1988, when anti-HEV seroprevalence among the oldest donors reached 76%. In hindsight, some cases of posttransfusion non-A, non-B hepatitis in those days may have been caused by HEV, not by hepatitis C virus. HEV transmission may be a threat to the safety of blood, especially if immunosuppressed recipients are considered. To our knowledge, universal donor screening for HEV is only performed in some areas of Japan. Because of the high incidence of community-acquired HEV infection, the screening of blood donors for HEV will only prevent part of HEV infections among vulnerable patients. Irrespective of donor screening for HEV, certain immunosuppressed patients should be monitored for HEV infection. At present, the elucidation of the transmission routes in community-acquired HEV infection, enabling the institution of control measures, seems more relevant than the introduction of blood donor screening for HEV.
Acknowledgments
We thank Femmeke Prinsze for help with setting up and querying relevant data from databases containing results and data from the repository sample archive.
Glossary
ABBREVIATION:
- OD/CO
optical density divided by cutoff value
Conflict of Interest
The authors have disclosed no conflicts of interest.
References
- 1.Schlauder GG, Desai SM, Zanetti AR, et al. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J Med Virol. 1999;57:243–251. doi: 10.1002/(sici)1096-9071(199903)57:3<243::aid-jmv6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Clemente-Casares P, Pina S, Buti M, et al. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis. 2003;9:448–454. doi: 10.3201/eid0904.020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton HR, Hunter JG, Bendall RP. Hepatitis E. Curr Opin Infect Dis. 2013;26:471–478. doi: 10.1097/01.qco.0000433308.83029.97. [DOI] [PubMed] [Google Scholar]
- 4.Zaaijer HL, Kok M, Lelie PN, et al. Hepatitis E in the Netherlands: imported and endemic. Lancet. 1993;341:826. doi: 10.1016/0140-6736(93)90599-c. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef L, Koopmans M, Duizer E, et al. Seroprevalence of hepatitis E antibodies and risk profile of HEV seropositivity in The Netherlands, 2006-2007. Epidemiol Infect. 2012;140:1838–1847. doi: 10.1017/S0950268811002913. [DOI] [PubMed] [Google Scholar]
- 6.Bendall R, Ellis V, Ijaz S, et al. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 7.Pas SD, Streefkerk RH, Pronk M, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58:629–634. doi: 10.1016/j.jcv.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Slot E, Hogema B, Riezebos-Brilman A, et al. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013;18:pii: 20550. doi: 10.2807/1560-7917.es2013.18.31.20550. [DOI] [PubMed] [Google Scholar]
- 9.Ijaz S, Vyse AJ, Morgan D, et al. Indigenous hepatitis E virus infection in England: more common than it seems. J Clin Virol. 2009;44:272–276. doi: 10.1016/j.jcv.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Christensen PB, Engle RE, Hjort C, et al. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: a potential zoonosis in Denmark. Clin Infect Dis. 2008;47:1026–1031. doi: 10.1086/591970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C, Wang RY, Schechterly CA, et al. An assessment of hepatitis E virus (HEV) in US blood donors and recipients: no detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion. 2013;53:2505–2511. doi: 10.1111/trf.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaaijer HL, Appelman P, Frijstein G. Hepatitis C virus infection among transmission-prone medical personnel. Eur J Clin Microbiol Infect Dis. 2012;31:1473–1477. doi: 10.1007/s10096-011-1466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber MS, Wenzel JJ, Jilg W, et al. Hepatitis E virus seroprevalence among adults, Germany. Emerg Infect Dis. 2012;18:1654–1657. doi: 10.3201/eid1810.111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansuy JM, Bendall R, Legrand-Abravanel F, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–2312. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berto A, Backer JA, Mesquita JR, et al. Prevalence and transmission of hepatitis E virus in domestic swine populations in different European countries. BMC Res Notes. 2012;5:190–196. doi: 10.1186/1756-0500-5-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutjes SA, Lodder WJ, Bouwknegt M, et al. Increased hepatitis E virus prevalence on Dutch pig farms from 33 to 55% by using appropriate internal quality controls for RT-PCR. J Virol Methods. 2007;143:112–116. doi: 10.1016/j.jviromet.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a “nonhyperendemic” country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 18.Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, France. Emerg Infect Dis. 2007;13:648–649. doi: 10.3201/eid1304.061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–1375. doi: 10.1111/j.1537-2995.2008.01722.x. [DOI] [PubMed] [Google Scholar]


