Summary
In the context of transfusion medicine, alloimmunization most often refers to the development of antibodies to non-ABO red blood cell (RBC) antigens following pregnancy, transfusion, or transplantation. The development of RBC alloantibodies can have important clinical consequences, particularly in patients who require chronic transfusions. It has been suggested that alloimmunization is more common in some clinical circumstances and patient populations than in others. As such, individuals that develop alloantibodies are frequently referred to as ‘responders’ in the medical literature. In contrast, individuals that do not develop alloantibodies despite repeated exposures to non-self blood group antigens have been referred to as ‘non-responders'. The purpose of this article is to review the phenomenon of RBC alloimmunization in the context of responders and non-responders to: i) establish a basic framework for alloimmunization as reported across several diverse patient populations; ii) more fully explore literature reports which support the concept of responders/non-responders regarding blood group antigen alloimmunization; iii) summarize the mechanisms that have been shown to predispose an individual to alloimmunization to determine how these factors may differentiate ‘responders’ from ‘non-responders'; and iv) briefly discuss some practical approaches to prevent alloimmunization in patients who may be prone to alloantibody development.
Keywords: Alloimmunization, Responders, Blood group antigens, Immunohematology
Introduction
In addition to the major blood group antigens (A and B), red blood cells (RBCs) also express scores of other antigens on their surface. These non-A, non-B antigens (sometimes referred to as ‘minor’ antigens), existing in either carbohydrate or polypeptide forms, serve many structural and physiologic functions for RBC homeostasis. However, it is their ability to induce alloimmune responses which typically draws the greatest attention from immunohematologists and transfusion medicine specialists. While antibodies to the ‘major’ A and B antigens appear ‘naturally’ in the serum after the first few months of life [1], antibodies to minor RBC antigens most frequently arise following a foreign exposure, usually as a result of transfusion, pregnancy, or transplantation.
Alloimmunization to non-ABO blood group antigens is a clinically relevant issue and can result in complications in the medical management of patients. One significant problem associated with blood group antibodies is difficulty in procuring compatible RBCs for transfusion. Delays in providing compatible RBC products can result in complications for symptomatically anemic patients requiring transfusion. In addition antibodies that are not detected during pre-transfusion laboratory screening can result in incompatible transfusions and resultant acute hemolytic transfusion reactions [2]. Moreover, blood group alloantibodies are well known to disappear from detection over time. In such circumstances, re-exposure to the antigen(s) during subsequent transfusions can induce an anamnestic antibody response resulting in delayed hemolytic transfusion reactions. Acute/delayed hemolytic transfusion reactions are common causes of morbidity and mortality. In fact, data from the Food and Drug Administration indicate that non-ABO antibodies were the second leading cause of mortality associated with transfusion in the USA from 2005 to 2013 [3]. Similarly, data from the Serious Hazards of Transfusion (SHOT) database in the UK identified hemolytic transfusion reactions and alloimmunization as the second most common pathologic complication of transfusion in 2012 [4].
Because of its critical clinical significance, the phenomenon of blood group alloimmunization has been investigated in a variety of settings. As a result of data from these studies (and many anecdotal observations) it has been suggested that some individuals may be more susceptible to developing alloantibodies to non-ABO blood group antigens than others. This group of patients, dubbed alloantibody ‘responders’ in the medical/transfusion literature, represents a substantial proportion of patients with underlying blood group alloantibodies. Therefore, from a practical (albeit hypothetical) standpoint, identifying ‘responders’ to RBC antigens a priori would allow transfusion services and blood banks to provide RBCs lacking antigens against which such individuals could mount an antibody response [5]. If such an assay existed, then performance of this testing in the pre-transfusion period (ideally prior to an initial exposure to any foreign RBCs) could greatly reduce alloimmunization rates and improve transfusion safety. Accordingly, much effort has been made in attempting to characterize the responder population and, moreover, to identify clinical and/or biologic markers which may identify a transfusion recipient as an ‘alloantibody responder’.
Given the numerous articles published in the medical literature on alloimmunization in general, and responder subgroups in particular, the primary purpose of this article is to concisely, but thoroughly summarize the existing data regarding these topics. Our specific aims are: i) to review alloimmunization rates reported among different clinical groups in order to explain how the idea of responder subgroups emerged from this literature; ii) to summarize the clinical studies which have attempted to establish the existence of responder subgroups; iii) to examine proposed explanations and/or biomarkers which may account for responders; and iv) to provide brief recommendations which may help to prevent or limit alloimmunization in responders.
An Aside about Definitions and Responder Models
Before engaging in a detailed discussion of alloimmunization rates, responder subgroups, and alloimmunization mitigation approaches, it is first important to define our terms. Perhaps the most pressing question is: how does one exactly define a responder in the context of alloimmunization associated with RBC transfusion? Unfortunately, there is no standard definition. Therefore, let us examine two potential definitions or models which can be derived from interpretations of previously published data. One way of defining responders is as a distinct subgroup of individuals capable of developing an alloantibody. In this scenario, where alloimmunization is a selective process, a ‘responder’ is defined as any individual who has become alloimmunized to at least one non-ABO antigen. This selective model also implies the existence of a distinct group of individuals incapable of responding to any non-ABO antigens despite repeated RBC exposures (a group typically dubbed ‘non-responders’). An alternative model posits that there is no specific selectivity and that virtually all individuals are capable of being alloimmunized to at least one non-ABO antigen upon exposure. Therefore, in this second model, ‘responders’ are typically viewed as individuals who exuberantly develop multiple alloantibodies following transfusion, sometimes after very few antigenic exposures.
Are either of these models or definitions completely sufficient to match alloimmunization patterns reported in the medical literature? There are certainly data to support the notion that only selected subsets of individuals are capable of responding to non-ABO antigens. For instance, there are several studies which demonstrate that human leukocyte antigen (HLA) class II restriction is an important determinant of whether individuals can present antigens such as K, Jka, and Fya (this will be discussed in greater detail later in this paper). There are also reports indicating groups of individuals who never appear to develop RBC alloantibodies despite hundreds of exposures. Therefore, at least for some antigens, a selective responder model seems appropriate. However, this definition does not seem to be readily applicable to all antigens. As an example, large numbers of Rh(D)-negative individuals (in some cases upwards of 85–90% of subjects) have been noted to develop anti-D following exposure. Moreover, both clinical studies and anecdotal observations suggest that within groups of alloimmunized patients there are individuals who are multiply alloimmunized, sometimes developing – more than 5 alloantibodies. As such, these latter observations lend some credence to the idea that: i) selectivity may not apply to every blood group antigen and ii) there are subsets of individuals even within the alloimmunized groups whose capability for developing alloantibodies may exceed even that of a ‘typical’ responder.
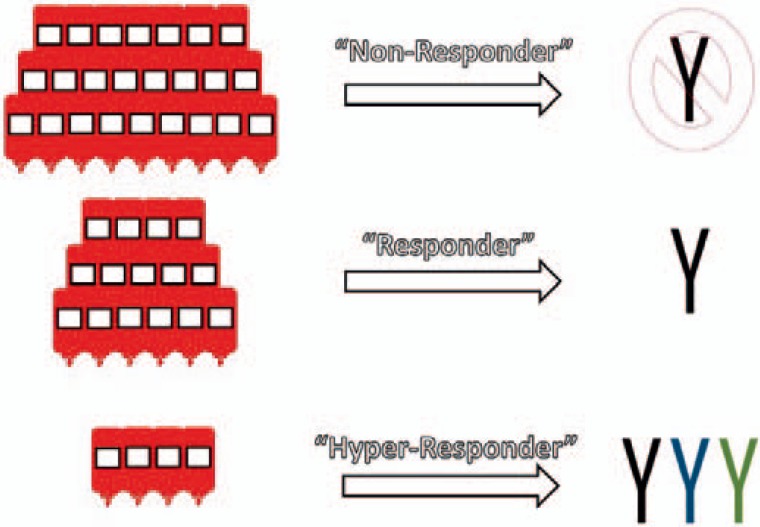
Given the above considerations, the ideal definition of a responder is likely one which incorporates aspects of both of the earlier frameworks (and is somewhat more expansive in its terminology). As demonstrated in figure 1, we propose a hypothetical model that categorizes transfused individuals in one of three ways: i) ‘non-responders’ (i.e., individuals who never develop any alloantibodies despite repeated exposures); ii) ‘responders’ (i.e., individuals who develop 1 alloantibody with one or more exposures), and iii) ‘hyper-responders’ (i.e., individuals who develop >1 alloantibody with one or more exposures). At present, we believe that there are some clinical data supporting such a model system. Below, we will more thoroughly review the studies which led to our creation of this model structure. Nonetheless, it will be important for the transfusion medicine community to continue to perform studies to more fully validate the concepts above, particularly regarding the existence of a hyperresponder population.
Fig. 1.
A proposed model for defining the ‘responder’ phenotype. Red bag icons represent RBC transfusions. Y represents alloantibodies; different colors represent alloantibodies with different specificities.
Incidence of Minor RBC Antigen Alloimmunization in Various Clinical Scenarios
In this section, we will review the rates of alloimmunization reported in hospitalized (non-oncology) patients, healthy volunteers, children less than 1 year of age, patients with hemoglobin disorders, hematology/oncology patients, and solid organ transplant recipients. The content of this discussion is also summarized in table 1.
Table 1.
Approximate alloimmunization rates to non-ABO blood group antigens reported in various patient populations
| Population | Most common antibody specificity | Approximate alloimmunization rate | Exposure | Other notes/comments |
|---|---|---|---|---|
| General transfused population [6, 7, 8, 9, 10] | K, E | <1–4% | RBC transfusion | primarily retrospective studies; alloimmunization rates of 8–10% reported in prospective transfusion studies |
| Rh-negative healthy volunteers [21, 22] | D | 83–93% | intravenous RBC infusion | anti-E, anti-C and/or anti-G also detected in some volunteers |
| Young children [23, 24, 25, 26, 27, 28] | K, E | vanishingly rare | RBC transfusion | may be associated with severe infection or treatment with infliximab. |
| Hospitalized, non-oncology patients [14, 15] | primarily C, E, K | 20–30% | RBC transfusion | military combat veterans may be at increased risk compared to civilians |
| SCD [29, 30, 32, 33] | primarily C, E, K | Up to 47% | RBC transfusion | several guidelines recommend the provision of C, E and K matched RBCs for transfusion in this population |
| Myelodysplastic syndromes [40, 41, 42, 43] | Rh and K | Up to 58.6% | RBC transfusion | most studies utilized FAB criteria for diagnosis of MDS |
| Thalassemias [34, 35, 36, 37] | Rh and K | Up to 37% | RBC transfusion | |
| Pregnancy (prior to RhIg) [86] | D | 7.2% | fetal-maternal hemorrhage | substantially reduced by the introduction of Rh(D) immune globulin |
| AIDS [87] | – | none reported | RBC transfusion | further study needed |
AABB = American Association of Blood Banks; FAB = French American British classification of hematologic diseases
MDS = myelodysplastic syndromes; SCD = sickle cell disease.
General Hospital-Based Patient Populations
Alloimmunization studies of general, hospital-based patients arguably constitute the largest subset of clinical studies regarding non-ABO alloantibody formation rates. The overall incidence of alloimmunization in this group is reported to range from slightly less than 1% up to about 3% of patients undergoing type-and-screen testing [6, 7, 8, 9, 10, 11]. It is important to note that these data arise primarily from retrospective analyses of patients at single institutions undergoing transfusion service antibody screening. However, the likelihood of a particular patient becoming immunized after a particular blood transfusion is known to be highly variable and in many instances is related to a number of surrounding circumstances such as: the patient's clinical condition and underlying inflammatory status [12], the probability of exposure to foreign antigens, and the immunogenicities of the antigens seen [13]. In addition, there are several analytical factors which influence alloantibody detection and alloimmunization rates such as the performance of antibody screen testing following transfusion exposures and the persistence of antibody detection. Thus, while alloimmunization rates of about 1–3% are commonly cited in textbooks, several studies with regular antibody screening following transfusion have shown higher antibody development rates in general patient groups. Reports have indicated that about 20–30% of hospital-based, Rh(D)-negative patients form anti-D after an exposure, [14, 15] while about 8-10% of transfused patients will develop non-ABO, non-Rh(D) antibodies following transfusions matched for the Rh(D)-antigen [16, 17, 18]. Thus, these studies indicate that i) the number of individuals developing blood group antibodies even after a single transfusion is higher than retrospective studies suggest, and ii) large percentages of general patients appear to be non-responders even in prospective studies.
In addition to providing baseline data regarding alloimmunization rates, studies of alloimmunized populations also allow for examination of possible hyper-responder subgroups within alloimmunized patient cohorts. For instance, a study of concurrent alloimmunization found that about 22% of alloimmunized patients formed more than 1 alloantibody [19]. Other groups have found similar results, with about one quarter to one third of alloimmunized patients demonstrating concurrent alloantibodies [6, 20]. These findings indicate that among alloimmunized groups, the majority of patients tend to form only one alloantibody. If this group is defined as a responder population, then those with two or more alloantibodies are likely best characterized as a hyper-responder subset.
Rh-Negative, Healthy Volunteers
It has been established that a large proportion of healthy, Rh(D)-negative volunteers exposed to Rh(D)-positive RBCs are immunized to the D antigen. One study of six healthy volunteers found that 83% (5 out of the 6 volunteers) had detectable anti-D in their serum 150 days after being exposed to a single dose of only 0.5 ml of RBCs [21]. Another study of 28 healthy, Rh-negative volunteers found that 86% (24 out of the 28 volunteers) had detectable anti-D in their serum after one exposure, and 93% (26 out of the 28 volunteers) had detectable anti-D in their serum after two exposures [22]. Thus, these studies help to lend some credence to the notion that not all non-ABO antigens can be considered the same from the standpoint of responder versus non-responder status, as certain antigens appear to be associated with alloimmunization in the vast majority of exposed patients.
Neonates and Children Less than 1 Year of Age
In contrast to healthy adults, neonates are known to be highly unlikely to alloimmunize to any minor RBC antigens, even after numerous RBC transfusions [23, 24]. This is thought to be due to the immaturity of the neonatal immune system, as even anti-A and anti-B are not detectable during the first few months of life [1]. Due to the exceedingly low likelihood of alloimmunization, American Association of Blood Banks (AABB) Standards allows for neonates to be transfused for up to 4 months without any additional compatibility testing [23]. Indeed, reports of neonatal alloimmunization are rare [25]. Two reports of neonatal alloimmunization have been associated with concomitant severe bacterial or fungal infection [26, 27]. In addition, there is one report of a 10-month-old developing an alloantibody while undergoing infliximab treatment for juvenile rheumatoid arthritis [28]. These observations lend support to the idea that underlying inflammation or immunomodulation can greatly influence responder status. This hypothesis and other associated studies will be explored later in this review.
Hemoglobin Disorders
The rate of alloimmunization to minor RBC antigens among patients with sickle cell disease (SCD) is higher than that reported in most studies of the general population [29], a finding which has been frequently touted as evidence of a responder phenotype. An early study identified an alloimmunization rate of about 8% among 245 pediatric and young adult SCD patients, with alloimmunization being more common among patients with a higher number of cumulative lifetime RBC transfusions [30]. However, this study used a relatively insensitive antibody detection technique and the study design did not consider the length of time between the most recent transfusion and the antibody screen. Both of these factors may have led to an underestimation of the true incidence of alloimmunization in the study population. A subsequent, larger study that incorporated historical records of alloimmunization and multiple post-transfusion antibody screens found that 18.6% of SCD patients were alloimmunized [31]. This study confirmed that patients with higher cumulative number of lifetime transfusions were more likely to be alloimmunized [31]. Smaller studies have shown even higher rates of alloimmunization ranging up to nearly 50% of subjects [29, 32, 33], particularly when there is a mismatch between the antigen frequency of C, E, and K between the donor and recipient [29]. Indeed, it has repeatedly been observed that antibodies to the C, E, and K antigens are among the most common antibodies observed in patients with SCD [30, 31, 32].
Historically, patients with transfusion-dependent thalassemia are reported to have a somewhat similar alloimmunization rate to patients with SCD [34, 35]. More recently, a study performed in Kuwait identified an alloimmunization rate of approximately 30% in an Arab population of thalassemia major patients transfused with ABO- and Rh(D)-compatible RBCs [36]. The most frequently encountered antibodies were reactive to K and Rh antigens [36], similar to what is observed among patients with SCD. Another recent study conducted in Taiwain identified a 37% alloimmunization rate among transfusion-dependent thalassemia patients [37].
Studies of transfused patients with SCD and thalassemia, which demonstrate two- to fourfold higher alloimmunization rates than those reported in even prospective studies of general, transfused patients, are striking from the perspective of alloantibody development. One possible interpretation of these findings is that the underlying disease state may potentially predispose an individual to responder status, perhaps via associated inflammation or genetic polymorphisms. Alternatively, another possible interpretation of these findings is that, with large numbers of SCD and thalassemia patients undergoing chronic transfusion and regular follow-up testing, responder subgroups are more easily unveiled and identified than they would be in general patient groups, which may undergo antibody screening and transfusion less frequently and at more random intervals. Clearly, more work remains to better determine the basis for increased alloantibody development in patients with hemoglobin disorders.
Hematology/Oncology Patients
Investigations of another chronically transfused patient population (i.e., patients with malignancies) also show great variation with regard to alloimmunization rates. A study of hospitalized Rh(D)-negative patients, mainly with surgical and oncology diagnoses, found that 21.4% alloimmunized to Rh antigens after transfusion with Rh-positive RBCs [38]. This study also reported that the rate of alloimmunization among patients with hematological malignancies was higher (41.6%) than among other patient populations (20.9%) [38]. Furthermore, among patients with hematologic malignancies transfused with Rh–matched RBCs, the alloimmunization incidence is approximately 9% [39].
Two frequently studied hematological populations are patients with myelodysplastic syndromes (MDS) and patients undergoing bone marrow transplant (BMT). Patients with MDS have been shown to have a relatively high rate of alloimmunization to minor blood group antigens, in part due to their relatively higher utilization of blood transfusions and partly because of changes in the immune system associated with MDS [40]. The rate of alloimmunization in MDS patients has been reported to be up to 58.6% [41], although other studies have shown rates of alloimmunization that are similar to non-oncology hospitalized patients [42, 43]. Similar to patients with SCD, alloimmunization in MDS patients is associated with a higher number of cumulative (lifetime) transfusions [43].
Patients undergoing BMT are at risk for alloimmunization and hemolysis [5, 44, 45]. Although BMT patients are profoundly immunosuppressed after myeloablation, minor RBC antibodies may occur either as a result of passenger lymphocytes from the graft [44, 46], myeloablation-resistant host lymphocytes [5, 46], or a primary alloimmune response to RBC transfusion [45, 46]. In the event of a primary alloimmune response, the source of minor RBC antigens in current practice is most likely RBC transfusions [47]. However, the rate of alloimmunization – even to the highly immunogenic Rh(D) antigen – among Rh(D)-negative BMT patients receiving Rh(D)-positive platelet concentrates is low [47].
It is difficult to conclude with any certainty whether patients with hematological or oncological disorders constitute a distinct responder subgroup. Their underlying disease-associated immunosuppression, in association with chemotherapeutic regimens, may suppress alloantibody development. Nonetheless, data from patients with MDS indicate a potential predisposition to alloantibody development, particularly when rates from these studies are compared to those of general patient groups. As with hemoglobin disorders, more investigation is needed to determine whether any factors may predispose patients with malignancies to form alloantibodies at higher rates than other transfused groups.
Solid Organ Transplant
The immunosuppressive regimens associated with solid organ transplantation are believed to substantially reduce the incidence of alloimmunization, even to Rh(D) [48, 49, 50]. Although cases of alloimmunization in the context of solid organ transplant are occasionally reported, the development of alloantibodies may be evidence of incomplete immunosuppression or possibly of the reappearance of a previously evanescent antibody. In the event that a new alloantibody is detected in this patient population, the possibility of immunosuppressive treatment failure should be considered. In the context of this review, findings from these studies suggest that interventions such as immunosuppression may be one tool to combat alloantibody formation in a responder subgroup.
Evidence Supporting the Existence of a Responder Subgroup
In addition to reviewing the vast literature on alloimmunization in general, it is important to consider investigations which have directly examined for the existence of responder subgroups. One of the best known of these studies found that the observed distribution of patients with alloimmunization is not consistent with the concept that all patients were equally likely to form an antibody after RBC transfusion. Specifically, if there was an equal 4% risk of alloimmunization after every transfusion for every patient, then, mathematically, it would be expected that half of patients who had received 17 or more transfusions would have alloantibodies to minor RBC antigens [10]. However, a mathematical model showed that more than 190 transfusions would be required before half of transfusion recipients would be predicted to be alloimmunized, indicating that a small percentage of the population must be far more likely to be alloimmunized than the population at large, independent of the number of transfusions they receive [10].
Another study reported that non-hematology patients who formed a single alloantibody were approximately 29 times more likely to form additional alloantibodies compared to patients who had not already alloimmunized, suggesting the existence of a responder subgroup [51]. Recently, a similar tendency towards alloimmunization among patients with pre-existing alloantibodies compared to those without pre-existing alloantibodies was demonstrated in a primarily male patient population where most antibodies were attributable to transfusion [52]. Thus, it would appear that the formation of at least one alloantibody would be a sufficient criterion to identify an individual as a responder as this patient subgroup appears much more likely to be alloimmunized than transfused control groups. While these studies are convincing for the existence of responders, they provide little insight into what biological factor(s) may predispose an individual to making one or more blood group antibodies. Hence, we will next consider the existing evidence and data on variables which may influence the formation of blood group antibodies within responder populations.
Recipient Factors Which May Correlate with Responder Status
In the paragraphs below, we will summarize the roles of various factors (e.g., genetics, inflammation, sex, and age) in potentially predisposing an individual to alloimmunization. Both clinical states and biological factors which influence alloimmunization are also outlined schematically in figure 2. Of course, it is important to note that in order to be capable of responding to any non-ABO antigen, by definition an individual must i) lack the corresponding antigen and ii) be exposed to that antigen in sufficient dosage to provoke an alloimmune response. Thus, a fundamental need for any individual to actually be classified as a responder is for that person to be exposed to an antigen(s) that they lack on their RBCs.
Fig. 2.

Schematic representation of factors that are believed to influence the likelihood of alloimmunization.
Genetics
One hypothesis accounting for responder populations is that genetic differences between individuals impact inter-individual variation in alloimmunization rates. An obvious genetic difference is antigenic diversity between donors and recipients. Theoretically, a donor population that has substantial variation in the expression of non-ABO antigens in comparison to recipients establishes the possibility for greater numbers of antigenic mismatches and, therefore, higher numbers of alloantibodies induced overall. This factor may help to explain, in part, the higher rates of alloimmunization seen in SCD – when donors and recipients are more closely matched for non-ABO antigens, alloimmunization rates have been noted to dramatically decrease [53]. However, the genetics of non-ABO antigenic discrepancies are not likely to account for all cases of responsiveness, even in the setting of SCD. A recent study has shown that RBC alloimmunization persisted at high rates even using Rh-matched minority donors [54]. Therefore, there are likely other genetic factors at play which may influence alloantibody development.
Another reasonable genetic consideration for examining an individual's responder status is the HLA system. It is possible that only certain individuals are capable of presenting a given blood group antigen due to their HLA inheritance [55]. In fact, certain class II HLA alleles have been associated with alloimmunization to some minor RBC antigens – alloimmunization to antigens within the Diego system as well as to Mia, Fya, Jka, K, E, and S have been shown to be dependent on recipient HLA class II polymorphisms [56, 57, 58, 59, 60]. In addition, a recent study suggested that the HLA-DRB1*15 allele was present in nearly half of individuals with multiple alloantibodies across a number antigen groups, indicating that HLA status may also be capable of identifying an individual as a general responder [59]. Thus, there is growing evidence to suggest that HLA allele restriction can play an important role in helping to determine whether an individual is capable of recognizing a blood group antigen as foreign and therefore may help to account for observations of responder subgroups. However, since many individuals who possess a certain HLA genoor phenotype apparently never develop an alloantibody after RBC exposure, the question arises whether there are other genetic factors that contribute to the responder phenotype.
While much ongoing work remains to definitively answer the question posed above, there are emerging data indicating that other forms of genetic inheritance may be useful in identifying a responder population. For example, a study of SCD patients found that single nucleotide polymorphisms in the CD81 gene were associated with alloimmunization [61]. CD81 polymorphisms, which are associated with B-cell signal modulation, therefore represent a potentially interesting alternative genetic target for examining responders. Of course, it is important to note that most genetic studies are small and are typically geographically limited in their scope. As such, further investigations are needed to not only identify additional genetic targets, but to better understand how polymorphic differences in two disparate systems (such as HLA class II and CD81) may act in concert to promote alloantibody formation.
Recipient Inflammation
With the possible exception of the Rh(D) antigen, minor RBC antigens are considered to be of relatively low immunogenicity [13, 62]. Given that multiple diseases are associated with increases in alloimmunization (see table 1), some investigators have hypothesized that responders suffer from systemic inflammatory conditions that increase the tendency of the immune system to ‘recognize’ foreign RBC antigens [26, 27]. One study of a murine model of alloimmunization found that treatment with an inflammatory RNA molecule increased the immune response to a model blood group antigen [63]. It is believed that viral-type inflammation causes enhanced trafficking of transfused RBCs to splenic dendritic cells (rather than macrophages), leading to CD4+ T-cell proliferation and alloantigen-specific immunity [64]. Interestingly, immunity appears to be suppressed when the inflammatory source is lipopolysaccharide, a bacterial mediator of inflammation, rather than an inflammatory RNA molecule which induces a viral type of infection [65].
In addition to the above animal models, there are several human studies supporting the notion that an underlying inflammatory disorder may predispose an individual to develop blood group antibodies. One investigation found a high rate of formation of antibodies to low-incidence and low-immunogenicity antigens among individuals with autoimmune disorders [66]. Another study of patients with inflammatory bowel disease indicated a about 2–3 fold increased risk for alloantibody development among this cohort in comparison to a control group [67]. Finally, a third group observed that patients experiencing febrile transfusion reactions were much more likely to develop alloantibodies than similarly transfused patients who had no concomitant inflammatory stimulus [68]. There have also been a series of case reports wherein individuals receiving immunomodulatory therapies [28] or those with severe infectious disorders [27] have developed multiple alloantibodies following small RBC exposures. Interestingly, in many of these latter cases, the antibodies were developed in young children, a group that typically responds poorly to RBC antigen challenges. As such, these findings raise the intriguing possibility that an underlying inflammatory process can predispose an individual to developing blood group alloantibodies, or perhaps even turn a non-responder into a responder. Clearly, more work is needed to determine if this phenomenon is occurring in individuals already primed to make alloantibodies, or whether inflammation itself is a key component to the responder phenotype.
Sex
It is generally observed that women have more robust immune responses than men [69]. The basis for this observation is not known, although it was recently hypothesized that the effect of testosterone on lipid metabolism is responsible for the blunted immune response in male seasonal influenza vaccine recipients [69]. Nonetheless, the influence of sex in minor RBC antigen alloimmunization remains controversial. Some studies have found that females are more likely to alloimmunize compared to males [8, 12, 70], but not all studies have found a statistically significant relationship between female sex and risk of alloimmunization [71]. In fact, a study reporting one of the highest general patient alloimmunization rates arose from the study of a predominantly male cohort of military veterans [11]. At present, there is no compelling evidence to consider male sex to be a significant protective factor against alloimmunization, nor female sex to be a predictor of responsiveness [72].
Age
It is known that the process of aging has measurable effects on the immune system overall [73], which has led to speculation that aging may also affect RBC alloimmunization rates. For example, it has been reported that age > 77 years is associated with a decreased rate of blood group antigen alloimmunization [38]. On the other end of the age spectrum, and as discussed earlier, neonates and young children are generally considered poor responders to RBC antigens. In fact, some studies have even found that patients who are transfused at a young age are less likely to develop antibodies compared to patients who start transfusion later in life [35]; one hypothesis to account for this latter phenomenon is the induction of tolerance to RBC antigens seen at an early age. Unfortunately, testing this hypothesis in a prospective clinical trial would be challenging in that it would likely involve intentionally exposing young patients to numerous RBC antigens. Nonetheless, this possibility in preventing alloimmunization remains intriguing and requires further investigation, perhaps first at the basic science level [71].
Practical Clinical Approaches to Limit Alloimmunization and Its Consequences
Since RBC alloimmunization can have serious clinical consequences, it is widely accepted that alloimmunization should be avoided as much as possible. This is particularly true in female patients of childbearing potential (due to the risk for hemolytic disease of the fetus and newborn) and for patients that may require extended transfusion support and/or stem cell transplantation [5, 44, 45, 46]. An extensive review of RBC mitigation strategies, both current and experimental, has recently been published [74]. As such, we will only briefly summarize some practical proposals intended to prevent or limit RBC alloimmunization in a responder subgroup.
Antigen-Matched RBCs
The most reliable approach to limit minor RBC alloimmunization is to provide RBCs for transfusion with the minimum possible number of antigen mismatches. To accomplish this, hospitals may pheno- or genotype recipients for various minor RBC antigens and then attempt to procure RBCs from donors who are matching. This approach has been shown to be very promising for the prevention of alloimmunization in multiply transfused patient populations [35, 51, 75, 76]. Although such a practice has been proposed to be cost-effective in some settings [77], it is believed to be cost-prohibitive in others, especially in patient populations that are unlikely to alloimmunize due to underlying severe immunosuppression [39]. At present, many large centers in the USA provide antigen-matched (for at least C, E, and K antigens) RBCs for patients with SCD, consistent with national recommendations [78]. However, even such limited antigen matching may not be protective for alloimmunization against rare partial or variant antigens, particularly those within the Rh system [54].
The logistics of establishing extended antigen-matched RBCs can be challenging for blood banks and transfusion services. As such, some administrative factors that would likely ease the procurement of antigen-matched RBCs would be i) the development and maintenance of national or international blood bank antibody records, so that a recipient's phenotype could be determined once and then used for reference as needed, and ii) the provision of a RBC donor phenotype, if known by the blood collection entity, to the transfusing hospital. Together, these proposals would ease the process of providing more antigen-matched units in more situations and would likely help to reduce alloimmunization rates among responders.
Conservative RBC Transfusion
Of course, the most effective mechanism to prevent alloimmunization is the elimination of RBC transfusions that are not clinically necessary. The AABB has published clinical guidelines based on several large, prospective trials to help to achieve this goal [79]. Implementation of such guidelines with reasonable enforcement (e.g., via prospective utilization audits) is an effective way to curb unnecessary transfusions, and thereby limit resultant alloimmunization.
Leukoreduction and Other Modifications
The efficacy of leukoreduction at preventing alloimmunization is controversial. According to one study, Rh-negative patients transfused with prestorage leukoreduced RBCs had a lower (13%) alloimmunization rate compared to patients transfused with non-leukoreduced RBCs (22%) [14]. However, this finding was based on a small number (n = 8) of patients, and has not been confirmed by other studies [42, 43, 80, 81]. Of course, it is important to note that the leukoreduction of RBCs does substantially reduce the likelihood of alloimmunization to HLA antigens, a potentially important issue for chronic transfusion recipients [80]. Regarding other clinical modifications available in most blood banks/transfusion services (e.g., irradiation, washing, or saline replacement), there is no evidence at present to suggest that any of these approaches has an effect on alloimmunization rates.
Antibody Evanescence and Record Portability
In addition to preventing or limiting alloimmunization, one other concern for ‘responders’ is limiting the clinical consequences of antibodies that they have already developed. This can be complex because blood group antibodies formed against most antigens tend to fade over time and may eventually become undetectable, a phenomenon known as antibody evanescence. It is has been observed that approximately two thirds of minor RBC alloantibodies will become undetectable in the months to years following their initial detection [11, 82, 83, 84]. At present, there is no laboratory test available to stimulate evanesced antibodies in vitro, and the lack of centralized blood bank record systems in the USA and other regions further hampers the identification of historical antibodies. Thus, patients with evanesced alloantibodies are at risk for the development of delayed hemolytic transfusion reactions with subsequent transfusions. As such, one other important consideration for a responder or hyper-responder would be the provision of portable antibody records (e.g., a wallet card or alert bracelet) in case such individuals seek transfusion-related care at more than one facility [85].
Conclusions
Although the association between exposure to foreign RBC antigens and alloimmunization has been known for decades, the specific factors that cause some individuals to immunize while others do not remain poorly understood. Nonetheless, a growing body of clinical literature supports the concept that there are a subset of non-ABO alloantibody ‘responders’ and even ‘hyper-responders’, i.e., transfused individuals who are capable of forming one or more antibodies after RBC exposure. Clinical and biological factors such as disease state, HLA polymorphisms, underlying inflammation, and patient age may all be contributory to the ‘responder’ phenotype. However, much investigation remains to better define the variables which predispose transfusion recipients to developing non-ABO antibodies. Ultimately, a primary goal of future studies should be to develop tools to allow the identification of responder patients such that appropriate resources can be allocated for the provision of antigen-matched RBCs in order to minimize the risk for alloimmunization and its consequences.
Disclosure Statement
No relevant conflicts of interest for the authors.
References
- 1.Fong SW, Qaqundah BY, Taylor WF. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex, and maternal isoagglutinins. Transfusion. 1974;14:551–559. doi: 10.1111/j.1537-2995.1974.tb04576.x. [DOI] [PubMed] [Google Scholar]
- 2.Davenport RD. Pathophysiology of hemolytic transfusion reactions. Semin Hematol. 2005;42:165–168. doi: 10.1053/j.seminhematol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.United States Food and Drug Administration. CBER. 2012. www.fda.gov/biologicsbloodvaccines/bloodbloodproducts/ (last accessed October 16, 2014)
- 4.Serious Hazards of Transfusion. Annual report. 2012. www.shotuk.org (last accessed October 16, 2014)
- 5.Booth GS, Gehrie EA, Savani BN. Minor RBC Ab and allo-SCT. Bone Marrow Transplant. 2014;49:456–457. doi: 10.1038/bmt.2013.196. [DOI] [PubMed] [Google Scholar]
- 6.Winters JL, Pineda AA, Gorden LD, Bryant SC, Melton LJ, 3rd, Vamvakas EC, Moore SB. RBC alloantibody specificity and antigen potency in Olmsted county, Minnesota. Transfusion. 2001;41:1413–1420. doi: 10.1046/j.1537-2995.2001.41111413.x. [DOI] [PubMed] [Google Scholar]
- 7.Spielmann W, Seidl S. Prevalence of irregular red cell antibodies and their significance in blood transfusion and antenatal care. Vox Sang. 1974;26:551–559. doi: 10.1111/j.1423-0410.1974.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–45. [PubMed] [Google Scholar]
- 9.Thakral B, Saluja K, Sharma RR, Marwaha N. Red cell alloimmunization in a transfused patient population: a study from a tertiary care hospital in North India. Hematology. 2008;13:313–318. doi: 10.1179/102453308X343419. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 11.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 12.Bauer MP, Wiersum-Osselton J, Schipperus M, Vandenbroucke JP, Briet E. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007;47:2066–2071. doi: 10.1111/j.1537-2995.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 13.Tormey CA, Stack G. Immunogenicity of blood group antigens: a mathematical model corrected for antibody evanescence with exclusion of naturally occurring and pregnancy-related antibodies. Blood. 2009;114:4279–4282. doi: 10.1182/blood-2009-06-227793. [DOI] [PubMed] [Google Scholar]
- 14.Yazer MH, Triulzi DJ. Detection of anti-D in D-recipients transfused with D+ red blood cells. Transfusion. 2007;47:2197–2201. doi: 10.1111/j.1537-2995.2007.01446.x. [DOI] [PubMed] [Google Scholar]
- 15.Frohn C, Dumbgen L, Brand JM, Gorg S, Luhm J, Kirchner H. Probability of anti-D development in D-patients receiving D+ RBCs. Transfusion. 2003;43:893–898. doi: 10.1046/j.1537-2995.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- 16.Redman M, Regan F, Contreras M. A prospective study of the incidence of red cell allo-immunisation following transfusion. Vox Sang. 1996;71:216–220. doi: 10.1046/j.1423-0410.1996.7140216.x. [DOI] [PubMed] [Google Scholar]
- 17.Alves VM, Martins PR, Soares S, Araujo G, Schmidt LC, Costa SS, Langhi DM, Moraes-Souza H. Alloimmunization screening after transfusion of red blood cells in a prospective study. Rev Bras Hematol Hemoter. 2012;34:206–211. doi: 10.5581/1516-8484.20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schabel A, Konig AL, Schiebel MR, Sugg U. Incidence and persistence of anti-Kell after transfusion of Kell-positive blood. Beitr Infusionsther Transfusionsmed. 1994;32:175–178. [PubMed] [Google Scholar]
- 19.Tormey CA, Stack G. The characterization and classification of concurrent blood group antibodies. Transfusion. 2009;49:2709–2718. doi: 10.1111/j.1537-2995.2009.02337.x. [DOI] [PubMed] [Google Scholar]
- 20.Schonewille H, Brand A. Does an alloimmune response to strong immunogenic red blood cell antigens enhance a response to weaker antigens? Transfusion. 2008;48:958–963. doi: 10.1111/j.1537-2995.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 21.Gunson HH, Stratton F, Cooper DG, Rawlinson VI. Primary immunization of Rh-negative volunteers. BMJ. 1970;i:593–595. doi: 10.1136/bmj.1.5696.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbaniak SJ, Robertson AE. A successful program of immunizing Rh-negative male volunteers for anti-D production using frozen/thawed blood. Transfusion. 1981;21:64–69. doi: 10.1046/j.1537-2995.1981.21181127486.x. [DOI] [PubMed] [Google Scholar]
- 23.Floss AM, Strauss RG, Goeken N, Knox L. Multiple transfusion fail to provoke antibodies against blood cell antigens in human infants. Transfusion. 1986;26:419–422. doi: 10.1046/j.1537-2995.1986.26587020115.x. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsen CW, Jr, Swanson JL, Thompson TR, McCullough J. The failure of neonates to form red blood cell alloantibodies in response to multiple transfusions. Am J Clin Pathol. 1987;87:250–251. doi: 10.1093/ajcp/87.2.250. [DOI] [PubMed] [Google Scholar]
- 25.DePalma L, Criss VR, Roseff SD, Luban NL. Presence of the red cell alloantibody anti-E in an 11-week-old infant. Transfusion. 1992;32:177–179. doi: 10.1046/j.1537-2995.1992.32292180151.x. [DOI] [PubMed] [Google Scholar]
- 26.Marsh WL, Nichols ME, Oyen R, Thayer RS, Deere WL, Freed PJ, Schmelter SE. Naturally occurring anti-Kell stimulated by E. coli enterocolitis in a 20-day-old child. Transfusion. 1978;18:149–154. doi: 10.1046/j.1537-2995.1978.18278160576.x. [DOI] [PubMed] [Google Scholar]
- 27.Hata JL, Johnson MS, Booth GS. Neonatal alloimmunization: a rare case of multiple alloantibody formation in a patient with disseminated histoplasmosis. Transfusion. 2013;53:1140–1141. doi: 10.1111/trf.12132. [DOI] [PubMed] [Google Scholar]
- 28.Tyler LN, Harville TO, Blackall DP. Multiple alloantibodies after transfusion in an infant treated with infliximab. N Engl J Med. 2007;357:2092–2093. doi: 10.1056/NEJMc070741. discussion 2093. [DOI] [PubMed] [Google Scholar]
- 29.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 30.Sarnaik S, Schornack J, Lusher JM. The incidence of development of irregular red cell antibodies in patients with sickle cell anemia. Transfusion. 1986;26:249–252. doi: 10.1046/j.1537-2995.1986.26386209381.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. the Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 32.Orlina AR, Unger PJ, Koshy M. Post-transfusion alloimmunization in patients with sickle cell disease. Am J Hematol. 1978;5:101–106. doi: 10.1002/ajh.2830050204. [DOI] [PubMed] [Google Scholar]
- 33.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 34.Coles SM, Klein HG, Holland PV. Alloimmunization in two multitransfused patient populations. Transfusion. 1981;21:462–466. doi: 10.1046/j.1537-2995.1981.21481276005.x. [DOI] [PubMed] [Google Scholar]
- 35.Spanos T, Karageorga M, Ladis V, Peristeri J, Hatziliami A, Kattamis C. Red cell alloantibodies in patients with thalassemia. Vox Sang. 1990;58:50–55. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 36.Ameen R, Al-Shemmari S, Al-Humood S, Chowdhury RI, Al-Eyaadi O, Al-Bashir A. RBC alloimmunization and autoimmunization among transfusion-dependent Arab thalassemia patients. Transfusion. 2003;43:1604–1610. doi: 10.1046/j.1537-2995.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang LY, Liang DC, Liu HC, Chang FC, Wang CL, Chan YS, Lin M. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfus Med. 2006;16:200–203. doi: 10.1111/j.1365-3148.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Porras JR, Graciani IF, Perez-Simon JA, Martin-Sanchez J, Encinas C, Conde MP, Nieto MJ, Corral M. Prospective evaluation of a transfusion policy of D+ red blood cells into D-patients. Transfusion. 2008;48:1318–1324. doi: 10.1111/j.1537-2995.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- 39.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39:763–771. doi: 10.1046/j.1537-2995.1999.39070763.x. [DOI] [PubMed] [Google Scholar]
- 40.Arriaga F, Bonanad S, Larrea L, de la Rubia J, Lopez F, Sanz MA, Sanz G, Marty ML. Immunohematologic study in 112 patients with myelodysplastic syndromes: 10-year analysis (in Spanish) Sangre. 1995;40:177–180. [PubMed] [Google Scholar]
- 41.Novaretti MC, Sopelete CR, Velloso ER, Rosa MF, Dorlhiac-Llacer PE, Chamone DA. Immunohematological findings in myelodysplastic syndrome. Acta Haematol. 2001;105:1–6. doi: 10.1159/000046525. [DOI] [PubMed] [Google Scholar]
- 42.Stiegler G, Sperr W, Lorber C, Fabrizii V, Hocker P, Panzer S. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Ann Hematol. 2001;80:330–333. doi: 10.1007/s002770100308. [DOI] [PubMed] [Google Scholar]
- 43.Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53:710–715. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 44.Young PP, Goodnough LT, Westervelt P, Diersio JF. Immune hemolysis involving non-ABO/RhD alloantibodies following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:1305–1310. doi: 10.1038/sj.bmt.1703074. [DOI] [PubMed] [Google Scholar]
- 45.de La Rubia J, Arriaga F, Andreu R, Sanz G, Jimenez C, Vicente A, Carpio N, Marty ML, Sanz MA. Development of non-ABO RBC alloantibodies in patients undergoing allogeneic HPC transplantation. Is ABO incompatibility a predisposing factor? Transfusion. 2001;41:106–110. doi: 10.1046/j.1537-2995.2001.41010106.x. [DOI] [PubMed] [Google Scholar]
- 46.Ting A, Pun A, Dodds AJ, Atkinson K, Biggs JC. Red cell alloantibodies produced after bone marrow transplantation. Transfusion. 1987;27:145–147. doi: 10.1046/j.1537-2995.1987.27287150186.x. [DOI] [PubMed] [Google Scholar]
- 47.Cid J, Ortin X, Elies E, Castella D, Panades M, Martin-Vega C. Absence of anti-D alloimmunization in hematologic patients after D-incompatible platelet transfusions. Transfusion. 2002;42:173–176. doi: 10.1046/j.1537-2995.2002.00038.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramsey G, Hahn LF, Cornell FW, Boczkowski DJ, Staschak S, Clark R, Hardesty RL, Griffith BP, Starzl TE. Low rate of Rhesus immunization from Rh-incompatible blood transfusions during liver and heart transplant surgery. Transplantation. 1989;47:993–995. doi: 10.1097/00007890-198906000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casanueva M, Valdes MD, Ribera MC. Lack of alloimmunization to D antigen in D-negative immunosuppressed liver transplant recipients. Transfusion. 1994;34:570–572. doi: 10.1046/j.1537-2995.1994.34794330009.x. [DOI] [PubMed] [Google Scholar]
- 50.Yuan S, Davis R, Lu Q, Goldfinger D, Ziman AF. Low risk of alloimmunization to the D antigen in D-orthotopic liver transplant recipients receiving D+ RBCs perioperatively. Transfusion. 2008;48:2653–2655. doi: 10.1111/j.1537-2995.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 51.Schonewille H, van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: is it time to take precautionary measures? Transfusion. 2006;46:630–635. doi: 10.1111/j.1537-2995.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 52.Tormey CA, Stack G. Pre-existing non-ABO blood group alloantibodies identify a ‘responder’ subgroup prone to subsequent red cell alloimmunization. Transfusion. 2013;53(suppl 2):150a. [Google Scholar]
- 53.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 55.Brantley SG, Ramsey G. Red cell alloimmunization in multitransfused HLA-typed patients. Transfusion. 1988;28:463–466. doi: 10.1046/j.1537-2995.1988.28588337338.x. [DOI] [PubMed] [Google Scholar]
- 56.Chu CC, Ho HT, Lee HL, Chan YS, Chang FJ, Wang CL, Lin M. Anti-‘Mia’ Immunization is associated with HLA-DRB1*0901. Transfusion. 2009;49:472–478. doi: 10.1111/j.1537-2995.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 57.Reviron D, Dettori I, Ferrera V, Legrand D, Touinssi M, Mercier P, de Micco P, Chiaroni J. HLA-DRB1 alleles and Jka immunization. Transfusion. 2005;45:956–959. doi: 10.1111/j.1537-2995.2005.04366.x. [DOI] [PubMed] [Google Scholar]
- 58.Baleotti W, Jr, Ruiz MO, Fabron A, Jr, Castilho L, Giuliatti S, Donadi EA. HLA-DRB1*07:01 allele is primarily associated with the Diego a alloimmunization in a Brazilian population. Transfusion. 2014;54:2468–2476. doi: 10.1111/trf.12652. [DOI] [PubMed] [Google Scholar]
- 59.Schonewille H, Doxiadis II, Levering WH, Roelen DL, Claas FH, Brand A. HLA-DRB1 associations in individuals with single and multiple clinically relevant red blood cell antibodies. Transfusion. 2014;54:1971–1980. doi: 10.1111/trf.12624. [DOI] [PubMed] [Google Scholar]
- 60.Noizat-Pirenne F, Tournamille C, Bierling P, Roudot-Thoraval F, Le Pennec PY, Rouger P, Ansart-Pirenne H. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46:1328–1333. doi: 10.1111/j.1537-2995.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 61.Tatari-Calderone Z, Tamouza R, Le Bouder GP, Dewan R, Luban NL, Lasserre J, Maury J, Lionnet F, Krishnamoorthy R, Girot R, Vukmanovic S. The association of CD81 polymorphisms with alloimmunization in sickle cell disease. Clin Dev Immunol. 2013;2013:937846. doi: 10.1155/2013/937846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631–635. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 63.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 64.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 65.Hendrickson JE, Roback JD, Hillyer CD, Easley KA, Zimring JC. Discrete Toll-like receptor agonists have differential effects on alloimmunization to transfused red blood cells. Transfusion. 2008;48:1869–1877. doi: 10.1111/j.1537-2995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 66.Ramsey G, Smietana SJ. Multiple or uncommon red cell alloantibodies in women: association with autoimmune disease. Transfusion. 1995;35:582–586. doi: 10.1046/j.1537-2995.1995.35795357881.x. [DOI] [PubMed] [Google Scholar]
- 67.Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, Eser A, Mikulits A, Mayr WR, Kormoczi GF. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. Am J Med. 125:717 e711–718. doi: 10.1016/j.amjmed.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 68.Yazer MH, Triulzi DJ, Shaz B, Kraus T, Zimring JC. Does a febrile reaction to platelets predispose recipients to red blood cell alloimmunization? Transfusion. 2009;49:1070–1075. doi: 10.1111/j.1537-2995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 69.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giblett ER. Blood group alloantibodies: an assessment of some laboratory practices. Transfusion. 1977;17:299–308. doi: 10.1046/j.1537-2995.1977.17477216857.x. [DOI] [PubMed] [Google Scholar]
- 71.Fluit CR, Kunst VA, Drenthe-Schonk AM. Incidence of red cell antibodies after multiple blood transfusion. Transfusion. 1990;30:532–535. doi: 10.1046/j.1537-2995.1990.30690333485.x. [DOI] [PubMed] [Google Scholar]
- 72.Verduin EP, Brand A, Schonewille H. Is female sex a risk factor for red blood cell alloimmunization after transfusion? A systematic review. Transfus Med Rev. 26:342–353. doi: 10.1016/j.tmrv.2011.12.001. 353 e1-5. [DOI] [PubMed] [Google Scholar]
- 73.Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- 74.Hendrickson JE, Tormey CA, Shaz BH. Red blood cell alloimmunization mitigation strategies. Transfus Med Rev. 2014;28:137–144. doi: 10.1016/j.tmrv.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Castro O, Sandler SG, Houston-Yu P, Rana S. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: theoretical and practical implications. Transfusion. 2002;42:684–690. doi: 10.1046/j.1537-2995.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 76.Ambruso DR, Githens JH, Alcorn R, Dixon DJ, Brown LJ, Vaughn WM, Hays T. Experience with donors matched for minor blood group antigens in patients with sickle cell anemia who are receiving chronic transfusion therapy. Transfusion. 1987;27:94–98. doi: 10.1046/j.1537-2995.1987.27187121485.x. [DOI] [PubMed] [Google Scholar]
- 77.Lau FY, Wong R, Chan NP, Chui CH, Ng E, Ng MH, Cheng G. Provision of phenotype-matched blood units: no need for pre-transfusion antibody screening. Haematologica. 2001;86:742–748. [PubMed] [Google Scholar]
- 78.Osby M, Shulman IA. Phenotype matching of donor red blood cell units for nonalloimmunized sickle cell disease patients: a survey of 1182 North American laboratories. Arch Pathol Lab Med. 2005;129:190–193. doi: 10.5858/2005-129-190-PMODRB. [DOI] [PubMed] [Google Scholar]
- 79.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 80.Sanz C, Ghita G, Franquet C, Martinez I, Pereira A. Red-blood-cell alloimmunization and female sex predict the presence of HLA antibodies in patients undergoing liver transplant. Vox Sang. 2010;99:261–266. doi: 10.1111/j.1423-0410.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 81.Schonewille H, Brand A. Alloimmunization to red blood cell antigens after universal leucodepletion. A regional multicentre retrospective study. Br J Haematol. 2005;129:151–156. doi: 10.1111/j.1365-2141.2005.05408.x. [DOI] [PubMed] [Google Scholar]
- 82.Ramsey G, Larson P. Loss of red cell alloantibodies over time. Transfusion. 1988;28:162–165. doi: 10.1046/j.1537-2995.1988.28288179022.x. [DOI] [PubMed] [Google Scholar]
- 83.Ramsey G, Smietana SJ. Long-term follow-up testing of red cell alloantibodies. Transfusion. 1994;34:122–124. doi: 10.1046/j.1537-2995.1994.34294143938.x. [DOI] [PubMed] [Google Scholar]
- 84.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49:505–512. doi: 10.1111/j.1537-2995.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 85.Unni N, Peddinghaus M, Tormey CA, Stack G. Record fragmentation due to transfusion at multiple health care facilities: a risk factor for delayed hemolytic transfusion reactions. Transfusion. 2014;54:98–103. doi: 10.1111/trf.12251. [DOI] [PubMed] [Google Scholar]
- 86.Chown B. Prevention of Rh immunization. Can Med Assoc J. 1969;100:869. [PMC free article] [PubMed] [Google Scholar]
- 87.Boctor FN, Ali NM, Mohandas K, Uehlinger J. Absence of D-alloimmunization in AIDS patients receiving D-mismatched RBCs. Transfusion. 2003;43:173–176. doi: 10.1046/j.1537-2995.2003.00289.x. [DOI] [PubMed] [Google Scholar]