Summary
Background
Many patients with autoimmune hemolytic anemia (AIHA) do not respond to standard therapy and/or may develop severe complications which can be of fatal outcome. There is some evidence that erythropoiesis-stimulating agents (ESAs) may be helpful in the management of such patients.
Methods
We describe the effect of ESAs in 12 new patients with therapy-refractory AIHA (7 of warm type and 5 of cold type) and review 5 previously reported cases. Serological testing was performed using standard methods.
Results
All patients responded well to treatment with ESAs. At least 5 of the 17 patients demonstrated complete recovery, and none of the patients developed significant adverse reactions due to treatment with ESAs.
Conclusion
The mechanism by which ESAs improves hemolysis in AIHA is not completely clear. In addition to increased production and prolonged RBC survival, it may inhibit eryptosis (programmed cell death). ESAs represent a new option in the treatment of decompensated and/or refractory AIHA of warm and cold type. However, more information is required to assess which patients can be treated with ESAs.
Keywords: AIHA, Autoimmune hemolytic anemia, Erythropoietin, EPO
Introduction
Autoimmune hemolytic anemia (AIHA) is one of the best characterized autoimmune diseases. It is related to complement and non-complement activating autoantibodies of the IgG, and less commonly, of the IgM and/or IgA classes. IgGand/or C3b-coated red blood cells (RBCs) are eliminated by phagocytosis (extravascular hemolysis), and in cases where the antibodies are capable of activating the terminal complement components (C5b-9), the target cells are lysed in the circulation (intravascular hemolysis) [1, 2, 3].
Until now, treatment of AIHA is not performed in analogous manner, and there are no generally accepted guidelines. Current therapy is largely based on relatively small and older retrospective studies which indicate, without exception, the use of steroids as the first-line treatment option in affected patients. However, this treatment is only effective in less than 20% of patients. A second-line treatment is not well-defined and may include azathioprine, cyclophosphamide, mycophenolate mofetil, cyclosporine, splenectomy, and during the last decade increasingly rituximab [4, 5, 6, 7, 8, 9, 10]. These drugs are not invariably effective, and in several cases hemolysis cannot be compensated even by the use of a combination of drugs. In addition, many treated patients develop severe side effects which result in significant morbidity and mortality. Finally, some of these drugs are not licensed for the treatment of AIHA [4, 5, 6, 7, 8, 9, 10]. Thus, the need for alternative treatment options is highly evident. Recently, we have reported on two AIHA patients who have been successfully treated with erythropoiesis-stimulating agents (ESAs) [11]. Here, we describe the effect of ESAs in 7 new patients with therapy-refractory AIHA of the warm type (WAIHA) and 5 patients with chronic AIHA of the cold type (CAIHA).
Patients and Methods
Seven of the 12 new patients (table 1) had therapy-refractory WAIHA (patients no. 1–7), and 5 had CAIHA (no. 8–12). Hemolysis was decompensated in all cases, despite intensive treatment in patients with WAIHA and standard care in patients with CAIHA (table 2). Co-existing underlying diseases such as lymphoma, other autoimmune diseases or related disorders were excluded in 10 patients. One of the patients with WAIHA (no. 3) had chronic lymphocytic leukemia (CLL), and one of those with CAIHA (no. 9) had B-cell lymphoma that was detected following the administration of ESAs. The patients were admitted to various hospitals in Germany without a unanimous form of treatment in effect for all patients. However, all patients required further treatment either due to a failure in response to conventional therapy, due to a chronic form of the disease or due to side effects as a result of the administered drugs. Dependent on the local hospital, patient's health insurance and costs, different ESAs were used.
Table 1.
Patients’ data prior to treatment with ESAs*
| Patient no. | Age, years | Sex | Interval to first admission | DAT prior to / post ESAs | Erythropoietin (4.3–29 U/l) | Diagnosis | |||
|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | C3d | ||||||
| 1* | 76 | m | 15 years | 4+ / 3+ | – / – | – / – | – / – | n.t. | WAIHA |
| 2* | 55 | f | few days | 1+ / – | – / – | – / – | 1+ / – | n.t. | WAIHA |
| 3 | 79 | f | 2 years | 4+ / 4+ | – / – | – / – | 1+ / 1+ | 16.7 | WAIHA, CLL |
| 4 | 35 | m | few days | 2+ / – | – / – | – / – | 2+ / – | n.t. | WAIHA |
| 36 | m | 1 year | – / – | – / – | 4+ / 3+ | – / – | 37.7 | WAIHA | |
| 5 | 80 | f | 17 years | 4+ / 1+ | – / – | – / – | 3+ / 1+ | n.t. | WAIHA |
| 6 | 78 | f | 2 years | 4+ / 4+ | – / – | – / – | – / – | n.t. | WAIHA |
| 7 | 52 | f | 3 months | 4+ / 2+ | – / – | – / – | – / – | 59.2 | WAIHA |
| 8 | 75 | m | > years | – / – | – / – | – / – | 4+ / 4+ | n.t. | CAIHA |
| 9 | 62 | m | > 15 years | – / – | – / – | – / – | 4+ / 4+ | 210 | CAIHA, lymphoma |
| 10 | 90 | f | many years | – / – | – / – | – / – | 4+ / 4+ | n.t. | CAIHA |
| 11 | 80 | f | > 4 years | – / – | – / – | – / – | 4+ / 4+ | 62.9 | CAIHA |
| 12 | 58 | f | > 3 years | – / – | – / – | – / – | 4+ / 4+ | 60.0 | CAIHA |
DAT = Direct antiglobulin test; CLL = chronic lymphocytic leukemia; n.t. = not tested.
Prior to treatment, histological investigations demonstrated increased erythropoiesis in all cases.
Table 2.
Effect of ES As on hemolysis in patients with WAIHA and CAIHA
| Patient no. | Treatment | Erythropoietin | Prior to /1–2 weeks after treatment with ESAs* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| previously | currently | preparation | dose (within) | Hb, g/dl | retis, % | LDH, U/l | Bili, mg/dl | last medication | |
| 1 | P, D, C, A, M, R, Bit | P+ | Aranesp® | 20× 20 µg/(4w) | 9.2/11.7 | 53/2.9 | 414/322 | 1.5/1.2 | P (7.5 mg/day) |
| 2 | P, D, C, IVIG, Blt (n = 43) | C, P | Aranesp® | 2× 500 µg/(1w) | 5.0/12.7 | 6.4/13 | 718/192 | 2.41/0.39 | C (50 mg/day) |
| 3 | P,C, Bend, Blt, IV IgG | EPO® | 4× 20,000 IU/(2w) | 11.3/12.9 | 18.7/27.3 | 248/306 | 0.8/0.8 | - | |
| 4 | P, D, C, Bit (n=38) | P, C | E-poietin® | 5× 40,000 IU/(3w) | <7.0/15.0 | 4.8/6.0 | 779/204 | 8.8/0.86 | - |
| D | P+ | E-poietin® | 1× 40,000 IU | 8.9/13.3 | n.t. | 613/283 | n.t. | P (10 mg/day) | |
| 5 | P, A, C, D, M | P+ | Silapo® | 12× 40,000 IU/(2y) | 7.9/10.5 | 3.3/13 | 392/233 | 1.1/0.32 | P (5 mg/day) |
| 6 | P, A, R, Blt | C, Blt | Silapo® | 3× 40,000 IU/(2w) | 8.9/11.5 | 11.4/4.7 | 429/351 | 2.3/0.97 | C (100 mg/day) |
| 7 | D, Blt, C, P | C, P, Blt | EPO® | 2× 20,000 U/(1w) | 8.6/12.4 | 10.8/4.5 | 611/353 | 1.24/0.37 | C (50 mg/day), P (15 mg/day) |
| 8 | P, A, Blt | P, A | EPO® | 3× 20,000 IU/(2w) | 5.2/8.4 | 7.07/7.86 | 308/310 | /2.86 | – |
| 9 | IVIgG, Blt | Silapo® | 2× 40,000 IU/(1w) | 8.5/7.3 | 6.5/8.3 | 1,294/1,396 | 1.9/2.4 | – | |
| IVIgG | Silapo® | 2× 40,000 IU/(1w) | 8.5/11.0 | S.2/3.2 | 1,396/419 | 23/1.1 | – | ||
| 10 | Blt | E-poietin® | 1× 10,000 IU | 8.6/9.2 | n.t/3.4 | n.t. | n.t. | – | |
| 11 | P, A, C, Blt | Blt | EPO® | 2× 20,000 U/(1w) | 9.0/10.6 | 3.5/5.0 | 717/359 | 2.29/2.9 | – |
| 12 | Silapo® | 2× 20,000 U/(1w) | 8.7/9.9 | 4.5/5.9 | 388/795 | 1.62/2.89 | – | ||
Bili = Total bilirubin; retis = reticulocytes; HP = haptoglobin; CR = creatinine; w = week; P = prednisolone; D = Dexamethasone; C = cyclophosphamide; A = azathioprine; M = mycophenolate moftefil; R = rituximab; Blt = red blood transfusion; Bend = bendamustin; y = year.
Haptoglobin was undetecable in all cases prior to treatment with EPO, and increased to normal value only in two cases following treatment with EPO (patients nos. 4, 5) + low dose (= 10 mg/day). Creatinine was increased only in patient no 1 (1.4 mg/dl) prior to treatment with EPO, and has been normalized (1.01 mg/dl) thereafter.
This study was approved by the institutional ethics review board (EA2/058112), and informed consent was obtained from all treated patients. Red blood cell serology was performed using standard methods [12].
Results
The history and clinical pictures of the patients were variable. Patient no. 1 was suffering from WAIHA since 15 years. During observation, hemolysis remained decompensated and the patient developed mild renal failure (creatinine increased to 1.4 mg/dl; normal value 1.1 mg/dl). Prior to treatment with ESAs, the patient underwent treatment with cyclophosphamide (100 mg/day) and prednisolone (10 mg/day). When cyclophosphamide was replaced by ESAs, hemolysis significantly improved and renal failure resolved (table 2), but direct antiglobulin test (DAT) remained positive (prior treatment 4+ and post treatment 3+).
Patient no. 2 had acute WAIHA. Erythropoietin was administered twice in an interval of 1 week, and hemoglobin levels gradually increased from 5.0 to 12.7 g/dl, as observed upon a control visit 2 months later. This beneficial effect cannot solely be attributed to cyclophosphamide administration. Initially, the patient received 700 mg by intravenous route and then per os (100 mg/day) for 4 weeks prior to treatment with ESAs. Unfortunately, serological examination was not performed on that control.
Patient no. 3 was suffering from chronic lymphocytic leukemia which was pre-treated by chemotherapy (9 cycles in 6 months). Nevertheless, hemolysis remained active and decompensated. The natural ESA level was 16.7 U/l (normal 4.9–29 U/l). Treatment with ESAs (20,000 IU/week) was started. During observation over a 2-week period, the patient's reticulocytes increased from 4.8 to 6.0%. In addition, the level of hemoglobin increased from 11.3 to 12.9 g/dl (table 2). Notably, the patient did not receive any other specific treatment during this period of time, and further administration of ESAs was not required. The positivity in DAT did not change during observation.
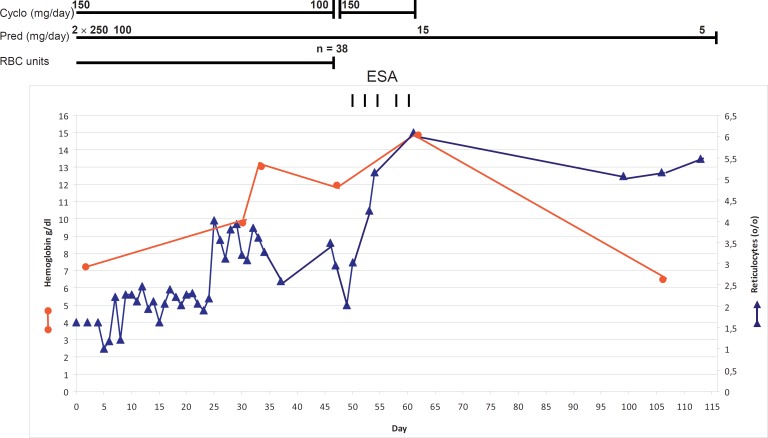
Patient no. 4 suffered from acute hemolysis that could not be compensated either by high-dose prednisolone (2× 250 mg/day) or by a combination of prednisolone and cyclophosphamide (100 mg/day and 150 mg/day, respectively). After 4 weeks of treatment, the patient developed leukopenia, and the cyclophosphamide dosage was reduced to 100 mg/day (table 2). Two weeks of intensive treatment with ESAs was commenced with the administration of 40,000 IU every 3 days (5 times). The patient abruptly recovered and DAT became negative (fig. 1). One year later, he again developed acute hemolysis which could be compensated by the administration of 40,000 IU of ESAs rather than by dexamethasone (40 mg on 4 consecutive days). Subsequently, he received 10 mg prednisolone, and hemoglobin concentration remained normal (≥13 g/dl). Interestingly, the causative autoantibody belonged to the IgA class (table 2). The DAT was strongly positive prior to treatment and remained moderately positive (3+) during observation.
Fig. 1.
Course of treatment in patient no. 4. Abrupt improvement occurred following treatment with ESA.
Patient no. 5 had therapy-refractory WAIHA since 1992. The patient continuously required treatment with prednisolone in combination with azathioprine, mycophenolate moftil, or cyclophosphamide, in addition to blood transfusion. Prior to the first administration of ESAs in March 2012, the level of hemoglobin was observed to be 7.9 g/dl, and hemolysis was active as reflected by a low haptoglobin level and an increase in lactate dehydrogenase (LDH) (373 U/l; normal <250 U/l). Hemoglobin levels increased to 10.8 g/dl in July 2012 and remained stable until December 2012 (table 3). ESAs were administered in June and October 2012, and hemoglobin level was normal (13.2 g/dl) though treatment with cyclophosphamide was discontinued. At the beginning of September 2013, hemoglobin decreased to 10.6 g/dl, which was followed by the administration of 40,000 IU of ESAs twice. Thereafter, hemoglobin was observed to increase to 11.8 g/dl without signs of hemolysis as indicated by normal values for haptoglobin, LDH, and reticulocytes.
Table 3.
Course of patient no. 5. This patient has been suffering from WAIHA since 1992, and her hemolysis could not be compensated by standard therapy (table 2), but following treatment with ES As. Prior to the latter treatment, she was receiving low-dose prednisolone and cyclophosphamide since 2005
| Date | Current therapy, mg/day | ESA, IU | Hb, g/dl | Retis, χ103/μ1 | LDH, U/l | DAT | ||
|---|---|---|---|---|---|---|---|---|
| Pred | Cyclo | IgG | C3d | |||||
| March 15, 2012 | 7.5 | 100 | – | 7.9 | 77 | 373 | 4+ | 3+ |
| March 22, 2012 to May 4, 2012 | 7.5 | 100 | 3×40,000 U/w | n.t. | n.t. | n.t. | n.t. | n.t. |
| April 26, 2012 | 7.5 | – | – | 9.8 | 84 | 290 | 3+ | 3+ |
| June 14, 2012 | 7.5 | – | 9.9 | 108 | 288 | 4+ | 3+ | |
| June 18, 2012 to June 25, 2012 | 7.5 | – | 3× 40,000 U/w | n.t. | n.t. | n.t. | n.t. | n.t. |
| December, 7, 2012 | 7.5 | – | – | 10.8 | 99 | 4+ | 3+ | |
| October 25, 2012 to December 12, 2012 | 10 | – | 3× 40,000 U | 10.5 | 99 | 277 | n.t. | n.t. |
| January 24, 2013 | 7.5 | – | – | 13.2 | 54 | 254 | 3+ | 3+ |
| March 5, 2013 | 7.5 | – | 40,000 | 11.9 | ||||
| March 14, 2012 | 7.5 | – | – | 12.3 | ||||
| April 30, 2013 | 5 | – | – | 12.3 | 40 | 254 | 3+ | 1+ |
| September 2, 2013 | 5 | 40,000 | 10.6 | 33 | 2+ | 2+ | ||
| September 18, 2013 | 5 | 40,000 | 11.8 | 49 | 252 | 1+ | 1+ | |
Pred = Prednisolone; Cyclo = cyclophosphamide; Hb = hemoglobin; Retis = reticulocytes; LDH = lactate dehydrogenase; DAT = direct antiglobulin test.
Patient no. 6 had active WAIHA, and treatment with prednisolone resulted in the development of diabetes mellitus. In addition, the patient's hemolysis was refractory to a previous treatment with azathioprine and rituximab. The attempt to quickly compensate hemolysis only by the administration of ESAs was initially abortive. One week after the first dose of ESAs (40,000 IU), hemolysis appeared to continue, and a blood transfusion was indicated. The patient received two RBC units and treatment with cyclophosphamide was commenced. Within 2 weeks, the patient's condition significantly improved, and hemoglobin levels were observed to increase from 8.5 g/dl (post transfusion) to 11.7 g/dl after treatment with ESAs and cyclophosphamide. Based on our experience, the abrupt beneficial effect of treatment in this case might be explained by the combination of both drugs. During observation, the hemolysis remained stable under treatment with cyclophosphamide, though the positivity in DAT did not change.
Patient no. 7 had WAIHA that was refractory to treatment with dexamethasone (4× 40 mg/day), prednisolone (50 mg/day) and cyclophosphamide (150 mg/day for more than 2 weeks). Since blood transfusions were repeatedly required and hemolysis appeared to continue, we decided to add ESAs in the treatment. In fact, hemoglobin was observed to increase despite a continuous dose-reduced regimen of prednisolone and cyclophosphamide (table 2). Upon control 2 months later, the hemoglobin concentration was normal (13.4 g/dl). On the latter control, all relevant laboratory parameter which include LDH, haptoglobin, bilirubin, and reticulocytes were normal, and DAT was moderately positive (2+).
Patient no. 8 had a chronic CAIHA which decompensated following a bacterial infection. Initially, the patient was treated with prednisolone (100 mg/day) and RBC transfusion under warm conditions. Though the patient was kept warm during hospitalization, anemia could not be successfully improved. Therefore treatment with ESAs was undertaken. Following the first administration of 20,000 IU, the patient's hemoglobin increased from 6 to 9.0 g/dl. Following a period of 3 days, the patient received an additional 20,000 IU without complication and was discharged from hospital with a hemoglobin concentration of 9.2 g/dl.
Patient no. 9 was suffering from CAIHA since 1994. In October 2013, the hemolysis rapidly deteriorated despite strict avoidance of cold expositions. Treatment with ESAs was initiated. Due to an ongoing exacerbation of the hemolysis, the patient received two units of RBCs following a period of 3 days and intravenous IgG (IVIgG) twice (2× 20 g in 4 weeks). However, hemolysis improved during further treatment with ESAs. The question why hemolysis initially deteriorated could not be answered. Subsequent histological investigation of the bone marrow (BM) revealed an increased erythropoiesis and B-cell infiltration of the BM, indicating the development of B-cell lymphoma which could not be characterized. Unexpectedly, 1 month later hemolysis further improved, and the hemoglobin concentration increased to 14.0 g/dl.
Patient no. 10 had been suffering from CAIHA since many years and frequently required RBC transfusion (2 units / 1–2 weeks). The patient received ESAs once, and hemoglobin increased from 8.6 to 9.2 g/dl. The hemolysis was observed to significantly improve as was reflected by the frequency of blood transfusion (1–2 units / 2–3 weeks). Unfortunately, treatment with ESAs was not continued, and further treatment was solely based on blood transfusions.
Patient no. 11 was suffering from severe CAIHA that repeatedly decompensated every winter despite strict avoidance of any exposure to the cold. After the last hemolysis attack, the patient received a blood transfusion, and hemoglobin levels increased from 6.5 to 9.0 g/dl. Following the administration of ESAs on two occasions (2× 20,000 U) within 2 weeks, the patient felt well and the hemoglobin level increased to a ‘record’ high within the last 5 years (table 2).
Patient no. 12 was suffering from CAIHA similar to that of patient no. 11. Following the administration of ESAs, hemoglobin appeared to slightly increase (table 2) as measured by LDH (388 vs. 795 U/l). In addition, the patient observed a mild lumbar pain which was associated with the treatment of ESAs. It is worth noting, that the patient had been suffering from a bad cold which could have influenced the course of hemolysis during this phase of treatment.
Discussion
Many patients with WAIHA require long-term treatment with immunosuppressive drugs which are often ineffective or are associated with severe side effects. Several patients may also require highly toxic drugs with or without autologous hematopoietic stem cell rescue that may result in a significantly increased mortality risk [6, 7, 8, 9, 10]. Unlike WAIHA, treatment of CAIHA is largely dependent on strict avoidance of cold expositions. In these patients, the administration of drugs remains questionable, and blood transfusions are indicated in decompensated cases [2, 8, 10].
Recombinant ESAs are used for the treatment of anemia in patients with various underlying diseases [13], but has not yet been extensively investigated in patients with AIHA. The reason behind why this agent has not been used in the treatment of AIHA might be due to the fact that erythropoiesis in these patients is usually increased, leading to the assumption that in such cases ESAs are not indicated. However, some patients with AIHA may develop reticulocytopenia or even hypoplasia of the BM [1, 3]. It is unknown whether ESAs might be reduced in these patients.
Recently, we successfully treated 2 AIHA patients with ESAs and hypothesized that most AIHA patients may respond to this treatment [11]. This is clearly supported by the results obtained in the present study although it is limited by several drawbacks including the retrospective character, the heterogeneity of patients, the difference between treating physicians, the lack of a study protocol, and the lack of systematic measurement of natural ESA levels in treated patients. Nevertheless, all 12 treated patients were found to respond well, and none were observed to develop significant adverse reactions. Only in patient no. 12 a mild lumbar pain was observed, which appeared to be associated with ESA administration. This might be related to the increased erythrocytopoiesis in the BM rather than to the hemolysis per se. Most intriguingly, treatment with ESAs may not only compensate hemolysis but may also result in complete recovery in a few patients with WAIHA (table 2, patient's no. 4 and 5). In addition, ESA treatment may also be helpful in the management of CAIHA as demonstrated in patients no. 7–12. All these patients had CAIHA and histories of hemolytic decompensations in previous winters, leading to blood transfusions in some cases. Similarly, their anemia was significant and could be improved by treatment with ESAs. This finding is highly evident and may help to amend the management of severely affected patients, at least during periods associated with decompensation hemolysis (cold seasons).
Our results are supported by two abstracts which were identified during the preparation of this article. The first abstract described 2 adult patients who had therapy-refractory WAIHA [14]. One of these patients appeared to have entered into complete remission following treatment with ESAs (initially 10,000 IU 3 days a week, which was followed by chloramucil plus ESAs). The second patient had AIHA associated with a T-lymphocyte clonal proliferation. This patient received 40,000 U ESAs per week, and hemoglobin increased to 11.1 g/dl 1 month after the commencement of treatment with ESAs [14]. In the other abstract the case of a HIV-infected child with AIHA was described in whom AIHA failed to response to steroid treatment and IVIgG, and required blood transfusion on a daily basis until reticulocyte counts increased following ESA and cyclosporine therapy [15].
It could be largely excluded that the response described in our AIHA patients is attributed to a delayed response to previous treatment. The majority of the patients (table 1, patients no.1, 3, 5, 6, 8, 9, and 11) had chronic AIHA for long periods of time (2–17 years), and none of the used drugs or drug combinations resulted in a significant improvement of hemolysis/anemia as it has been observed following the administration of ESAs. The second question whether ESAs could be used alone in patients with WAIHA, likewise thrombopoietin (TPO) in immune thrombocytopenic purpura (ITP), remains to be answered by treatment of selected patients with stable hemolysis.
The mechanism responsible for the remarkable effect of ESAs in AIHA patients remains speculative. There are various possible explanations. Although erythropoiesis is usually increased in patients with AIHA, natural ESAs might be diminished, at least in some patients. Until now, ESA concentrations have only been measured in a few patients with AIHA; thus no conclusions can be drawn from these results [16]. The notion that ESAs might be rather decreased in AIHA is supported by findings in ITP patients where TPO has been observed to be decreased rather than increased [17]. Several studies have demonstrated that TPO receptor agonists (romiplostim and eltrombopag) are highly effective in the treatment of ITP [18, 19], which is quite similar to AIHA in many aspects such as production of autoantibodies and cell destruction. Similarly, granulocyte colony-stimulating factor (G-CSF) has also observed to be highly effective in the treatment of autoimmune neutropenia [20, 21]. Thus, the effectivity of ESAs in the treatment of AIHA was not surprising. Unfortunately, the natural level of ESAs was only measured in 5 patients prior to treatment with ESAs. It was found to be increased in 4 patients (no. 7, 9, 11, and 12), and normal in 1 patient with secondary CAIHA (no. 3). Based on these findings, high levels of natural ESAs do not exclude a beneficial effect of exogenous ESAs.
A second explanation might be related to the decrease in the total amount of autoantibodies present on each RBC due to an increase in the total number of RBCs following ESA treatment [11].
A third possibility may be the direct effect of ESAs on RBCs. It has been demonstrated that ESAs binds to RBCs, thereby prolonging their life span [22]. Thus, it may contribute to an enhancement in RBC numbers during ESA therapy in dialysis patients.
Finally, RBCs have been shown to undergo suicidal death (eryptosis) resulting in their engulfment by macrophages [23, 24, 25]. Eryptosis can be stimulated by several endogenous and exogenous mediators, and erythropoietin is a potent inhibitor of eryptosis [26]. Although eryptosis has not yet been described in AIHA, there is evidence that RBCs are, in part, eliminated from the circulation by eryptosis and not by hemolysis or Fc/C3b-mediated phagocytosis. At the acute phase of any hemolytic attack, the blood picture of affected patients is frequently characterized by anisocytosis, microcytosis, and spherocytosis. All these defective cells may undergo eryptosis as has been observed in various types of hemolysis including iron deficiency, sickle cell disease, thalassemia, hereditary spherocytosis, glucose-6-phosphate dehydrogenase deficiency, malaria, and other diseases [26]. This is also supported by the fact that oxidative stress may induce eryptosis [26, 27] and thereby AIHA [27].
Whatever the mechanism may be which triggers the described beneficial effects of ESAs in AIHA treatment, its use in these patients is well justified due to the fact that it is less toxic than current drugs which are still applied in this field. In addition, treatment with ESAs could, in total, be more cost-effective than other treatment regimens, including RBC transfusions, in therapy-refractory cases. Most importantly, further studies are required in order to determine the optimal doses required for therapy and to clarify the mechanisms leading to this effect, including antibody class and concentration, complement activation, treatment, underlying diseases, the concentration of natural ESAs, and the extent of eryptosis prior to and after treatment with ESAs.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Dacie J. Auto-immune haemolytic anaemia (AIHA): warm antibody syndromes II: ‘idiopathic’ types: haematological and biochemical findings. In: Dacie J, editor. The Haemolytic Anaemias. ed 3. Vol. 3. New York: Churchill Livingstone; 1992. pp. 54–93. [Google Scholar]
- 2.Salama A, Ahrens N, Kiesewetter H. Serological and clinical aspects of autoimmune hemolytic anemias. Infus Ther Transfus Med. 2002;29:206–217. [Google Scholar]
- 3.Petz LD, Garratty G. Immune Hemolytic Anemias. 2nd ed. Philadelphia: Churchill Livingstone; 2004. [Google Scholar]
- 4.Allgood JW, Chaplin H., Jr Idiopathic acquired autoimmune hemolytic anemia. A review of fortyseven cases treated from 1955 through 1965. Am J Med. 1967;43:254–273. doi: 10.1016/0002-9343(67)90168-4. [DOI] [PubMed] [Google Scholar]
- 5.Murphy S, LoBuglio AF. Drug therapy of autoimmune hemolytic anemia. Semin Hematol. 1976;13:323–334. [PubMed] [Google Scholar]
- 6.King KE. Review: pharmacologic treatment of warm autoimmune hemolytic anemia. Immunohematology. 2007;23:120–129. [PubMed] [Google Scholar]
- 7.Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008;22:17–31. doi: 10.1016/j.blre.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 9.Crowther M, Chan YL, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036–4040. doi: 10.1182/blood-2011-05-347708. [DOI] [PubMed] [Google Scholar]
- 10.Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- 11.Arbach O, Funck R, Seibt F, Salama A. Erythropoietin may improve anemia in patients with autoimmune hemolytic anemia associated with reticulocytopenia. Transfus Med Hemother. 2012;39:221–223. doi: 10.1159/000339260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer B, Yürek S, Kiesewetter H, Salama A. Mixed-type autoimmune hemolytic anemia: differential diagnosis and a critical review of reported cases. Transfusion. 2008;48:2229–2234. doi: 10.1111/j.1537-2995.2008.01805.x. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair AM. Erythropoiesis stimulating agents: approaches to modulate activity. Biologics. 2013;7:161–174. doi: 10.2147/BTT.S45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández JJ. Two cases of refractory autoimmune hemolytic anemia (AIHA) treated with Epoetin alpha (EPO) Haematologica. 2007;92(suppl 2):533. abstract 1500. [Google Scholar]
- 15.Chadwick EG, DiMichele D, Yogev R. Characteristics of autoimmune hemolytic anemia (AIHA) in HIV-infected children. 1995. The Second National Conference on Human Retroviruses and Related Infections, Washington, D.C., January.
- 16.Roque ME, Sandoval MJ, Aggio MC. Serum erythropoietin and its relation with soluble transferrin 26 receptor in patients with different types of anaemia in a locally defined reference population. Clin Lab Haematol. 2001;23:291–295. doi: 10.1046/j.1365-2257.2001.00413.x. 27. [DOI] [PubMed] [Google Scholar]
- 17.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 18.Stasi R, Bosworth J, Rhodes E, Shannon MS, Willis F, Gordon-Smith EC. Thrombopoietic agents. Blood Rev. 2010;24:179–190. doi: 10.1016/j.blre.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Hallam S, Provan D, Newland AC. Immune thrombocytopenia – what are the new treatment options? Expert Opin Biol Ther. 2013;13:1173–1185. doi: 10.1517/14712598.2013.801451. [DOI] [PubMed] [Google Scholar]
- 20.Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther. 2005;7:208–214. doi: 10.1186/ar1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhtari M, Curtis B, Waller EK. Autoimmune neutropenia in adults. Autoimmun Rev. 2009;9:62–66. doi: 10.1016/j.autrev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Myssina S, Huber SM, Birka C, Lang PA, Lang KS, Friedrich B, Risler T, Wieder T, Lang F. Inhibition of erythrocyte cation channels by erythropoietin. J Am Soc Nephrol. 2003;14:2750–2757. doi: 10.1097/01.asn.0000093253.42641.c1. [DOI] [PubMed] [Google Scholar]
- 23.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 25.Kempe DS, Lang PA, Duranton C, Akel A, Lang KS, Huber SM, Wieder T, Lang F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006;20:368–370. doi: 10.1096/fj.05-4872fje. [DOI] [PubMed] [Google Scholar]
- 26.Lang E, Qadri SM, Lang F. Killing me softly – suicidal erythrocyte death. Int J Biochem Cell Biol. 2012;44:1236–1243. doi: 10.1016/j.biocel.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Iuchi Y, Kibe N, Tsunoda S, Suzuki S, Mikami T, Okada F, Uchida K, Fujii J. Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radic Biol Med. 2010;48:935–944. doi: 10.1016/j.freeradbiomed.2010.01.012. [DOI] [PubMed] [Google Scholar]