Summary
Red blood cell (RBC) alloimmunization may occur following transfusion or pregnancy/delivery. Although observational human studies have described the immunogenicity of RBC antigens and the clinical significance of RBC alloantibodies, studies of factors influencing RBC alloimmunization in humans are inherently limited by the large number of independent variables involved. This manuscript reviews data generated in murine models that utilize transgenic donor mice, which express RBC-specific model or authentic human blood group antigens. Transfusion of RBCs from such donors into nontransgenic but otherwise genetically identical recipient mice allows for the investigation of individual donor or recipient-specific variables that may impact RBC alloimmunization. Potential donor-related variables include methods of blood product collection, processing and storage, donor-specific characteristics, RBC antigen-specific factors, and others. Potential recipient-related variables include genetic factors (MHC/HLA type and polymorphisms of immunoregulatory genes), immune activation status, phenotype of regulatory immune cell subsets, immune cell functional characteristics, prior antigen exposures, and others. Although murine models are not perfect surrogates for human biology, these models generate phenomenological and mechanistic hypotheses of RBC alloimmunization and lay the groundwork for follow-up human studies. Long-term goals include improving transfusion safety and minimizing the morbidity/mortality associated with RBC alloimmunization.
Keywords: RBC, Alloimmunization, Transfusion, Murine models
Introduction
Red blood cell (RBC) alloantibodies can develop after exposure to foreign RBC antigens in the context of transfusion therapy or pregnancy/delivery. Hemolytic transfusion reactions due to non-ABO antibodies have been the 2nd or 3rd leading cause of transfusion-associated death reported to the FDA over the last 5 years [1, 2, 3], with non-US countries also reporting a number of adverse events resulting from alloantibodies [4]. In addition to mortality, RBC alloantibodies may lead to morbidity in the forms of hemolytic transfusion reactions, bystander hemolysis, and renal failure. Patients with multiple RBC alloantibodies or antibodies against high-incidence antigens may experience complications of anemia due to lengthy delays prior to the location of compatible RBC units for transfusion; some may even die if compatible RBCs cannot be located. Finally, in addition to being detrimental in a transfusion setting, RBC alloantibodies may also be detrimental to developing fetuses [5].
Much effort has been dedicated over the past century to describing the structure and function(s) of human blood group antigens [6]. There have been significant strides made in understanding the relative immunogenicity of these antigens in transfusion and pregnancy situations, the impact of cognate antigen/alloantibody interactions, and the patterns of evanescence of alloantibodies against individual alloantigens [7, 8, 9]. As more information about antibody evanescence patterns emerges, it becomes clear that a larger number of patients than previously appreciated are likely alloimmunized, with many antibodies falling over time below the level of detection by conventional blood bank methodologies.
As data has been gathered and knowledge in the field of transfusion medicine has evolved, interest in responder/non-responder patient populations has grown [10]. The percentage of transfused patients who become alloimmunized varies by study, study design, and patient population, with numbers ranging from 5–50% [11, 12]. It is thought that certain patients are responders and make RBC alloantibodies in response to multiple transfusions; such patients were defined by Higgins and Sloan [13] using stochastic modeling. It is also thought that disease status may impact RBC alloimmunization. For example, patients with sickle cell disease are known to have high rates of RBC alloimmunization [14]; however, other factors (including phenotypic/genotypic differences between donor and recipient) must also be taken into consideration in interpreting these data [15]. Recently, GWAS studies have begun to investigate immunogenetics of responder/non-responder patients, with a goal of predicting responder patients prior to RBC exposure and enabling personalized transfusion therapy based on these profiles.
Although human studies are clearly necessary to reveal factors contributing to responder/non-responder status, there are many variables that have the potential to confound the interpretation of data generated by such studies. These variables include the number of antigenic differences between donor and recipient during each transfusion event, the HLA differences in recipients (some RBC antigens are thought to be HLA-restricted) [16, 17, 18], the broader genetic differences between recipients other than HLA, epigenetic variables (e.g. the microbiome), donor differences in RBC storage, and the health status of the recipient at the time of the transfusion; few transfusions are given to ‘healthy’ individuals. RBC collection and processing methodologies, which are not fully standardized between collection centers or between countries, could also impact recipient immune responses to RBC antigens.
Logistical issues have prevented in-depth studies of RBC antigen consumption, antigen processing/presentation, and localization of B-cell responses in humans. However, general humoral immune responses to transfused human RBCs are typically thought to be T-cell dependent, with IgG responses predominating over IgM responses soon after antigen exposure [19]. The antigen presenting cells usually described to consume RBCs are macrophages [20], though RBC consumption by dendritic cells also occurs. As described further within this review, factors on both the donor and recipient sides presumably impact not only rates of initial antigen consumption by antigen-presenting cells but also co-stimulatory/co-inhibitory signals present at the time of antigen presentation. Any of these factors may impact T-cell receptor responses to the presented antigen and, ultimately, B-cell stimulation.
Differences between murine and human immunobiology notwithstanding, the fundamental underpinnings of human immunology were essentially all discovered from using mice and other animal systems [21]. Thus, there are considerable benefits to studying RBC alloimmunization in reductionist animal systems. In recognition of the contribution of these reductionist systems to the current understanding of immune responses to RBCs, this review is dedicated to discussing factors that influence RBC alloimmunization in murine models. Murine models of RBC alloimmunization developed over the past few decades have generally utilized either model antigens (such as hen egg lysozyme; HEL) [22], or authentic human blood group antigens (such as KEL2) [23], expressed on murine RBCs. These models allow for analysis of single blood group antigenic differences between donor and recipient in otherwise genetically identical recipients – thus controlling for the above mentioned genetic variability intrinsic to human populations; such systems are powerful tools to investigate specific donor or recipient factors that influence RBC alloimmunization. The systems also allow for investigation of factors influencing maternal RBC alloimmunization during pregnancy, a topic that will only briefly be discussed in this review. A number of factors known to influence RBC alloimmunization identified in murine models are in the process of being investigated in humans. It is the hope that such bench to bedside and back approaches will benefit patient care by leading to strategies to mitigate the morbidity and mortality associated with RBC alloimmunization.
Donor- or Product-Specific Factors
Historically, the focus of donor studies has largely centered on issues of infectious disease and quality control. Though some studies have investigated associations between product characteristic and general recipient ‘immunomodulation’, few have addressed hypotheses that donor-specific, or product-specific variables correlated with or affected recipient RBC alloimmune responses. Population-based RBC alloimmunization studies are difficult to design and execute in humans, given the myriad of potential contributing variables to consider on both the donor and recipient sides of the equation. The first portion of this review will focus on what has been learned from murine studies regarding the impact of donoror product-specific variables on RBC alloimmunization, with potential variables and unanswered questions to be considered further outlined in table 1.
Table 1.
Donor- or product-specific variables that may impact RBC alloimmunization
Product collection/processing
|
Storage considerations
|
RBC clearance rates after transfusion
|
RBC antigen specific factors
|
Product Collection/Processing
The ‘non-RBC’ contents of transfused products may be important variables to consider when evaluating recipient immune responses to RBC antigens: white blood cells (WBCs) and their remnants, platelets and their remnants, and soluble factors including cytokines are co-transfused along with RBCs in each unit of ‘RBCs’. There is much variability among blood collection centers in their methodologies of blood collection and processing, including the use of different anti-coagulant preservative solutions and amounts of residual plasma in units, lengths of holding time prior to processing, leukoreduction filters/techniques, and centrifugation speeds.
Any of these variables may potentially impact recipient immune response to transfused RBC antigens, though human studies investigating the effects of such variables on outcome measures are logistically difficult. One study has demonstrated that human blood held overnight prior to leukoreduction contains more proinflammatory cytokines and microparticles than blood collected by apheresis or blood processed soon after collection [24]; thus it would not be illogical to predict that such variables may affect immune responses. Microparticles are cellular fragments derived from RBCs, leukocytes, platelets, and other cells that are known to exist in stored blood components [24, 25]. Emerging evidence indicates that these vesicles are capable of inducing a proinflammatory response, driving T-cell proliferation [26]. Whether they contribute to RBC alloimmune responses is not known, but this possibility warrants investigation. Other studies have shown that an overnight holds of human blood prior to processing may decrease post-transfusion RBC recovery [27], or may decrease leukoreduction efficiency [28]. Yet others have shown variation in residual platelet numbers and platelet-derived cytokines in human RBC units, depending on the leukoreduction filter used [29].
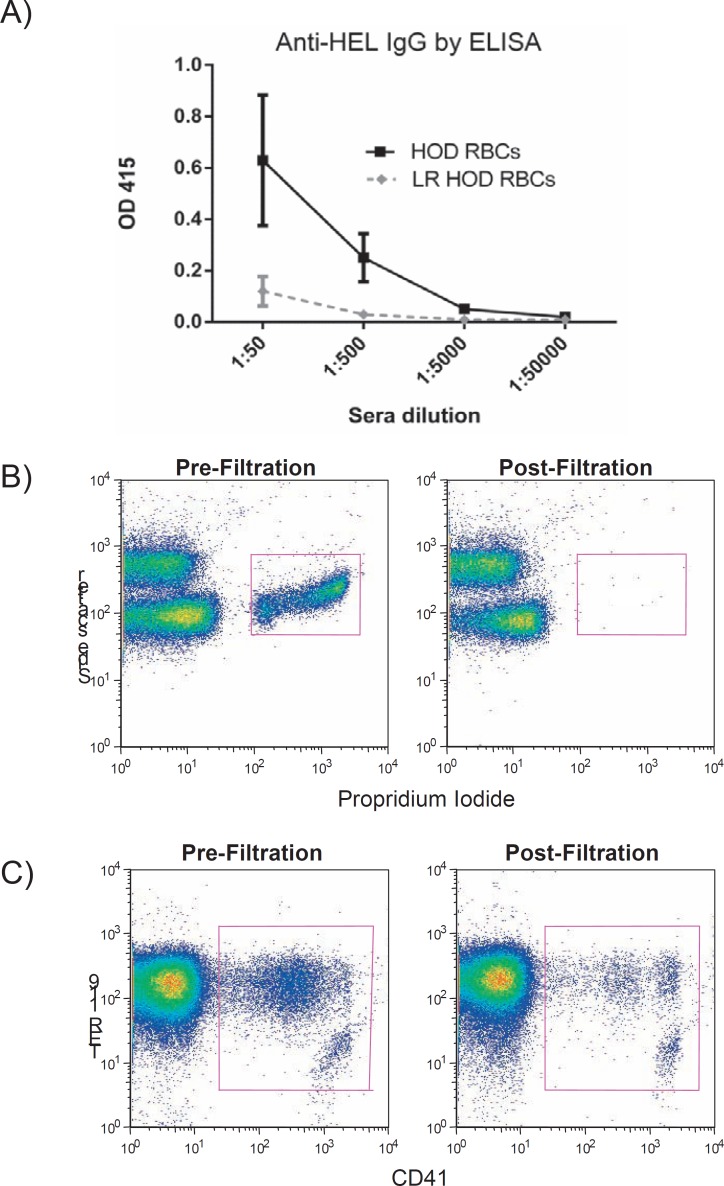
Much attention has been paid to the role of contaminating WBCs in human RBC units, though this focus has been due to an interest in decreasing febrile transfusion reactions, infectious disease transmission, and HLA alloimmunization [30]. Controversy exists in the literature regarding whether contaminating WBCs impact RBC alloimmunization in humans, with some studies suggesting leukoreduced RBCs are less immunogenic than non-leukoreduced RBCs [31, 32, 33] and others suggesting that WBCs may not influence RBC alloimmunization [34, 35]. Reductionist murine studies have been completed in the HOD system, in which donor RBCs express a fusion protein containing hen egg lysozyme (HEL), ovalbumin, and the human Duffy(b) antigen. These studies have shown significantly decreased immune responses in recipient C57BL/6 mice transfused with donor HOD RBCs on an FVB (Friend Virus B) genetic background that have been passed over a Pall neonatal leukoreduction filter, as compared to mice transfused with non-leukoreduced RBCs [36]. Figure 1A shows anti-HEL IgG responses detected by ELISA in one of six representative experiments with 5 animals/group, two weeks after transfusion (p < 0.05 between groups in 5/6 experiments). Murine blood is efficiently leukoreduced using Pall neonatal leukoreduction filters [22, 37], with propridium iodide staining showing very few nucleated cells remaining after leukoreduction (fig. 1B). These filters also decrease the number of murine platelets in the RBC units (fig. 1C), but not as efficiently as they decrease human platelets in RBC units. Although these data demonstrate that HOD.FVB RBCs passed over a Pall neonatal leukoreduction filter are less immunogenic than non-filtered RBCs, it is not yet clear whether this decreased immunogenicity is due to a decrease in WBCs, in platelets, or in other variables. Furthermore, it is not yet known whether these findings will be observed in other RBC antigen systems, or in other donor/recipient strains.
Fig. 1.
Transgenic HOD RBCs on an FVB background were leukoreduced using a Pall neonatal leukoreduction filter, with the equivalent of 1 human ‘unit’ of RBCs transfused into C57BL/6 recipients.
A Anti-HEL responses were measured in sera 2 weeks post-transfusion. B Nucleated cells were evaluated pre and post-filtration, using propridium iodide staining. C Platelets were evaluated pre and post-filtration, using CD41 staining (and trucount beads).
Storage Considerations
Over the past four decades, there has been a waxing and waning interest in the RBC ‘storage lesion’ and its impact on recipient health. Although beyond the scope of this review, RBC storage characteristics may impact many recipient outcomes other than alloantibodies. Until fairly recently, studies in animal models of RBC storage were limited by technical abilities to preserve RBC integrity during storage. However, the use of CPDA-1 as an anticoagulant-preservative storage solution has now been shown to allow for leukoreduced murine RBCs on a C57BL/6 background to be stored for 2 weeks, with an approximately 75% post-transfusion recovery [38].
Building upon this model of murine RBC storage, leukoreduced murine HOD RBCs on a FVB background stored for 2 weeks were shown to be significantly more immunogenic than freshly collected leukoreduced RBCs [39]. This increase in immunogenicity was not due to obvious changes in antigen expression or integrity, as determined by flow cytometry. Unlike the 75% post-transfusion recovery reported on stored RBCs on a C57BL/6 background, however, HOD.FVB RBCs stored for 2 weeks had post-transfusion recovery rates closer to 30–40% [39]. Recent studies have highlighted strain-specific differences in storage characteristics, with RBCs from mice on an FVB background having inferior storage compared to RBCs from mice on a C57BL/6 background. Metabolomics studies juxtaposing these two strains of mice have identified differences in lipid peroxidation, natural anti-oxidants, and cytidine levels [40]. Other human studies have shown differences in RBC storage characteristics by donor gender, with RBCs from female donors exhibiting less mechanical fragility than those from male donors [41]; murine studies investigating female versus male RBC storage characteristics are ongoing.
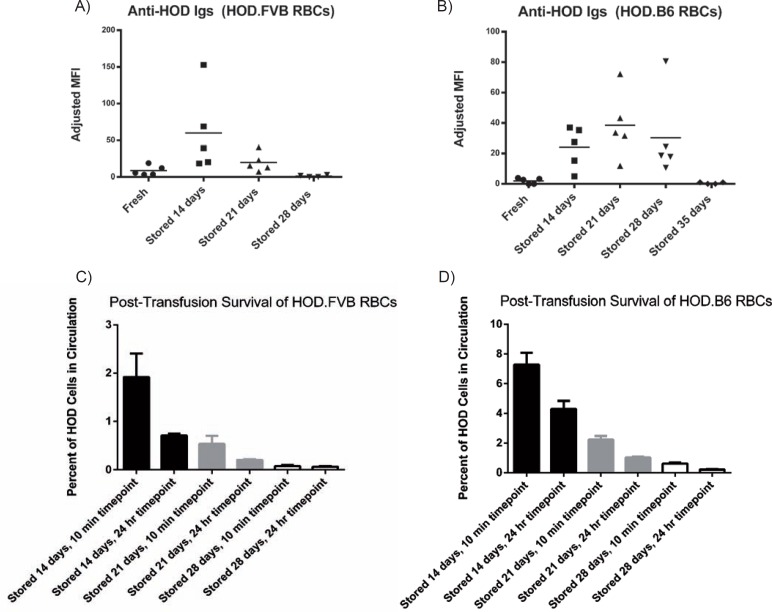
Backcrossing of the HOD mouse (which was generated on an FVB background) onto a C57BL/6 background allowed for evaluation of the impact of donor strain on alloimmunogenicity. Freshly collected, leukoreduced RBCs from HOD.FVB donors result in slightly higher degrees of anti-HOD alloantibodies upon transfusion into C57BL/6 recipients than do freshly collected, leukoreduced RBCs from HOD.B6 donors transfused into C57BL/6 recipients. Over the storage duration, however, differences in immunogenicity between HOD. FVB and HOD.B6 RBCs become more apparent. HOD.FVB RBCs have a peak of immunogenicity after approximately 10–14 days of storage (fig. 2A), compared to a peak noted around 21 days of storage in HOD.B6 animals (fig. 2B). These differences in peaks of immunogenicity correlate with posttransfusion recovery rates (fig. 2C, D), with decreases in immunogenicity noted once few intact RBCs are recovered posttransfusion; three out of three experiments had similar result (one representative experiment is shown). These observations laid the groundwork for clearance studies investigating the impact of post-transfusion recovery on recipient alloimmune responses, detailed below.
Fig. 2.
Blood from transgenic HOD.FVB or HOD.B6 animals was leukoreduced and stored for 28–35 days. A, B The equivalent of 1 human ‘unit’ was transfused into C57BL/6 mice, with recipient anti-HOD Ig immune responses measured by flow cytometric cross-match 14 days post-transfusion. C, D Post-transfusion RBC survival and recovery studies were completed, using monoclonal antibodies against Fy3 to track the transfused HOD RBCs.
Human studies have not noted an association between the duration of RBC storage and recipient alloimmune responses [42, 43, 44], although one recent study has shown a correlation between storage time and in vitro phagocytosis [45]. Potentially important considerations in the interpretation of these studies, however, include the definition of an ‘older’ RBC unit as well as whether the recipients received fresh RBCs in combination with older RBCs. Murine studies in the HOD.FVB system have shown that a fresh HOD.FVB unit is able to abrogate the enhanced alloimmunogenicity of a stored HOD. FVB unit [46]. The mechanism(s) behind this observation are not clear, but these data highlight potentially important biology. An additional variable that warrants investigation in storage/alloimmunization studies is the nature of the RBC antigen itself.
MicroRNAs and Damage-Associated Molecular Patterns
There is an emerging body of literature, largely consisting of in vitro studies of human-derived blood components including RBCs and platelets that suggests that microRNAs (miRNAs), small noncoding RNA molecules involved in regulating gene/protein expression through multiple mechanisms, are produced in varying quantities and with varying kinetics during storage of blood components [47, 48, 49, 50]. More and more evidence suggests that miRNAs may be involved in regulating immune responses, specifically by influencing T helper cell differentiation [51]; their potential role in influencing RBC alloimmune responses is an area of interest. Similarly, cellular injury incurred during the collection, processing, and storage of blood components likely results in the release of inflammatory cellular components, namely mitochondrial DNA and formyl peptides, termed damage-associated molecular patterns (DAMPs) [52, 53]. Some groups have implicated these DAMPs as being involved in transfusion-related acute lung injury (TRALI) reactions, though there is ongoing debate regarding this association [52, 54]. The role of DAMPs in inducing inflammation is well accepted [53], and their role in influencing RBC alloimmune responses is also an area of interest.
Clearance Rates of RBCs
Clearance rates of transfused RBCs and length of exposure to transfused RBC antigens are variables that likely influence recipient immune responses. These clearance rates may be impacted by donor- or recipient-specific variables. One study, for example, has shown that malaria infection impacts RBC clearance rates [55]. Murine studies have been completed in which RBCs were damaged with oxidative stress (phenylhydrazine) or with heat prior to transfusion. Neither of these forms of damage obviously altered the HOD antigen expression, yet both treatments simultaneously increased the rate of HOD.FVB RBC clearance and the magnitude of recipient anti-HOD alloantibody responses [56]. Similar to what was observed after HOD RBCs were stored for lengthy intervals, extreme amounts of RBC damage using phenylhydrazine or heat (in which RBCs were instantly cleared following transfusion) resulted in very low recipient alloantibody responses. These studies demonstrate that RBC clearance rates impact recipient alloimmune responses to at least one model RBC antigen and raise the question of whether clearance rates, due to intrinsic properties of the RBCs themselves or due to recipient factors, also contribute to alloimmunization to other RBC antigens.
Antigen-Specific Factors
Structural differences among human RBC antigens have been appreciated for many years [6]. Antigenic structural complexity has contributed, at least in part, to difficulties in generating ‘one bead, one antigen’ screening methodologies for RBC alloantibodies [57, 58]. Without question, the immunogenicity of RBC antigens is in part dependent on their structural characteristics, including the degree to which recipients recognize an antigen as foreign. Rh(D), for example, is one of the more immunogenic RBC antigens. This is partially a result of Rh(D)-positive donors expressing an entire gene product and recipients lacking it. Further, the size of the Rh(D) antigen is such that most recipients are capable of presenting a portion of the foreign antigen on their HLA molecules [59]. Conversely, antithetic antigens that differ by a single amino acid polymorphism from donor to recipient (which is true for most antigens other than RhD), may be less immunogenic than RhD due to either an inability of the recipient to present a portion of the antigen on their HLA/MHC (discussed in more detail later in this paper) or due to other factors.
As more transgenic murine models have been developed, differences in immunogenicity based on antigen structure/type are becoming apparent. For example, recipient immune responses to transfused leukoreduced mHEL RBCs are significantly lower in magnitude than responses to transfused HOD RBCs, despite the humoral response being anti-HEL in both instances [60]. It is hypothesized that these differences in the magnitude of the anti-HEL alloantibody response may be due in part to the inclusion of a portion of the OVA antigen in the HOD construct, which is able to elicit additional recipient CD4+ T-cell help [37]. Described in greater detail by Desmarets et al. [37], the HOD triple fusion protein was generated using the entire open reading frame of HEL, the portion of the OVA open reading frame encoding amino acids 251–349, and the entire open reading frame of the human Duffyb RBC antigen.
One additional consideration is that the density of the HEL antigen on mHEL versus HOD RBCs may also be a factor in the differences in recipient responses, with mHEL RBCs [22] having lower levels of HEL expression than HOD RBCs. RBC copy number on transfused RBCs likely impacts recipient immune responses in other antigen systems, as evidenced by the differences in immune responses to weak Rh(D) or Rh(D) RBCs in humans. For example, Rh(D)-negative recipients transfused with RBCs from weak Rh(D) donors have low rates of anti-D formation compared to those transfused with RBCs from Rh(D) donors [61]. Similar findings have been reported in abstract format in the murine KEL2 system: recipients transfused with RBCs from ‘weak’ KEL2 donors fail to make anti-KEL glycoprotein alloantibodies, but essentially all recipients transfused with RBCs from KEL2 donors with moderate levels of antigen expression form anti-KEL glycoprotein alloantibodies [62].
RBC antigen characteristics not only influence the development of recipient alloantibodies, they also can at least partially determine the clinical significance of RBC-specific alloantibodies. For example, anti-HEL alloantibodies are fairly clinically insignificant, due in part to antigen down-modulation that is known to occur following engagement of the anti-HEL alloantibody with the HEL antigen [63, 64, 65]. In contrast, monoclonal antibodies against the hGPA antigen are clinically significant, in that they lead to hemolytic transfusion reactions [66, 67] through a complement- and Fcy receptor-independent process [68, 69]. Polyclonal antibodies against the KEL2 antigen are clinically significant in both transfusion and pregnancy scenarios: hemolytic transfusion reactions are mediated by both complement and Fcy receptors [70], while hemolytic disease of the fetus and newborn appears to be due at least in part to suppression of erythropoiesis by anti-KEL glycoprotein alloantibodies [71].
Recipient Factors
The human responder/non-responder literature suggests that recipient factors, be they genetic or non-genetic, are quite critical in determining alloantibody development [10, 13]. Within a given population that is predisposed to respond, however, donor factors may play key roles in determining alloantibody responses. Indeed, studies in murine models support that both donor and recipient factors play a role in recipient RBC alloimmune responses. Genetically identical recipients respond differently to the same antigen depending on numerous factors, including those depicted in table 2 and further reviewed below.
Table 2.
Recipient variables that may impact RBC alloimmunization
Genetic Factors
|
Immune status
|
Prior antigen exposures
|
Genetic Factors
One genetic factor that has a clear influence on recipient immunity in general are variability in HLA, which affects the ability of recipients to process and present particular peptides (derived from RBC antigens) by class I and class II MHC. RBC antigen presentation has been investigated in a few human studies, and it is now thought that HLA restriction does exist for some RBC antigens, such as Fya [16, 72] and potentially Kell [73], but not for others, in particular Rh(D) [59]. Certain HLA types may also be more likely to be associated with a ‘responder’ phenotype [74]. The ability to predict subsets of patients who may benefit from RBCs phenotypically matched at certain loci would be a powerful tool, and one that could ultimately conserve resources [75].
Although questions of MHC restriction for RBC antigens in animal models are just beginning to be investigated, many studies investigating MHC presentation of the model humoral antigen HEL have been completed over the past 40 years [76, 77, 78]. Certain recipient mouse strains (including C57BL/6 (H-2b MHC)) have low-level or no responses to the HEL antigen, whereas other strains (including B10.BR, H-2k MHC) have higher-level responses. Differences in donor responses to the same antigen are thought to involve variable affinity of specific peptide epitopes for different MHC molecules as well as differences in recognition of the peptide/MHC complex by the T-cell receptor.
In addition to HLA/MHC differences, polymorphisms of immunoregulatory genes may also influence RBC alloimmunization. Polymorphisms in TRIM 21 (also known as Ro52), an immunoregulatory element in close proximity to the human β-globin gene, have been proposed to impact immune response to transfused RBCs in patients with sickle cell disease [79]. Follow-up studies in reductionist animal models, however, showed that TRIM 21 knock-out animals and wild-type recipients had similar humoral immune responses to transfused HOD RBCs [80]. It is possible that different results may have been observed if the TRIM 21 knock-out animals had also had sickle cell disease, if the transfused RBC antigen had been different, or if recipients had low levels of TRIM 21 expression instead of completely lacking this gene. In the absence of such studies, however, the results from murine models suggest that decreased TRIM 21 expression may not, in and of itself, enhance RBC alloimmunization.
A recent study investigating the SNPs of responder and non-responder human patients with sickle cell disease has implicated CD81 polymorphisms as potentially contributing to recipient immune responses [81]. These CD81 polymorphisms may have myriad immunological consequences, including signal modulations of B lymphocytes and altered functionality of dendritic cells. Although there have been no follow-up animal studies as of yet, a growing body of published and unpublished data in murine RBC alloimmunization models suggests that B cells and dendritic cells are integral in generating immune responses to transfused RBCs [82, 83].
An additional genetic recipient factor that warrants discussion is the impact of sickle cell disease on RBC alloimmunization. A single glutamine to valine substitution in the β-globin gene results in a disease with many clinical manifestations. Ongoing studies are investigating which disease manifestations can be attributed solely to the altered β-globin gene and resultant RBC sickling, and which may be due to co-inheritance of immunoregulatory or other genes along with the sickle globin gene. It is well recognized that this patient population has amongst the highest levels of RBC alloimmunization following transfusion of any patient population [84, 85, 86]. However, there is much debate surrounding the reasons for the high rates of RBC alloimmunization [15, 87, 88], with potential factors including transfusion burden, RBC phenotypic differences between donors and recipients, and RBC genotypic variants in the sickle patients themselves. Sickle cell-associated vascular disease and chronic inflammation [89], as well as immune dysregulation [90, 91], may also potentially contribute to the high rates of RBC alloimmunization in patients with sickle cell disease.
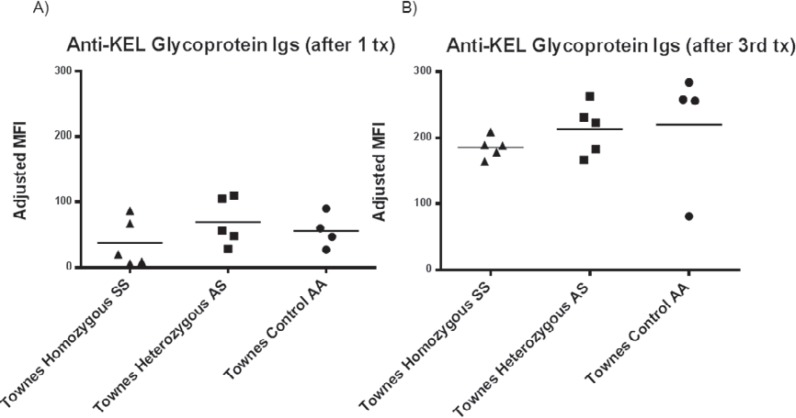
To investigate the impact of the sickle β-globin gene in a reductionist model, transgenic animals with sickle cell disease were transfused with transgenic RBCs expressing the HOD antigen, and alloimmune responses were measured longitudinally [92]. Animals with sickle cell disease (including Berkeley and Townes animals, which express the human sickle β-globin gene) had similar responses to transfused HOD RBCs as did littermate controls with sickle cell trait or hemoglobin AA. Furthermore, no increases in recipient humoral alloimmune responses to transfused HOD RBCs above that of control mice were observed after inflammation of the Hgb SS mice with poly (I:C) [92]. These experiments have since been repeated using transfused KEL2B RBCs [93] to investigate whether the lack of observed differences was inherent to HOD RBC exposure. Similar findings can now be reported, with animals that express the human sickle β-globin gene demonstrating similar responses to littermate controls without sickle cell disease following single or multiple transfusions of KEL2 RBCs (fig. 3A, B). Given the results of these murine experiments, it is possible that factors beyond the expression of sickle β-globin itself may be responsible for the high rates of RBC alloimmunization observed in patients with sickle cell disease. It is also possible, however, that immune responses to transfused RBCs may be different in recipients with acute chest syndrome/hypoxia, or in those with acute vaso-occlusive crises. Likewise, as sickle cell disease patients are often chronically transfused, and thus have altered iron biology, chronic transfusion status may affect alloimmunization as well.
Fig. 3.
Transgenic RBCs expressing the KEL2B antigen were transfused every 4 weeks (for a total of 3 transfusions) into Townes mice homozygous for Hgb SS, heterozygous for Hgb S (AS), or homozygous for Hgb A (AA). A Anti-KEL glycoprotein Igs were measured by flow cytometric crossmatch 28 days after the first transfusion, and B measured again 28 days after the 3rd and final transfusion.
Recipient Inflammatory Status
The immunology literature contains many reports indicating that the presence of a ‘danger’ signal at the time of antigen exposure influences immune responses to antigens [94], though much debate surrounds what determines a response to ‘non-self’ and what defines a ‘danger’ signal [94, 95]. It is curious that recipients are exposed to hundreds of foreign (non-self) antigens with each RBC unit transfused, yet fewer than 10% make detectable humoral alloimmune responses. Conversely, it could be viewed as equally interesting that even 10% of recipients make detectable alloantibody responses, given that each RBC unit is presumably sterile, and thus has no obvious danger signal, at least not of microbial origin. Compared to other more widely studied model humoral antigens, RBC antigens are unique in their structure, route of administration, quantity/volume of antigens accessible to recipient immune cells, and duration of exposure. In addition to the recipient-specific danger signals discussed in this section, it is possible that the RBC units themselves contain elements (such as co-stimulatory molecules, inflammatory cytokines, or free heme, among others) that may predispose a transfusion recipient to generate an alloimmune response.
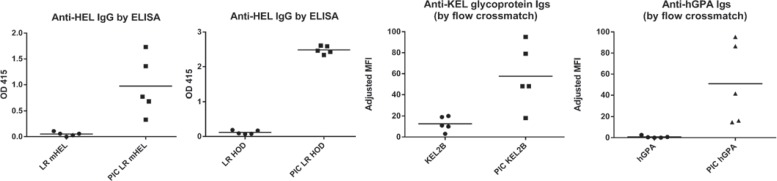
The fact that responder patients tend to make multiple RBC alloantibodies after repeated RBC exposures has led to the suggestion that genetic factors influence responder status [13]. However, studies in reductionist animal models, which have the advantage of genetically identical recipients, have shown that environmental/inflammatory factors also influence RBC alloimmune responses. In every murine model of RBC alloimmunization described to date, recipient inflammation induced by the double stranded RNA poly (I:C) around the time of RBC exposure has been shown to increase the degree or the magnitude of humoral immune responses. Figure 4 shows antigen-specific recipient immune responses after a single transfusion of the equivalent of one ‘unit’ of leukoreduced mHEL, leukoreduced HOD, KEL2B, or hGPA RBCs, in the presence or absence of pre-treatment with 100 μg of i.p. poly (I:C) from Amersham/GE Healthcare; data from representative experiments are shown, and each study has been repeated many times with similar results. Poly (I:C) increases the magnitude of alloantibody responses in the mHEL, HOD, and KEL2 systems, whereas poly (I:C) turns non-responders to responders following hGPA RBC transfusion [22, 39, 96, 97]. Ongoing studies are investigating the mechanism(s) through which poly (I:C) increase alloimmunization, with antigen-presenting cell type/function [82] under investigation.
Fig. 4.
The equivalent of 1 human ‘unit’ of leukoreduced mHEL or HOD RBCs, or KEL2B or hGPA RBCs were transfused into wild-type recipients, in the presence or absence of recipient poly (I:C) pre-treatment. Alloantibodies were measured 2–4 weeks post-transfusion by HEL specific ELISA or by flow cytometric cross-match using transfused and wild-type RBCs as targets.
The increased immune responses observed in the presence of poly (I:C) are not unique to this immunostimulant molecule, as other forms of recipient inflammation have also been shown to impact recipient alloimmune responses. For example, co-transfusion of a different TLR agonist, CpG, increases recipient immune responses to hGPA RBCs [98, 99]. In addition, recipient inflammation with the bacterial endotoxin LPS influences immune responses to transfused transgenic RBCs, though, for reasons still under investigation, LPS enhances recipient alloimmune responses to RBC antigens in some systems (HOD, hGPA), while it inhibits alloimmune responses in others (mHEL, KEL) ([100, 101] and unpublished data). Although many murine studies have focused on the impact of discrete TLR agonists on RBC alloimmunization, at least one has shown that authentic viral infections also increase the magnitude of RBC alloimmune responses [60]. Human studies are beginning to investigate the impact of different types of inflammation on RBC alloimmunization, with one suggesting that febrile transfusion reactions may be associated with subsequent RBC alloantibody formation [102], one showing that inflammatory bowel disease may be a risk factor for alloimmunization [103], and another implying that transfusion at the time of an acute inflammatory event (such as acute chest syndrome) may be more likely to result in alloantibody formation than transfusion in the absence of acute illness [89].
It is often stated, as an experimental concern, that one needs to add an adjuvant (e.g. poly (I:C) as a danger signal) in order to get a strong alloimmune response to transfused RBCs in mice. This is seen as an artificial difference between mice and humans, as human responders are clearly not ‘given’ an adjuvant at time of transfusion. However, it is worth noting that careful examination of the data in the literature demonstrates that control mice (not given inducer of inflammation) have a wide range of responses, with many animals showing weak or no response and others showing strong responses (as above, the pattern changes somewhat depending upon the RBC antigen being studied). Indeed, this is the response pattern seen in human transfusion recipients. Because the animals are genetically identical and are all transfused with the same blood, it is presumably an environmental factor that is regulating response. It is worth noting that there is no such thing as an ‘uninflamed’ mouse, as mice fight with each other and have everyday encounters that may inflame them. While the addition of a danger signal for the experimental purposes of studying the nature of a response in a given situation is a powerful scientific maneuver, it is not required for RBC alloimmunization in many of the antigen systems described.
Other Features of Recipient Immune Status
In addition to recipient inflammatory status, other recipient immune factors may affect RBC alloimmunization. Regulatory T cells are known to suppress the activation and effector functions of many different cell types, in many different situations. The group of Yazdanbakhsh have explored this scenario with respect to RBC antigens in mice and humans, with the conclusion that certain phenotypes of regulatory T cells and B cells may influence responses to transfused RBC antigens [90, 91, 98, 99]. Another group, however, failed to find functional differences in regulatory T cells in alloimmunized or non-alloimmunized humans with sickle cell disease [104]. Additional studies are needed in this area, and it is possible that therapeutic approaches to optimize the function of such regulatory cell subsets, or to alter the way the immune system ‘sees’ foreign RBC antigens, may be effective in decreasing rates of RBC alloimmunization in recipients at highest risk for this complication.
One potential therapeutic approach involves eliminating the organ thought to be responsible for filtering RBCs. In the absence of a spleen, transfused RBCs are shunted to the liver, an organ thought to be more tolerogenic than immunogenic [105]. Recent studies in mice have demonstrated that a spleen is critical for primary immune responses to transfused RBCs [106], though non-responsiveness may not equate to long-term tolerance. These findings are consistent with studies completed many years ago, using sheep RBCs instead of murine RBCs as immunogens [107]. Of note, animals splenectomized after an initial transgenic murine RBC antigen exposure have immunologic memory and are able to mount anamnestic responses in an antigen-specific manner [108]. It must also be appreciated that splenectomy has many potential adverse immunologic and hematologic/vascular sequelae [109, 110] beyond RBC immune responses to RBC antigens, especially over the long term. The human literature concerning the spleen's role in RBC alloimmunization is mixed: some studies have found that splenectomy has no statistically significant impact on RBC alloimmunization rates, or that it decreases alloimmunization [13, 111, 112, 113], while others suggest that splenectomy may increase RBC alloimmunization rates [32, 33, 114, 115]. Such findings are likely due in part to the large number of confounding variables involved and, as above with animal studies, may be affected by the history of RBC transfusion and whether the recipient was first exposed to foreign RBCs before or after splenectomy.
Therapies that target specific immune cell subsets, with goals of minimizing RBC alloimmunization rates, are on the horizon [116]. A better understanding of the most critical steps in immune responses to transfused RBC antigens would be advantageous, in considering the development of such potential therapies. It is possible that these steps will vary by specific RBC antigen or by recipient health status at the time of antigen exposure. For example, preliminary animal studies have suggested that T helper cell responses are important in primary immune responses to some RBC antigens, but not to others [117]. Future experiments will more clearly define how specific immune cell subsets interact to lead to RBC alloantibody formation, and the results of these studies will guide rational therapeutic strategies to minimize RBC alloimmunization.
Prior Exposures to Non-RBC Antigens
For many years, it has been appreciated that certain bacteria (including some strains of Escherichia coli and Shigella) express ‘RBC-like’ antigens that may be capable of inducing humoral antibody responses, independent of RBC exposure [118]. Additionally, increasing evidence suggests that past exposures to pathogens may influence subsequent immune responses to transfused RBCs, without the pathogen exposures alone resulting in appreciable humoral immune responses that react with RBC antigens. For example, a search of the BLAST database has revealed that Haemophilus influenzae, Yersinia pestis, and Bordetella parapertussis share a degree of orthology with the Kell, Duffy, and Kidd RBC antigens [60]. Thus, exposure to these pathogens may prime an individual (presumably at the T-cell level) to respond more vigorously upon subsequent exposure to RBC antigens with overlapping peptide sequences. Because the pathogens have orthology only at the level of linear peptides, and not three-dimensional proteins, exposure will not induce alloantibodies detected by immunohematology, but will rather prime a recipient such that subsequent transfusion will result in a robust and rapid humoral response to a given RBC alloantigen.
Evidence for past non-RBC exposure priming for subsequent responses to RBC antigens exists in humans [73] and in animals [60]. Peripheral blood mononuclear cells from humans with no detectable anti-KEL alloantibodies were stimulated with overlapping KEL peptides, with evidence of T-cell reactivity present in subjects with no prior RBC exposure [73]. This reactivity appeared to be a memory response, given the thymidine incorporation observed in CD45 RO-positive T cells after peptide stimulation. Animal studies using a model RBC antigen have also demonstrated this concept: sequences contained within non-RBC antigens (in this case an ovalbumin sequence contained within a polyoma virus) have been shown to prime a recipient to generate a robust response upon subsequent exposure to a shared epitope within a RBC antigen [60]. Of interest (as above) is the fact that traditional antibody-focused blood bank screens would not detect this prior ‘priming’ phenomenon. In theory, priming may lead to rapid and robust alloantibody responses following primary RBC exposure, which may result in early ‘delayed’ hemolytic transfusion reactions.
Tolerance to RBC Antigens
It is possible that non-responders to RBC antigens are actually tolerized (through mechanisms not yet defined), though this hypothesis is difficult to test in humans given relatively low baseline rates of alloimmunization with each transfusion event. Young recipient age at the time of initial RBC exposure has been shown to influence rates of RBC alloimmunization in patients with sickle cell disease [14, 79] and thalassemia major [115], leading to a hypothesis that relative ‘tolerance’ to RBC antigens may be possible in young transfusion recipients. To date, only one animal study has been published investigating the relationship between recipient age at initial RBC exposure and RBC alloimmunization, with no or very low levels of anti-HOD alloantibodies observed in juvenile animals (3 weeks of age) compared to adult animals [80]. However, these studies did not evaluate repeat antigen exposure, as it has been shown that subsequent HEL antigen exposures do not result in immunologic boosting [96] for reasons that remain under investigation. Ongoing experiments using KEL transgenic RBCs, which are capable of generating memory and boostable responses in C57BL/6 animals [97], are investigating the impact of RBC exposure as neonates and subsequent responses when these same animals are re-transfused as adults.
Characteristics of the transfused RBC antigens themselves also play key roles in determining recipient responsiveness versus non-responsiveness. For example, non-responsiveness/tolerance to the hGPA antigen occurs when the initial antigen exposure takes place in the absence of an adjuvant [96]. This non-responsiveness is antigen-specific, with non-responders to the hGPA antigen being fully capable of responding to other distinct RBC antigens. RBC antigen copy number may contribute to whether a particular antigen is capable of inducing an immune response following transfusion, as suggested by studies that have shown antigen density to be a key determinant of immunologic responsiveness to non-RBC antigens [119, 120, 121]. Although hGPA copy number has not been formally evaluated, flow-cytometric cross-matching of these RBCs with monoclonal anti-hGPA results in a 3–4 log shift and in vitro agglutination, suggesting that the copy number is very high. Ongoing studies are comparing recipient immune responses to transfused RBCs expressing high, mid, and low levels of the human KEL2 antigen.
Studies in animals suggest that soluble antigen (outside of the context of RBC immunology) may be capable of inducing non-responsiveness, and potentially even tolerance, depending on the route of exposure [122, 123]. Furthermore, animal studies have shown that primary antigen exposure via the nasal mucosa decreases secondary responses to subsequently transfused RBC antigens [73, 124]. Such studies have been completed using immunodominant Rh(D) peptides as well as immunodominant KEL peptides. One study has suggested that there may be antigen-specific mechanisms for reducing T-cell responsiveness with immunodominant peptides: following a primary i.v. transfusion of RBCs with a secondary intranasal peptide exposure to an immunodominant peptide of an antigen expressed on the RBC surface, the authors were able to decrease the T-cell response [73]. Other murine studies have recently explored the use of RBCs as vehicles to induce tolerance to non-RBC antigens, with antigen-specific tolerance to the OVA antigen observed following immunization with OVA-entrapped RBCs [121].
RBC Exposure via Transfusion or Pregnancy
Although this review has focused on factors that may influence immune responses to transfused RBCs, exposure to paternally derived foreign RBC antigens may also occur during pregnancy. In the KEL2 murine model, anti-KEL glycoprotein alloantibodies develop not only following transfusion of KEL2 RBCs into C57BL/6 mice [97] but also after pregnancy in C57BL/6 female mice bred with KEL2 transgenic males [71]. The titers of anti-KEL glycoprotein immunoglobulins increase with repeat antigen exposure, whether the exposure is due to multiple RBC transfusions or due to multiple pregnancies/deliveries [71, 97]. All IgG subtypes are generated in response to KEL2 RBC exposure by both pregnancy and transfusion, with these antibodies being clinically significant in both settings. Ongoing experiments are investigating differences in immune responses generated by pregnancy and transfusion, with attention being paid to the duration of RBC exposure, the state of pregnancy itself, and other variables that may impact the magnitude of the anti-KEL response.
Conclusions
Studies in murine models have answered fundamental questions of transfusion immunobiology and have raised new questions to be studied in humans. As more tools have been developed and more studies have been completed, it has become clear that murine immune responses to RBC antigens are dependent on antigen properties as well as on donor and recipient factors. Although these variables increase the complexity of the experimental biology, the variables reflect that what has also been observed in human transfusion immunology. The murine models reviewed provide a tractable experimental landscape in which to pursue mechanistic knowledge, though practical and specific instructions on translational strategies to mitigate RBC alloimmunization in humans will require additional research. Being cognizant of similarities and differences in murine versus human biology, it is hoped that the translation of knowledge gained in murine models may ultimately help to decrease rates of RBC alloimmunization and to mitigate the dangers of existing RBC alloantibodies in humans, in both transfusion and pregnancy scenarios.
Disclosure Statement
Conflict of interest – none relevant to this manuscript.
Acknowledgement
Reviewed studies were funded in part by NIH/NHLBI (K08 HL092959, R21 HL11569) and by the Emory Egleston Children's Research Center, to JEH.
References
- 1.Bolton-Maggs PH, Cohen H. Serious hazards of transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163:303–314. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Fatalities Reported to the FDA following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2013. 2014. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm391574.htm.
- 3.US Food and Drug Administration. Fatalities Reported to FDA Following Blood Collection and Transfusion. Annual Summary for Fiscal Year 2011. 2012. www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM300764.pdf.
- 4.Annual SHOT Report, 2012. 2012. www.shotuk.org/wp-content/uploads/2013/08/SHOT-Annual-Report-2012.pdf.
- 5.Moise KJ, Jr, Argoti PS. Management and prevention of red cell alloimmunization in pregnancy: a systematic review. ObstetGynecol. 2012;120:1132–1139. doi: 10.1097/aog.0b013e31826d7dc1. [DOI] [PubMed] [Google Scholar]
- 6.Reid M, Lomas-Francis C. The Blood Group Antigen Facts Book. 2nd ed. Amsterdam: Elsevier; 2004. [Google Scholar]
- 7.Tormey CA, Stack G. Immunogenicity of blood group antigens: a mathematical model corrected for antibody evanescence with exclusion of naturally occurring and pregnancy-related antibodies. Blood. 2009;114:4279–4282. doi: 10.1182/blood-2009-06-227793. [DOI] [PubMed] [Google Scholar]
- 8.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 9.Schonewille H, Haak HL, van Zijl AM. RBC antibody persistence. Transfusion. 2000;40:1127–1131. doi: 10.1046/j.1537-2995.2000.40091127.x. [DOI] [PubMed] [Google Scholar]
- 10.Hendrickson JE, Tormey CA, Shaz BH. Red blood cell alloimmunization mitigation strategies. Transfus Med Rev. 2014;28:137–144. doi: 10.1016/j.tmrv.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Redman M, Regan F, Contreras M. A prospective study of the incidence of red cell allo-immunisation following transfusion. Vox Sang. 1996;71:216–220. doi: 10.1046/j.1423-0410.1996.7140216.x. [DOI] [PubMed] [Google Scholar]
- 12.Schonewille H, van de Watering LM, Loomans DS, Brand A. Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006;46:250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 14.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 15.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 16.Picard C, Frassati C, Basire A, Buhler S, Galicher V, Ferrera V, Reviron D, Zappitelli JP, Bailly P, Chiaroni J. Positive association of DRB1 04 and DRB1 15 alleles with Fya immunization in a southern European population. Transfusion. 2009;49:2412–2417. doi: 10.1111/j.1537-2995.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- 17.Reviron D, Dettori I, Ferrera V, Legrand D, Touinssi M, Mercier P, de Micco P, Chiaroni J. HLA-DRB1 alleles and Jk(a) immunization. Transfusion. 2005;45:956–959. doi: 10.1111/j.1537-2995.2005.04366.x. [DOI] [PubMed] [Google Scholar]
- 18.Schonewille H, Doxiadis II, Levering WH, Roelen DL, Claas FH, Brand A. HLA-DRB1 associations in individuals with single and multiple clinically relevant red blood cell antibodies. Transfusion. 2014;54:1971–1980. doi: 10.1111/trf.12624. [DOI] [PubMed] [Google Scholar]
- 19.Mollison's Blood Transfusion in Clinical Medicine. 11th ed. Oxford: Blackwell; 2005. [Google Scholar]
- 20.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 21.Nagy ZA. A History of Modern Immunology: The Path toward Understanding. Amsterdam: Elsevier; 2014. [Google Scholar]
- 22.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith NH, Henry KL, Cadwell CM, Bennett A, Hendrickson JE, Frame T, Zimring JC. Generation of transgenic mice with antithetical KEL1 and KEL2 human blood group antigens on red blood cells. Transfusion. 2012;52:2620–2630. doi: 10.1111/j.1537-2995.2012.03641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radwanski K, Garraud O, Cognasse F, Hamzeh-Cognasse H, Payrat JM, Min K. The effects of red blood cell preparation method on in vitro markers of red blood cell aging and inflammatory response. Transfusion. 2013;53:3128–3138. doi: 10.1111/trf.12143. [DOI] [PubMed] [Google Scholar]
- 25.Jy W, Ricci M, Shariatmadar S, Gomez-Marin O, Horstman LH, Ahn YS. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion. 2011;51:886–893. doi: 10.1111/j.1537-2995.2011.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, Heitman JW, Norris PJ. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–696. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroff G, AuBuchon JP, Pickard C, Whitley PH, Heaton WA, Holme S. Evaluation of the properties of components prepared and stored after holding of whole blood units for 8 and 24 hours at ambient temperature. Transfusion. 2011;51(suppl 1):7s–14s. doi: 10.1111/j.1537-2995.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 28.Thibault L, Beausejour A, de Grandmont MJ, Lemieux R, Leblanc JF. Characterization of blood components prepared from whole-blood donations after a 24-hour hold with the platelet-rich plasma method. Transfusion. 2006;46:1292–1299. doi: 10.1111/j.1537-2995.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 29.Glenister KM, Sparrow RL. Level of platelet-derived cytokines in leukoreduced red blood cells is influenced by the processing method and type of leukoreduction filter. Transfusion. 2010;50:185–189. doi: 10.1111/j.1537-2995.2009.02353.x. [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg N, Heal JM, Gettings KF. WBC reduction of RBC transfusions is associated with a decreased incidence of RBC alloimmunization. Transfusion. 2003;43:945–952. doi: 10.1046/j.1537-2995.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 32.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–3373. [PubMed] [Google Scholar]
- 33.Hussein E, Desooky N, Rihan A, Kamal A. Predictors of red cell alloimmunization in multitransfused Egyptian patients with beta-thalassemia. Arch Pathol Lab Med. 2014;138:684–688. doi: 10.5858/arpa.2013-0016-OA. [DOI] [PubMed] [Google Scholar]
- 34.van de Watering L, Hermans J, Witvliet M, Versteegh M, Brand A. HLA and RBC immunization after filtered and buffy coat-depleted blood transfusion in cardiac surgery: a randomized controlled trial. Transfusion. 2003;43:765–771. doi: 10.1046/j.1537-2995.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 35.Schonewille H, Brand A. Alloimmunization to red blood cell antigens after universal leucodepletion. A regional multicentre retrospective study. Br J Haematol. 2005;129:151–156. doi: 10.1111/j.1365-2141.2005.05408.x. [DOI] [PubMed] [Google Scholar]
- 36.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Leukoreduction decreases alloimmunogenicity of transfused murine RBCs. Blood. 2009;114:646. [Google Scholar]
- 37.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114:2315–2322. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilson CR, Kraus T, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cells storage and post-transfusion in vivo survival. Transfusion. 2009;48:1456–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroidspecific model antigen. Transfusion. 2010;50:642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, Hod EA, Hendrickson JE, Roback JD, Spitalnik SL. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–148. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, Kameneva MV, Yazer MH. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–331. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 42.Strauss RG, Cordle DG, Quijana J, Goeken NE. Comparing alloimmunization in preterm infants after transfusion of fresh unmodified versus stored leukocyte-reduced red blood cells. J Pediatr Hematol Oncol. 1999;21:224–230. doi: 10.1097/00043426-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Yazer MH, Triulzi DJ. Receipt of older RBCs does not predispose D-negative recipients to anti-D alloimmunization. Am J Clin Pathol. 2010;134:443–447. doi: 10.1309/AJCP2J8SVWOXRLRB. [DOI] [PubMed] [Google Scholar]
- 44.Zalpuri S, Schonewille H, Middelburg R, van de Watering L, de Vooght K, Zimring J, van der Bom JG, Zwaginga JJ. Effect of storage of red blood cells on alloimmunization. Transfusion. 2013;53:2795–2800. doi: 10.1111/trf.12156. [DOI] [PubMed] [Google Scholar]
- 45.Veale MF, Healey G, Sparrow RL. Longer storage of red blood cells is associated with increased in vitro erythrophagocytosis. Vox Sang. 2014;106:219–226. doi: 10.1111/vox.12095. [DOI] [PubMed] [Google Scholar]
- 46.Hendrickson JE, Hod EA, Hudson KE, Spitalnik SL, Zimring JC. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–2702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kannan M, Atreya C. Differential profiling of human red blood cells during storage for 52 selected microRNAs. Transfusion. 2010;50:1581–1588. doi: 10.1111/j.1537-2995.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- 48.Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, Ma L, Fortina P, Kunapuli S, Holinstat M, McKenzie SE, Dong JF, Shaw CA, Bray PF. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123:e37–45. doi: 10.1182/blood-2013-12-544692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kannan M, Mohan KV, Kulkarni S, Atreya C. Membrane array-based differential profiling of platelets during storage for 52 miRNAs associated with apoptosis. Transfusion. 2009;49:1443–1450. doi: 10.1111/j.1537-2995.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- 50.Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, Lopez JA, Yang L, Jin Y, Bray MS, Leal SM, Dong JF, Bray PF. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Land WG. Transfusion-related acute lung injury: the work of DAMPs. Transfus Med Hemother. 2013;40:3–13. doi: 10.1159/000345688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YL, King MB, Gonzalez RP, Brevard SB, Frotan MA, Gillespie MN, Simmons JD. Blood transfusion products contain mitochondrial DNA damage-associated molecular patterns: a potential effector of transfusion-related acute lung injury. J Surg Res. 2014;191:286–289. doi: 10.1016/j.jss.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ing R, Segura M, Thawani N, Tam M, Stevenson MM. Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol. 2006;176:441–450. doi: 10.4049/jimmunol.176.1.441. [DOI] [PubMed] [Google Scholar]
- 56.Hendrickson JE, Hod EA, Cadwell CM, Eisenbarth SC, Spiegel DA, Tormey CA, Spitalnik SL, Zimring JC. Rapid clearance of transfused murine red blood cells is associated with recipient cytokine storm and enhanced alloimmunogenicity. Transfusion. 2011;51:2445–2454. doi: 10.1111/j.1537-2995.2011.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seltsam A, Blasczyk R. Recombinant blood group proteins for use in antibody screening and identification tests. Curr Opin Hematol. 2009;16:473–479. doi: 10.1097/MOH.0b013e3283319a06. [DOI] [PubMed] [Google Scholar]
- 58.Seltsam A, Wagner F, Lambert M, Bullock T, Thornton N, Scharberg EA, Grueger D, Schneeweiss C, Blasczyk R. Recombinant blood group proteins facilitate the detection of alloantibodies to high-prevalence antigens and reveal underlying antibodies: results of an international study. Transfusion. 2014;54:1823–1830. doi: 10.1111/trf.12553. [DOI] [PubMed] [Google Scholar]
- 59.Brantley SG, Ramsey G. Red cell alloimmunization in multitransfused HLA-typed patients. Transfusion. 1988;28:463–466. doi: 10.1046/j.1537-2995.1988.28588337338.x. [DOI] [PubMed] [Google Scholar]
- 60.Hudson KE, Lin E, Hendrickson JE, Lukacher AE, Zimring JC. Regulation of primary alloantibody response through antecedent exposure to a microbial T-cell epitope. Blood. 2010;115:3989–3996. doi: 10.1182/blood-2009-08-238568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flegel WA. Molecular genetics and clinical applications for Rh. Transfus Apher Sci. 2011;44:81–91. doi: 10.1016/j.transci.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendrickson J, Smith N, Girard-Pierce K, Tormey C, Henry K, Zimring J, Stowell S. A murine model of weak KEL: Similarities to weak Rh(D) Blood. 2012;120:842. [Google Scholar]
- 63.Zimring JC, Cadwell CM, Chadwick TE, Spitalnik SL, Schirmer DA, Wu T, Parkos CA, Hillyer CD. Nonhemolytic antigen loss from red blood cells requires cooperative binding of multiple antibodies recognizing different epitopes. Blood. 2007;110:2201–2208. doi: 10.1182/blood-2007-04-083097. [DOI] [PubMed] [Google Scholar]
- 64.Zimring JC, Cadwell CM, Spitalnik SL. Antigen loss from antibody-coated red blood cells. Transfus Med Rev. 2009;23:189–204. doi: 10.1016/j.tmrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Cadwell CM, Zimring JC. Cross-linking induces non-haemolytic antigen-loss from transfused red blood cells: a potential role for rheumatoid factor. Vox Sang. 2008;95:159–162. doi: 10.1111/j.1423-0410.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 66.Hod EA, Cadwell CM, Liepkalns JS, Zimring JC, Sokol SA, Schirmer DA, Jhang J, Spitalnik SL. Cytokine storm in a mouse model of IgG-mediated hemolytic transfusion reactions. Blood. 2008;112:891–894. doi: 10.1182/blood-2008-01-132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hod EA, Zimring JC, Spitalnik SL. Lessons learned from mouse models of hemolytic transfusion reactions. Curr Opin Hematol. 2008;15:601–605. doi: 10.1097/MOH.0b013e328311f40a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liepkalns JS, Hod EA, Stowell SR, Cadwell CM, Spitalnik SL, Zimring JC. Biphasic clearance of incompatible red blood cells through a novel mechanism requiring neither complement nor Fcy receptors in a murine model. Transfusion. 2012;52:2631–2645. doi: 10.1111/j.1537-2995.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- 69.Liepkalns JS, Cadwell CM, Stowell SR, Hod EA, Spitalnik SL, Zimring JC. Resistance of a subset of red blood cells to clearance by antibodies in a mouse model of incompatible transfusion. Transfusion. 2013;53:1319–1327. doi: 10.1111/j.1537-2995.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 70.Girard-Pierce KR, Stowell SR, Smith NH, Arthur CM, Sullivan HC, Hendrickson JE, Zimring JC. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood. 2013;122:1793–1801. doi: 10.1182/blood-2013-06-508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stowell SR, Henry KL, Smith NH, Hudson KE, Halverson GR, Park JC, Bennett AM, Girard-Pierce KR, Arthur CM, Bunting ST, Zimring JC, Hendrickson JE. Alloantibodies to a paternally derived RBC KEL antigen lead to hemolytic disease of the fetus/newborn in a murine model. Blood. 2013;122:1494–1504. doi: 10.1182/blood-2013-03-488874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noizat-Pirenne F, Tournamille C, Bierling P, Roudot-Thoraval F, Le Pennec PY, Rouger P, Ansart-Pirenne H. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46:1328–1333. doi: 10.1111/j.1537-2995.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 73.Stephen J, Cairns LS, Pickford WJ, Vickers MA, Urbaniak SJ, Barker RN. Identification, immunomodulatory activity, and immunogenicity of the major helper T-cell epitope on the K blood group antigen. Blood. 2012;119:5563–5574. doi: 10.1182/blood-2012-02-410324. [DOI] [PubMed] [Google Scholar]
- 74.Schonewille H, Doxiadis II, Levering WH, Roelen DL, Claas FH, Brand A. HLA-DRB1 associations in individuals with single and multiple clinically relevant red blood cell antibodies. Transfusion. 2014;54:1971–1980. doi: 10.1111/trf.12624. [DOI] [PubMed] [Google Scholar]
- 75.Kacker S, Ness PM, Savage WJ, Frick KD, Shirey RS, King KE, Tobian AA. Economic evaluation of a hypothetical screening assay for alloimmunization risk among transfused patients with sickle cell disease. Transfusion. 2014;54:2034–2044. doi: 10.1111/trf.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz RH. Immune response (IR) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- 77.Kim BS, Jang YS. Constraints in antigen processing result in unresponsiveness to a T cell epitope of hen egg lysozyme in C57BL/6 mice. Eur J Immunol. 1992;22:775–782. doi: 10.1002/eji.1830220322. [DOI] [PubMed] [Google Scholar]
- 78.Adorini L, Miller A, Sercarz EE. The fine specificity of regulatory T cells. I. Hen egg-white lysozymeinduced suppressor T cells in a genetically nonresponder mouse strain do not recognize a closely related immunogenic lysozyme. J Immunol. 1979;122:871–877. [PubMed] [Google Scholar]
- 79.Tatari-Calderone Z, Minniti CP, Kratovil T, Stojakovic M, Vollmer A, Barjaktarevic I, Zhang E, Hoang A, Luban NL, Vukmanovic S. Rs660 polymorphism in Ro52 (SSA1; TRIM21) is a marker for age-dependent tolerance induction and efficiency of alloimmunization in sickle cell disease. Mol Immunol. 2009;47:64–70. doi: 10.1016/j.molimm.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 80.Patel SR, Hendrickson JE, Smith NH, Cadwell CM, Ozato K, Morse HC, 3rd, Yoshimi R, Zimring JC. Alloimmunization against RBC or PLT antigens is independent of TRIM21 expression in a murine model. Mol Immunol. 2011;48:909–913. doi: 10.1016/j.molimm.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tatari-Calderone Z, Tamouza R, Le Bouder GP, Dewan R, Luban NL, Lasserre J, Maury J, Lionnet F, Krishnamoorthy R, Girot R, Vukmanovic S. The association of CD81 polymorphisms with alloimmunization in sickle cell disease. Clin Dev Immun. 2013;2013:937846. doi: 10.1155/2013/937846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 83.Girard-Pierce K, Stowell SR, Smith NH, Henry KL, Zimring JC, Hendrickson JE. Marginal zone B cells mediate alloantibody formation to a clinically significant human RBC antigen in a murine model. Blood (ASH Annual Meeting Abstracts) 2012;120:843. [Google Scholar]
- 84.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 85.Chou ST. Transfusion therapy for sickle cell disease: a balancing act. Hematology Am Soc Hematol Educ Program. 2013;2013:439–446. doi: 10.1182/asheducation-2013.1.439. [DOI] [PubMed] [Google Scholar]
- 86.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 88.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 89.Fasano RM. Booth GS, Miles M.R., Du L, Koyama T, Meier ER, Luban NL. RBC alloimmunization is influenced by inflammatory status at the time of transfusion in patients with sickle cell disease. Blood. 2013;122:40. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 90.Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, Lee MT, Sheth S, Shi P, Yazdanbakhsh K. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am J Hematol. 2013;88:736–740. doi: 10.1002/ajh.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bao W, Zhong H, Li X, Lee MT, Schwartz J, Sheth S, Yazdanbakhsh K. Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and beta-thalassemia major. Am J Hematol. 2011;86:1001–1006. doi: 10.1002/ajh.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hendrickson JE, Hod EA, Perry JR, Ghosh S, Chappa P, Adisa O, Kean LS, Ofori-Acquah SF, Archer DR, Spitalnik SL, Zimring JC. Alloimmunization to transfused HOD red blood cells is not increased in mice with sickle cell disease. Transfusion. 2012;52:231–240. doi: 10.1111/j.1537-2995.2011.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith NH, Henry KL, Cadwell CM, Bennett A, Hendrickson JE, Frame T, Zimring JC. Generation of transgenic mice with antithetical KEL1 and KEL2 human blood group antigens on red blood cells. Transfusion. 2012;52:2620–2630. doi: 10.1111/j.1537-2995.2012.03641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 95.Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:287. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith NH, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE. Transfusion in the absence of inflammation induces antigen-specific tolerance to murine RBCs. Blood. 2012;119:1566–1569. doi: 10.1182/blood-2011-09-382655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stowell SR, Girard-Pierce KR, Smith NH, Henry KL, Arthur CM, Zimring JC, Hendrickson JE. Transfusion of murine red blood cells expressing the human KEL glycoprotein induces clinically significant alloantibodies. Transfusion. 2014;54:179–189. doi: 10.1111/trf.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bao W, Yu J, Heck S, Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood. 2009;113:5624–5627. doi: 10.1182/blood-2008-12-193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. Am J Hematol. 2007;82:691–696. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hendrickson JE, Hillyer CD, Zimring JC. Effects of inflammation on alloimmunization to 3 unique red blood cell specific antigens. Transfusion. 2008;48:185A. doi: 10.1111/j.1537-2995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 101.Hendrickson J, Roback JD, Hillyer CD, Easley KA, Zimring, JC. Discrete toll like receptor agonists have differential effects on alloimmunization to red blood cells. ransfusion. 2008. pp. 1869–1877. [DOI] [PubMed]
- 102.Yazer MH, Triulzi DJ, Shaz B, Kraus T, Zimring JC. Does a febrile reaction to platelets predispose recipients to red blood cell alloimmunization? Transfusion. 2009;49:1070–1075. doi: 10.1111/j.1537-2995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 103.Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, Eser A, Mikulits A, Mayr WR, Kormoczi GF. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. Am J Med. 2012;125:717e711–718. doi: 10.1016/j.amjmed.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 104.Vingert B, Tamagne M, Desmarets M, Pakdaman S, Elayeb R, Habibi A, Bernaudin F, Galacteros F, Bierling P, Noizat-Pirenne F, Cohen J. Partial dysfunction of TREG activation in sickle cell disease. Am J Hematol. 2014;89:261–266. doi: 10.1002/ajh.23629. [DOI] [PubMed] [Google Scholar]
- 105.Holz LE, McCaughan GW, Benseler V, Bertolino P, Bowen DG. Liver tolerance and the manipulation of immune outcomes. Inflamm Allergy Drug Targets. 2008;7:6–18. doi: 10.2174/187152808784165225. [DOI] [PubMed] [Google Scholar]
- 106.Hendrickson JE, Saakadze N, Cadwell CM, Upton JW, Mocarski ES, Hillyer CD, Zimring JC. The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion. 2009;49:1678–1684. doi: 10.1111/j.1537-2995.2009.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benner R, van Oudenaren A. Antibody formation in mouse bone marrow. IV. The influence of splenectomy on the bone marrow plaque-forming cell response to sheep red blood cells. Cell Immunol. 1975;19:167–182. doi: 10.1016/0008-8749(75)90201-4. [DOI] [PubMed] [Google Scholar]
- 108.Hendrickson J, Stowell SR, Smith NH, Girard-Pierce KR, Hudson KE, Zimring, JC. Transfused RBCs can be immunogenic in splenectomized mice: of inflammation, adjuvants, and anamnestic responses. Transfusion. 2012;52(suppl):P1–030A. [Google Scholar]
- 109.Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114:2861–2868. doi: 10.1182/blood-2009-04-210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schilling RF. Risks and benefits of splenectomy versus no splenectomy for hereditary spherocytosis – a personal view. Br J Haematol. 2009;145:728–732. doi: 10.1111/j.1365-2141.2009.07694.x. [DOI] [PubMed] [Google Scholar]
- 111.Pahuja S, Pujani M, Gupta SK, Chandra J, Jain M. Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–177. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 112.Ho HK, Ha SY, Lam CK, Chan GC, Lee TL, Chiang AK, Lau YL. Alloimmunization in Hong Kong southern Chinese transfusion-dependent thalassemia patient. Blood. 2001;97:3999–4000. doi: 10.1182/blood.v97.12.3999. [DOI] [PubMed] [Google Scholar]
- 113.McPherson ME, Anderson AR, Castillejo MI, Hillyer CD, Bray RA, Gebel HM, Josephson CD. HLA alloimmunization is associated with RBC antibodies in multiply transfused patients with sickle cell disease. Pediatr Blood Cancer. 2010;54:552–558. doi: 10.1002/pbc.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson AA, Cunningham MJ, Singer ST, Neufeld EJ, Vichinsky E, Yamashita R, Giardina P, Kim HY, Trachtenberg F, Kwiatkowski JL. Red cell alloimmunization in a diverse population of transfused patients with thalassaemia. Br J Haematol. 2011;153:121–128. doi: 10.1111/j.1365-2141.2011.08576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vichinsky E, Neumayr L, Trimble S, Giardina PJ, Cohen AR, Coates T, Boudreaux J, Neufeld EJ, Kenney K, Grant A, Thompson AA. Transfusion complications in thalassemia patients: a report from the Centers for Disease Control and Prevention (CME) Transfusion. 2014;54:972–981. doi: 10.1111/trf.12348. quiz 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zalpuri S, Evers D, Zwaginga JJ, Schonewille H, de Vooght KM, le Cessie S, van der Bom JG. Immunosuppressants and alloimmunization against red blood cell transfusions. Transfusion. 2014;54:1981–1987. doi: 10.1111/trf.12639. [DOI] [PubMed] [Google Scholar]
- 117.Stowell SR, Girard-Pierce KR, Arthur CM, Smith NH, Zimring JC, Hendrickson JE. KEL RBC transfusion induces IgG anti-KEL antibodies independent of CD4 T cells. Blood. 2013;122:41. [Google Scholar]
- 118.Savalonis JM, Kalish RI, Cummings EA, Ryan RW, Aloisi R. Kell blood group activity of Gramnegative bacteria. Transfusion. 1988;28:229–232. doi: 10.1046/j.1537-2995.1988.28388219149.x. [DOI] [PubMed] [Google Scholar]
- 119.Sasamoto Y, Kawano YI, Bouligny R, Wiggert B, Chader GJ, Gery I. Immunomodulation of experimental autoimmune uveoretinitis by intravenous injection of uveitogenic peptides. Invest Ophthalmol Vis Sci. 1992;33:2641–2649. [PubMed] [Google Scholar]
- 120.Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: ROLE of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- 121.Cremel M, Guerin N, Horand F, Banz A, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013;443:39–49. doi: 10.1016/j.ijpharm.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 122.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 123.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 124.Hall AM, Cairns LS, Altmann DM, Barker RN, Urbaniak SJ. Immune responses and tolerance to the RhD blood group protein in HLA-transgenic mice. Blood. 2005;105:2175–2179. doi: 10.1182/blood-2004-04-1554. [DOI] [PubMed] [Google Scholar]