Summary
The search for genetic determinants of alloimmunization in sickle cell disease transfusion recipients was based on two premises: i) that polymorphisms responsible for stronger immune and/or inflammatory responses and hemoglobin βS mutation were co-selected by malaria; and ii) that stronger responder status contributes to development of lupus. We found a marker of alloimmunization in the gene encoding for Ro52 protein, also known as Sjögren syndrome antigen 1 (SSA1) and TRIM21. Surprisingly, the nature of the association was opposite of that with lupus; the same variant of a polymorphism (rs660) that was associated with lupus incidence was also associated with induction of tolerance to red blood cell antigens during early childhood. The dual function of Ro52 can explain this apparent contradiction. We propose that other lupus/autoimmunity susceptibility loci may reveal roles of additional molecules in various aspects of alloimmunization induced by transfusion as well as during pregnancy.
Keywords: Alloimmunization, Antibodies, Autoimmunity, Red blood cells
Introduction
About 8% of African Americans are heterozygous carriers of hemoglobin S and 1/500 has sickle cell disease (SCD). In 1973, the average life span of a patient with SCD was 14 years. The development of comprehensive care has decreased early mortality and increased life expectancy to 50 years. Transfusion is a key component in the management of SCD patients [1], and its use has increased over time for prevention of stroke [2] and other complications.
RBC transfusion therapy is complicated by the development of antibodies specific for allelic (alloantibodies) or self (autoantibodies) RBC determinants. Alloantibodies are more frequent than autoantibodies, the clinical significance of which remain questionable. The presence of anti-RBC antibodies may cause delay in finding suitable blood for transfusion, which can result in life-threatening anemia. In addition, anti-RBC antibodies may cause delayed hemolytic transfusion reactions resembling sickle cell crises that can be lethal [3, 4, 5]. Finally, anti-HLA antibodies promoting rejection of hematopoietic cell grafts are more frequent in patients with anti-RBC antibodies [6]. Anti-RBC antibodies develop in 18–47% of patients with SCD [7, 8, 9, 10, 11], usually after receiving a small number of transfusions (responders; Rs), while other patients remain antibody-free (nonresponders; NRs), despite extensive exposure to donor RBC antigens. RBC-specific antibodies make selection of RBC and assurance of cross-match compatibility extremely complicated. Further, transfusion of incompatible blood when no other options exist may result in increased hyper-hemolysis and poor in vivo survival of the transfused RBCs. The use of antigen-matched blood has been suggested to prevent alloimmunization, decrease the risk of delayed hemolytic transfusion reactions, and reduce morbidity in transfused SCD patients. This, however, has two drawbacks: i) utilizing antigen-matched blood for all patients, even if they are NRs; and ii) a lifetime commitment of ensuring antigen-matched blood, which is impractical for all Rs, given the cost and resources needed.
Factors Influencing the (Non-)Responder Status
The rates of alloimmunization in SCD patients (18–47%) are considerably higher than those found in transfused patients without SCD (0.2–2.8%) [12, 13, 14, 15]. This may be due to antigenic disparity, i.e., different blood group antigen distribution between predominantly Caucasian blood donors and SCD patients who are of African or African-Caribbean descent [16]. This concept is supported by reduced alloimmunization frequency in SCD patients in Saudi Arabia (13.7%), Uganda (6.1%), Egypt (21.4%), and Tunisia (16.6%) where blood donors and SCD patients are from similar ethnic background [17, 18, 19, 20]. Even lower alloimmunization rates (2.6%) were noted in a Jamaican patient cohort [21], but might have been secondary to low number of units received (1–2 per patient). However, even these reduced rates are higher than the ‘background’ rate of 0.2–2.8%, suggesting that additional factor(s) may influence alloimmunization.
There are NR patients with documented multiple exposures to RBC antigens. So, what other factor may have a decisive influence on alloimmunization? Stochastic modeling suggested that a subgroup of transfusion recipients has genetically determined increased risk of alloimmunization [22]. Genetic factors controlling inflammatory responses are possible candidates, as the state of inflammation in recipients may activate the innate immune system and convert an inert or even a tolerogenic event into an immunogenic one [23, 24]. Indeed, in the SCD mouse model, recipient inflammation induced with poly (I:C) treatment augmented humoral immune response to transfused antigens [23]. However, not every form of inflammation promotes alloimmunization in mice, as lipopolysaccharide failed to induce the same effect as poly (I:C) [25]. Not surprisingly, elevated levels of cytokines are not markers for alloimmunization [26] and only some forms of pro-inflammatory events are associated with alloimmunization (Fasano et al. submitted).
Selection of High Responders in Africa
If inflammatory signals contribute to alloimmunization and SCD subjects have higher rates of alloimmunization, then individuals with SCD (or people of African descent, in general) should be prone developing stronger inflammatory and immune responses then other transfused subjects. This, indeed, appears to be the case, as SCD patients display increased inflammation and activation of innate immunity [27, 28] and increased levels of serum cytokines [29, 30, 31, 32]. Evidence for higher rates of inflammatory conditions in African Americans in general also exists. Thus, incidence of lupus [33, 34, 35], asthma [36], hypertension [37], type 2 diabetes mellitus [38], obesity [39], necrotizing enterocolitis [40], and keloid formation [41] is higher in African Americans. In addition, African Americans display a significantly higher rate of arthritis and uveitis associated with Crohn's disease [42] and higher rates of acute graft rejection [43], and they require higher doses of immunosuppressive drugs post transplantation [44, 45]. Although such ethnic differences have traditionally been dismissed as a mix of environmental, social, cultural, and economic factors, evidence at the cellular and molecular level points to a contributing genetic component. Thus, African Americans have more robust cellular immune responses [46, 47, 48] and express higher levels of CD80 and CD86 co-stimulatory molecules [49, 50] compared to other ethnic groups. Furthermore, polymorphisms in immunomodulatory genes that favor expression of variants or levels of encoded molecules that promote stronger immune responses are more frequent in Africans/African Americans. These include multiple cytokine genes [51, 52, 53], CTLA-4 and PD1 co-stimulatory molecules [53], Duffy antigen receptor molecule [54] proposed to act as a ‘chemokine sink’ [55], and CD1d molecule [56] that presents antigens to NKT cells.
Why do Africans have stronger immune/inflammatory response? Although selective pressures of evolution on the human genome are very small [57, 58], there are several welldocumented examples. Most of them involve selection by infectious agents, except for the intestinal lactase variant selected for amongst the dairy farmers [59]. Thus, sickle cell trait was one of the earliest recognized traits selected for. HbβS heterozygosity confers about 10-fold increase in protection against life-threatening forms of the malaria induced by Plasmodium falciparum [60]. Similar patterns of heterozygosity advantage are apparently conferred by other hemoglobinopathies as well as by glucose-6-phosphate dehydrogenase deficiency [61]. Distribution pattern of cystic fibrosis mutation (higher frequency in northwest than in the south east Europe) suggests that heterozygosity may have been protective against cholera [62]. Perhaps the most striking example of selection is the Duffy antigen receptor molecule which is absent from the RBCs in almost 100% West Africans and serves as a receptor for Plasmodium vivax [63].
In addition to the RBC antigens, malaria infection has also exerted pressure for the development of strong immune responses. Polymorphic variants in HLA-B, HLADR, IL-4, CD40L, FcGR2A, TNF-α, and genes encoding other molecules affecting immune responses are more frequent in defined populations with increased resistance to malaria [64]. We therefore reasoned that there may be other as yet undiscovered malaria-selected polymorphisms that promote stronger immune responsiveness, and that some of them are likely to be close to Hbβ. The neighboring immune response-modifying genetic markers are more likely to segregate with HbβS than those located further away on the same chromosome, or those located on other chromosomes.
Polymorphism(s) in Ro52 and Alloimmunization
Based on the above discussion we hypothesized that two linked malaria-protective polymorphisms were co-selected: the HbβS and an allele of a near-by gene, encoding a molecule with immunomodulatory function. The consequence of this co-selection would be stronger immune responses in HbβS homozygous individuals, reflected in a high incidence of antibody responses following transfusion. The near-by allele may not be the only locus favoring strong immune responses in SCD patients. Nevertheless, we felt that investigating this possibility would be an important step in understanding alloimmunization and, by extension, human immune responsiveness.
The best candidate gene in the vicinity of Hbβ on chromosome 11p15 was found 832 kb away. The gene encodes for Ro52 protein, also known as Sjögren syndrome antigen 1 (SSA1) and lately as tripartite motif (TRIM)21 [65]. Ro52 is best known as the target for antibodies that develop in lupus erythematosus and Sjögren syndrome, two autoantibody-mediated autoimmune diseases characterized by overall increased antibody production. Ro52 is part of an RNA-protein complex consisting of four small RNA molecules, Ro52 and two additional proteins, Ro60 and La [66, 67]. Mice deficient in Ro60 develop lupus [68] confirming that the molecule targeted by the autoantibody can have an active role in developing or protecting against lupus. Interestingly, Ro52 gene harbors an SNP, designated rs660, with association with lupus in African Americans [69], but not in the Japanese population [70].
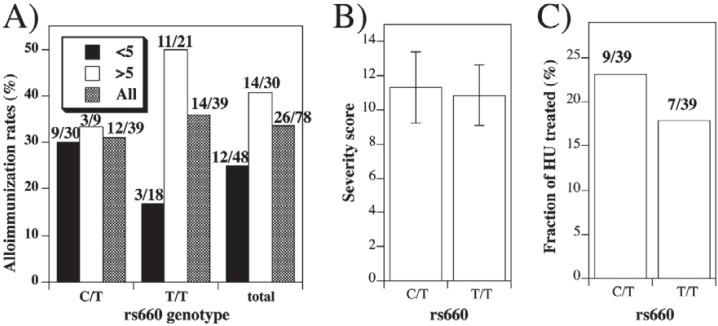
We therefore tested whether the rs660 genotype may be associated with alloimmunization in SCD. We recruited 83 patients of African American background homozygous for hemoglobin S (HbS) who received at least two ABO- and RhD-matched, cross-match-compatible leukodepleted RBC transfusions, documented by blood bank review. Overall distribution of rs660 alleles was unbalanced – 39 SCD patients were each of C/T and T/T, with only 5 patients with C/C genotype [71], similar to the frequencies previously observed in African Americans [70]. Consequently, comparisons were made between rs660C/T and rs660T/T patients. The frequency of alloimmunization in rs660C/T and rs660T/T patients was similar – 31% and 36%, respectively (fig. 1A). In addition, the markers of disease severity [72] (fig. 1B), the number of patients undergoing hydroxyurea treatment (fig. 1C), and the average total number of RBC transfusions received were indistinguishable (p = 0.45) in patients with rs660 C/T (84.3 ± 13.9) or T/T (101.2 ± 17.5) genotype (see also fig. 2D). Thus, rs660 does not associate with an overall rate of alloimmunization, or with indicators of severity of SCD.
Fig. 1.
Alloimmunization rates and rs660 genotype. A Frequency of alloimmunization in patients that received first transfusion before (closed bars) or after (open bars) the age of 5 (or all ages – shaded bars) in the function of rs660 genotype. The differences between alloimmunization rates in patients first transfused before or after the age of 5 were significant (Fisher's exact test) for T/T (p = 0.043), but not for C/T (p = 1.00) genotype, or for both groups together (0.195). Exact patient numbers are given above the bars. B Mean (± SE) SCD severity scores in patients with C/T or T/T genotype. Scores represent cumulative score B, calculated as described C Frequency of hydroxyurea treatment in patients with C/T or T/T genotype.
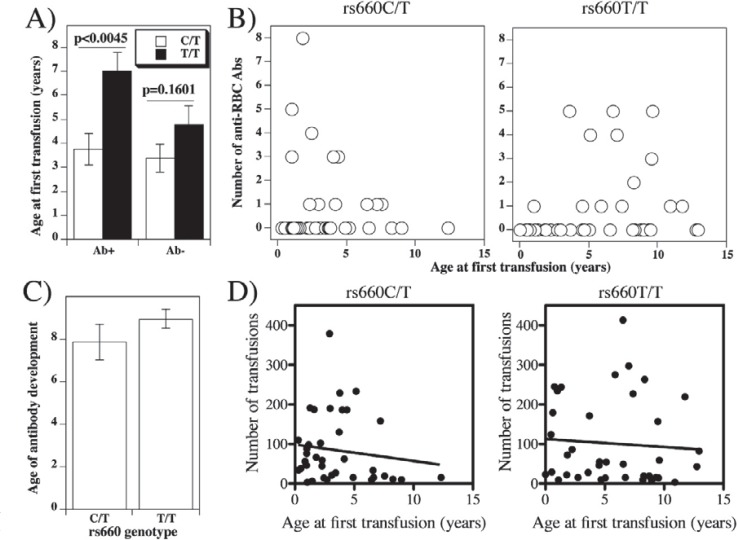
Fig. 2.
Anti-RBC antibody production as a function of patient age at first transfusion. A Average age (± SE) of antibody positive or negative patients with rs660C/T or rs660T/T genotype when they received first transfusion. B The number of distinct anti-RBC antibodies detected in each patient in the function of the age at first transfusion of individual SCD patients with C/T (left) or T/T (right) genotype.C Average age (±SE) of patients with rs660C/T or rs660T/T genotype when individual antibodies were detected (differences are not significant; p = 0.3615). D Correlation between age at first transfusion with total number of transfusions in patients with rs660C/T or rs660T/T genotype. Correlation coefficients were: r = 0.1364 for rs660C/T and r = 0.0712 for rs660T/T patients (not significant in either case). Best-fit straight lines were obtained by linear regression analysis and are represented by the following formulas: y = −23.7 −4.17x (rs660C/T) and y = 55.73 – 2.021x for rs660T/T. Deviations from zero were not significant (p = 0.414 for rs660C/T and p = 0.671 for rs660T/T genotype. Mean numbers of total transfusions received were 84.29 ± 13.92 for rs660C/T and 101.2 ± 17.47 for rs660T/T (p = 0.451).
Age at first transfusion is an important contributor to alloimmunization risk [73]. Mean age at first transfusion of SCD patients that developed at least one anti-RBC antibody was significantly lower in patients with rs660C/T than in patients with T/T genotype (fig. 2A; p = 0.045). In other words, relative to the rs660C/T patients, patients with rs660T/T genotype in general become alloimmunized if they are first exposed to RBC antigens late in life. The difference is not due to early initiation of transfusion in patients with the C/T genotype, since the average age of the first transfusion in antibody-negative patients was not significantly different (fig. 2A; p = 0.1601). When age at first transfusion was plotted for individual patients against the numbers of detected alloantibodies, it became clear that the age of 5 years represented the turning point (fig. 2B). The rate of alloimmunization in patients first transfused before 5 years of age was 30% if they were of C/T, and 17% if they were of T/T genotype (fig. 1A).
An important related issue is the patient age at antibody detection. Is the age at first transfusion an indicator of the age when antibodies develop? Age of subjects with C/T or T/T genotype when anti-RBC antibodies was detected was not significantly different (fig. 2C). This finding shows that patients with the C/T genotype are capable of producing anti-RBC antibodies after the age of 5, just like those with T/T genotype. In other words, the time it takes from the first RBC exposure to production of anti-RBC antibodies is much longer in patients with rs660C/T than in patients with rs660 T/T genotype. However, the latter respond mostly when RBCs transfusion is introduced beyond infancy and early childhood. Further, there is no correlation between total number of transfusions received and age at first transfusion in patients with either genotype (fig. 2D). Therefore, low alloimmunization rate in subjects with rs660T/T genotype when first transfused within the first 5 years of life suggests that they develop tolerance to RBCs more efficiently than rs660C/T subjects. The breakdown by antibody specificities suggests that tolerance is relatively equally inducible for several blood group antigens [71], excluding ABO and RhD antigens.
The term tolerance in immunology implies an active process induced by antigen. So, should the absence of antibody responses in SCD patients be designated ‘tolerance’, or perhaps a more passive term ‘non-responsiveness’ is better suited? The findings observed in patients with rs660C/T genotype clearly fit the ‘non-responsiveness’ designation: the patients developed antibodies to RBC transfusions after the age of 5 irrespective of whether they received transfusions before the age of 5. Thus, for this subset of patients exposure to RBC antigens during the early childhood did not alter the immune system responses to the same antigens later in life. However, administration of RBC transfusions before the age of 5 in patients with rs660T/T genotype clearly influenced non-responsiveness to RBC antigens after the age of 5. Although the exact timings of distinct RBC antigen exposures remain to be determined, it is evident that the ‘non-responsiveness’ in patients with rs660T/T genotype is induced, which in turn, is an operational definition of tolerance. Thus, we will in this review use the word ‘tolerance’, bearing in mind that it relates only to a subset of patients.
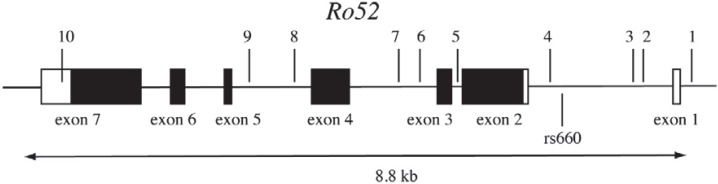
How can rs660 contribute to neonatal tolerance? Given that there appear to be differences in the levels of Ro52 expression in cell lines homozygous for rs660C and rs660T [71], one possibility is that rs660 is involved in enhancing or silencing the Ro52 expression. Ro52 gene consists of seven exons (fig. 3) spanning 8.8 kb (www.ncbi.nlm.nih.gov accession number NC_000011). The rs660 polymorphic site is located about 600 bp upstream of the initiation codon [70] in the first intron [74]. Bioinformatics analysis using MatInspector software (www.genomatix.de) indicated that there may be a potential for differential binding of three transcription factors to the sequence around and containing the rs660 SNP. These factors include nuclear receptor subfamily 2, peroxisome proliferator-activated receptor and Myt1 C2HC zinc finger protein that can all potentially bind to rs660C, but not to rs660T. Interestingly, this pattern of binding, if confirmed, would be suggestive of silencing function of the surrounding DNA element. Another possibility is that rs660 is in linkage disequilibrium with another polymorphism in Ro52 gene, or even outside the Ro52, that is directly involved in regulating Ro52 expression.
Fig. 3.
Exon/intron structure of the Ro52 gene and location of rs660. The coding and non-coding sequences are represented by closed and open rectangles, respectively. Indicated are the positions of rs660 below the line and ten arbitrarily chosen HapMap-validated SNPs above the line (1-rs1426378; 2-rs928914; 3-rs928915; 4-rs7947461; 5-rs926101; 6-rs2855142; 7-rs890419; 8-rs2554933; 9-rs2599586; 10-rs4144331).
Extended Neonatal Tolerance Concept
The finding of tolerance in early childhood is not a new concept. The infants’ immune system has long been thought to be prone to induction of tolerance, rather than immunity, as suggested by the classic neonatal tolerance experiments of Billinghamet al. [75]. They noted that tolerance to paternal antigens was acquired during pregnancy. These observations were inspired by Owen's studies of tolerance induction of antibody responses through in utero exposure to red blood cell alloantigens in cattle [76].
The concept of tolerance has been revisited frequently, and the original findings were confirmed in other species, including humans, and were extended beyond the neonatal into the early childhood period. In addition, most investigators now agree that neonates, infants, and children up to 5 years of age respond to antigens, but less efficiently than adults [77, 78, 79, 80]. Examples include responses to malaria [80, 81, 82], factor VIII in patients with hemophilia [83], RBC antigens in patients with SCD [84] and thalassemia [84, 85], response to vaccines [80, 86], and the intensity of immune cell infiltration in tumors [87]. Further, prolonged replication of HBV and human CMV [88, 89], and more rapid progression to AIDS [90, 91] when infections occur in early life suggest less efficient pathogen clearance by the immune system.
What are the mechanism(s) for suboptimal responses in neonatal and early childhood period? At least a part of the answer may lie in the dynamics of T-cell receptor repertoire generation. T cells are generated in the thymus, and 1–2% of total thymocytes are each day exported to the periphery as mature T cells, referred to as recent thymic emigrants [92]. In young adult mice, about 20% of the peripheral T-cell repertoire represents recent thymic emigrants, while in the young mice (up to 3 weeks of age – corresponding to the early childhood in humans) this number is close to 100% [93]. The important aspect of recent thymic emigrants is their functional potential – they are less functionally competent than the long-term peripheral naïve resident T lymphocytes and require post-thymic maturation in the peripheral lymphoid organs to acquire full competency [94]. Therefore, a larger fraction of functionally less competent T cells repopulate peripheral lymphoid organs of neonates and young children. Hence, lower level responses are not surprising.
Another factor that may contribute to relatively weaker neonatal responses is the ontogeny of terminal deoxynucleotidyl transferase (TdT). This enzyme makes template-independent additions at the junctions of variable, diversity and joining junctions during T-cell receptor and immunoglobulin rearrangement. This is a critical step in generating the diversity of immune receptors – about 90–95% of the T-cell receptor diversity was attributed to TdT [95]. However, in most species neonatal T cells do not express TdT. In humans, TdT expression is first noticeable around weeks 18–19 [96]. The consequence of this pattern is that the neonatal T-cell receptor repertoire is suboptimal, with relatively lower avidity for antigens [97].
The function of B cells is also suboptimal in early childhood. Human neonatal B cells express lower levels of the costimulatory molecules CD40, CD80 and CD86, which decreases their interaction with T cells [98]. Further, marginal zone B cells are present in lower numbers and display lower levels of CD21 (also known as complement receptor 2) that cripples their responses to thymus-independent 2 antigens (polysaccharides from encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitides, Haemophilus influenzae) in children under 2 years of age [99, 100, 101]. This results in lower levels of IgG2 and IgG4 isotypes that reach the adult levels not until the age of 5–10 [102, 103].
Finally, innate pro-inflammatory immune responses are also attenuated during neonatal and early childhood period, whereas anti-inflammatory responses (e.g. IL-10 secretion) are enhanced [104]. Some suggested mechanisms include lower expression of TLR4, CD14, MyD88, and IRF5 [105, 106, 107, 108]. Clearly, more research needs to be done on the functioning of the innate immune system in children, especially that related to TLR independent receptors like nucleotide oligomerization receptors (NOD) and retinoic acid-inducible gene I-like receptors (RLR) [109]. All in all, however, it is clear that both adaptive and innate functions of the immune system respond suboptimally during the neonatal period, and slowly progress towards adult levels during the early childhood.
Of Mice and Men
Our study implied a potential role for Ro52 in promoting the neonatal tolerance to RBC antigens. However, direct evidence was missing. To address this question Patel et al. [110] examined the role of Ro52 in a mouse model of alloimmunization using the Ro52 knock-out mice. They transfused wild-type controls and Ro52 knock-outs with RBCs expressing the HOD transgene (a fusion molecule containing hen egg lyzozyme, portion of ovalbumin and human Duffy antigen receptor complex) and tested anti-HOD antibody production 2 weeks post transfusion. They found that juvenile mice transfused at 3 weeks of age failed to produce specific antibodies, while adult mice (10–16 weeks old) produced antibodies with maximal frequency, irrespective of their Ro52 genotype. These results confirmed the suboptimal alloimmunization rates in young individuals, but according to the authors, did not support the role of Ro52 in promoting the early childhood tolerance.
So, what are the reasons for the discrepancy between the human and the mouse model? First, rs660 may be a marker of an adjacent functional gene other than Ro52. The association of rs660 with early childhood tolerance is through linkage disequilibrium with the causative genetic element that may, or may not lie, within the Ro52 gene. If it lies outside, then negative result is expected if the Ro52 is knocked out.
Species differences in the immune system have been described that could account for distinctive experimental observations [111]. Although at this point no differences in the function of the human and mouse Ro52 were noted, it remains possible that human and mouse alloimmunization models are (partly) unique.
The impact of the complete absence of a protein (such is the case in the mouse knock-out model) may be different from the impact of changing the protein levels (which is the case in SCD patient cohort), even within the same species.
Another possible explanation may be related to the immunogenicity of RBC antigens. The HOD 586 amino acids long fusion protein contains three antigens foreign to the mouse immune system, whereas differences between RBC donors and recipients are in general less significant. The most drastic differences include 417 amino acids when RhD-negative subjects respond to RhD antigens (due to RhD matching; however, this occurrence is extremely rare in contemporary transfusion medicine), or 338 amino acids when Duffy-negative recipients respond to Duffy-positive transfusion. Mostly, however, the antigenic differences are much smaller and can sometimes be only one amino acid [112, 113]. Thus the strength of the antigenic stimulation in the mouse system may have over-ridden any relatively small impact of Ro52 that might become notable only after a more subtle antigenic challenge.
The apparent absence of the impact of the Ro52 in the mouse model may be viewed in an entirely different light if antibody levels are taken as a key measurement instead of alloimmunization rates. Thus, Patel et al. [110] noted that the levels of anti-HOD antibodies were significantly (p = 0.02) lower in Ro52 knock-out than in the wild-type mice. Perhaps, with a less immunogenic stimulation the lower response in Ro-52-deficient mice would be recognized as a reduced alloimmunization rate.
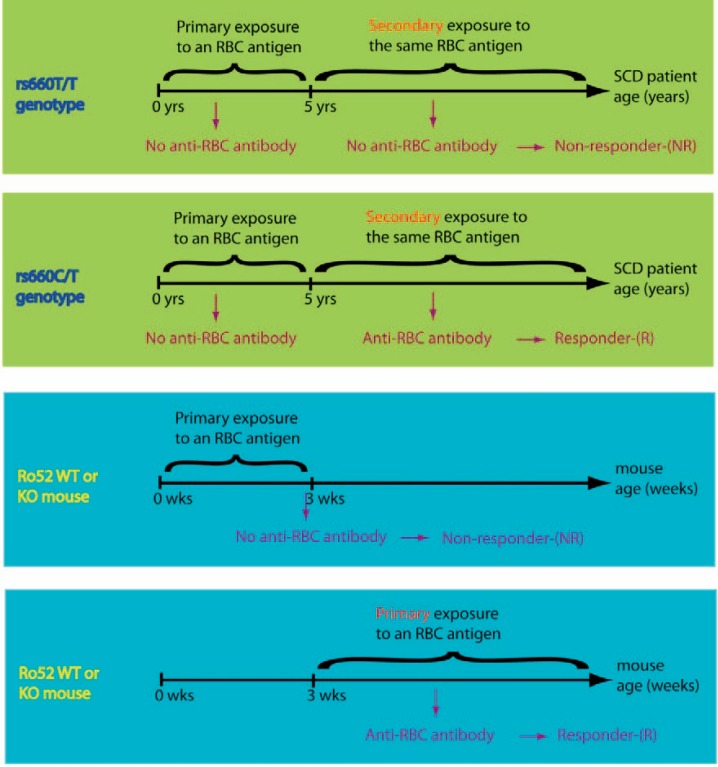
Finally, there are significant differences in the design between the mouse and human experimental models. Patients were exposed to RBC antigens during the early childhood period at least once, while the adult mice used in the study were exposed to the fusion antigen for the first time as adults (fig. 4). Thus, the mouse model does not replicate conditions observed in our study with SCD patients.
Fig. 4.
Similarities and differences between the studies using experimental mice and human subjects.
How Could Ro52 Induce Tolerance and Promote Lupus?
Assuming that rs660 association with both lupus and early childhood tolerance reflects a function of the Ro52 protein, we raise the following question: how can the same molecule be involved in apparently opposing outcomes? Ro52 can perform two opposing functions at the cellular level. Ro52 is an ubiquitin-conjugating E3 ligase [114] targeting various substrates for proteasome-mediated degradation. The best known substrates are IRF-3, IRF-5, IRF-7, and IRF-8 [115], hence the overall effect of Ro52 is inhibition of type I interferon production [116]. The overall anti-inflammatory role of Ro52 was confirmed by experiments in Ro52-deficient mice which mount excessive immune responses, characterized by production of autoantibodies and tissue pathology reminiscent of lupus [117].
However, Ro52 can also serve as an intracellular receptor for antigen-antibody complexes internalized via cell surface receptors that infectious agents use to enter the cells. This intracellular interaction results in activation of intracellular immune pathways, such as NF-κB, AP-1, IRF3, IRF5, and IRF7 [118]. This activates the production of pro-inflammatory cytokines, which promote resistance to viruses and intracellular bacteria [119]. Thus, depending on the context, Ro52 can have proinflammatory or anti-inflammatory actions. RBC antigens following transfusion are unlikely internalized as part of complexes with antibodies, hence anti-inflammatory function of Ro52 prevails. We would have to hypothesize that at least some antigen-antibody complexes that are abundantly formed in lupus are internalized, activating the intracellular immune reaction.
Conclusions and Future Directions
The present findings provide a proof of principle that lupus susceptibility locus rs660 may also be a marker of early childhood tolerance. There is a high possibility that both associations are mediated through the opposing functions of the same molecule – Ro52 – in regulating inflammation. In a similar manner, other lupus susceptibility loci [120], especially those that overlap with susceptibility to other autoimmune/inflammatory diseases [121], may be involved in promoting (anti-)inflammatory conditions favorable for producing antibodies and/or promoting tolerance following transfusion. Formal proof of linkage disequilibrium of rs660 with an element in Ro52 gene remains to be established, as well as a more detailed cellular and molecular mechanism of early childhood tolerance.
The implications of determining the molecular and cellular basis of R-NR status in alloimmunization go beyond the transfusion medicine. Thus, although the backbone of therapy for solid organ transplantation is directed toward altering the function of T cells, it is becoming increasingly clear that T cells are responsible mainly for acute rejection, while the antibodies are the primary cause of chronic transplant rejection [122, 123, 124]. Furthermore, biopharmaceuticals such as factors VIII and IX, growth hormone, erythropoietin, and IFN-α can also induce antibodies that interfere with their therapeutic efficacy [125]. Finally, alloimmunization may occur naturally during pregnancy and cause a host of pathological conditions for the fetus as well as consequences for subsequent conception. The neonatal conditions linked to alloimmunization include fetal and neonatal alloimmune thrombocytopenia [126, 127], fetal and neonatal hemolytic anemia [128], alloimmune neonatal neutropenia [129], hydrops fetalis [130], neonatal hemochromatosis [131], biliary atresia [132], and neonatal glomerulopathy [133]. Alloimmunization was also proposed to at least partially explain implantation failure, recurrent pregnancy loss and pre-eclampsia/eclampsia [134, 135] as well as inflammatory lesions of the placental villi during pregnancy [136]. It is therefore clear that the lessons learned from genetics of lupus and autoimmunity in general may have a broader impact than initially thought.
Disclosure Statement
The authors declare no conflict of interest with the pharmaceutical industry or elsewhere.
References
- 1.Smith-Whitley K. Alloimmunization in patients with sickle cell disease. In: Herman JH, Manno CS, editors. Pediatric Transfusion Therapy. Bethesda: AABB Press; 2002. pp. 249–282. [Google Scholar]
- 2.Pegelow CH, Adams RJ, McKie V, Abboud M, Berman B, Miller ST, Olivieri N, Vichinsky E, Wang W, Brambilla D. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. J Pediatr. 1995;126:896–899. doi: 10.1016/s0022-3476(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 3.Petz LD, Calhoun L, Shulman IA, Johnson C, Herron RM. The sickle cell hemolytic transfusion reaction syndrome. Transfusion. 1997;37:382–392. doi: 10.1046/j.1537-2995.1997.37497265338.x. [DOI] [PubMed] [Google Scholar]
- 4.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, Johnson CS, Rogers ZR, Smith-Whitley K, Wang WC, Telen MJ. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85:6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson ME, Anderson AR, Castillejo MI, Hillyer CD, Bray RA, Gebel HM, Josephson CD. HLA alloimmunization is associated with RBC antibodies in multiply transfused patients with sickle cell disease. Pediatr Blood Cancer. 2010;54:552–558. doi: 10.1002/pbc.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piomelli S. Chronic transfusions in patients with sickle cell disease. Indications and problems. Am J Pediatr Hematol Oncol. 1985;7:51–55. [PubMed] [Google Scholar]
- 8.Orlina AR, Unger PJ, Koshy M. Post-transfusion alloimmunization in patients with sickle cell disease. Am J Hematol. 1978;5:101–106. doi: 10.1002/ajh.2830050204. [DOI] [PubMed] [Google Scholar]
- 9.Luban NL. Variability in rates of alloimmunization in different groups of children with sickle cell disease: effect of ethnic background. Am J Pediatr Hematol Oncol. 1989;11:314–319. [PubMed] [Google Scholar]
- 10.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322:1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 11.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42:37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 12.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688–693. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinkerton PH, Coovadia AS, Goldstein J. Frequency of delayed hemolytic transfusion reactions following antibody screening and immediate-spin crossmatching. Transfusion. 1992;32:814–817. doi: 10.1046/j.1537-2995.1992.32993110751.x. [DOI] [PubMed] [Google Scholar]
- 14.Heddle NM, Soutar RL, O'Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 15.Vamvakas EC, Pineda AA, Reisner R, Santrach PJ, Moore SB. The differentiation of delayed hemolytic and delayed serologic transfusion reactions: incidence and predictors of hemolysis. Transfusion. 1995;35:26–32. doi: 10.1046/j.1537-2995.1995.35195090655.x. [DOI] [PubMed] [Google Scholar]
- 16.Storry JR. Human blood groups: Inheritance and importance in transfusion medicine. J Infus Nurs. 2003;26:367–372. doi: 10.1097/00129804-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Bashawri LA. Red cell alloimmunization in sickle-cell anaemia patients. East Mediterr Health J. 2007;13:1181–1189. doi: 10.26719/2007.13.5.1181. [DOI] [PubMed] [Google Scholar]
- 18.Natukunda B, Schonewille H, Ndugwa C, Brand A. Red blood cell alloimmunization in sickle cell disease patients in Uganda. Transfusion. 2010;50:20–25. doi: 10.1111/j.1537-2995.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 19.Aly R, El-Sharnoby MR, Hagag AA. Frequency of red cell alloimmunization in patients with sickle cell anemia in an Egyptian referral hospital. Transfus Apher Sci. 2012;47:253–257. doi: 10.1016/j.transci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Ben Amor I, Louati N, Khemekhem H, Dhieb A, Rekik H, Mdhaffar M, Gargouri J. Red blood cell immunization in haemoglobinopathies: about 84 cases (in French) Transfus Clin Biol. 2012;19:345–352. doi: 10.1016/j.tracli.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Olujohungbe A, Hambleton I, Stephens L, Serjeant B, Serjeant G. Red cell antibodies in patients with homozygous sickle cell disease: a comparison of patients in Jamaica and the United Kingdom. Br J Haematol. 2001;113:661–665. doi: 10.1046/j.1365-2141.2001.02819.x. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 24.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631–635. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 25.Hendrickson JE, Roback JD, Hillyer CD, Easley KA, Zimring JC. Discrete toll-like receptor agonists have differential effects on alloimmunization to transfused red blood cells. Transfusion. 2008;48:1869–1877. doi: 10.1111/j.1537-2995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 26.Tatari-Calderone Z, Fasano RM, Miles MR, Pinto LA, Luban NL, Vukmanovic S. High multi-cytokine levels are not a predictive marker of alloimmunization in transfused sickle cell disease patients. Cytokine. 2014;68:59–64. doi: 10.1016/j.cyto.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Gee BE, Mohamed AI, Buchanan ID, Al-Mahmoud A, Stiles JK. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med. 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol. 1994;87:592–597. doi: 10.1111/j.1365-2141.1994.tb08318.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SC, Shacks SJ, Qu Z, Wiley P. Type 2 cytokine serum levels in healthy sickle cell disease patients. J Natl Med Assoc. 1997;89:753–757. [PMC free article] [PubMed] [Google Scholar]
- 31.Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Hematol. 1998;61:49–54. doi: 10.1111/j.1600-0609.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 32.Raghupathy R, Haider MZ, Azizieh F, Abdelsalam R, D'Souza TM, Adekile AD. Th1 and Th2 cytokine profiles in sickle cell disease. Acta Haematol. 2000;103:197–202. doi: 10.1159/000041049. [DOI] [PubMed] [Google Scholar]
- 33.Mohan C. Environment versus genetics in autoimmunity: a geneticist's perspective. Lupus. 2006;15:791–793. doi: 10.1177/0961203306070005. [DOI] [PubMed] [Google Scholar]
- 34.Molokhia M, McKeigue P. Systemic lupus erythematosus: genes versus environment in high risk populations. Lupus. 2006;15:827–832. doi: 10.1177/0961203306070007. [DOI] [PubMed] [Google Scholar]
- 35.Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus. 2006;15:715–719. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 36.Barnes KC. Genetic epidemiology of health disparities in allergy and clinical immunology. J Allergy Clin Immunol. 2006;117:243–254. doi: 10.1016/j.jaci.2005.11.030. quiz 255-246. [DOI] [PubMed] [Google Scholar]
- 37.Jamerson KA. The disproportionate impact of hypertensive cardiovascular disease in African Americans: getting to the heart of the issue. J Clin Hypertens (Greenwich) 2004;6:4–10. doi: 10.1111/j.1524-6175.2004.03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowie CC, Harris MI, Silverman RE, Johnson EW, Rust KF. Effect of multiple risk factors on differences between blacks and whites in the prevalence of non-insulin-dependent diabetes mellitus in the United States. Am J Epidemiol. 1993;137:719–732. doi: 10.1093/oxfordjournals.aje.a116732. [DOI] [PubMed] [Google Scholar]
- 39.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 40.Carter BM, Holditch-Davis D. Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv Neonatal Care. 2008;8:285–290. doi: 10.1097/01.ANC.0000338019.56405.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly AP. Keloids. Dermatol Clin. 1988;6:413–424. [PubMed] [Google Scholar]
- 42.Basu D, Lopez I, Kulkarni A, Sellin JH. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100:2254–2261. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 43.Padiyar A, Augustine JJ, Bodziak KA, Aeder M, Schulak JA, Hricik DF. Influence of African-American ethnicity on acute rejection after early steroid withdrawal in primary kidney transplant recipients. Transplant Proc. 2010;42:1643–1647. doi: 10.1016/j.transproceed.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 44.Gaston RS, Hudson SL, Deierhoi MH, Barber WH, Laskow DA, Julian BA, Curtis JJ, Barger BO, Shroyer TW, Diethelm AG. Improved survival of primary cadaveric renal allografts in blacks with quadruple immunosuppression. Transplantation. 1992;53:103–109. doi: 10.1097/00007890-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Neylan JF. Immunosuppressive therapy in high-risk transplant patients: dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1997;64:1277–1282. doi: 10.1097/00007890-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 46.Kerman RH, Kimball PM, Van Buren CT, Lewis RM, Kahan BD. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51:338–342. doi: 10.1097/00007890-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17:564–572. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 48.Willwerth BM, Schaub B, Tantisira KG, Gold DR, Palmer LJ, Litonjua AA, Perkins DL, Schroeter C, Gibbons FK, Gillman MW, Weiss ST, Finn PW. Prenatal, perinatal, and heritable influences on cord blood immune responses. Ann Allergy Asthma Immunol. 2006;96:445–453. doi: 10.1016/S1081-1206(10)60912-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchings A, Purcell WM, Benfield MR. Peripheral blood antigen-presenting cells from African-Americans exhibit increased CD80 and CD86 expression. Clin Exp Immunol. 1999;118:247–252. doi: 10.1046/j.1365-2249.1999.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchings A, Purcell WM, Benfield MR. Increased costimulatory responses in African-American kidney allograft recipients. Transplantation. 2001;71:692–695. doi: 10.1097/00007890-200103150-00021. [DOI] [PubMed] [Google Scholar]
- 51.Zabaleta J, Schneider BG, Ryckman K, Hooper PF, Camargo MC, Piazuelo MB, Sierra RA, Fontham ET, Correa P, Williams SM, Ochoa AC. Ethnic differences in cytokine gene polymorphisms: potential implications for cancer development. Cancer Immunol Immunother. 2008;57:107–114. doi: 10.1007/s00262-007-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am J Epidemiol. 2004;160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 53.Martin AM, Athanasiadis G, Greshock JD, Fisher J, Lux MP, Calzone K, Rebbeck TR, Weber BL. Population frequencies of single nucleotide polymorphisms (SNPs) in immuno-modulatory genes. Hum Hered. 2003;55:171–178. doi: 10.1159/000073201. [DOI] [PubMed] [Google Scholar]
- 54.Nickel RG, Willadsen SA, Freidhoff LR, Huang SK, Caraballo L, Naidu RP, Levett P, Blumenthal M, Banks-Schlegel S, Bleecker E, Beaty T, Ober C, Barnes KC. Determination of Duffy genotypes in three populations of African descent using PCR and sequence-specific oligonucleotides. Hum Immunol. 1999;60:738–742. doi: 10.1016/s0198-8859(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 55.Fukuma N, Akimitsu N, Hamamoto H, Kusuhara H, Sugiyama Y, Sekimizu K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem Biophys Res Commun. 2003;303:137–139. doi: 10.1016/s0006-291x(03)00293-6. [DOI] [PubMed] [Google Scholar]
- 56.Chen QY, Jackson N, Vargas A, Chalew S, Rao J, Batzer M, Lan MS, Chang YH, Mokhashi M, Liu D. Identification of three genomic haplotypes 5’ to the human CD1d gene and their distribution in four ethnic groups. Tissue Antigens. 2003;62:442–448. doi: 10.1034/j.1399-0039.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 57.Varki A, Geschwind DH, Eichler EE. Explaining human uniqueness: genome interactions with environment, behaviour and culture. Nat Rev Genet. 2008;9:749–763. doi: 10.1038/nrg2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris EE. Nonadaptive processes in primate and human evolution. Am J Phys Anthropol. 2010;143(suppl 51):13–45. doi: 10.1002/ajpa.21439. [DOI] [PubMed] [Google Scholar]
- 59.Itan Y, Powell A, Beaumont MA, Burger J, Thomas MG. The origins of lactase persistence in Europe. PLoS Comput Biol. 2009;5:e1000491. doi: 10.1371/journal.pcbi.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954;4857:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omenn GS. Evolution in Health and Medicine Sackler Colloquium: Evolution and public health. Proc Natl Acad Sci U S A. 2010;107(suppl 1):1702–1709. doi: 10.1073/pnas.0906198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morral N, Bertranpetit J, Estivill X, Nunes V, Casals T, Gimenez J, Reis A, Varon-Mateeva R, Macek M, Jr, Kalaydjieva L, et al. The origin of the major cystic fibrosis mutation (delta F508) in European populations. Nat Genet. 1994;7:169–175. doi: 10.1038/ng0694-169. [DOI] [PubMed] [Google Scholar]
- 63.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 64.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005. pp. 171–192. [DOI] [PMC free article] [PubMed]
- 65.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- 66.Itoh K, Itoh Y, Frank MB. Protein heterogeneity in the human Ro/SSA ribonucleoproteins. The 52- and 60-kd Ro/SSA autoantigens are encoded by separate genes. J Clin Invest. 1991;87:177–186. doi: 10.1172/JCI114968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruijn GJ, Simons FH, van Venrooij WJ. Intracellular localization and nucleocytoplasmic transport of Ro RNP components. Eur J Cell Biol. 1997;74:123–132. [PubMed] [Google Scholar]
- 68.Xue D, Shi H, Smith JD, Chen X, Noe DA, Cedervall T, Yang DD, Eynon E, Brash DE, Kashgarian M, Flavell RA, Wolin SL. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proc Nat Acad Sci U S A. 2003;100:7503–7508. doi: 10.1073/pnas.0832411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank MB, Itoh K, Fujisaku A, Pontarotti P, Mattei MG, Neas BR. The mapping of the human 52-kD Ro/Ssa autoantigen gene to human chromosome 11, and its polymorphisms. Am J Hum Genet. 1993;52:183–191. [PMC free article] [PubMed] [Google Scholar]
- 70.Tsugu H, Horowitz R, Gibson N, Frank MB. The location of a disease-associated polymorphism and genomic structure of the human 52-kDa Ro/SSA locus (ssa1) Genomics. 1994;24:541–548. doi: 10.1006/geno.1994.1664. [DOI] [PubMed] [Google Scholar]
- 71.Tatari-Calderone Z, Minniti CP, Kratovil T, Stojakovic M, Vollmer A, Barjaktarevic I, Zhang E, Hoang A, Luban NLC, Vukmanovic S. Rs660 polymorphism Ro52 (SSA1; TRIM 21) is a marker for age-dependent tolerance induction and efficiency of alloimmunization in sickle cell disease. Mol Immunol. 2009;47:64–70. doi: 10.1016/j.molimm.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 72.van den Tweel XW, van der Lee JH, Heijboer H, Peters M, Fijnvandraat K. Development and validation of a pediatric severity index for sickle cell patients. Am J Hematol. 2010;85:746–751. doi: 10.1002/ajh.21846. [DOI] [PubMed] [Google Scholar]
- 73.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 74.Zhao B, Bepler G. Transcript map and complete genomic sequence for the 310 kb region of minimal allele loss on chromosome segment 11p15.5 in non-small-cell lung cancer. Oncogene. 2001;20:8154–8164. doi: 10.1038/sj.onc.1205027. [DOI] [PubMed] [Google Scholar]
- 75.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 76.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 77.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 79.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 80.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 81.Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner PG. Frequency of cytokine-producing T cells in patients of different age groups with Plasmodium falciparum malaria. J Infect Dis. 1999;179:209–216. doi: 10.1086/314571. [DOI] [PubMed] [Google Scholar]
- 82.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol. 2002;169:3200–3207. doi: 10.4049/jimmunol.169.6.3200. [DOI] [PubMed] [Google Scholar]
- 83.Zhang AH, Skupsky J, Scott DW. Factor VIII inhibitors: risk factors and methods for prevention and immune modulation. Clin Rev Allergy Immunol. 2009;37:114–124. doi: 10.1007/s12016-009-8122-5. [DOI] [PubMed] [Google Scholar]
- 84.Sarnaik S, Schornack J, Lusher JM. The incidence of development of irregular red cell antibodies in patients with sickle cell anemia. Transfusion. 1986;26:249–252. doi: 10.1046/j.1537-2995.1986.26386209381.x. [DOI] [PubMed] [Google Scholar]
- 85.Spanos T, Karageorga M, Ladis V, Peristeri J, Hatziliami A, Kattamis C. Red cell alloantibodies in patients with thalassemia. Vox Sang. 1990;58:50–55. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 86.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 87.Vakkila J, Jaffe R, Michelow M, Lotze MT. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: a major nosologic difference with adult tumors. Clin Cancer Res. 2006;12:2049–2054. doi: 10.1158/1078-0432.CCR-05-1824. [DOI] [PubMed] [Google Scholar]
- 88.Bortolotti F. Chronic viral hepatitis in childhood. Baillieres Clin Gastroenterol. 1996;10:185–206. doi: 10.1016/s0950-3528(96)90002-0. [DOI] [PubMed] [Google Scholar]
- 89.Stagno S, Pass RF, Dworsky ME, Britt WJ, Alford CA. Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig Artic Ser. 1984;20:65–85. [PubMed] [Google Scholar]
- 90.Goulder PJ, Jeena P, Tudor-Williams G, Burchett S. Paediatric HIV infection: correlates of protective immunity and global perspectives in prevention and management. Br Med Bull. 2001;58:89–108. doi: 10.1093/bmb/58.1.89. [DOI] [PubMed] [Google Scholar]
- 91.Luzuriaga K, Sullivan JL. Pediatric HIV-1 infection: advances and remaining challenges. AIDS Rev. 2002;4:21–26. [PubMed] [Google Scholar]
- 92.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fink PJ. The biology of recent thymic emigrants. Annu Rev Immunol. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 95.Cabaniols JP, Fazilleau N, Casrouge A, Kourilsky P, Kanellopoulos JM. Most alpha/beta T cell receptor diversity is due to terminal deoxynucleotidyl transferase. J Exp Med. 2001;194:1385–1390. doi: 10.1084/jem.194.9.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janossy G, Thomas JA, Bollum FJ, Granger S, Pizzolo G, Bradstock KF, Wong L, McMichael A, Ganeshaguru K, Hoffbrand AV. The human thymic microenvironment: an immunohistologic study. J Immunol. 1980;125:202–212. [PubMed] [Google Scholar]
- 97.Rudd BD, Venturi V, Smith NL, Nzingha K, Goldberg EL, Li G, Nikolich-Zugich J, Davenport MP. Acute neonatal infections ‘lock-in’ a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 2013;9:e1003572. doi: 10.1371/journal.ppat.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 99.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- 100.Rijkers GT, Sanders EA, Breukels MA, Zegers BJ. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16:1396–1400. doi: 10.1016/s0264-410x(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 101.Klein Klouwenberg P, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clin Dev Immunol. 2008;2008:628963. doi: 10.1155/2008/628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zegers BJ, van der Giessen M, Reerink-Brongers EE, Stoop JW. The serum IgG subclass levels in healthy infants of 13-62 weeks of age. Clin Chim Acta. 1980;101:265–269. doi: 10.1016/0009-8981(80)90252-1. [DOI] [PubMed] [Google Scholar]
- 103.Kutukculer N, Karaca NE, Demircioglu O, Aksu G. Increases in serum immunoglobulins to age-related normal levels in children with IgA and/or IgG subclass deficiency. Pediatr Allergy Immunol. 2007;18:167–173. doi: 10.1111/j.1399-3038.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 104.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES, 3rd, Kollmann TR. Ontogeny of toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLOS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, Pollak A, Spittler A. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 106.Henneke P, Osmers I, Bauer K, Lamping N, Versmold HT, Schumann RR. Impaired CD14-dependent and independent response of polymorphonuclear leukocytes in preterm infants. J Perinat Med. 2003;31:176–183. doi: 10.1515/JPM.2003.024. [DOI] [PubMed] [Google Scholar]
- 107.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 108.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med. 2007;30:E44–53. doi: 10.25011/cim.v30i2.979. [DOI] [PubMed] [Google Scholar]
- 109.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel SR, Hendrickson JE, Smith NH, Cadwell CM, Ozato K, Morse HC, 3rd, Yoshimi R, Zimring JC. Alloimmunization against RBC or PLT antigens is independent of TRIM21 expression in a murine model. Mol Immunol. 2011;48:909–913. doi: 10.1016/j.molimm.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 112.Klein HG, Anstee DJ. The Rh blood group system (and LW); in Mollison's Blood Transfusion in Clinical Medicine. Oxford: Blackwell; 2005. pp. 163–208. [Google Scholar]
- 113.Klein HG, Anstee DJ. Other red cell antigens; in Mollison's Blood Transfusion in Clinical Medicine. Oxford: Blackwell; 2005. pp. 209–252. [Google Scholar]
- 114.Espinosa A, Zhou W, Ek M, Hedlund M, Brauner S, Popovic K, Horvath L, Wallerskog T, Oukka M, Nyberg F, Kuchroo VK, Wahren-Herlenius M. The Sjogren's syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J Immunol. 2006;176:6277–6285. doi: 10.4049/jimmunol.176.10.6277. [DOI] [PubMed] [Google Scholar]
- 115.Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. 2012;39:77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 116.Higgs R, Ni Gabhann J, Ben Larbi N, Breen EP, Fitzgerald KA, Jefferies CA. The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J Immunol. 2008;181:1780–1786. doi: 10.4049/jimmunol.181.3.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, Sjostrand M, Eloranta ML, Ni Gabhann J, Winqvist O, Sundelin B, Jefferies CA, Rozell B, Kuchroo VK, Wahren-Herlenius M. Loss of the lupus autoantigen Ro52/TRIM21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206:1661–1671. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol. 2013;14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watkinson RE, McEwan WA, James LC. Intracellular antibody immunity. J Clin Immunol. 2014;34(suppl 1):30–34. doi: 10.1007/s10875-014-0017-4. [DOI] [PubMed] [Google Scholar]
- 120.Grossman JM, Tsao BP. Genetics and systemic lupus erythematosus. Curr Rheumatol Rep. 2000;2:13–18. doi: 10.1007/s11926-996-0063-x. [DOI] [PubMed] [Google Scholar]
- 121.Becker KG, Simon RM, Bailey-Wilson JE, Freidlin B, Biddison WE, McFarland HF, Trent JM. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc Natl Acad Sci U S A. 1998;95:9979–9984. doi: 10.1073/pnas.95.17.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 123.Colvin RB. Pathology of chronic humoral rejection. Contrib Nephrol. 2009;162:75–86. doi: 10.1159/000170814. [DOI] [PubMed] [Google Scholar]
- 124.Colvin RB, Smith RN. Antibody-mediated organallograft rejection. Nat Rev Immunol. 2005;5:807–817. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 125.Tamilvanan S, Raja NL, Sa B, Basu SK. Clinical concerns of immunogenicity produced at cellular levels by biopharmaceuticals following their parenteral administration into human body. J Drug Target. 2010;18:489–498. doi: 10.3109/10611861003649746. [DOI] [PubMed] [Google Scholar]
- 126.Kaplan C. Foetal and neonatal alloimmune thrombocytopaenia. Orphanet J Rare Dis. 2006;1:39. doi: 10.1186/1750-1172-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skogen B, Killie MK, Kjeldsen-Kragh J, Ahlen MT, Tiller H, Stuge TB, Husebekk A. Reconsidering fetal and neonatal alloimmune thrombocytopenia with a focus on screening and prevention. Expert Rev Hematol. 2010;3:559–566. doi: 10.1586/ehm.10.49. [DOI] [PubMed] [Google Scholar]
- 128.Egbor M, Knott P, Bhide A. Red-cell and platelet alloimmunisation in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;26:119–132. doi: 10.1016/j.bpobgyn.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 129.Maheshwari A, Christensen RD, Calhoun DA. Immune neutropenia in the neonate. Adv Pediatr. 2002;49:317–339. [PubMed] [Google Scholar]
- 130.Joshi DD, Nickerson HJ, McManus MJ. Hydrops fetalis caused by homozygous alpha-thalassemia and Rh antigen alloimmunization: report of a survivor and literature review. Clin Med Res. 2004;2:228–232. doi: 10.3121/cmr.2.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitington PF. Neonatal hemochromatosis: a congenital alloimmune hepatitis. Semin Liver Dis. 2007;27:243–250. doi: 10.1055/s-2007-985069. [DOI] [PubMed] [Google Scholar]
- 132.Muraji T, Suskind DL, Irie N. Biliary atresia: a new immunological insight into etiopathogenesis. Expert Rev Gastroenterol Hepatol. 2009;3:599–606. doi: 10.1586/egh.09.61. [DOI] [PubMed] [Google Scholar]
- 133.Ronco P, Debiec H. Podocyte antigens and glomerular disease. Nephron Exp Nephrol. 2007;107:e41–46. doi: 10.1159/000107708. [DOI] [PubMed] [Google Scholar]
- 134.Robertson SA, Sharkey DJ. The role of semen in induction of maternal immune tolerance to pregnancy. Semin Immunol. 2001;13:243–254. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 135.Christiansen OB, Steffensen R, Nielsen HS. Anti-HY responses in pregnancy disorders. Am J Reprod Immunol. 2011;66(suppl 1):93–100. doi: 10.1111/j.1600-0897.2011.01038.x. [DOI] [PubMed] [Google Scholar]
- 136.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]