Abstract
The genes encoding group IIE phospholipase A2, abbreviated as IIE PLA2, and its 5' and 3' flanking regions of Crotalinae snakes such as Protobothrops flavoviridis, P. tokarensis, P. elegans, and Ovophis okinavensis, were found and sequenced. The genes consisted of four exons and three introns and coded for 22 or 24 amino acid residues of the signal peptides and 134 amino acid residues of the mature proteins. These IIE PLA2s show high similarity to those from mammals and Colubridae snakes. The high expression level of IIE PLA2s in Crotalinae venom glands suggests that they should work as venomous proteins. The blast analysis indicated that the gene encoding OTUD3, which is ovarian tumor domain-containing protein 3, is located in the 3' downstream of IIE PLA2 gene. Moreover, a group IIA PLA2 gene was found in the 5' upstream of IIE PLA2 gene linked to the OTUD3 gene (OTUD3) in the P. flavoviridis genome. It became evident that the specified arrangement of IIA PLA2 gene, IIE PLA2 gene, and OTUD3 in this order is common in the genomes of humans to snakes. The present finding that the genes encoding various secretory PLA2s form a cluster in the genomes of humans to birds is closely related to the previous finding that six venom PLA2 isozyme genes are densely clustered in the so-called NIS-1 fragment of the P. flavoviridis genome. It is also suggested that venom IIA PLA2 genes may be evolutionarily derived from the IIE PLA2 gene.
Keywords: group IIE phospholipase A2, venom, evolution, gene cluster, comparative genomics
1. Introduction
Protobothrops genus snakes (Crotalinae, Viperidae) are distributed in the southwestern islands of Japan, P. flavoviridis and Ovophis okinavensis in Amami-Oshima, Tokunoshima, and the Okinawa islands, P. tokarensis in the Tokara islands, and P. elegans in the Sakishima islands. The venoms of Protobothrops snakes are produced and stored in the venom glands, which are assumed to share an original developmental organ with the mammalian submaxillary glands. The injection of the venom through tubular front fangs causes various severe lesions in humans, such as myonecrosis, hemorrhage, and edema [1,2,3].
Phospholipase A2 (PLA2) [EC 3.1.1.4] catalyzes the hydrolysis of glycerophospholipid at the sn-2 position to produce free fatty acids and lysophospholipids [4]. As various forms of PLA2s work in almost whole organs in the body [5], they are divided into three categories: secretory, cytosolic, and Ca2+-independent PLA2s, based on the working modes [6]. Furthermore, novel transcriptome analysis in mammals showed that secretory PLA2s are classified into 11 groups: IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA, and XIIB, according to the primary structures and the organs to be expressed [7]. Snake venoms also contain PLA2 isoforms as major toxic components. With regard to the primary structures and the modes of disulfide bond pairings [8], snake venom PLA2s are classified into group IA found in Elapidae (Elapinae and Hydrophiinae) venoms and group II found in Viperidae (Viperinae and Crotalinae) venoms [9]. Group II venom PLA2s are further divided into group IIA PLA2s ([Asp49]PLA2 forms) and group IIB PLA2s ([Lys49]PLA2 forms) [10,11]. P. flavoviridis (Crotalinae) group IIA venom PLA2 genes form a multi-gene family of 16~32 copies per haploid [12] and are located at two loci on a microchromosome [13]. The mathematical analysis of their nucleotide sequences delineated that they have evolved in an accelerated manner to acquire isozymes with diverse physiological activities [14,15,16]. Recently, the nucleotide sequence of the 31,348 bp genome fragment of P. flavoviridis was completely deciphered. It showed that six PLA2 isozyme genes are aligned in series and four of them are linked with the fragment of CR1 long interspersed nuclear element (LINE), named PcRTF (PLA2 gene-coupled reverse transcriptase fragment), at the 3' terminus [13]. We call this fragment, composed of six PLA2 isozyme genes, NIS-1 (Figure 5 and Figure 6). Fry et al. (2012) found that group IIE PLA2 was expressed in the venom glands of Colubridae snakes and proposed that it is a component of Colubridae snake venoms [17].
Figure 5.
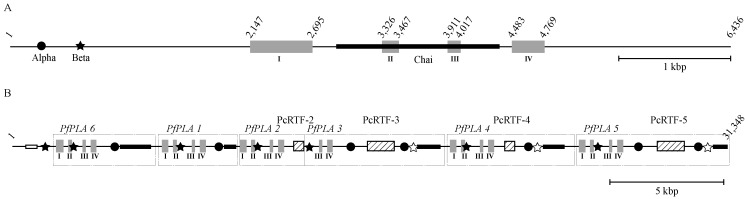
Diagrammatic representation of secretory PLA2 genes in human, mouse, chicken, and snake genomes. The names of the organisms and the numbers of chromosomes are shown at left. Bold arrows indicate the areas of the genes in the chromosomes and the direction of arrows indicates the transcribing direction of the genes. Dashed lines indicate the regions where the nucleotide sequences are not determined. Organisms and genome information: H. sapiens chr. 1 (NC000001.10); M. musculus chr. 4 (NC000070.6); G. gallus chr. 21 (NC006108.3); O. hannah scaffold 1015.1 (AZIM01001014); P. flavoviridis NIS-1 (AB440236), PfPLA 6 (AB588615), and PfIIEPLA2 (this work, KM488539).
Figure 6.
The schematic representation of the locations of three typical nucleotide segments, named Alpha, Beta, and Chai, in the PfIIEPLA2 gene (A); and in six IIA PLA2 genes in the NIS-1 fragment [13,29] of P. flavoviridis (B). Alpha, Beta, and Chai segments are shown by closed circle, closed star, and closed box, respectively. Gray boxes indicate exons of the PLA2 gene and their numbers are shown as Roman numerals below the boxes. Boxes filled with oblique lines indicate the retroelements named PcRTFs [13]. The nucleotide position numbers are the same as those in Figure 1 and those reported previously [13]. The open star and open box mean the antisense nucleotide segments of Beta and Chai segments, respectively. The genome fragment, which encompasses from the venom IIA PLA2 isozyme genes with or without PcRTF segment in the 3' terminus to the Alpha and Chai segments, is bracketed as a unit.
In the work reported here, we sequenced the segment-harboring novel IIE PLA2 gene linked to OTUD3, which codes for the ovarian tumor domain-containing protein (OTUD) 3, in the P. flavoviridis genome. Moreover, the IIA PLA2 gene was found in the 5' upstream of IIE PLA2 gene. It became evident that the linear arrangement of the IIA PLA2 gene, the IIE PLA2 gene, and OTUD3, in this order, is common in the genomes of humans to snakes. It is also found that the clusters of the genes encoding various PLA2s in the 5' upstream region of IIE PLA2 gene in the genomes of humans to birds possibly correspond to those of six PLA2 isozyme genes in NIS-1 fragment of P. flavoviridis genome. Possible conversion of the IIE PLA2 gene to the IIA PLA2 gene and its multiplication in Crotalinae snake genomes are discussed.
2. Materials and Methods
2.1. Materials
P. flavoviridis (Amami-Oshima Island, Japan), P. tokarensis, P. elegans, and O. okinavensis specimens were provided from the Institute of Medical Sciences of the University of Tokyo. High molecular weight genomic DNAs were prepared from the livers or the venom glands of the snakes according to the method of Blin and Stafford (1976) [18]. Total RNAs were prepared from various organs of the snakes according to the protocol of ISOGEN (Nippon Gene, Toyama, Japan). Restriction endonucleases and KOD plus DNA polymerase were purchased from Nippon Gene and TOYOBO (Osaka, Japan), respectively. The other reagents and antibiotics were from Nacalai Tesque (Kyoto, Japan) and TAKARA BIO (Shiga, Japan). Specific oligonucleotide primers were synthesized by GENNET (Fukuoka, Japan). All relevant ethical safeguards have been met in relation to animal experimentation.
2.2. Cloning and Sequencing of the Genome Segments Harboring IIE PLA2 Gene and Its 5' and 3' Flanking Regions of Crotalinae Snakes and of Their IIE PLA2 cDNAs
The personal expressed sequence tags (ESTs) database was constructed to unite the snake ESTs collected from Genbank and the ESTs of P. flavoviridis venom glands supplied from the Medical Institute of Biodefense of Kyushu University (Fukumaki and Shibata, unpublished) by utilizing the NCBI C++ Toolkit (National Center for Biotechnology Information, Bethesda, MD, USA). The tblastx analysis of the database was carried out with the nucleotide sequences of Homo sapiens IIE PLA2 gene (NM_014589) [19] and Mus musculus IIE PLA2 gene (NM_012044) [20] as query. The 1085 bp candidate subject, named isotig03504, was acquired. Isotig is a subsequence of an isogroup. An isogroup is an assembled transcription sequence approximately equivalent to that of a gene. Based on the nucleotide sequences of its 5' and 3' ends, the sense primer named SPII-3, 5'-gTA gAC TgC gCg TAA TTT gTA g-3', and the antisense primer named SPII-2, 5'-ggC CgA gTC CgT CgT AgC T-3', were designed (Figure 1). Genomic PCRs with these primers were carried out against the P. flavoviridis, P. tokarensis, P. elegans, and O. okinavensis genomes as the templates. Thus, about 2.6 kbp DNA fragments, which contain four exons coding for IIE PLA2s, were obtained. Moreover, to acquire the nucleotide sequences of its 5' and 3' flanking regions, adaptor ligation PCR, designated as “Ligation-Mediated PCR (LM-PCR)” (Takara Bio, Shiga, Japan), was conducted. The genomic DNA obtained by digestion with Hind III or Pst I was ligated with the adaptor nucleotide fragments with Hind III- or Pst I-terminus, designated as a “cassette.” Then, PCR was done with a C1 primer, 5'-gTA CAT ATT gTC gTT AgA ACg CgT AAT ACg ACT CA-3', which can anneal to the “cassettes,” and SPII-2 primer to amplify the genome fragment containing the 5' flanking region or SPII-3 primer to amplify the genome fragment containing the 3' flanking region (Figure 1). Moreover, to ensure the validity of the PCR, another PCR was conducted with the C2 primer, 5'-CgT TAg AAC gCg TAA TAC gAC TCA CTA TAg ggA gA-3', which can anneal to the “cassette” at the internal portion of the C1 primer and SPII-8 primer, 5'-CAg TCC TTC CAT AAA gCT C-3', to amplify the genome fragment corresponding to the 5' flanking region, or SPII-10 primer, 5'-CTT gCA CgT CTC Cgg ATT gTg-3', to amplify the genome fragment corresponding to the 3' flanking region to be overlapped to the fragments prepared as described above (Figure 1). Amplified genome fragments were ligated to pCR™-Blunt II-TOPO® vector (Life Technologies, Carlsbad, CA, USA), and transformed with DH5α competent cells (Takara Bio). The nucleotide sequences were determined with an ABI 3130xl capillary sequencer. The nucleotide sequences of Crotalinae IIE PLA2 genes and their 5' and 3' flanking regions are available in the Genbank/EMBL/DDBJ databases under Accession Nos. KM488538-KM488542.
Figure 1.
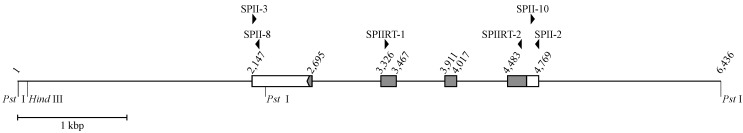
The schematic representation of the genome segment harboring the Crotalinae IIE PLA2 gene. The nucleotide positions are numbered. Closed boxes represent open reading frames (ORFs) and open boxes untranslated regions (UTRs). Vertical bars indicate the positions of restriction enzyme sites. Arrow heads show the positions of primers.
2.3. Acquisition of the Genome Segment Harboring IIA PLA2 Gene, IIE PLA2 Gene, and OTUD3 of P. flavoviridis
Long genomic PCR was carried out with CHO5, 5'-gAT TCg ggA ggA TgA ggA CTC TC-3' [21], which anneals to the 5' UTR of the IIA PLA2 gene, and OTUD3-1, 5'-CCT Tgg TAg CCT CTT TgC CAT CAg-3', which anneals to the middle portion of intron 7 of OTUD3, against the P. flavoviridis genome in order to confirm whether the IIA PLA2 gene is located in the 5' upstream of the IIE PLA2 gene linked to OTUD3.
2.4. Expression Analysis by Semi-Quantitative RT-PCR of Crotalinae IIE PLA2 mRNA
The first strand cDNA of snake body organs was synthesized by reverse transcription and primer extension of the SMART cDNA Library Construction Kit (Clontech Laboratories, Mountain View, CA, USA). Based on the nucleotide sequences of the genes encoding the IIE PLA2s of Crotalinae snakes, the sense primer SPIIRT-1, 5'-CAC ATC ATC RAg CAC TTg AC-3', which commonly anneals to the middle portion of exon 2, was designed. The antisense primers SPIIRT-2 (5'-TCC TTC gCA CAg gCg gTT A-3', which can anneal specifically to the middle portion of exon 4 of the P. flavoviridis IIE PLA2 gene) and SPIIRT-3 (5'-TCC TTC gCA CAg gCg gTT A-3', which can anneal specifically to the middle portion of exon 4 of the O. okinavensis IIE PLA2 gene) were designed. The cDNA of β-actin, designated as ACTB, was amplified as an internal standard with the sense primer SHU7, 5'-CAg AgC AAg AgA ggT ATC CN-3' (N = G, A, T, C), and the antisense primer SHU8, 5'-TAg ATg ggC ACA gTg Tgg gN-3', as described previously [22]. The intensities of the bands of the amplified DNA fragments were estimated with Image J (NIH, Bethesda, MD, USA) and corrected relative to those of ACTB. The vertical numerals of the histogram are the values relative to that of the lung of P. flavoviridis, taken as one.
2.5. Phylogenetic Analysis of Secretory PLA2s
A phylogenetic tree was constructed based on the amino acid sequences of the mature proteins of the secretory PLA2s from various organisms (Homo sapiens, Mus musculus, Gallus gallus, Ornithorhynchus anatinus, Macaca mulatta, Pan troglodytes, Oryctolagus cuniculus, Canis lupus familiaris, Bos taurus, Laticauda semifasciata, Leioheterodon madagascariensis, Dispholidus typus, P. flavoviridis, P. tokarensis, P. elegans, and O. okinavensis) with the maximum likelihood method of the RAxML program [23]. The degrees of confidence for internal lineage in the phylogenetic tree were determined by the bootstrap confidence [24] using Kimura’s (1969) method to compute a distance matrix with 1000 replicates [25].
2.6. Comparative Structural Analysis of the Cluster Domains of Secretory PLA2 Genes in the Genomes
The BLAST analysis done with the nucleotide sequences of the cDNA encoding human secretory IIA, IIC, IID, IIE, IIF, and V PLA2s [5], against the draft genome databases of H. sapiens (GRCh37P.p13), M. musculus (GRCm38.p2), and G. gallus (Gallus_gallus-4.0), deciphered that secretory PLA2s are distributed within a 300 kb genome segment of H. sapiens chromosome 1 (NC_000001 GPC_000000025), 200 kb of M. musculus chromosome 4 (NC_000070 GPC_000000777), and 21 kb of G. gallus chromosome 21 (NC_006108 GPC_000000738) (Figure 5). The OTUD3 (NP_056022 for H. sapiens, NP_082729 for M. muculus, XP_424363 for G. gallus) was found in the 3' downstream of a series of PLA2 genes in these regions. In the case of Ophiophagus hannah, all the draft genome data (AZIM00000000.1) [26] were downloaded and made it personal O. hannah genome database. Referring to these gene arrangements in H. sapiens, M. musculus, and G. gallus, the contig harboring secretory PLA2 genes was constructed through tblastn analysis against the O. hannah personal genome database. Then, the chromosomal loci of the secretory PLA2 genes and OTUD3 were mapped on their scaffolds and compared with the P. flavoviridis genome segments harboring the IIE PLA2 gene and OTUD3 (this study) and the NIS-1 fragment composing of six consecutive IIA PLA2 genes [13].
3. Results and Discussion
3.1. The Structure of a 6436 bp P. flavoviridis Genome Segment Containing the IIE PLA2 Gene
The tblastx analysis of the personal EST database gave three subjects, two of which were the wrong transcripts; one contained a stop codon with the redundant mutations and the other was fused with an irrelevant nucleotide fragment. As the deduced amino acid sequence encoded by the remaining subject, isotig03504, is similar to those of human (59%) and mouse IIE PLA2 proteins (61%)—in particular, the positions of the half-cystine residues and the sequences of the Ca2+ binding site and the catalytic site are identical—this subject is thought to be the transcript derived from the gene encoding P. flavoviridis IIE PLA2, designated as PfIIEPLA2. Genomic PCR with SPII-2 and SPII-3 primers, which can anneal to the 5' and 3' terminal portions, respectively, of isotig03504, of the Amami-Oshima P. flavoviridis genome gave a 2616 bp fragment, which covers from the 5' portion of the first exon to the 3' terminal portion of the fourth exon of the PfIIEPLA2 gene. In addition, LM-PCR of the Amami-Oshima P. flavoviridis genome gave a 6436 bp genome fragment harboring the PfIIEPLA2 gene and its 5' and 3' flanking regions (Figure 1). Moreover, genomic PCR of P. tokarensis, P. elegans, and O. okinavenesis genome DNA also gave the genome fragments harboring the PtIIEPLA2, PeIIEPLA2, and OoIIEPLA2 genes, together with their 5' and 3' flanking regions.
3.2. The Characteristic Primary Structures of Snake IIE PLA2 Proteins
The deduced amino acid sequences of Crotalinae IIE PLA2s are aligned with those of the IIE PLA2s from two Colubridae genus snakes [17], from H. sapiens (NP_055404) [19] and M. musculus (NP_036174) [20], as well as those from the venom IIA PLA2s from P. flavoviridis [27,28,29], venom IA PLA2 from Laticauda semifasciata [30], and pancreatic IB PLA2 from P. flavoviridis [31] (Figure 2). This alignment confirms that four PLA2s, PfIIEPLA2, PtIIEPLA2, PeIIEPLA2, and OoIIEPLA2, from Crotalinae genus snakes are clearly classified into group IIE. Although the amino acid sequences of IIE and IIA PLA2s are similar to one another, the C-terminal amino acid sequences from the 133th residue are distinct between them. The phylogenetic analysis including other group PLA2s, such as IA, IB, IIC, IID, IIF, and V PLA2s, also shows that the IIE PLA2s, including four novel Crotalinae PLA2s, form an independent clade separated from other group PLA2s (Figure 3). Moreover, IIE PLA2s are further divided into those from snakes or mammals, in accordance with the differences in their C-terminal sequences.
Figure 2.
The aligned amino acid sequences of IIE, IIA, IA and IB PLA2s from snakes and mammals. The positions are numbered from the first residue of the signal peptides. The half-cystines are shown in shaded letters. Abbreviations: Dt, Dispholidus typus; Hs, Homo sapiens; Lm, Leioheterodon madagascariensis; Ls, Laticauda semifasciata; Mm, Mus musculus; Oo, Ovophis okinavensis; Pe, Protobothrops elegans; Pf, P. flavoviridis; and Pt, P. tokarensis. References: PfIIEPLA2 (this work); PtIIEPLA2 (this work); PeIIEPLA2 (this work); OoIIEPLA2 (this work); DtDis-1 (AFH66958) [17]; HsIIEPLA2 (NP_055404) [19]; LmLei-1 (AFH66960) [17]; LsLsPLA2cPm09 (BAB03302) [32]; MmIIEPLA2 (NP_036174) [20]; PfPLA-B (BAG82670) [13]; PfPLA-N (BAG82669) [13]; PfPLA 6 (BAJ84552) [29]; and PfPancPLA2 (BAN08536) [31]. Numerals in parentheses show the identities of the amino acid sequences against those of the mature protein of PfIIEPLA2.
Figure 3.
The phylogenetic tree constructed for the secretory PLA2s of snakes and mammals, based on the amino acid sequences of their mature proteins. The numerals at the nodes represent bootstrap confidence values and the branch lengths represent the numbers of amino acid substitutions per site. Abbreviations: Bt, Bos taurus; Cf, Canis lupus familiaris; Gg, Gallus gallus; Mc, Macaca mulatta; Oa, Ornithorhynchus anatinus; Oc, Oryctolagus cuniculus; and Pn, Pan troglodytes. References: BtIIEPLA2 (NP_001179015) [33], CfIIEPLA2 (XP_544525) (automated computational prediction by GNOMON); DtDis-2 (AFH66959) [17]; GgIBPLA2 (NP_001138961) [34]; GgIIEPLA2 (NP_001171878) [35]; HsIBPLA2 (NP_000919) [36]; HsIIAPLA2 (NP_001155199) [37]; HsIICPLA2 (NP_001099042) [38]; HsIIDPLA2 (NP_036532) [39]; HsIIFPLA2 (NP_073730) [40]; HsVPLA2 (NP_000920) [38]; LmLei-2 (AFH66961) [17]; LmLei-3 (AFH66962) [17]; McIIEPLA2 (XP_001094364) (automated computational prediction by GNOMON); MmIBPLA2 (NP_035237) [41]; MmIIAPLA2 (NP_001076000) [42]; MmIICPLA2 (NP_032894) [41]; MmIIDPLA2 (NP_035239) [39]; MmIIFPLA2 (NP_036175) [20]; MmVPLA2 (NP_001116426) [41]; OaIIEPLA2 (XP_001505559) (automated computational prediction by GNOMON); OcIIEPLA2 (XP_002716050) (automated computational prediction by GNOMON); OoIIEPLA2 (this work); PeIIEPLA2 (this work); PfIIEPLA2 (this work); PnIIEPLA2 (XP_001163677) (automated computational prediction by GNOMON); and PtIIEPLA2 (this work).
3.3. Venom Gland-Specific Expression of IIE PLA2s in Crotalinae Snakes
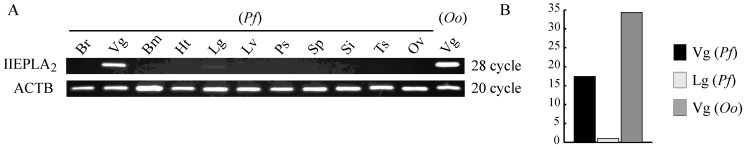
In general, mammalian IIE PLA2s are non-venomous somatic molecules. On the other hand, as mRNA-encoding IIE PLA2s, abbreviated as Lei-1, 2, and 3 and Dis-1 and 2, were found in the venom glands of Colubridae snakes Leioheterodon madagascariensis and Dispholidus typus, respectively, it was proposed that the IIE PLA2 of Colubridae snakes may work as a venom protein [17]. In the case of Crotalinae genus snakes, the expression analysis by semi-quantitative RT-PCR performed on several organs of P. flavoviridis and O. okinavensis showed that the IIE PLA2s of P. flavoviridis and O. okinavensis are expressed at remarkably high levels in the venom glands (Figure 4). These results suggest that Crotalinae IIE PLA2s are also venom proteins. Its expression in the lungs, though at a low level, may show that they act as an immune factor to neutralize bacteria infected through the air [43,44].
Figure 4.
(A) Electrophoretograms of RT-PCR products of IIE PLA2 mRNA and β-actin mRNA (ACTB, as the internal standard) for various organs of P. flavoviridis and O. okinavensis; (B) The histogram showing the relative intensities of the bands of IIE PLA2s from (A). Abbreviations: Bm, Buccinator muscle; Br, Brain; Ht, Heart; Lg, Lung; Lv, Liver; Ov, Ovary; Ps, Pancreas; Si, Small intestine; Sp, Spleen; Ts, Testis; and Vg, Venom gland.
3.4. The Genome Structures Harboring Secretory PLA2 Genes Are Conserved from Human to Snake
The BLAST analysis showed that OTUD3 is located in the 3' downstream of the PfIIEPLA2 gene in the P. flavoviridis genome (Figure 5). Based on the linear arrangement of the IIE PLA2 gene and OTUD3 and the nucleotide sequences of the cDNA encoding human secretory IIA, IIC, IID, IIE, IIF, and V PLA2s [5], BLAST analysis was made against the H. sapiens draft-genome database [45]. Then, it was found that the secretory PLA2 genes are aligned in the 5' upstream of OTUD3 within the 300-kb genome segment of H. sapiens chromosome 1. Interestingly, similar genome structures were found in the M. musculus, G. gallus, and O. hannah genomes (Figure 5). Particularly, it should be noted that the linear arrangement of the IIA PLA2 gene, the IIE PLA2 gene, and OTUD3, that is, the triplet genes, in this order is common in the genomes of human, mouse, and snake. In the case of O. hannah, it was found that three PLA2 genes, that is, the PfPLA 6-like gene, the IID PLA2 gene, and the IIF PLA2 gene, are aligned in the 5' upstream of the IIE PLA2 gene and OTUD3. The PfPLA 6 gene is contained in the P. flavoviridis NIS-1 fragment [13,29]. In the case of the G. gallus genome, the IIA PLA2 gene is found in the 5' upstream of IIE PLA2 gene, but the IIA and IIE PLA2 genes are interrupted by the V PLA2 gene. This unexpected location of the V PLA2 gene in G. gallus is thought to be specific to birds. The alignment of secretory PLA2 genes in the 5' upstream of OTUD3 should be highly conserved among the vertebrates. In this work, we also acquired an 11 kb genome fragment of P. flavoviridis, which encompasses from the gene encoding venom IIA PLA2, called PLA-B', at the 5' terminus to intron 7 of OTUD3 at the 3' terminus, by genomic PCR with CHO5, which anneals to the 5' UTR of the venom IIA PLA2 gene, and OTUD3-1, which specifically anneals to the middle portion of intron 7 of OTUD3 (data not shown). The alignment of six IIA PLA2 isozyme genes in the P. flavoviridis NIS-1 fragment is shown in Figure 5 [13]. PfPLA 6 codes for a novel basic [Asp49]PLA2 [29], PfPLA 1(Ψ) is 91% similar in sequence to PfPLA 6 with 10 nucleotide deletions, PfPLA 2 [Lys49]PLA2 called BPII, PfPLA 3(Ψ) is a fragment from the second intron to the fourth exon of the G6D49PLA2 gene found in the Trimeresurus stejnegeri snake [46], PfPLA 4 is a neurotoxic [Asp49]PLA2 called PLA-N, and PfPLA 5 is a basic [Asp49]PLA2 called PLA-B. PLA-B and PLA-B' are the same isozymes with only one amino acid substitution at position 53, Glu or Gly, respectively [47,48]. It could be assumed that a cluster of IIA PLA2 isozyme genes like NIS-1 is located in the 5' upstream of the triplet genes, that is, the PLA-B' gene, the IIE PLA2 gene, and OTUD3, in the P. flavoviridis genome.
3.5. The Structural Relationship between the IIE PLA2 Gene and IIA PLA2 Genes in the P. flavoviridis Genome
Two-BLAST analysis showed that the three highly homologous nucleotide segments, named Alpha, Beta, and Chai, are commonly contained in both the PfIIEPLA2 gene (Figure 6A) and venom IIA PLA2 isozyme genes clustered in the NIS-1 fragment (Figure 6B). Alpha, Beta, and Chai segments are about 0.4, 0.3, and 1.4 kbps in length with 69%–94% aligned scores. Their locations in the genes are distinctive. In the PfIIEPLA2 gene (Figure 6A), the Alpha segment is found in the 5' flanking region, the Beta segment in the 3' downstream of the Alpha segment in the 5' flanking region, and the Chai segment encompasses from the middle portion of intron 1 to the posterior portion of intron 3. The NIS-1 fragment consists of a series of IIA PLA2 isozyme genes with or without PcRTF segment in the 3' terminus, each of which is bracketed as a unit in Figure 6B. Here, the Alpha and Chai segments are located in the 3' flanking region in this order and the Beta segment is in the anterior portion of intron 2 (Figure 6B). Therefore, it could be thought that after the prototype of venom IIA PLA2 gene containing the three segments had been formed, its multiplication occurred as seen in NIS-1 fragment. Since the Alpha, Beta, and Chai segments are found at the particular locations, it is hard to imagine that the three segments had been introduced after multiplication of the venom IIA PLA2 genes. On the other hand, it could be thought that the IIA PLA2 gene had been converted from a IIE PLA2 gene as a precursor with unknown mechanism.
3.6. Different Multiplication Processes between Non-Venomous Secretory PLA2 Genes and P. flavoviridis Venom IIA PLA2 Genes
As OTUD3 is a single-copy gene and codes for an ordinary non-venomous protein, structural and functional boundaries must exist between the IIE PLA2 gene and OTUD3. The genome domain harboring the cluster of various PLA2 genes seems to be easily multiplied, unlike that harboring OTUD3. Thus, it could be assumed in the human to snake genomes that a series of secretory PLA2 genes have multiplied toward the 5' upstream direction from the IIE PLA2 gene as the ancestor and diversified to various PLA2 gene species (Figure 5). However, the multiplication pattern of P. flavoviridis venom IIA PLA2 isozyme genes in the NIS-1 fragment is considerably different from those of non-venomous secretory PLA2 genes. The IIA PLA2 isozyme genes of the NIS-1 fragment are periodically and densely repeated, whereas the non-venomous secretory PLA2 genes are considerably scattered and the proteins encoded are structurally diversified so as to be classified into IIA, IIC, IID, IIF, and V PLA2s (Figure 5). The two mechanisms may be considered for multiplication of PLA2 genes. As the two nucleotide sequences in Chai segments, named Chai-1 and Chai-2, can be predicted to form stem-loop structures (Figure 7A,B), which could be the scaffolding of the gene recombination [49,50], it appears that such gene recombination might have been involved in the multiplication of non-venomous secretory PLA2 genes. On the other hand, Castoe et al. (2011) pointed out that the quantities of retroelements like SINEs and LINEs in venomous snake genomes are much higher than those in nonvenomous snake genomes [51]. In fact, the associated forms between PLA2 genes and CR1 LINEs were found in the P. flavoviridis NIS-1 fragment as mentioned above [13]. This suggests that retrotransposition, such as 3'-transduction [52,53], with CR1 LINE has participated in the multiplication of venom IIA PLA2 isozyme genes.
Figure 7.
The constructed stem-loop structures of Chai-1 (A) and Chai-2 segments (B). The secondary structures are deduced based on their nucleotide sequences via DNA folding form of the mfold Web Server. The numerals at both termini of the segments are the position numbers of the corresponding nucleotides in Figure 6A.
Acknowledgments
This work was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in Aid for Scientific Research (C), 2014-2016 (26340095, Takahito Chijiwa).
Abbreviations
- CR 1
chicken repeat 1
- EST
expressed sequence tag
- LINE
long interspersed nuclear element
- OTUD3
ovarian tumor domain-containing protein 3
- Oo
Ovophis okinavensis
- ORF
open reading frame
- P
Protobothrops
- PLA2
phospholipase A2
- SINE
short interspersed nuclear element
- UTR
untranslated region
Author Contributions
Kazuaki Yamaguchi and Takahito Chijiwa conceived and designed the experiments; Kazuaki Yamaguchi performed the experiments and analyzed the data; Naoki Ikeda, Hiroki Shibata, Yasuyuki Fukumaki, Naoko Oda-Ueda, and Shosaku Hattori contributed reagents/materials/analysis tools; Kazuaki Yamaguchi, Takahito Chijiwa, and Motonori Ohno wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tateno I., Suzuki S., Kitamoto O., Chiku N., Sawai Y. Anticoagulant activity of habu snake venom (Trimeresurus flavoviridis) I. Anticoagulant activity of crude habu venom. Jpn. J. Exp. Med. 1960;30:409–419. [PubMed] [Google Scholar]
- 2.Tateno I., Suzuki S., Chiku N., Kitamoto O. Anticoagulant activity of habu snake (Trimeresurus flavoviridis) venom. II. Effect of crude venom on blood vessels and fibrin clots. Jpn. J. Exp. Med. 1960;30:421–426. [PubMed] [Google Scholar]
- 3.Mitsuhashi S., Maeno H., Sato I., Tanaka T., Kawakami M., Yagi S., Okonogi T., Sawai Y., Ono T., Matsushita J. Studies on Trimeresurus venom. 1b) Comparison of the toxicological action of the venoms of Trimeresurus flavoviridis Hallowell, Trimeresurus elegans Gray and Trimeresurus okinavensis Boulenger. Nihon Saikingaku Zasshi. 1961;16:904–908. doi: 10.3412/jsb.16.904. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra B.W., Kalk K.H., Hol W.G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7 Å resolution. J. Mol. Biol. 1981;147:97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M., Taketomi Y., Miki T., Sato H., Hirabayashi T., Yamamoto K. Recent progress in phospholipase A2 research: From cells to animals to humans. Prog. Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Balsinde J., Winstead M.V., Dennis E.A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M., Taketomi Y., Girard C., Yamamoto K., Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie. 2010;92:561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Dufton M.J., Hider R.C. Classification of phospholipases A2 according to sequence. Evolutionary and pharmacological implications. Eur. J. Biochem. 1983;137:545–551. doi: 10.1111/j.1432-1033.1983.tb07860.x. [DOI] [PubMed] [Google Scholar]
- 9.Kini R.M. In: Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism. Kini R.M., editor. John Wiley & Sons; Hoboken, NJ, USA: 1997. [Google Scholar]
- 10.Maraganore J.M., Merutka G., Cho W., Welches W., Kezdy F.J., Heinrikson R.L. A new class of phospholipase A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J. Biol. Chem. 1984;259:13839–13843. [PubMed] [Google Scholar]
- 11.Maraganore J.M., Heinrikson R.L. The lysine-49 phopholipase A2 from the venom of Agkistrodon piscivorus piscivorus. Relation of structure and function to other phospholipase A2. J. Biol. Chem. 1986;261:4797–4804. [PubMed] [Google Scholar]
- 12.Nakashima K., Ogawa T., Oda-Ueda N., Hattori S., Sakaki Y., Kihara H., Ohno M. Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proc. Natl. Acad. Sci. USA. 1993;90:5964–5968. doi: 10.1073/pnas.90.13.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda N., Chijiwa T., Matsubara K., Oda-Ueda N., Hattori S., Matsuda Y., Ohno M. Unique structural characteristics and evolution of a cluster of venom phospholipase A2 isozyme genes of Protobothrops flavoviridis snake. Gene. 2010;461:15–25. doi: 10.1016/j.gene.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Ohno M., Ménez R., Ogawa T., Danse J.M., Shimohigashi Y., Fromen C., Ducancel F., Zinn-Justin S., le Du M.H., Boulain J.C., et al. Molecular evolution of snake toxins: Is the functional diversity of snake toxins associated with a mechanism of accelerated evolution? Prog. Nucleic Acid Res. Mol. Biol. 1998;59:307–364. doi: 10.1016/s0079-6603(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 15.Ohno M., Ogawa T., Oda-Ueda N., Chijiwa T., Hattori S. Perspectives in Molecular Toxinology. John Wiley & Sons; Hoboken, NJ, USA: 2002. Accelerated and regional evolution of snake venom gland isozymes; pp. 387–400. [Google Scholar]
- 16.Ohno M., Chijiwa T., Oda-Ueda N., Ogawa T., Hattori S. Molecular evolution of myotoxic phospholipases A2 from snake venom. Toxicon. 2003;42:841–854. doi: 10.1016/j.toxicon.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Fry B.G., Scheib H., de L.M., de Azevedo I.J., Silva D.A., Casewell N.R. Novel transcripts in the maxillary venom glands of advanced snakes. Toxicon. 2012;59:696–708. doi: 10.1016/j.toxicon.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Blin N., Stafford D.W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M., Fujii N., Kawamoto K., Hanasaki K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A2s. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 20.Valentin E., Ghomashchi F., Gelb M.H., Lazdunski M., Lambeau G. On the diversity of secreted phospholipase A2. Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- 21.Chijiwa T., Deshimaru M., Nobuhisa I., Nakai M., Ogawa T., Oda-Ueda N., Nakashima K., Fukumaki Y., Shimohigashi Y., Hattori S., et al. Regional evolution of venom-gland phospholipase A2 isoenzymes of Trimeresurus flavoviridis snakes in the southwestern islands of Japan. Biochem. J. 2000;347:491–499. doi: 10.1042/0264-6021:3470491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chijiwa T., Nakasone H., Irie S., Ikeda N., Tomoda K., Oda-Ueda N., Hattori S., Ohno M. Structural characteristics and evolution of the Protobothrops elegans pancreatic phospholipase A2 gene in contrast with those of Protobothrops genus venom phospholipase A2 genes. Biosci. Biotechnol. Biochem. 2013;77:97–102. doi: 10.1271/bbb.120595. [DOI] [PubMed] [Google Scholar]
- 23.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M. The rate of molecular evolution considered from the standpoint of population genetics. Proc. Natl. Acad. Sci. USA. 1969;63:1181–1188. doi: 10.1073/pnas.63.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonk F.J., Casewell N.R., Henkel C.V., Heimberg A.M., Jansen H.J., McCleary R.J., Kerkkamp H.M., Vos R.A., Guerreiro I., Calvete J.J., et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA. 2013;110:20651–20656. doi: 10.1073/pnas.1314702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chijiwa T., Hamai S., Tsubouchi S., Ogawa T., Deshimaru M., Oda-Ueda N., Hattori S., Kihara H., Tsunasawa S., Ohno M. Interisland mutation of a novel phospholipase A2 from Trimeresurus flavoviridis venom and evolution of Crotalinae group II phospholipases A2. J. Mol. Evol. 2003;57:546–554. doi: 10.1007/s00239-003-2508-4. [DOI] [PubMed] [Google Scholar]
- 28.Chijiwa T., Abe K., Ogawa T., Nikandrov N.N., Hattori S., Oda-Ueda N., Ohno M. Amino acid sequence of a basic aspartate-49-phospholipase A2 from Trimeresurus flavoviridis venom and phylogenetic analysis of Crotalinae venom phospholipases A2. Toxicon. 2005;46:185–195. doi: 10.1016/j.toxicon.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Chijiwa T., Ikeda N., Masuda H., Hara H., Oda-Ueda N., Hattori S., Ohno M. Structural characteristics and evolution of a novel venom phospholipase A2 gene from Protobothrops flavoviridis. Biosci. Biotechnol. Biochem. 2012;76:551–558. doi: 10.1271/bbb.110848. [DOI] [PubMed] [Google Scholar]
- 30.Fujimi T.J., Tsuchiya T., Tamiya T. A comparative analysis of invaded sequences from group IA phospholipase A2 genes provides evidence about the divergence period of genes groups and snaked families. Toxicon. 2002;40:873–884. doi: 10.1016/S0041-0101(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 31.Chijiwa T., Nakasone H., Ikeda N., Irie S., Oda-Ueda N., Hattori S., Ohno M. Structural characterization and evolution of pancreatic phospholipase A2 from Crotalinae snakes. Submitted to the EMBL/GenBank/DDBJ databases, Bethesda, MD, USA, BAN08536.
- 32.Tamiya T., Fujimi T.J. Laticauda semifasciata phospholipase A2 cDNA clone LsPLA2cPm09. Submitted to the EMBL/GenBank/DDBJ databases, Bethesda, MD, USA, BAB03302.
- 33.Zimin A.V., Delcher A.L., Florea L., Kelley D.R., Schatz M.C., Puiu D., Hanrahan F., Pertea G., van Tassell C.P., Sonstegard T.S., et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10:R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karray A., Ben A.Y., Boujelben J., Amara S., Carriere F., Gargouri Y., Bezzine S. Drastic changes in the tissue-specific expression of secreted phospholipase A2 in chicken pulmonary disease. Biochimie. 2012;94:451–460. doi: 10.1016/j.biochi.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karray A., Frikha F., Ben A.Y., Gargouri Y., Bezzine S. Purification and biochemical characterization of a secreted group IIA chicken intestinal phospholipase A2. Lipids Health Dis. 2011;10:27. doi: 10.1186/1476-511X-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seilhamer J.J., Randall T.L., Yamanaka M., Johnson L.K. Pancreatic phospholipase A2: Isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986;5:519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- 37.Seilhamer J.J., Randall T.L., Johnson J.K., Heinzmann C., Klisak I., Sparkes R.S., Lusis A.J. Novel gene exon homologous to pancreatic phospholipase A2: Sequence and chromosomal mapping of both human genes. J. Cell. Biochem. 1989;39:327–337. doi: 10.1002/jcb.240390312. [DOI] [PubMed] [Google Scholar]
- 38.Tischfield J.A., Xia Y.R., Shih D.M., Klisak I., Chen J., Engle S.J., Siakotos A.N., Winstead M.V., Seilhamer J.J., Allamand V., et al. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333. doi: 10.1006/geno.1996.0126. [DOI] [PubMed] [Google Scholar]
- 39.Ishizaki J., Suzuki N., Higashino K., Yokota Y., Ono T., Kawamoto K., Fujii N., Arita H., Hanasaki K. Cloning and characterization of novel mouse and human secretory phospholipase A2s. J. Biol. Chem. 1999;274:24973–24979. doi: 10.1074/jbc.274.35.24973. [DOI] [PubMed] [Google Scholar]
- 40.Valentin E., Singer A.G., Ghomashchi F., Lazdunski M., Gelb M.H., Lambeau G. Cloning and recombinant expression of human group IIF-secreted phospholipase A2. Biochem. Biophys. Res. Commun. 2000;279:223–228. doi: 10.1006/bbrc.2000.3908. [DOI] [PubMed] [Google Scholar]
- 41.Dennis E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 42.Moser A.R., Dove W.F., Roth K.A., Gordon J.I. The Min (multiple intestinal neoplasia) mutation: Its effect on gut epithelial cell differentiation and interaction with a modifier system. J. Cell Biol. 1992;116:1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami M., Yoshihara K., Shimabara S., Lambeau G., Singer A., Gelb MH., Sawada M., Inagaki N., Nagai H., Kudo I. Arachidonate release and eicosanoid generation by group IIE phospholipase A2. Biochem. Biophys. Res. Commun. 2002;292:689–696. doi: 10.1006/bbrc.2002.6716. [DOI] [PubMed] [Google Scholar]
- 44.Touqui L., Wu Y.Z. Interaction of secreted phospholipase A2 and pulmonary surfactant and its pathophysiological relevance in acute respiratory distress syndrome. Acta Pharmacol. Sin. 2003;24:1292–1296. [PubMed] [Google Scholar]
- 45.Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A., et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 46.Tsai I.H., Wang Y.M., Chen Y.H., Tsai T.S., Tu M.C. Venom phospholipase A2 of bamboo viper (Trimeresurus stejnegeri): Molecular characterization, geographic variations and evidence of multiple ancestries. Biochem. J. 2004;377:215–223. doi: 10.1042/BJ20030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chijiwa T., Yamaguchi Y., Ogawa T., Deshimaru M., Nobuhisa I., Nakashima K., Oda-Ueda N., Fukumaki Y., Hattori S., Ohno M. Interisland evolution of Trimeresurus flavoviridis venom phospholipase A2 isozymes. J. Mol. Evol. 2003;56:286–293. doi: 10.1007/s00239-002-2400-7. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi Y., Shimohigashi Y., Chijiwa T., Nakai M., Ogawa T., Hattori S., Ohno M. Characterization, amino acid sequence and evolution of edema-inducing, basic phospholipase A2 from Trimeresurus flavoviridis venom. Toxicon. 2001;39:1069–1076. doi: 10.1016/S0041-0101(00)00250-6. [DOI] [PubMed] [Google Scholar]
- 49.Lemoine F.J., Degtyareva N.P., Lobachev K., Petes T.D. Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites. Cell. 2005;120:587–598. doi: 10.1016/j.cell.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 50.Koszul R., Fischer G. A prominent role for segmental duplications in modeling eukaryotic genomes. C. R. Biol. 2009;332:254–266. doi: 10.1016/j.crvi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Castoe T.A., Hall K.T., Guibotsy Mboulas M.L., Gu W., de Koning A.P., Fox S.E., Poole A.W., Vemulapalli V., Daza J.M., Mockler T., et al. Discovery of highly divergent repeat landscapes in snake genomes using high-throughput sequencing. Genome Biol. Evol. 2011;3:641–653. doi: 10.1093/gbe/evr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moran J.V., DeBerardinis R.J., Kazazia H.H., Jr. Exon shuffling by L1 retrotransposition. Science. 1999;283:1530–1534. doi: 10.1126/science.283.5407.1530. [DOI] [PubMed] [Google Scholar]
- 53.Xing J., Wang H., Belancio V.P., Cordaux R., Deininger P.L., Batzer M.A. Emergence of primate genes by retrotransposon-mediated sequence transduction. Proc. Natl. Acad. Sci. USA. 2006;103:17608–17613. doi: 10.1073/pnas.0603224103. [DOI] [PMC free article] [PubMed] [Google Scholar]