Summary
Background
Data from cross-sectional and short-term longitudinal studies have suggested that children infected with HIV-1 might have cardiovascular abnormalities. We aimed to investigate this hypothesis in a long-term cohort study.
Methods
We measured cardiovascular function every 4–6 months for up to 5 years in a birth cohort of 600 infants born to women infected with HIV-1. We included 93 infants infected with HIV-1 and 463 uninfected infants (internal controls) from the same cohort. We also included a cross-sectionally measured comparison group of 195 healthy children born to mothers who were not infected with HIV-1 (external controls).
Findings
Children infected with HIV-1 had a significantly higher heart rate at all ages (mean difference 10 bpm, 95% CI 8–13) than internal controls. At birth, both cohort groups of children had similar low left ventricular (LV) fractional shortening. At 8 months, fractional shortening was similar in internal and external controls, whereas in children infected with HIV-1, fractional shortening remained significantly lower than in controls for the first 20 months of life (mean difference from internal controls at 8 months 3·7%, 2·3–5·1). LV mass was similar at birth in both cohort groups, but became significantly higher in children with HIV-1 from 4–30 months (mean difference 2·4 g at 8 months, 0·9–3·9).
Conclusions
Vertically-transmitted HIV-1 infection is associated with persistent cardiovascular abnormalities identifiable shortly after birth. Irrespective of their HIV-1 status, infants born to women infected with HIV-1 have significantly worse cardiac function than other infants, suggesting that the uterine environment has an important role in postnatal cardiovascular abnormalities.
Introduction
The number of children born to women infected with HIV-1 worldwide continues to increase rapidly as the rate of HIV-1 infection rises in women of childbearing age1–3 and improved care lengthens survival in children infected with HIV-1 in developed nations.1–3 As the number of children infected with HIV-1 increases so does the importance of understanding the course of paediatric HIV-1 disease.
Results from the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection (P2C2 HIV) study have shown that subclinical cardiac abnormalities develop early in children infected with HIV-1, and that they are frequent, persistent, and often progressive.4–7 Common abnormalities include dilated cardiomyopathy (decreased left ventricular [LV] contractility and LV dilation) and inappropriate LV hypertrophy (high LV mass with decreased height and weight).4 Also, LV dysfunction and increased wall thickness at baseline are risk factors for death, independent of low CD4 cell count, HIV-1 viral load, and neurological disease.6 During the first 2 years of the P2C2 HIV study, about 10% of children infected with HIV-1 developed congestive heart failure, required treatment with cardiac drugs, or both, and 20% developed cardiac dilation or dysfunction.5 Cardiac disease was the underlying cause of 12% of HIV-1-related deaths and was a contributing cause in an additional 22%.7 We have previously reported the first 2 years of follow-up data from a second cohort of children infected with HIV-1, who were enrolled at a median age of 2 years.4–6 However, enrolment of the cohort might have been subject to referral bias because of the presence of symptomatic HIV-1 infection or pre-existing cardiopulmonary disease or because of early mortality.
We aimed to assess LV structure and function characterised by echocardiography during the first 5 years of life in children born to women infected with HIV-1. Our long-term, prospective study supplements 2-year follow-up data from this cohort and results from cross-sectional and short-term studies of cardiac abnormalities in children infected with HIV-1.
Methods
Participants and procedures
The methods of the P2C2 HIV study, a prospective natural history study of cardiac and pulmonary complications of vertically-transmitted HIV-1 infection at five US clinical centres, have been reported in detail.8 Children were enrolled at Houston, TX, USA; Boston, MA; New York, NY; and Los Angeles, CA. We enrolled 600 infants born to mothers infected with HIV-1. All infants were enrolled before birth (n=432) or in the first 28 days of life (n=168) between May, 1990, and January, 1994, and followed up until January, 1997.
We excluded 44 infants from the analysis: nine died, none of whom had cardiomyopathy as a cause or contributor to their death; and 35 were lost to follow-up before HIV-1 status could be established. We included 93 infants infected with HIV-1 and 463 uninfected individuals (cohort groups). We randomly selected 216 uninfected infants to remain in the study as internal controls with a random number list generated by the coordinating centre, stratified by clinical centre.8 We used the mother as the unit of randomisation to account for mothers with more than one enrolled child.
All children underwent protocol-directed echocardio-graphic tests at predetermined 4–6 month intervals, irrespective of their clinical status. We used sedation if necessary in children younger than 3 years.4,6,9 Functional LV data were gathered prospectively and analysed centrally by technicians unaware of the child’s clinical status or treatment. LV fractional shortening is a measurement of overall LV systolic performance that is affected by contractility, preload, afterload, and heart rate. We measured afterload as meridional end-systolic LV wall stress. We calculated LV mass from M-mode measurements.4,6,9 The study was approved by the institutional review board at each centre and written informed consent was obtained from all mothers.
When significant differences between children infected with HIV-1 and controls were noted, we decided to try to establish whether the cardiac function of either group was similar to healthy children born to mothers who were not infected with HIV-1. Therefore, we recruited an external comparison group (external controls) of 195 infants and children who were healthy volunteers or who had been assessed for cardiac disease but in whom cardiac disease had been ruled out. The echocardiograms from this group were measured cross-sectionally at the same echocardiography laboratory in the same manner as those from the P2C2 HIV study children.
Statistical analysis
We compared baseline clinical characteristics between groups with the Wilcoxon rank-sum test for continuous variables and with the χ2 or Fisher’s exact test for proportions. Repeated-measures analyses for each of the seven echocardiographic measurements were done with a means model with SAS Proc Mixed software (version 6) providing separate estimates of the means by age and HIV-1 group. A heterogeneous compound symmetry variance-covariance form in repeated measurements was assumed for each outcome, and robust estimates of the SEs of parameters10 were used to do tests and construct 95% CIs. The repeated measures models for LV end-diastolic dimension and mass also included a covariate for body-surface area. For each age category, the adjusted mean for a cohort subgroup (HIV-1 infected or internal control) was defined as the mean response obtained by evaluating the statistical model at the mean body-surface area of the two subgroups. p values are two-sided. The level of significance was 0·05 for the repeated-measures analysis of each echocardiographic measurement.
Role of the funding source
The National Institutes of Health funded this study and participated in discussions of study design; the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Baseline characteristics
| Children with HIV-1 (n=93)
|
Internal controls (n=463)
|
||||
|---|---|---|---|---|---|
| Total n for whom data available | Baseline characteristics | Total n for whom data available | Baseline characteristics | p | |
|
|
|
|
|
|
|
| Sex | |||||
| Male | 93 | 44 (47%) | 463 | 249 (54%) | 0·25 |
| Female | 93 | 49 (53%) | 463 | 214 (46%) | ·· |
| Race | |||||
| White | 93 | 15 (16%) | 463 | 54 (12%) | 0·40 |
| Black | 93 | 41 (44%) | 463 | 245 (53%) | ·· |
| Hispanic | 93 | 32 (34%) | 463 | 138 (30%) | ·· |
| Other | 93 | 5 (5%) | 463 | 26 (6%) | ·· |
| Most severe maternal CDC symptom at age 3 months | |||||
| Symptom free | 93 | 59 (63%) | ·· | ·· | ·· |
| Mild (category A) | 93 | 16 (17%) | ·· | ·· | ·· |
| Moderate (category B) | 93 | 11 (12%) | ·· | ·· | ·· |
| Severe (category C) | 93 | 7 (8%) | ·· | ·· | ·· |
| Any antiretroviral therapy at 3 months’ gestation | 93 | 17 (18%) | 421 | 40 (10%) | 0·015 |
| Mother smoked tobacco during pregnancy | 87 | 34 (39%) | 397 | 149 (38%) | 0·79 |
| Illicit drug use during pregnancy | 85 | 30 (35%) | 399 | 120 (30%) | 0·35 |
| Zidovudine use during pregnancy | 86 | 26 (30%) | 395 | 135 (34%) | 0·48 |
| Gestational age (weeks)* | 91 | 37·7 (2·9) | 457 | 38·4 (2·6) | 0·01 |
| Birthweight (g)* | 91 | 2926 (651) | 440 | 3042 (634) | 0·16 |
| Birthweight z score*† | 90 | −0·12 (0·45) | 436 | −0·13 (0·52) | 0·50 |
| Birth length (cm)*† | 87 | 48·7 (3·6) | 427 | 49·1 (3·3) | 0·56 |
| Birth length z score*† | 86 | −0·06 (0·58) | 424 | 0·16 (0·66) | 0·17 |
| CD4 cell count (cells/μL), 0–7 days* | 42 | 2291 (1143) | 194 | 2354 (1220) | 0·72 |
| CD4 cell count z score, 0–7 days*‡ | 42 | −1·08 (0·65) | 194 | −1·05 (0·67) | 0·70 |
| CD4 cell count, (cells/μL), 8–45 days* | 37 | 2767 (1121) | 189 | 3239 (1143) | 0·07 |
| CD4 cell count z score, 8–45 days‡ | 37 | −0·64 (0·72) | 189 | −0·34 (0·72) | 0·07 |
CDC=US Centers for Disease Control and Prevention. Data are number (%) or *mean (SD).
Adjusted for gestational age.
Adjusted for age.
Results
The table shows baseline characteristics. 286 (51%) infants were black, 170 (31%) were Hispanic; 293 (53%) were male. 29 (31%) children infected with HIV-1 and one (0·2%) internal control died during follow-up. Infants infected with HIV-1 had a lower mean gestational age than controls. More than 90% of children infected with HIV-1 took antiretroviral drugs at some time during the study. Zidovudine and didanosine were the most common drugs; eight children took protease inhibitors (ritonavir, nelfinavir, saquinavir, or indinavir), all starting after 2 years of age. 26 (28%) infants infected with HIV-1 received intravenous immunoglobulin at some time during the study.9,11
Measurements of LV end-diastolic dimension and fractional shortening were available in 86 (92%) children infected with HIV-1 and from 428 (92%) internal controls. We excluded data for LV function in two children with congenital heart abnormalities (one infected with HIV-1, one internal control) and six children with wall-motion or septal-motion abnormalities (one infected with HIV-1, five internal controls). A further five children infected with HIV-1 could not be included in the analysis: three were lost to follow-up with no echocardiogram data, one had an echocardiogram that could not be digitised, and one was lost to follow-up with no systolic function data. A further 29 internal controls could not be included in the analysis: eight had echocardiograms that could not be digitised, two had a missing strip chart, six were randomised out of the study, and 13 were lost to follow-up with no systolic function data.
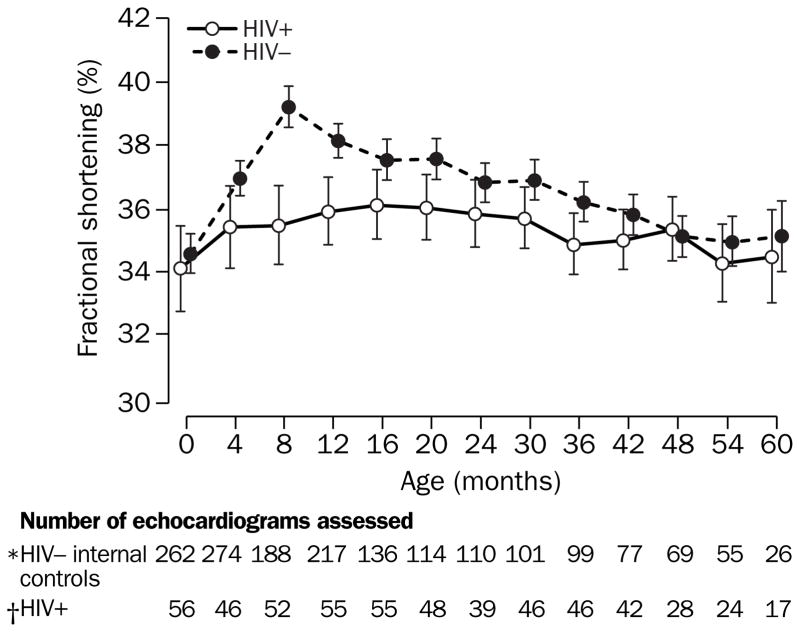
Fractional shortening in study cohorts changed in significantly different ways during follow-up (p=0·002, test for interaction between age and HIV-1 infection status). Mean fractional shortening was similar in both cohort groups at birth (p=0·54), but became significantly higher in internal controls at 8 months’ (39·2% vs 35·5%, p<0·0001) and 1 year’s follow-up (38·1% vs 35·9%, p=0·0003). Mean differences at 8 months’ and 1 year’s follow-up were 3·7% (95% CI 2·3–5·1) and 2·2% (1·0–3·4), respectively. These differences became smaller by 2 years’ follow-up as the fractional shortening for internal controls followed a natural decline with age, whereas the fractional shortening for the children with HIV-1 remained stable (figure 1).
Figure 1. Longitudinal change in mean left ventricular fractional shortening in children born to women infected with HIV-1.
*n=428 internal controls. †n=86 children with HIV-1 infection. Vertical bars=95% CIs.
At birth, mean (95% CI) fractional shortening of children infected with HIV-1 and internal controls were lower compared with the mean of 37 external controls younger than 3 months (34·1% [32·7–35·5] and 34·6% [33·9–35·2] vs 38·3% [37·5–39·2], p<0·0001 for each comparison), suggesting an in-utero effect. Fractional shortening in children infected with HIV-1 remained below that of external controls until 20 months’ follow-up, before natural declines in fractional shortening in external controls brought the groups together. By contrast, internal controls showed an increase in fractional shortening at 4-months’ follow-up and then followed the same pattern as external controls for the remainder of the follow-up period.
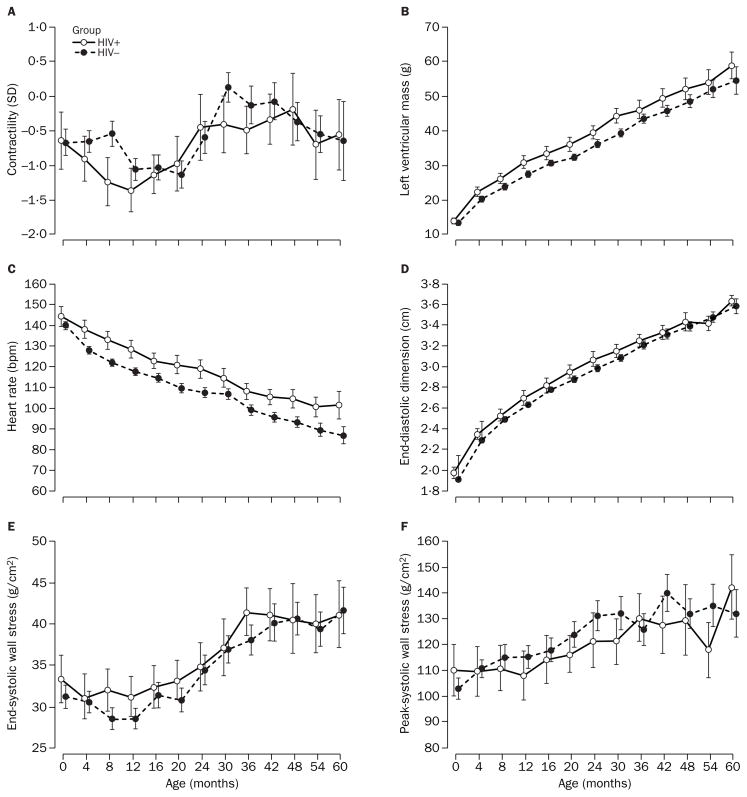
Contractility for study cohorts also changed in significantly different ways during follow-up (p=0·03, test for interaction between age and HIV-1 infection status). However, as figure 2A shows, there was no consistent pattern in either group, although contractility was lower more often in children infected with HIV-1 (p=0·0004 at age 8 months, mean difference −0·71, 95% CI −1·11 to −0·32). Both study cohorts tended to have lower mean contractility than external controls at all ages. Mean contractility was about 1 SD lower for children infected with HIV-1 compared with external controls. The mean difference in contractility pooled across ages was −1·01 (95% CI −1·26 to −0·76).
Figure 2. Longitudinal change in echocardiographic measurements in children born to women infected with HIV-1.
Contractility was measured by the stress-velocity index. Left ventricular mass and end-diastolic dimension were adjusted for body surface area.
LV mass changed in significantly different patterns with age in study cohorts (p=0·02, test for interaction between age and HIV-1 infection status). Children infected with HIV-1 and internal controls had very similar mean LV masses at study entry (13·7 g [95% CI 12·8–14·5] vs 13·2 g [12·8–13·5], respectively, p=0·30), but from 1 year’s to 30 months’ follow-up, mean LV mass became significantly and consistently higher by about 3–5 g in children with HIV-1 (figure 2B).
Heart rates in study cohorts were consistently different (p<0·0001), with children infected with HIV-1 having a significantly higher heart rate than internal controls at every age except birth. Mean difference in heart rate pooled across ages was 10 beats per minute (bpm) (95% CI 8–13). Interaction between age and study group was not significant for heart rate (p=0·56), indicating that both cohorts followed the same natural pattern of decline in heart rate (figure 2C). Both study cohorts had generally higher mean heart rates than external controls. Mean differences were 13 bpm (10–16) and 3 bpm (1–5), respectively.
Although end-diastolic dimension was consistently higher in children infected with HIV-1 than in internal controls (apart from at 54 months; figure 2D), neither the pattern of change of this variable (p=0·34) nor the difference between groups over time was significant (p=0·06). Similarly, end-systolic wall stress was usually higher in children infected with HIV-1 than in internal controls (figure 2E). However, differences were not significant, either when considered as a pattern of change (p=0·54) or as a persistent difference between groups over time (p=0·12). Figure 2F shows that peak-systolic wall stress tended to be higher in internal controls than in children infected with HIV-1. However, this relation was not consistent (p=0·04, test for interaction between age and HIV-1 infection status), and was significant only at 54 months’ follow-up (p=0·01).
Discussion
Children infected with HIV-1 have faster heart rates, higher LV mass, and lower LV function than uninfected children born to women with HIV-1 infection. Unlike healthy children whose mothers do not have known HIV-1 infection, children born to women infected with HIV-1 have LV dysfunction including reduced contractility indices that may persist over time. This effect could be attributable to the uterine environment of the HIV-1-infected mother, as it occurred irrespective of HIV-1-infection status of the infant. We have previously reported that fetal echocardiograms for many P2C2 study patients showed significant abnormalities of cardiovascular structure and function, irrespective of whether the children were later confirmed to have HIV-1 infection.12 We concluded that the maternal environment had important cardiovascular effects on the fetus. Our results extend that conclusion to the postnatal period.
The significant differences in heart rate, LV mass, and LV function between children with and without HIV-1 infection could indicate autonomic dysfunction and a hyperadrenergic state. We have previously reported that tachycardia, increased LV mass, and hyperdynamic LV function are typically seen in this setting, and are consistent with an increased risk of clinically significant cardiovascular events including congestive heart failure, high-grade arrhythmias, and death.4–7,9,12–14 This profile may suggest an HIV-1 effect on the central and peripheral nervous systems. We have previously described a strong association of the development of HIV-1 encephalopathy and cardiovascular morbidity and mortality in this population.4,14 Our findings provide a basis for testing a hypothesis that treatment with β-adrenergic antagonist drugs could improve outcomes.
Our finding that dilated cardiomyopathy and inappropriate LV hypertrophy develop more in children infected with HIV-1 compared with uninfected children strengthens the findings of our previous studies, which did not have an appropriate uninfected control group.4–7,14 We can now confirm that these cardiovascular abnormalities relate to HIV-1-infection status in children, though whether this is caused by HIV-1 or is the result of other uncontrolled factors is unclear.
Although internal controls showed improved left ventricular fractional shortening during the first postnatal year, they continued to have lower contractility than did external controls throughout follow-up. Children with HIV-1 infection had persistent global LV dysfunction.
Do children born without HIV-1 infection to women infected with HIV-1 have significant abnormalities that should be followed up?9,15 Our study raises concerns about long-term myocardial function and suggests that all children born to mothers with HIV-1 infection should be followed up. Even mild LV dysfunction over time has been shown to effect mortality.6,16–18 These findings accord with the Barker fetal origins hypothesis, which states that future cardiovascular disorders are, in part, programmed by stimuli or insults at critical early periods of prenatal life, and that fetal responses and adaptations to the uterine environment permanently change the body’s structure, function, and metabolism.19–21
Our study has several limitations. We have relatively complete follow-up data from the first 36 to 42 months of life, but after that, losses to follow-up and deaths reduced the sample size. The resulting wide 95% CIs indicate that the pattern seen in the age-specific means does not necessarily reflect the trend for an individual patient. A second limitation stems from the selection of the comparison group. Our study was initially designed to recruit a control sample of mothers with similar baseline characteristics apart from no HIV-1 infection during pregnancy, but this was not feasible. As a result, we recruited an external control sample that may not be ideally matched for socioeconomic and other relevant factors, is cross-sectional, and has a restricted number of patients. Thus, some of our non-significant results could have resulted from small external control sample size. Comparison of longitudinal with cross-sectional data may also complicate interpretation of our results. The suggestion that the in-utero environment could have an important role in postnatal cardiovascular abnormalities requires confirmation in other studies, given the concerns about the external control group and the inconsistency of findings by age group. Studies in more contemporary cohorts in which antiretroviral prophylaxis was used extensively during pregnancy would also be of interest. A report by our group9 concluded that zidovudine was not associated with acute or chronic abnormalities in LV structure and function in infants exposed to the drug in the perinatal period.
In summary, dilated cardiomyopathy, inappropriate LV hypertrophy, and resting tachycardia seem related to HIV-1-infection status in children born to women infected with HIV-1. Since LV dysfunction is found in both infected and uninfected children born to women infected with HIV-1, the dysfunction could be related to the intrauterine environment. These environmental effects could result from HIV-1 and other infections, maternal and postnatal nucleoside analogue and other drug use, maternal nutrition, placental abnormalities, racial and ethnic differences, and mitochondrial dysfunction. LV dysfunction may persist beyond the delivery, even in uninfected children. We suggest that continuing follow-up and appropriate treatment strategies should be considered for all children born to women infected with HIV-1.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (NO1-HR-96037, NO1-HR-96038, NO1-HR-96039, NO1-HR-96040, NO1-HR-96041, NO1-HR-96042, NO1-HR-96043) and in part by the US National Institutes of Health (RR-00865, RR-00188, RR-02172, RR-00533, RR-00071, RR-00645, RR-00685, RR-00043).
Members of the P2C2 HIV Study Group
National Heart, Lung And Blood Institute—Hannah Peavy, Anthony Kalica, Elaine Sloand, George Sopko, Margaret Wu.
Clinical centres
Baylor College of Medicine, Houston TX—William Shearer, Nancy Ayres, J Timothy Bricker, Arthur Garson, Linda Davis, Paula Feinman, Mary Beth Mauer.
University of Texas—Debra Mooneyham, Teresa Tonsberg
Children’s Hospital/Harvard Medical School, Boston MA—Steven Lipshultz, Steven Colan, Lisa Hornberger, Steven Sanders, Marcy Schwartz, Helen Donovan, Janice Hunter, Ellen McAuliffe, Nandini Moorthy, Patricia Ray, Sonia Sharma.
Boston Medical Center—Suzanne Steinbach, Karen Lewis.
Mount Sinai School of Medicine, New York NY—Meyer Kattan, Wyman Lai, Diane Carp, Donna Lewis, Sue Mone.
Beth Israel Medical Center—Mary Anne Worth.
Presbyterian Hospital in the City of New York/Columbia University, New York NY—Robert Mellins, Fred Bierman, Welton Gersony, Jane Pitt, Thomas Starc, Anthony Brown, Margaret Challenger, Kimberly Geromanos.
UCLA School of Medicine, Los Angeles CA—Samuel Kaplan, Y Al-Khatib, Robin Doroshow, Josephine Isabel-Jones, Roberta Williams, Helene Cohen, Sharon Golden, Karen Simandle, Ah-Lin Wong.
Children’s Hospital, Los Angeles CA—Arno Hohn, Barry Marcus, Audrey Gardner, Toni Ziolkowski.
LAC/USC—Lynn Fukushima.
Clinical coordinating centre
The Cleveland Clinic Foundation—Kirk A Easley, Michael Kutner, Mark Schluchter, Johanna Goldfarb, Douglas Moodie, Cindy Chen, Scott Husak, Victoria Konig, Sunil Rao, Amrik Shah, Susan Sunkle, Weihong Zhang.
Policy, data, and safety monitoring board—Henrique Rigatto, Edward B Clark, Robert B Cotton, Vijay V Joshi, Paul S Levy, Norman S Talner, Patricia Taylor, Robert Tepper, Janet Wittes, Robert H Yolken, Peter E Vinks.
Footnotes
Conflict of interest statement
None declared.
Contributors
All authors helped conceive and design the study, draft and revise the manuscript, analyse and interpret data, obtain funding, and provide administrative, technical, or material support. S Lipshultz, S Kaplan, T Starc, T Bricker, W Lai, and S Colan obtained data. K Easley, E Orav, and M Schluchter provided statistical help.
References
- 1.NAIDS Joint United Nations Programme on HIV/AIDS. [accessed April 23, 2001];AIDS epidemic update. 2000 Dec; http://www.unaids.org/epidemic_update/report_dec00/index_dec.html#full.
- 2.UNAIDS Joint United Nations Programme on HIV/AIDS. [accessed March 25, 2001];Report on the global HIV/AIDS epidemic. 2000 Jun; http://www.unaids.org/epidemic_update/report/index.html.
- 3.US Centers for Disease Control and Prevention. [accessed March 25, 2002];HIV/AIDS surveillance report. 2000 7:1–43. http://www.cdcgov/hiv/stats/hasrsupp71.htm. [Google Scholar]
- 4.Lipshultz SE, Easley KA, Orav EJ, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Circulation. 1998;97:1246–56. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starc TJ, Lipshultz SE, Kaplan S, et al. Cardiac complications in children with human immunodeficiency virus infection. [accessed April 23, 2001];Pediatrics. 1999 104 (online):e14. doi: 10.1542/peds.104.2.e14. http://www.pediatrics.org/cgi/content/full/104/2/e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children: the prospective P2C2 HIV multicenter study. Circulation. 2000;102:1542–48. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langston C, Cooper ER, Goldfarb J, et al. Human immundeficiency virus-related mortality in infants and children: data from the pediatric pulmonary and cardiovascular complications of vertically transmitted HIV (P2C2) study. Pediatrics. 2001;107:328–38. doi: 10.1542/peds.107.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.P2C2 HIV Study Group. The pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus (P2C2 HIV) infection study: design and methods. J Clin Epidemiol. 1996;49:1285–94. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Easley KA, Orav EJ, et al. Absence of cardiac toxicity of zidovudine in infants. N Engl J Med. 2000;343:759–66. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- 10.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 11.Shearer WT, Lipshultz SE, Easley K, et al. Alterations in cardiac and pulmonary function in pediatric rapid human immunodeficiency virus type 1 disease progressors. [accessed March 25, 2002];Pediatrics. 2000 105 (suppl 1):1–8. doi: 10.1542/peds.105.1.e9. http://www.pediatrics.org/cgi/content/full/105/1/e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornberger LK, Lipshultz SE, Easley KA, et al. Cardiac structure and function in fetuses of mothers infected with HIV: the prospective P2C2 HIV multicenter study. Am Heart J. 2000;140:575–84. doi: 10.1067/mhj.2000.109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Orav EJ, Sanders SP, Rubin A, McIntosh K, Colan SD. Abnormalities of cardiac structure and function in HIV-infected children treated with zidovudine. N Engl J Med. 1992;327:1260–65. doi: 10.1056/NEJM199210293271802. [DOI] [PubMed] [Google Scholar]
- 14.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with symptomatic HIV infection. J Am Med Assoc. 1993;269:2869–75. [PubMed] [Google Scholar]
- 15.Culane M, Fowler MG, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS clinical trials group protocol 219–076 teams. JAMA. 1999;281:151–57. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin in childhood acute lymphoblastic leukemia. N Engl J Med. 1991;324:808–15. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 17.Lipshultz SE, Lipsitz S, Goorin AM, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 18.Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Prog Pediatr Cardiol. 2000;12:1–29. doi: 10.1016/s1058-9813(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJP. Fetal origins of cardiovascular and lung disease. New York: Marcel Dekker; 2001. [Google Scholar]
- 20.Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995;311:171–74. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Editorial. An overstretched hypothesis? Lancet. 2001;357:405. [PubMed] [Google Scholar]