Abstract
Background
Ischemia-reperfusion injury (IRI) is a common phenomenon occurring during liver surgery, transplantation, and trauma. IRI causes oxidative stress which plays a critical role in causing organ damage. The Nrf2 is the master regulator of numerous genes, encoding antioxidant, detoxifying, and cytoprotective molecules. Nrf2 dysfunction has been implicated in the pathogenesis of several inflammatory disorders, cancer, and aging. This study was undertaken to investigate the effect of Nrf2 pathway activator (dh404) on warm liver IRI in a rodent model.
Methods
Ten Sprague-Dawley rats were treated with dh404 or vehicle. Dh404 was dissolved in sesame oil and was given orally (1.5mg/kg) the night before and 5 hours before procedures. Rat livers were subjected to 60 minutes of 70% ischemia followed by 3 hours of reperfusion. Serum ALT and Malondialdehyde (MDA) were determined and liver tissue was processed for histological examination, and determination of apoptosis, myeloperoxidase (MPO) activity, ADP/ATP ratio, and expressions of Nrf2, eNOS, anti-oxidant enzymes, and inflammatory mediators.
Results
Serum ALT and MDA levels and tissue MPO activity were significantly lower, expression of the anti-oxidant enzyme, glutamate cysteine ligase were significantly higher, whereas expression of NFkB and COX-2 was unchanged in the dh404-treated group. Although the total Suzuki histology score did not differ significantly, the extent of sinusoidal congestion, vacuolization, and apoptosis was significantly reduced in the dh404 treated compared to the untreated group (P<0.01).
Conclusions
Pre-treatment with dh404 resulted in partial attenuation of hepatic ischemia reperfusion injury in rats.
Keywords: liver, ischemia reperfusion injury, Reactive Oxygen Species, oxidative stress, bardoxolone methyl analogue, inflammation
Introduction
Liver ischemia reperfusion injury (IRI) is a major contributor to tissue damage during liver transplantation, liver resection procedures, hypovolemic shock, and trauma. IRI is a major cause of primary non-function after liver transplantation and occurs when the blood supply is restored in the ischemic tissues.(1-3). Clamping of the hepatic artery and portal vein is a common procedure in liver transplantation. This leads to disruption of the cellular mitochondrial oxidative phosphorylation and accumulation of metabolic intermediates during the ischemic period and oxidative stress following the resumption of blood flow. The resulting oxidative stress results in cellular damage and release of chemotactic factors which amplify the inflammatory response during reperfusion (4, 5). Moreover oxidative stress impairs mitochondrial function which leads to further production ROS and amplification of oxidative stress and tissue injury (6). A major source of inflammation and ROS production in the early phase of reperfusion is Kpuffer cells, which release inflammatory mediators, such as TNF-α and IL-1. Release of these cytokines and chemokines, in turn, triggers activation of neutrophils and their infiltration in the liver tissue. (4). Together these events results in oxidative and inflammatory stress in the liver tissue.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor, which is widely expressed in many organs (5-8). It regulates expression of genes encoding antioxidant and phase II enzyme, including glutathione peroxidase (GPx), γ-glutamate-cysteine ligase catalytic (GCLC) and modifier (GCLM) subunit, catalase (CAT), NAD(P)H: quinone oxidoreductase 1 (NQO1), and heme oxygenase 1 (HO-1), by binding to the antioxidant response element (ARE) in the promoters segments of the corresponding genes. Therefore, Nrf2 plays a central role in cellular redox system. Natural Nrf2 activators have been used for centuries as Chinese herbal medications for the treatment of a number of inflammatory conditions (9). Recently, Ke B et al. reported that Nrf2 has a protective role against ischemia-induced hepatocellular damage using Nrf2 defficient mouse liver transplants (10).
The aim of the present study was to test the hypothesis that pre-treatment with a potent, synthetic Nrf2 activator, dh404, (CDDO-9,11-dihydro-trifluoroethyl amide [CDDO-dhTFEA]) may attenuate severity of liver IRI in a rodent model through upregulation of cellular antioxidant and anti-inflammatory machinery.
Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee (University of California, Irvine). Male Sprague-Dawley rats (200-250g) were purchased from Charles River (Wilmington, MA). The animals were housed in the animal care facility for acclimatization prior to the study. Animals were allowed free access to food and water, and were maintained on a 12 hour light-dark cycle. They were fasted 12 h prior to the start of the experiments. Dh404 was provided by Reata Pharmaceuticals, Inc. (Irving, TX). The chemical name for dh404 is CDDO-9,11-dihydro-trifluoroethyl amide (CDDO-dhTFEA) (11).
Experimental Design
The rats were randomly divided into 2 experimental groups: (1) Control group (n=9): weas subjected to 1 hour of ischemia followed by 3 hours of reperfusion. (2) Dh404 group (n=8) received dh404 (1.5mg / kg, twice / day) by oral gavage the night before and 5 hours prior to surgery. Dh404 was dissolved in sesame oil and given. The rats in the control group were given sesame oil alone.
Operative procedure
Under general isoflurane anesthesia, a midline incision was made. The left and median portal triad was occluded by a microvascular clamp to achieve 70% hepatic ischemia (12-14). After 1 hour, the clamp was removed and the abdominal wall was closed. Three hours later after declamping, blood samples and liver specimens were collected under the general anesthesia for analysis.
Histologic analysis
Liver tissue specimens were fixed with 10% natural buffered formalin and embedded in paraffin. The 5 micrometers sections were made and stained with hematoxylin and eosin. The severity of I/RI was graded blindly using modified Suzuki’s criteria (15). In this classification, sinusoidal congestion, hepatocyte necrosis and ballooning degeneration are graded on a scale of 0-4. No necrosis, congestion, or centrilobular ballooning is given a score of 0, whereas severe congestion/ballooning, and >60% lobular necrosis is given a value of 4.
Assessment of apoptosis in liver
Apoptosis was determined by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick-end labelling (TUNEL) technique (ApopTag Plus Peroxidase In Situ Apoptosis Fluorescein Detection Kit, Millipore, MA). Total 20 random fields were examined by confocal microscopy (x20 objective) for each section. An apoptotic index (the number of nuclei labeled by the TUNEL method/the number of total nuclei) was calculated (16).
Measurement of serum alanine aminotransferase (ALT) and malondialdehyde (MDA)
Blood samples were obtained from the right heart ventricle. Serum ALT level was determined to assess the liver injury by using commercial kits (Bio Vision, CA) according to the manufacturer’s instructions. Serum MDA formation assay was performed using the TBARS assay kit (Cayman, USA) according to the manufacturer’s instructions.
Measurement of liver myeloperoxidase (MPO)
The presence of MPO was used as an index of neutrophil accumulation in the liver using the MPO colorimetric Assay kit (BioVision, Milpitas, CA) according to the manufacturer’s instructions(17).
Western blotting analysis
The protein was extracted with CelLytic™ NuCLEAR™ Extraction Kit (Sigma) following the manufacturer’s instruction. The Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA) was used to determine protein concentration. Target proteins were measured by western blot analysis with the following antibodies: rabbit antibodies against Nrf2 (1:200 dilution, Abcam), GCLM (1:1000 dilution, abcam), GCLC (1:800 dilution, abcam), and GAPDH (1:5000 dilution, Sigma). Aliquots containing 50μg proteins were heated for 5 min at 55°C and were loaded on NuPAGE 4-12% Bis-Tris gel (Life Technologies, Grand Island, NY), and then transferred to a PVDF membrane (Pall Life Sciences, Ann Arbor, MI). The membrane was blocked in TBS-T (Thermo) containing 5% blocking grade non-fat dry milk (Bio-Rad), and incubated overnight with primary antibodies at 4°C. After washing, the membrane was incubated with HRP-conjugated goat anti-rabbit secondary antibody (Cell signals) at 1:3000 dilution at RT for 2 hours. Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Thermo Scientific, Rockford, IL) (8). Densitometric measurements were done with Image Quant (Molecular Dynamics). GAPDH bands, as a housekeeping protein, were used to normalize the expression of the target proteins.
Statistical Analysis
All results were presented as mean ± standard deviation (SD). Comparisons between the two experimental groups were performed with Student’s t-test, Mann-Whitney’s U test t, as appropriate using Stat View-J 5.0 software. Statistical significance was defined as p-value less than 0.05.
Result
Liver damage evaluation by histological assay
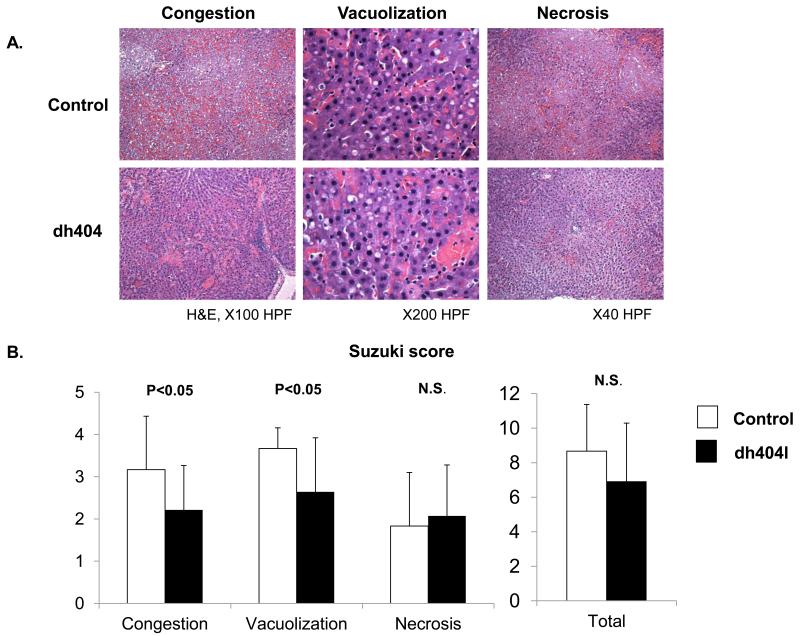
H&E staining was used to determine sinusoidal congestion, vacuolization, and focal necrosis (Fig. 1A). Scores were given using the Suzuki model (Fig. 1B). The level of congestion and vacuolization in the dh404 treated group was significantly improved compared to the control group (congestion: 2.2 ±1.1 v.s. 3.2 ±1.3; vacuolization 2.6 ±1.3 v.s.3.7 ±0.5, respectively, P<0.05) However, there was no significant difference in total score between the control and dh404 treated group (8.7 ±2.7 v.s. 6.9±3.4, N.S.).
Figure 1. Liver damage evaluation by histological assay.
(A). Liver tissue specimens were stained with hematoxylin and eosin. Representative specimens are shown (×40 - ×200). (B) Suzuki score The level of sinusoidal congestion and vacuolization in the dh404 treated group was significantly improved compared to the untreated group but not necrosis level. There was no significant difference in the total score. Data represent mean±S.D.
Assessment of hepatocellular apoptosis
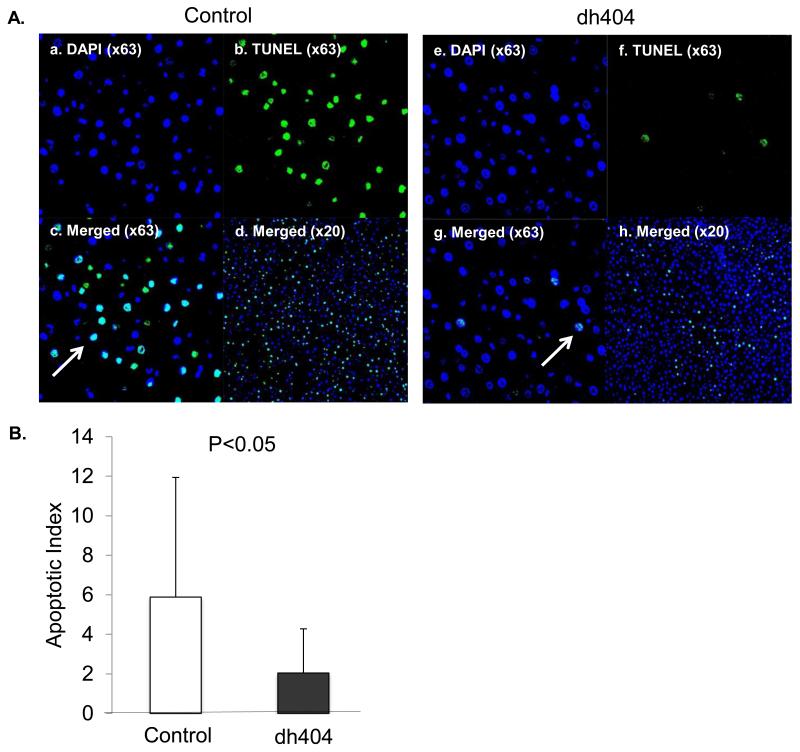
Fig. 2A shows representative confocal microscopy images of TUNEL (green fluorescence)-labeled hepatocytes. The apoptotic index in the dh404 group (2.1 ± 2.0) was significantly lower when compared to the control group 6.1 ± 5.9) (Fig. 2B, P<0.05).
Figure 2. TUNEL staining in liver post ischemia/reperfusion injury.
(A). a-d. TUNEL staining in the control group (a; DAPI ×63, b; TUNEL×63, c; Merged×63, d; Merged ×20). e-h. Dh404 group. (Arrows indicate apoptotic cells) Apoptotic index indicates that the dh404 treated group had significantly less TUNEL-positive cells when compared to the control group (P<0.05). (B). Apoptotic index. Data represent mean±S.D.
ALT and MDA in the serum, and MPO activity and ADP/ATP ratio in the liver
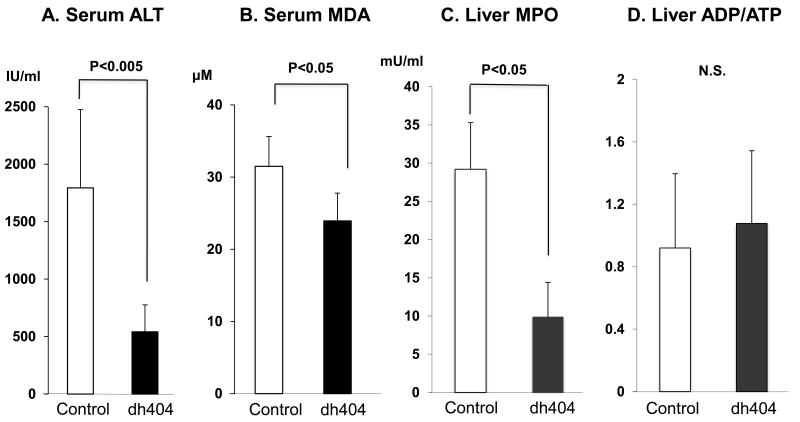
Serum ALT level in the dh404 treated rats (542±234 IU/ml) was significantly lower compared to the untreated group (1794±680 IU/ml, P<0.005, Fig. 3A). These findings may indicate that the liver experienced less damage from ischemic injury by dh404 administration.
Figure 3. Biochemical makers for liver damage.
The levels of serum ALT (A), MDA (B) and MPO(C) in the liver tissue were determined. All of them in the dh404 treated group showed significantly lower compared to the control group. However, there was no significant difference in ADP/ATP ratio (D).
Serum MDA which is a marker of oxidative stress was significantly lower in the dh404 treated rats (23.9±3.8μM) compared to the untreated (31.5±4.1, p<0.05) group (Fig 3B). Likewise, liver tissue MPO level in the dh404-treated group was significantly lower (9.9 ± 4.5 μM) compared to the untreated group (29.2± 6.1 μM, p<0.05) (Fig. 3C).These findings suggest that oxidative stress was reduced in the dh404-treated group. However, there was no significant difference in the ADP/ATP ratio between the two groups.
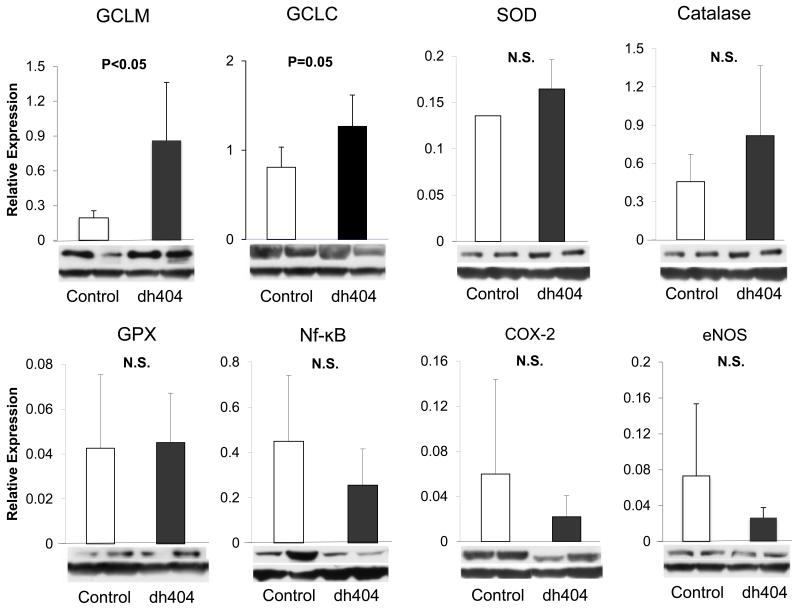
Nrf2, antioxidant and inflammatory mediators
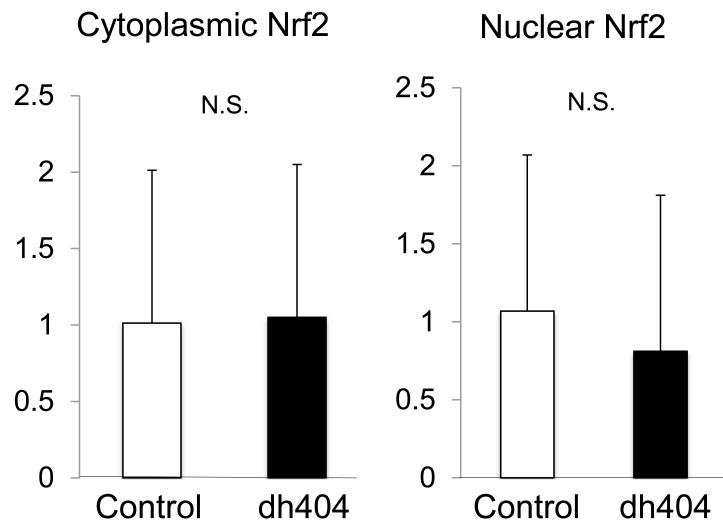
No significant difference was found in either the nuclear or cytoplasmic Nrf2 contents or expression of catalase, SOD, GPX, or eNOS between the two groups (Fig. 4, 5). Likewise no significant difference was found in expression of inflammatory factors including NF-κB, and Cox-2 between the two groups. However, GCLM and GCLC expressions were significantly increased in the dh404-treated group (Fig 4, 5).
Figure 4. Nrf2 expression in both cytoplasm and nucleus in the liver.
The data were expressed as relative values standardized by GAPDH for cytoplasmic protein and histone for nucleus protein. There was no significant difference in Nrf2 expression in both cytoplasm and nucleus.
Figure 5. Western blotting analysis.
The data were expressed as relative values standardized by GAPDH. The expression of GCLM and GCLC in the dh404 treated group was significantly increased. However, there was no significant difference in other Nrf2 target antioxidants. There were no significant reduction of both NF-κB, COX-2 and eNOS expressions in the dh404-treated animals’ liver tissues
Discussions
IRI occurs during liver surgery including liver resection, transplantation and trauma. Oxidant stress plays a critical role in the ischemia-reperfusion-induced organ damage. IRI is largely due to the accumulation of ROS and oxidative degradation of lipids, proteins, and nucleic acids in the affected tissue. Nrf2 regulates the antioxidant response element (ARE) which controls the expression of numerous antioxidant and detoxifying enzymes and related proteins and plays a major role in defense against oxidative stress and inflammation. Previous studies have shown that administration of dh404 derivatives confers cytoprotective effects in different organs and tissues by inducing activation of Nrf2 and upregulation of cytoprotective proteins (18-22). However, the effect of dh404 on hepatic IRI has not been defined. Given the critical role of oxidative stress in the pathogenesis of tissue injury in IRI and the central role of Nrf2 in host defense against oxidative stress, in this study, we investigated the effect of the Nrf2 inducer, dh404, in a rat model of hepatic IRI. The study revealed that the pretreatment with dh404 resulted in increased GCLM and GCLC expressions and modest reduction of hepatocyte damage.
Histological examination of the liver in the study animals revealed severe pathological alterations marked by sinusoidal congestion, necrosis, and severe endothelial injury and cytoplasmic vacuolization. Although severity of tissue damage in the dh404 treated rats was less than that found in the untreated animals using the unpaired t-test the difference did not reach statistical significance. However, semi-quantitative assessment of necrosis conducted according to the score system reported by Camino et al showed significant improvement in the dh404 treated group. In addition, serum ALT levels were significantly lower in the dh404 treated rats indicating a reduction in the intensity of liver damage in these animals. This was associated with a significant reduction of MDA levels pointing to attenuation of oxidative stress in the dh404-treated rats IRI results in acute inflammatory response characterized by accumulation of neutrophils which can intensify tissue damage by generating reactive oxygen and halogen species. In fact histological examination of tissues in the study animals revealed heavy accumulation of neutrophils in the liver which was confirmed by elevation of MPO activity. Pretreatment with dh404 resulted in significant reduction of neutrophil infiltration and MPO activity in the liver pointing to attenuation of inflammatory response in the treated rats.
Administration of dh404 resulted in a significant increase in the expression of glutamate cysteine ligase catalytic (GCLC) and glutamate cysteine ligase modifier (GCLM) subunits in the treated group. Glutamate cysteine ligase is the rate-limiting enzyme for biosynthesis of glutathione (GSH) which is the most abundant non-protein thiol that has potent antioxidant and detoxifying properties (23). The observed rise in expression of the subunits of this enzyme which are regulated by Nrf2 (24) must have contributed to attenuation of oxidative stress in the treated animals. It should be noted that no significant difference was found in either cytoplasmic or nuclear Nrf2 contents between the untreated and dh404-treated animals. However, similarity in expression and nuclear translocation of Nrf2 in two groups despite significant reduction of oxidant stress points to relative enhancement of the Nrf2 system in the treated group.
In conclusion, the present study demonstrated for the first time that pretreatment with Nrf2 inducer, dh404 confers partial protection against experimental hepatic IRI by attenuating oxidative stress and enhancing the antioxidant defense system.
Acknowledgments
Funding sources
This study was in part supported by grants from: NIH-NCRR UL1 TR000153, KL2 TR000147; the Juvenile Diabetes Research Foundation International 17-2011-609.
Reference
- 1.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. Am J Transplant. 2007;7(1):218–25. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Kan Q, Li J, Zhang X, Qi Y. Effect of neferine on liver ischemia-reperfusion injury in rats. Transplant Proc. 2011;43(7):2536–9. doi: 10.1016/j.transproceed.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Busuttil R. Liver ischaemia and reperfusion injury. Br J Surg. 2007;94(7):787–8. doi: 10.1002/bjs.5921. [DOI] [PubMed] [Google Scholar]
- 4.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–43. doi: 10.1084/jem.20042614. PMCID: 2213120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orkin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993;362(6422):722–8. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, et al. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–6. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 7.Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, Lloyd B, et al. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer. 2011;10:37. doi: 10.1186/1476-4598-10-37. PMCID: 3098205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298(3):F662–71. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 9.Zhao CR, Gao ZH, Qu XJ. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34(5):523–33. doi: 10.1016/j.canep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue S, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59(6):1200–7. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44(6):570–8. doi: 10.3109/00498254.2013.852705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes-Braz M, Elias-Miro M, Jimenez-Castro MB, Casillas-Ramirez A, Ramalho FS, Peralta C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J Biomed Biotechnol. 2012;2012:298657. doi: 10.1155/2012/298657. PMCID: 3357607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palladini G, Ferrigno A, Rizzo V, Boncompagni E, Richelmi P, Freitas I, et al. Lobe-specific heterogeneity and matrix metalloproteinase activation after ischemia/reperfusion injury in rat livers. Toxicol Pathol. 2012;40(5):722–30. doi: 10.1177/0192623312441403. [DOI] [PubMed] [Google Scholar]
- 14.Liang R, Nickkholgh A, Kern M, Schneider H, Benzing S, Zorn M, et al. Green tea extract ameliorates reperfusion injury to rat livers after warm ischemia in a dose-dependent manner. Mol Nutr Food Res. 2011;55(6):855–63. doi: 10.1002/mnfr.201000643. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, et al. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation. 1991;52(6):979–83. doi: 10.1097/00007890-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World journal of gastroenterology: WJG. 2004;10(13):1934–8. doi: 10.3748/wjg.v10.i13.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14(3):157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 18.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65(11):4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 19.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74(3):537–45. doi: 10.1021/np100826q. PMCID: 3064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 21.Bataille AM, Manautou JE. Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther. 2012;92(3):340–8. doi: 10.1038/clpt.2012.110. PMCID: 3704160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Liu XH, Fan JJ, Chen WF, Wang J, Zeng YJ, et al. Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway. Toxicology. 2014;318:22–31. doi: 10.1016/j.tox.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. doi: 10.1016/j.bbagen.2012.09.008. PMCID: 3549305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244(1):57–65. doi: 10.1016/j.taap.2010.01.013. PMCID: 2860427. [DOI] [PMC free article] [PubMed] [Google Scholar]