Significance
Early social experience is critical to developing appropriate adult behavior. Mothers are particularly important social partners but may also facilitate or restrict access to others. Studies investigating human parental facilitation are limited and confounded by culture. Comparative studies therefore provide invaluable insight into how parents provide social opportunities for offspring. We investigated maternal subgrouping patterns by infant sex in one of our closest relatives, chimpanzees, and found that mothers with sons were more gregarious. Infants themselves may influence patterns later in infancy, but differences were apparent early in life, when mothers and infants are in almost constant contact. Furthermore, mothers with sons associated more with adult males, potential behavioral models, early in life. These differences foreshadow sex-typical adult social behavior.
Keywords: infant socialization, maternal behavior, chimpanzees, fission–fusion species
Abstract
In many mammals, early social experience is critical to developing species-appropriate adult behaviors. Although mother–infant interactions play an undeniably significant role in social development, other individuals in the social milieu may also influence infant outcomes. Additionally, the social skills necessary for adult success may differ between the sexes. In chimpanzees (Pan troglodytes), adult males are more gregarious than females and rely on a suite of competitive and cooperative relationships to obtain access to females. In fission–fusion species, including humans and chimpanzees, subgroup composition is labile and individuals can vary the number of individuals with whom they associate. Thus, mothers in these species have a variety of social options. In this study, we investigated whether wild chimpanzee maternal subgrouping patterns differed based on infant sex. Our results show that mothers of sons were more gregarious than mothers of daughters; differences were especially pronounced during the first 6 mo of life, when infant behavior is unlikely to influence maternal subgrouping. Furthermore, mothers with sons spent significantly more time in parties containing males during the first 6 mo. These early differences foreshadow the well-documented sex differences in adult social behavior, and maternal gregariousness may provide sons with important observational learning experiences and social exposure early in life. The presence of these patterns in chimpanzees raises questions concerning the evolutionary history of differential social exposure and its role in shaping sex-typical behavior in humans.
Early socialization is critical to developing social competency later in life. In mammals, mothers have enormous influence on their offspring’s early social experience, with implications for adult social behavior. In humans, the relative contribution of parental and others’ social influence on the development of sex-typical behavior receives considerable attention and is often debated (1–4). Comparative research provides insight into the origins and development of sex differences in the absence of human cultural sex socialization. Decades of research in rodent and primate models have demonstrated that social deprivation curtails the development of species-appropriate behaviors (5–8) and cognition (9, 10). Primate mothers are critical for the normal social development of their infants (11–13), with classic studies demonstrating that maternal deprivation is associated with intense anxiety (14), inappropriate aggression (15), and an inability to form social relationships (16). The mother–infant relationship is therefore critical to proper social development; however, research also demonstrates the importance of the larger social milieu (17–20). For example, a recent study in mice found that early interactions with mothers and peers independently shape adult behavior (21). Likewise, some negative impacts of maternal deprivation are attenuated in macaques that are raised in peer groups (22).
Most primates rear their offspring in a stable social group in which mothers can influence infant interactions with conspecifics. Restrictive or protective mothering styles have been observed in some Old World monkey species, and style correlates with maternal rank, parity, offspring sex, and perceived risk to the infant (23–25). These patterns demonstrate maternal influence on the early social experiences of infants in species that live in cohesive groups. However, much less is known about how mothers influence infant social opportunities in species that live in fission–fusion social groups. Fission–fusion species, particularly those that have high fission–fusion dynamics such as humans and chimpanzees (26), are an excellent paradigm in which to consider individual differences in infant social exposure, as subgroup size and composition varies over time. This dynamic social system allows flexibility in the amount of social exposure that infants experience.
Here, we examine how maternal gregariousness varies by infant sex in wild chimpanzees (Pan troglodytes). Chimpanzees live in permanent communities or unit groups with multiple males and females. Temporary subgroups, or parties, form within the community and often change composition over the course of the day. Studies have demonstrated that party size is related to food availability and the presence of fertile females (27–29). The social flexibility characteristic of chimpanzees may allow mothers and infants to associate in parties of different sizes depending upon the optimal strategy for infant development.
Given well-documented differences between adult males and females, particularly regarding social behavior, it seems possible that maternal subgrouping patterns vary by infant sex in a manner that foreshadows adult sex-specific behavioral strategies. Adult male east African chimpanzees (Pan troglodytes schweinfurthii) are more gregarious and aggressive than females, as they compete for high dominance rank, which affords greater access to estrous females (28, 30). Males also cooperate with each other to form coalitions for dominance rank acquisition, community defense, and communal hunting (31), with some male–male relationships enduring for years (32). Although intersite variation exists (e.g., refs. 33, 34), east African female chimpanzees are generally less gregarious than males (35), and at Gombe National Park, Tanzania, Kasekela community females spend ∼40–70% of their time alone or with adult daughters and dependents (36–38). Females also exhibit comparatively low levels of physical aggression (28, 39–41). Emerging evidence suggests that sex differences in social behavior appear early in life. For example, male infants have significantly more social partners than their female counterparts when they first spend the majority of time out of reach from their mother (30–36 mo) (42). Specifically, males in this age class interact more with unrelated individuals, particularly adult males, than do females. However, it is unclear to what extent mothers mediate social exposure based on infant sex. Differences in maternal gregariousness may predispose male and female infants to different levels of independence and sociability very early in life, which may influence development. In chimpanzees and other fission–fusion species, mothers may join or leave parties, thereby affording or restricting social exposure. Once infants are able to travel without being carried by their mother, they may also influence maternal patterns by leading mothers to join or remain in parties (43).
As in most other primates, the chimpanzee mother–infant relationship is primary in early life. Infants are in almost constant contact with their mothers for the first 4–6 mo of life (28), when they have low levels of social interactions with nonmothers (this study). Mothers with infants later form nursery groups that contain several mothers (44–47). Infants only begin traveling under their own power more than they are carried by their mother around the age of 3.5 y (48). Infants begin eating solid foods by 6 mo of age (49) yet remain nutritionally dependent upon their mother until they are weaned between 4 and 5 y old (50, 51).
In this study, we investigated differences in maternal subgrouping patterns based on infant sex. We hypothesized that maternal gregariousness varies in ways that would foster sex-appropriate social development. Because male offspring will need to integrate into the adult male hierarchy and rely more on social skills and bonds for success as adults, whereas females will ultimately spend more of their time alone with dependent offspring, we predicted that mothers with male offspring would be more gregarious than mothers with female offspring. We tested this prediction with 37 y of data on maternal subgrouping among the wild chimpanzees at Gombe National Park, Tanzania. We considered three measures of gregariousness for mothers who were observed with both sons and daughters. The first measure was the time spent with another adult who is not an immediate female family member (mother or adult daughter). This measure allowed mother–adult daughter pairs to count as nonsocial time given that some study females do not emigrate and frequently associate with their mothers; excluding their time together allows us to address the question of infant exposure to the larger social milieu. Secondly, we examined average party size and composition. Specifically, we examined the average adult party size, as well as the average number of maternal kin (individual adults related through the matriline) and maternal nonkin (individual adults not related through the matriline) present in a mother’s party over the course of a day. Thirdly, we investigated subgrouping preferences by comparing the proportion of time mothers spent in mixed-sex (at least one adult male in the party) and female-only parties (at least one additional adult female, but no adult males, in the party). The second and third measures of gregariousness include mothers and adult daughters in the count of adults present to yield the actual adult party size and composition. We compared each of our metrics by infant sex during two time periods: the first 6 mo of life and from 6 mo to 3.5 y of age. Infants are in nearly constant contact with their mother during the first 6 mo of life such that they are unlikely to directly influence their mother’s subgrouping patterns, whereas patterns at older ages may reflect both maternal and infant social preferences and interactions. The first 6 mo postpartum is also distinct in that it corresponds to the period when mothers experience the highest metabolic costs of lactation (52), which may influence behavior. Females with infants less than 3.5 y of age in our study very rarely exhibited sexual swellings, which are known to influence female gregariousness (53). Finally, we investigated infant interactions with nonmothers across the entire infancy (infants aged ≤3.5 y) using a complementary 24-y dataset on infant behavior to investigate how maternal gregariousness relates to infant social interactions.
Results
All results on maternal subgrouping patterns are from analyses of data from well-sampled mothers who were observed with both sons and daughters as infants (n = 9 mothers with at least 10 follows during the first 6 mo of the infant’s life). This within-mother comparison allows for direct testing of our prediction; however, results based on a larger sample of mothers, including mothers that were just observed with offspring of one sex, are provided in SI Results and Table S1 to demonstrate the generality of the patterns. Categorical maternal rank (high versus low) was also included as a fixed factor in all analyses of maternal subgrouping patterns, as females of different ranks face different competitive pressures that may influence gregariousness. Rank results are included in Tables S2 and S3. Notably, in our dataset, high-ranking females were no more likely than low-ranking females to have a son versus a daughter (χ2 with Yates correction, ).
Maternal Time Spent with Others.
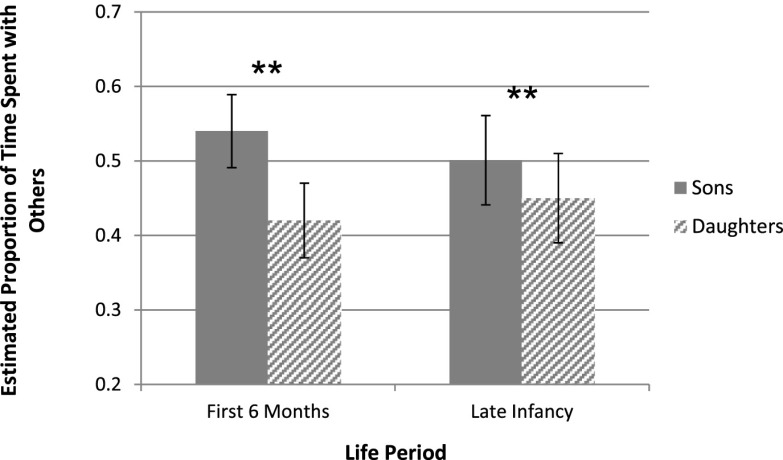
Infant sex was a significant predictor of the time spent with others beyond immediate female family members for both time periods [linear mixed model (LMM), first 6 mo: F1, 447 = 13.00, P = 0.0003; late infancy: F1, 1,824 = 9.90, P = 0.002; Fig. 1] (first 6 mo, 459 follows on nine mothers with 25 sons and 18 daughters; late infancy, 1,836 follows on nine mothers with 27 sons and 19 daughters). A post hoc test revealed within-female differences based on the sex of her offspring. The estimated least square mean proportion of time each mother spent with others when with her son(s) versus when with her daughter(s) was significantly different during the first 6 mo [paired t test, t(9) =3.23, P = 0.01; Fig. S1] and tended to be different over late infancy [t(9) = 2.00, P = 0.08]. In each period, mothers were more gregarious when they had sons than daughters.
Fig. 1.
Least square means for the time mothers spent with others were estimated from the LMM on well-sampled mothers observed with sons and daughters. First 6 mo, 459 follows on nine mothers with 25 sons and 18 daughters; late infancy (6 mo–3.5 y), 1,836 follows on nine mothers with 27 sons and 19 daughters. Error bars represent SEs. **P < 0.005.
Maternal Party Size and Composition.
Infant sex significantly predicted the daily average party size in both periods (LMM, first 6 mo: F1, 400 = 8.47, P = 0.004; late infancy: F1, 1,750 = 8.64, P = 0.003). The average party size in each period was higher for sons than for daughters (Table 1).
Table 1.
Estimated daily average party size and kin composition
| Life period | Offspring | Party size | Maternal kin | Maternal nonkin |
| First 6 mo | Sons | 7.49 | 1.30 | 5.89 |
| Daughters | 5.34 | 0.91 | 4.19 | |
| Late infancy | Sons | 5.11 | 0.96 | 4.15 |
| Daughters | 4.21 | 0.70 | 3.51 |
Least square means were generated from a model that included maternal rank and year nested in season. All comparisons between mothers with sons and mothers with daughters were significant (P < 0.05).
Infant sex predicted the daily average number of maternal kin (the average number of a mother’s maternal kin present in a mother’s party or parties per day) in both periods (LMM, first 6 mo: F1, 400 = 12.41, P = 0.0005; late infancy: F1, 1,750 = 24.92, P < 0.0001). Mothers with sons associated with a significantly higher average number of maternal kin than mothers with daughters (Table 1). It is important to note that there is no difference in the number of maternal kin available to mothers with sons versus mothers with daughters. There was also a significant difference by infant sex in the daily average number of maternal nonkin in the first 6 mo and late infancy (LMM, first 6 mo: F1, 400 = 6.36, P = 0.01; late infancy: F1, 1,750 = 5.45, P = 0.02). Mothers with sons associated with a higher number of maternal nonkin on average than mothers with daughters (Table 1).
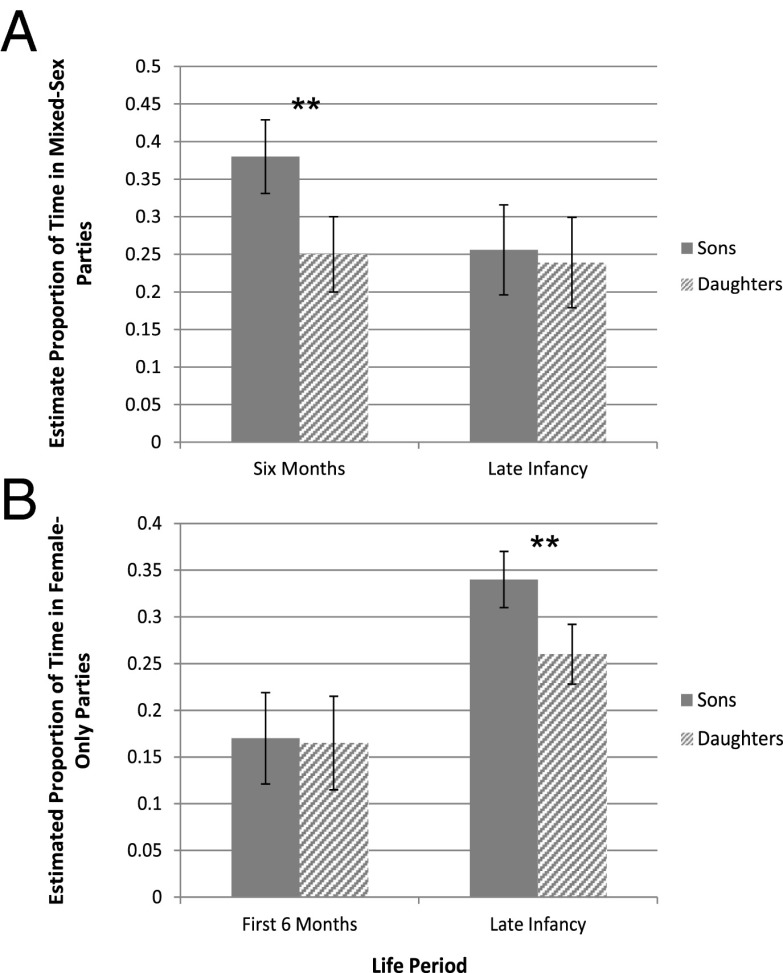
Mothers with sons spent significantly more time in mixed-sex parties than mothers with daughters during the first 6 mo (F1, 447 = 15.16, P = 0.0001), but there was no difference during late infancy (F1, 1,824 = 2.39, P = 0.12; Fig. 2A). Infant sex also predicted the proportion of time spent in female-only parties in late infancy (F1, 1,824 = 26.42, P < 0.0001), but not during the first 6 mo (F1, 447 = 0.13, P = 0.72; Fig. 2B). During late infancy, mothers spent a significantly higher proportion of their time in female-only parties with sons than with daughters.
Fig. 2.
Least square means for the proportion of time mother spent in (A) mixed-sex and (B) female-only parties were estimated from the LMM on mothers observed with sons and daughters. Error bars represent SEs. **P < 0.005.
Maternal Gregariousness and Infant Social Interactions.
Infants first interacted (groomed or played) with nonmothers (including siblings) at 0.43 ± 0.06 y old (mean ± SD; n = 14 infants with ≥10 h of observation before first interaction). The average proportion of minutes during a follow that an infant was observed interacting with nonmothers during the first 6 mo of life was 0.0006 (±0.0027 SD) and increased to peak during the infancy period at a proportion of 0.176 (±0.124 SD) at age 27–30 mo. The number of nonmothers an infant interacted with per day (hereafter referred to as social partners) significantly increased with both increasing infant age () and average daily party size including all age classes (). Although there was a main effect of infant sex with males having significantly more social partners than females (), there was also a significant interaction between infant sex and average daily party size (), with the number of social partners increasing with party size more for male infants than female infants (662 follows on 21 mothers, 29 male infants, and 17 female infants; Fig. S2).
Discussion
Mothers in species with a fission–fusion social structure can influence their offspring’s social environment through selective subgrouping. Because the social associates of infant chimpanzees are restricted to those individuals with whom the mother spends time, chimpanzee mothers are able to influence their offspring’s social experience to an even greater extent than females of fission–fusion species with more physically precocious infants (e.g., bottlenose dolphins) (54). In this study, we found that mothers with sons were more gregarious than mothers with daughters; they spent more time with others and had larger average party sizes, with a higher number of both maternal kin and nonkin present on average throughout the day. The percent difference in time spent with individuals beyond immediate female family members reported here translates to ∼2 more hours per day for male infants during early infancy, a substantial ∼25% increase in social exposure time.
It is noteworthy that mothers with daughters did not associate as much with maternal kin as mothers with sons, despite the fact that family parties should be safe social environments. This result suggests the intriguing possibility that social exposure in general is not as critical for females, which is in accord with the lower gregariousness observed in adult females compared with adult males. Association with others may carry costs in terms of increased competition, heightened risk of infanticide, and higher stress. Females, particularly those with low ranks, experience increased social stress in larger parties (55). Interestingly, mothers with infant daughters were likely opting out of a competitive and stressful context, whereas mothers with sons incur these costs. Additionally, compared with mothers with daughters, mothers with sons spent more time with adult males during the first 6 mo, indicating a preference for association with males early in their infant’s life. Although mothers with sons did not spend more time in female-only parties than mothers with daughters in the early months, they did so during late infancy. This pattern suggests that once males began interacting with others at appreciable levels, their mothers started spending more time in nursery groups comprised of females and dependent offspring; this pattern may have been driven in part by the infant males themselves.
In a previous study, we demonstrated that males had more social partners during the 30–36-mo age window, when they first spent the majority of time out of reach from the mother, but we did not detect differences in maternal party size (42). Here, our investigation over a broader range of infant ages revealed that mothers with sons were more gregarious than mothers with daughters, particularly early in the infant’s life. Additionally, our results here indicate that as offspring get older and begin moving farther from their mothers (48), they appear to make use of these social opportunities, as the number of social partners per day increased with offspring age. Compared with female infants, male infants also made greater use of the social opportunities provided them by interacting with more conspecifics as average party size increased. These results suggest both maternal facilitation and infant sociability are at play in the increased social interactions by young males.
Interestingly, even in the first 6 mo of life, when an infant is primarily in contact with the mother and cannot reasonably be expected to direct maternal subgrouping, mothers with sons were far more gregarious than mothers with daughters. During this first 6 mo of life, very low levels of infant interaction with nonmothers begin to occur, but are insufficient to drive the observed differences in maternal subgrouping. These early ages, however, may serve as an important period of social exposure and observational learning. Indeed, the greater gregariousness of mothers with sons compared with daughters may provide these young males with more of an opportunity to observe, and eventually interact with, members of the broader social milieu. Most intriguing is the possibility that the opportunity to observe adult models facilitates development of social skills important for success as an adult, particularly for males. Indeed, a previous study of the same population found a tendency for sons of mothers who more frequently associated with adult males during their immaturity to achieve higher rank as adults (37). Theory and empirical research predict that individuals will exhibit biases in which models they choose to observe to avoid unreliable information and maximize the transfer of useful information (56–58). Importantly, there is evidence that young male chimpanzees attend to same-sex models and sex-typical behavior. For example, juvenile and early adolescent males often watch their adult counterparts during copulations and displays, even following and imitating the behavior (59, 60). Thus, the opportunity to observe adult male behavior, particularly early in infancy, may influence social development in male infant chimpanzees and provide a future competitive advantage.
Although our results are consistent with our hypothesis that maternal subgrouping patterns provide infants with social exposure that fosters sex-appropriate social development, an alternative hypothesis is that mothers associate with adult males early in infancy to garner protection against aggression or infanticide (28, 61, 62). In terms of intercommunity aggression, killings occur more frequently when the victim’s party is greatly outnumbered (63); thus, the presence of males may provide some safety in numbers. However, Kasekela female core areas tend to be well within community boundaries (64), and evidence from the Kanyawara community in Uganda indicates females use boundary areas less than males (65). Thus, seeking protection from attacks by foreign males is unlikely to drive mothers to regularly associate more with adult males. There is evidence of adult males intervening in intracommunity female conflicts and attempting to prevent infanticide (e.g., refs. 61, 66, 67). However, in this case, the male protection hypothesis also seems unlikely to drive the observed patterns, as adult males themselves may present a risk to infants. There are documented cases of infanticide committed by community males (63); thus, association with adult males in general may be considered a risk to infant safety. The surest way to avoid intracommunity infanticide should be to avoid parties, which Kasekela mothers appear to do by spending up to 70% of their time alone or with close female kin (37, 38). A Kanyawara study found that mothers were less gregarious than nonmothers and less likely to group with adult males, which the authors suggest avoids the risk of injury to their infants by aggressive adult males (68). However, given the number of females who do not emigrate, future studies of the Kasekela community should investigate if natal mothers associate more with male relatives, which may allow them to expose infants to groups while mitigating risks.
Another possibility is that the sex-biased differences in maternal gregariousness are driven by others. Attraction to infants by conspecifics is well documented in a number of primate species (e.g., refs. 69–71). Community members may be more attracted to male infants than female infants, thereby remaining in the company of mothers with sons for longer periods. Young males will eventually integrate into the community, whereas young females may emigrate at adolescence. Attraction by others could also explain the general increase in time spent in female-only parties, as peers may draw mothers together as they play. Although attraction by others is an intriguing possibility, it should be noted that in the chimpanzee fission–fusion social system, mothers have the option to leave and must at least acquiesce to remaining in a party.
If we are correct that the patterns we describe result largely from the mother’s behavior, our results raise questions concerning the proximate mechanisms driving differences in maternal subgrouping patterns based on infant sex, particularly at early ages. An intriguing possibility concerns the role of maternal androgens. Despite mixed results, some studies in both humans and nonhuman primates have found higher levels of circulating androgens in mothers carrying a male rather than a female fetus, particularly in the second half of pregnancy (reviewed in ref. 72). Elevated maternal testosterone may influence the propensity to group, as studies have demonstrated a positive correlation between female testosterone and female aggression or competition (humans) (73, 74). Chimpanzee mothers may be more likely to remain in parties where levels of competition are higher, if their testosterone levels are high. Prenatal testosterone might also predispose male chimpanzee infants to greater physical activity levels than females, as has been observed in humans (reviewed in refs. 75, 76). This greater activity by males may prompt mothers to provide more external stimuli for their young male infants.
Given the close evolutionary relationship between chimpanzees and humans, how do these results compare with patterns observed in humans? The human literature provides some examples of differential parental behavior toward infants based on sex. For example, mothers communicate in a way that encourages risk-taking by boys and vulnerability perception by girls (77), and parents participate in more strenuous play with boys (78). However, evidence for sex differences in parental facilitation of social situations is surprisingly scant, despite a few studies that have reported no sex difference in parents arranging peer contacts (79, 80). Nevertheless, children of parents that initiate more social contacts have more consistent companionship and more play partners, whereas boys with more parent-initiated interactions have greater peer acceptance and lower levels of rejection; however, this advantage is not true for girls (80).
In humans, females are often considered more prosocial than males (e.g., refs. 81–85); however, boys are reported to play in larger same-sex groups than girls, and recent research found greater social tolerance among males as predicted by the evolutionary need for male–male cooperation (e.g., refs. 86–88). Likewise, east African adult male chimpanzees navigate a complicated social landscape involving both competition and cooperation (31). Although important differences in social structure exist (e.g., pair bonding in humans) (89), both humans and chimpanzees exhibit a fission–fusion social system, which affords mothers of both species the opportunity to influence their offspring’s social development by controlling exposure to potential models and social partners. Our results indicate that among the chimpanzees of Gombe, maternal gregariousness differs by infant sex in a manner that foreshadows sex differences in adult behavior. Future studies by our group will relate variation in maternal sociality to adult behavior and outcomes in general; however, the greater gregariousness of mothers with sons compared with daughters in particular may provide young males with the exposure to social behavior and skills important for success as an adult. Given early sex differences in behavior in chimpanzees and the persistent role of male cooperative hunting and defense in chimpanzees and humans, the question remains as to whether differential maternal grouping patterns based on infant sex were present during human evolution and contributed to the development of sex-typical behaviors.
Materials and Methods
Data were collected on the wild chimpanzees of Gombe National Park, Tanzania. The central Kasekela community has been under continuous study since 1960. We used two different datasets for these analyses: One focused on adults; the other focused on mothers and their offspring. Further detail on these datasets can be found in SI Materials and Methods. Briefly, both datasets contain detailed behavioral data collected using standard quantitative sampling techniques. For analyses of maternal subgrouping patterns, we analyzed data from 1974 to 2011 resulting from daily focal follows of individual adults. Notably, party composition scans were recorded every 15 min, with all adult community members indicated as present or absent (see SI Materials and Methods for more details). All Maternal Subgrouping × Infant Sex analyses were run during two different periods: the first 6 mo of life and late infancy (6 mo–3.5 y of age). We also accounted for covariates of maternal gregariousness, including maternal rank and season (SI Materials and Methods). We also tested for a main effect of infant age on maternal subgrouping patterns over the entire period of infancy; however, as infant age was not a significant predictor, it was removed from all analyses except the analysis of infant social partners (see below).
We calculated the daily proportion of time spent with others as the number of party composition scans during which the mother was with other adult individuals divided by the total number of party composition scans for the day. Because 50% of females in the Kasekela community remain in their natal community and associate at high levels with their mothers, we scored a mother associating with only her adult daughter (or vice versa) and her dependent offspring as alone.
We fit LMMs (PROC MIXED, SAS 9.3) that included maternal rank, infant sex, mother ID, and the interaction between infant sex and mother ID as fixed effects. Season was included as a random effect to control for temporal variation in food availability and subgrouping patterns. We then performed a post hoc test on the Sex × Mother ID interaction to compare each mother’s mean time spent with others by her infant’s sex after controlling for covariates. Specifically, we tested for significant within-female differences between the amounts of time spent with others with sons versus daughters with a paired t test. We also investigated whether the sex of the next oldest sibling influenced results using a larger sample of mothers (SI Materials and Methods). Sibling sex was not a significant predictor of time spent with others, and those results are included in SI Results.
To investigate differences in party size and composition, we first calculated the daily average party size as the sum of the number of adults (adult age ≥12 y) present at each party composition scan divided by the number of party composition scans that day. Thus, this measure of association incorporates both the number of individuals as well as the amount of time spent with them. We also examined the average number of adult maternal kin (individuals related through the mother; calculated as the sum of the number of maternal kin present during each party composition scan divided by the number of party composition scans that day) and the average number of adult nonmaternal kin (individuals not related through the mother) present in the mother’s party over the course of a day. A mother’s maternal kin included both males and females of all generations.
We further compared the daily proportion of time spent in female-only and mixed-sex parties by infant sex. Each party composition scan was scored based on the sex of all adults present. In this analysis, unlike the analysis of time spent in parties, adult daughters or mothers were counted. Thus, females were considered to be in female-only parties rather than alone when associating with just their mother or adult daughter. Mixed-sex parties contained the mother and at least one adult male (including adult sons). All daily proportions were calculated as the number of party composition scans in the party type divided by the total number of party composition scans. All measures of maternal subgrouping patterns were analyzed using LMMs with maternal rank and infant sex as fixed effects and mother ID as the random effect. The Proportion of Time × Party Type model also included season as a random effect, whereas the average party size and composition models included season nested in year to control for community size changes.
To examine the relationship between maternal gregariousness and infant social interactions, we used a separate behavioral dataset focused on mothers and their offspring (SI Materials and Methods). Specifically, we fit a generalized linear mixed model (GLMM) with poisson error distribution and logit link function. Fixed effects included infant sex, infant age in days, average daily party size (including individuals of all ages), and the interaction of sex and daily average party size. Random effects included season nested within year and infant ID nested within mother ID to control for repeated, uneven sampling of infants, and mothers with more than one infant in the dataset. We determined the significance of each fixed effect by using likelihood ratio tests to compare the full model to those that did not contain the variable of interest (90). GLMM analysis was conducted using the lme4 package (91) in R (version 3.0.1, R Core Development Team 2013).
This study was completely observational in nature; the subjects are well-habituated to human observation. Our research has approval from the Tanzanian governing bodies, including Tanzania National Parks, the Tanzanian Wildlife Research Institute, and the Tanzanian Commission for Science and Technology.
Supplementary Material
Acknowledgments
We are grateful to the numerous interns and research assistants who have helped digitize both behavioral datasets. We also thank L. Chandler and the George Washington University Primate Behavioral Ecology lab for invaluable discussions, as well as the editor and three anonymous reviewers for helpful comments. We thank Tanzania National Parks, the Tanzania Wildlife Research Institute, and the Tanzanian Commission for Science and Technology for granting us permission to work in Gombe National Park. We also thank the Jane Goodall Institute for funding long-term research and the Gombe Stream Research Centre staff for maintaining data collection. Data digitization was funded by National Institutes of Health Grants R00HD057992 and R01 AI050529; National Science Foundation Grants DBS-9021946, SBR-9319909, BCS-0452315, and LTREB-1052693; the Harris Steel Group; the Windibrow Foundation; the Carnegie Corporation; the University of Minnesota; Duke University; the Leo S. Guthman Foundation; and the National Geographic Society. KRW and JAM were supported by a department grant from NSF DGE-0801634. Behavioral analyses were supported by National Institutes of Health Grant R00HD057992.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.W. is a guest editor invited by the Editorial Board.
See Commentary on page 18106.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409507111/-/DCSupplemental.
References
- 1.Blakemore JEO, Berenbaum SA, Liben LS. Gender Development. Psychology Press; New York: 2013. [Google Scholar]
- 2.Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychol Bull. 2006;132(1):98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandell DL. Parents, peer groups, and other socializing influences. Dev Psychol. 2000;36(6):699–710. [PubMed] [Google Scholar]
- 4.Harris JR. Where is the child’s environment? A group socialization theory of development. Psychol Rev. 1995;102(3):458–489. [Google Scholar]
- 5.Mitchell GD, Raymond EJ, Ruppenthal GC, Harlow HF. Long-term effects of total social isolation upon behavior of rhesus monkeys. Psychol Rep. 1966;18(2):567–580. [Google Scholar]
- 6.Marriner KM, Drickamer LC. Factors influencing stereotyped behavior of primates in a zoo. Zoo Biol. 1994;13(3):267–275. [Google Scholar]
- 7.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 8.Latham NR, Mason GJ. Maternal deprivation and the development of stereotypic behaviour. Appl Anim Behav Sci. 2008;110(1):84–108. [Google Scholar]
- 9.Davenport RK, Rogers CM, Rumbaugh DM. Long-term cognitive deficits in chimpanzees associated with early impoverished rearing. Dev Psychol. 1973;9(3):343–347. [Google Scholar]
- 10.Bennett AJ, Pierre PJ. Contributions to understanding genetic and environmental influences on phenotypic outcomes across development. In: Hood KE, Halpern CT, Greenberg G, Lerner RM, editors. The Handbook of Developmental Science, Behavior, and Genetics. John Wiley & Sons; Hoboken, NJ: 2010. pp. 353–399. [Google Scholar]
- 11.Maestripieri D. Maternal influences on offspring growth, reproduction, and behavior in primates. In: Maestripieri D, Mateo JM, editors. Maternal Effects in Mammals. University of Chicago Press; Chicago: 2009. pp. 256–291. [Google Scholar]
- 12.Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. Pantheon Books; New York: 1999. [DOI] [PubMed] [Google Scholar]
- 13.Lonsdorf EV, Ross SR. Socialization and development of behavior. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 245–268. [Google Scholar]
- 14.Harlow HF, Suomi SJ. Induced depression in monkeys. Behav Biol. 1974;12(3):273–296. doi: 10.1016/s0091-6773(74)91475-8. [DOI] [PubMed] [Google Scholar]
- 15.Mineka S, Suomi SJ. Social separation in monkeys. Psychol Bull. 1978;85(6):1376–1400. [PubMed] [Google Scholar]
- 16.Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60(1):1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- 17.Parker JG, Asher SR. Peer relations and later personal adjustment: Are low-accepted children at risk? Psychol Bull. 1987;102(3):357–389. doi: 10.1037//0033-2909.102.3.357. [DOI] [PubMed] [Google Scholar]
- 18.Clarke MR. Behavioral development and socialization of infants in a free-ranging group of howling monkeys (Alouatta palliata) Folia Primatol (Basel) 1990;54(1-2):1–15. doi: 10.1159/000156422. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton SF. Apprenticeship for Adulthood: Preparing Youth for the Future. Free Press; New York: 1990. [Google Scholar]
- 20.Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Dev. 1994;65(2 Spec No):649–665. [PubMed] [Google Scholar]
- 21.Branchi I, et al. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38(4):522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins MS, Suomi SJ. Long-term effects of social rehabilitation in rhesus monkeys. Primates. 1976;17(1):43–51. [Google Scholar]
- 23.Fairbanks LA. Individual differences in maternal style: Causes and consequences for mothers and infants. Adv Stud Behav. 1996;25:579–611. [Google Scholar]
- 24.Altmann J. Baboon Mothers and Infants. Harvard Univ Press; Cambridge, MA: 1980. [Google Scholar]
- 25.Nguyen N, Gesquiere L, Alberts SC, Altmann J. Sex differences in the mother–neonate relationship in wild baboons: Social, experiential and hormonal correlates. Anim Behav. 2012;83(4):891–903. [Google Scholar]
- 26.Aureli F, et al. Fission-fusion dynamics. Curr Anthropol. 2008;49(4):627–654. [Google Scholar]
- 27.Mitani JC, Watts DP, Lwanga JS. Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge Univ Press; Cambridge, UK: 2002. [Google Scholar]
- 28.Goodall J. The Chimpanzees of Gombe. Harvard Univ Press; Cambridge, MA: 1986. [Google Scholar]
- 29.Matsumoto-Oda A, Hosaka K, Huffman MA, Kawanaka K. Factors affecting party size in chimpanzees of the Mahale Mountains. Int J Primatol. 1998;19(6):999–1011. [Google Scholar]
- 30.Muller MN. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge Univ Press; Cambridge, UK: 2002. pp. 112–124. [Google Scholar]
- 31.Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Adv Stud Behav. 2005;35:275–331. [Google Scholar]
- 32.Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77(3):633–640. [Google Scholar]
- 33.Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) Am J Primatol. 2009;71(10):840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- 34.Wakefield ML. Social dynamics among females and their influence on social structure in an East African chimpanzee community. Anim Behav. 2013;85(6):1303–1313. [Google Scholar]
- 35.Gilby IC, Wrangham RW. Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav Ecol Sociobiol. 2008;62(11):1831–1842. [Google Scholar]
- 36.Halperin SD. Temporary association patterns in free ranging chimpanzees: An assessment of individual grouping preferences. In: Hamburg DA, McCown ER, editors. The Great Apes. Benjamin Cummings Publishing Company; Menlo Park, CA: 1979. pp. 491–499. [Google Scholar]
- 37.Williams JM, Liu HY, Pusey AE. Costs and benefits of grouping for female chimpanzees at Gombe. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge Univ Press; Cambridge, UK: 2002. p. 192. [Google Scholar]
- 38.Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: Towards an ideal despotic distribution. Anim Behav. 2007;74(6):1795–1804. [Google Scholar]
- 39.Nishida T. Social interactions between resident and immigrant female chimpanzees. In: Heltne PG, Marquardt LA, editors. Understanding Chimpanzees. Harvard Univ Press; Cambridge, MA: 1989. pp. 68–89. [Google Scholar]
- 40.Wittig RM, Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. Int J Primatol. 2003;24(4):847–867. [Google Scholar]
- 41.Miller JA, et al. Competing for space: Female chimpanzees are more aggressive inside than outside their core areas. Anim Behav. 2014;87:147–152. doi: 10.1016/j.anbehav.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonsdorf EV, et al. Boy will be boys: Sex differences in wild infant chimpanzee social interactions. Anim Behav. 2014;88:79–83. doi: 10.1016/j.anbehav.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pusey AE. Mother-offspring relationships in chimpanzees after weaning. Anim Behav. 1983;31(2):363–377. [Google Scholar]
- 44.Kortlandt A. Chimpanzees in the wild. Sci Am. 1962;206:128–138. doi: 10.1038/scientificamerican0562-128. [DOI] [PubMed] [Google Scholar]
- 45.Tutin CEG, McGrew WC, Baldwin PJ. Social organization of savanna-dwelling chimpanzees, Pan troglodytes verus, at Mt. Assirik, Senegal. Primates. 1983;24(2):154–173. [Google Scholar]
- 46.Sakura O. Factors affecting party size and composition of chimpanzees (Pan troglodytes verus) Bossou, Guinea. Int J Primatol. 1994;15(2):167–183. [Google Scholar]
- 47.Boesch C. Social grouping in Tai chimpanzees. In: McGrew W, Marchant L, Nishida T, editors. Great Ape Societies. Cambridge Univ Press; Cambridge, UK: 1996. pp. 101–113. [Google Scholar]
- 48.Lonsdorf EV, et al. Sex differences in wild chimpanzee behavior emerge during infancy. PLoS ONE. 2014;9(6):e99099. doi: 10.1371/journal.pone.0099099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith TM, et al. First molar eruption, weaning, and life history in living wild chimpanzees. Proc Natl Acad Sci USA. 2013;110(8):2787–2791. doi: 10.1073/pnas.1218746110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark CB. A Preliminary Report on Weaning Among Chimpanzees of the Gombe National Park. Primate Bio-Social Dev; Tanzania: 1977. pp. 235–250. [Google Scholar]
- 51.van de Rijt-Plooij HH, Plooij FX. Growing independence, conflict and learning in mother-infant relations in free-ranging chimpanzees. Behaviour. 1987;101(1):1–86. [Google Scholar]
- 52.Emery Thompson M, Muller MN, Wrangham RW. The energetics of lactation and the return of fecundity in wild chimpanzees. Behav Ecol. 2012;23(6):1234–1241. [Google Scholar]
- 53.Wrangham RW, Smuts BB. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J Reprod Fertil Suppl. 1980;13(Suppl 28):13–31. [PubMed] [Google Scholar]
- 54.Gibson QA, Mann J. Early social development in wild bottlenose dolphins: Sex differences, individual variation and maternal influence. Anim Behav. 2008;76(2):375–387. [Google Scholar]
- 55.Markham AC, et al. Rank effects on social stress in lactating chimpanzees. Anim Behav. 2014;87:195–202. doi: 10.1016/j.anbehav.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyd R, Richardson P. Culture and the Evolutionary Process. University of Chicago Press; Chicago: 1985. [Google Scholar]
- 57.Laland KN. Social learning strategies. Learn Behav. 2004;32(1):4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- 58.Wood LA, Kendal RL, Flynn EG. Whom do children copy? Model-based biases in social learning. Dev Rev. 2013;33(4):341–356. [Google Scholar]
- 59.Pusey A. Behavioural changes at adolescence in chimpanzees. Behaviour. 1990;115(3-4):203–246. [Google Scholar]
- 60.Pusey A. 1978. The Physical and Social Development of Wild Adolescent Chimpanzees (Pan troglodytes schweinfurthii). PhD dissertation (Stanford University, Stanford, CA)
- 61.Pusey AE, et al. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. Int J Primatol. 2008;29(4):949–973. [Google Scholar]
- 62.Goodall J. Infant killing and cannibalism in free-living chimpanzees. Folia Primatol (Basel) 1977;28(4):259–289. doi: 10.1159/000155817. [DOI] [PubMed] [Google Scholar]
- 63.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513(7518):414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 64.Williams JM, et al. Female competition and male territorial behavior influence female chimpanzees’ ranging patterns. Anim Behav. 2002;63(2):347–360. [Google Scholar]
- 65.Chapman CA, Wrangham RW. Range use of the forest chimpanzees of Kibale: Implications for the understanding of chimpanzee social organization. Am J Primatol. 1993;31(4):263–273. doi: 10.1002/ajp.1350310403. [DOI] [PubMed] [Google Scholar]
- 66.Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim Behav. 2008;76(5):1497–1509. [Google Scholar]
- 67.Townsend SW, Slocombe KE, Emery Thompson M, Zuberbühler K. Female-led infanticide in wild chimpanzees. Curr Biol. 2007;17(10):R355–R356. doi: 10.1016/j.cub.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Otali E, Gilchrist JS. Why chimpanzee (Pan troglodytes schweinfurthii) mothers are less gregarious than nonmothers and males: The infant safety hypothesis. Behav Ecol Sociobiol. 2006;59(4):561–570. [Google Scholar]
- 69.Silk JB. Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Anim Behav. 1999;57(5):1021–1032. doi: 10.1006/anbe.1998.1065. [DOI] [PubMed] [Google Scholar]
- 70.Fruteau C, van de Waal E, Van Damme E, Noë R. Infant access and handling in sooty mangabeys and vervet monkeys. Anim Behav. 2011;81(1):153–161. [Google Scholar]
- 71.Price EC. The benefits of helpers: Effects of group and litter size on infant care in tamarins (Saguinus oedipus) Am J Primatol. 1992;26(3):179–190. doi: 10.1002/ajp.1350260304. [DOI] [PubMed] [Google Scholar]
- 72.Smith AS, Birnie AK, French JA. Prenatal androgens affect development and behavior in primates. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building Babies: Primate Development in Proximate and Ultimate Perspective. Springer; New York: 2013. pp. 103–131. [Google Scholar]
- 73.Harris JA, Rushton JP, Hampson E, Jackson DN. Salivary testosterone and self-report aggressive and pro-social personality characteristics in men and women. Aggress Behav. 1996;22(5):321–331. [Google Scholar]
- 74.Edwards DA, Kurlander LS. Women’s intercollegiate volleyball and tennis: Effects of warm-up, competition, and practice on saliva levels of cortisol and testosterone. Horm Behav. 2010;58(4):606–613. doi: 10.1016/j.yhbeh.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Archer J, Lloyd B. Sex and Gender. Cambridge Univ Press; London: 2002. [Google Scholar]
- 76.Eaton WO, Enns LR. Sex differences in human motor activity level. Psychol Bull. 1986;100(1):19–28. [PubMed] [Google Scholar]
- 77.Morrongiello BA, Dawber T. Mothers’ responses to sons and daughters engaging in injury-risk behaviors on a playground: Implications for sex differences in injury rates. J Exp Child Psychol. 2000;76(2):89–103. doi: 10.1006/jecp.2000.2572. [DOI] [PubMed] [Google Scholar]
- 78.MacDonald K, Parke RD. Parent-child physical play: The effects of sex and age of children and parents. Sex Roles. 1986;15(7-8):367–378. [Google Scholar]
- 79.Ladd GW, Golter BS. Parents’ management of preschooler’s peer relations: Is it related to children’s social competence? Dev Psychol. 1988;24(1):109–117. [Google Scholar]
- 80.Bhavnagri NP, Parke RD. Parents as direct facilitators of children’s peer relationships: Effects of age of child and sex of parent. J Soc Pers Relat. 1991;8(3):423–440. [Google Scholar]
- 81.Bakan D. The Duality of Human Existence. Rand McNally; Chicago: 1966. [Google Scholar]
- 82.Connellan J, Baron-Cohen S, Wheelwright S, Batki A, Ahluwalia J. Sex differences in human neonatal social perception. Infant Behav Dev. 2000;23(1):113–118. [Google Scholar]
- 83.Hall J, Carter J, Horgan T. In: Gender and Emotion: Social Psychological Perspectives. Fischer A, editor. Cambridge Univ Press; Cambridge, UK: 2000. pp. 97–117. [Google Scholar]
- 84.Eisenberg N, Fabes R, Spinrad T. In: Social, Emotional, and Personality Development. Eisenberg N, editor. John Wiley; Hoboken, NJ: 2006. pp. 646–718. [Google Scholar]
- 85.Taylor SE, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 86.Fabes RA, Martin CL, Hanish LD. Young children’s play qualities in same-, other-, and mixed-sex peer groups. Child Dev. 2003;74(3):921–932. doi: 10.1111/1467-8624.00576. [DOI] [PubMed] [Google Scholar]
- 87.Benenson JF, et al. Males’ greater tolerance of same-sex peers. Psychol Sci. 2009;20(2):184–190. doi: 10.1111/j.1467-9280.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 88.Benenson JF, Markovits H, Wrangham R. Rank influences human sex differences in dyadic cooperation. Curr Biol. 2014;24(5):R190–R191. doi: 10.1016/j.cub.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 89.Chapais B. Primeval Kinship: How Pair-Bonding Gave Birth to Human Society. Harvard Univ Press; Cambridge, MA: 2008. [Google Scholar]
- 90.Crawley M. The R Book. John Wiley; Chichester, UK: 2007. [Google Scholar]
- 91.Bates D, Maechler M, Bolker BM, Walker S. 2014. lme4: Linear Mixed-Effects Models Using Eigen and S4, R package version 1.1-4. http://CRAN.R-project.org/package=lme4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.