Significance
We have shown that the growth hormone-releasing hormone–growth hormone–insulin-like growth factor-1 (GHRH–GH–IGF1) axis is involved in experimentally induced acute inflammation in the iris and ciliary body, where its activation is associated with inflammatory responses. Blocking GHRH–GH–IGF1 axis activity by the GHRH receptor (GHRH-R) antagonist is effective in alleviating inflammatory responses. These findings demonstrate a novel activity of GHRH–GH–IGF1 axis signaling in ocular inflammation and suggest a potential therapeutic application of GHRH-R antagonist.
Keywords: GHRH–GH–IGF1 axis, experimental uveitis, GHRH analogs
Abstract
Disruptions in immunity and occurrence of inflammation cause many eye diseases. The growth hormone-releasing hormone–growth hormone–insulin-like growth factor-1 (GHRH–GH–IGF1) axis exerts regulatory effects on the immune system. Its involvement in ocular inflammation remains to be investigated. Here we studied this signaling in endotoxin-induced uveitis (EIU) generated by LPS. The increase in GHRH receptor (GHRH-R) protein levels was parallel to the increase in mRNA levels of pituitary-specific transcription factor-1, GHRH-R splice variant 1, GHRH, and GH following LPS insult. Elevation of GHRH-R and GH receptor was localized on the epithelium of the iris and ciliary body, and GHRH-R was confined to the infiltrating macrophages and leukocytes in aqueous humor but not to those in stroma. Treatment with GHRH-R antagonist decreased LPS-stimulated surges of GH and IGF1 in aqueous humor and alleviated inflammation by reducing the infiltration of macrophages and leukocytes and the production of TNF-α, IL-1β, and monocyte chemotactic protein-1. Our results indicate that inflammation in the iris and ciliary body involves the activation of GHRH signaling, which affects the recruitment of immune cells and the production of proinflammatory mediators that contribute to EIU pathogenesis. Moreover, the results suggest that GHRH-R antagonists are potential therapeutic agents for the treatment of acute ocular inflammation.
Growth hormone-releasing hormone (GHRH) is a hypothalamic hormone that belongs to the secretin superfamily of peptide hormones (1). The amino terminal sequence of 29 amino acids retains the full biological activity of GHRH (2). GHRH binds to its receptor (GHRH-R) on pituitary somatotrophs and activates synthesis and secretion of growth hormone (GH) (2, 3). GHRH peptide and GHRH-R also are expressed in extrapituitary tissues, including normal and malignant tissues, cancer cell lines, and immune cells (4–7). Moreover, truncated splice variant (SV-1) of GHRH-R also is found in tumors, pituitary, and peripheral tissues, implying a physiological or pathophysiological role unrelated to its normal endocrine function (8).
Extrahypothalamic GHRH in neoplastic and normal peripheral tissues plays a mitogenic role in stimulating cell proliferation and preventing apoptotic cell death, likely mediated by an autocrine and/or paracrine production of insulin-like growth factor-1 (IGF1) (9–11). GHRH and its downstream signaling are involved in the development of autoimmune disease. For example, GHRH-R–deficient mice are not susceptible to the induction of experimental autoimmune encephalomyelitis (12). On the other hand, pretreatment with GHRH reverses endotoxin-induced inflammatory hyperalgesia (13). GHRH antagonist suppressed the expression of inflammatory mediators, such as IL-1β, NF-κBp65, and COX-2, in prostatic hyperplasia (14). Furthermore, GHRH, GH, and IGF1 augment the differentiation of granulocytes from progenitor bone marrow cells into functional mature immune cells (15).
Uveitis is a common form of ocular inflammation. Repeat episodes can induce irreversible damages to ocular tissues leading to visual impairment (16). Administration of corticosteroid is the standard therapeutic strategy, but alternative treatments with less severe side effects are being sought (17). The GHRH-R antagonist MIA-602 suppressed the expression of inflammatory genes in breast cancer without obvious side effects (18). Thus, we hypothesized that the GHRH–GH–IGF1 axis is involved in pathogenesis of ocular inflammation and that GHRH-R antagonists can serve as alternative agents for treatment of uveitis. We attempted to address these issues in an animal model of endotoxin-induced uveitis (EIU), which we recently have characterized in adult rats (19).
Results
Gene Expression of GHRH-R and Its Related Signaling Molecules in Ocular Tissues After LPS Insult.
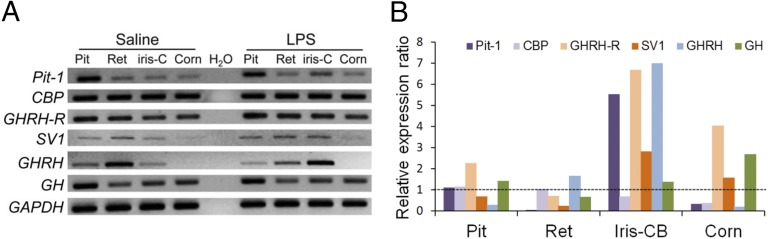
Transcripts of GHRH-R and its related signaling molecules in ocular tissues and the pituitary were determined using RT-PCR with the primer sets listed in Table S1. Tissues were collected 24 h after a unilateral footpad injection of LPS in adult Sprague–Dawley rats. In an adult rat model of EIU a significant increase in protein and cell contents in the aqueous humor occurred as early as 3 h after LPS injection and reached peak levels at 18–24 h (20). In control rats injected with saline, GHRH-R was detected in the retina, iris and ciliary body (iris-CB), and cornea, whereas its ligand GHRH and the receptor variant SV-1 were expressed in the retina and iris-CB but were expressed only slightly in the cornea (Fig. 1A). GH was detected, together with pituitary-specific transcription factor-1 (Pit-1) and CREB-binding protein (CBP), a ubiquitously expressed nuclear transcription factor and a cofactor of Pit-1 (21, 22). Transcripts of GHRH, GHRH-R, GH, and related signaling molecules also were detected in the pituitary. Injection of LPS, known to induce inflammatory changes in ocular tissues (19), caused a general increase in the expression of these genes, particularly in the iris-CB.
Fig. 1.
PCR analyses of gene expression of GHRH and its downstream molecules in normal and LPS-treated rats. (A) RT-PCR showed a single band for each gene in 2% agarose gel. The product size was as predicted (Table S1). Pit-1, GHRH-R, SV-1, and GHRH in iris-CB were up-regulated 24 h after LPS injection. (B) In qPCR, the relative expression ratio was the mean normalized expression value (Table 1) in LPS-treated rats versus control rats. The most obvious increases in expression of Pit-1, GHRH-R, SV-1, and GHRH were in iris-CB. GADPH served as the internal control. CBP, CREB-binding protein; Corn, cornea; iris-C or iris-CB, iris and ciliary body; Pit, pituitary; Ret, retina.
Changes in gene expression were analyzed using further quantitative real-time PCR (qRT-PCR) (Table 1). LPS induced a significant increase in the expression of GHRH-R, SV-1, GHRH, and Pit-1 in iris-CB (Fig. 1B). GHRH-R expression also was increased in cornea and pituitary, whereas CBP was down-regulated in iris-CB and cornea. GHRH-R, GHRH, Pit-1, and SV-1 expression was substantially higher in the iris-CB (a three- to sevenfold increase) than in the cornea and pituitary. No obvious change was observed in the retina.
Table 1.
Normalized expression values of Pit-1, CBP, GHRH-R, SV-1, GHRH, and GH in the tissues of normal and LPS-treated rats based on qRT-PCR analysis
| Sample | Saline+saline | LPS+saline |
| Pit-1 | ||
| Pituitary | 0.8018 ± 0.0832 | 0.8819 ± 0.0300 |
| Retina | 0.0442 ± 0.0396 | 0.0014 ± 0.0004 |
| Iris-CB | 0.0008 ± 0.0001 | 0.0042 ± 0.0006*** |
| Cornea | 0.0002 ± 0.0001 | 0.0010 ± 0.0004 |
| CBP | ||
| Pituitary | 0.2337 ± 0.0159 | 0.2663 ± 0.0155 |
| Retina | 0.1192 ± 0.0114 | 0.1235 ± 0.0082 |
| Iris-CB | 0.2243 ± 0.0190 | 0.1533 ± 0.0089*** |
| Cornea | 0.0798 ± 0.0090 | 0.0344 ± 0.0021*** |
| GHRH-R | ||
| Pituitary | 0.1525 ± 0.366 | 0.3442 ± 0.0320*** |
| Retina | 0.0029 ± 0.0005 | 0.0020 ± 0.0006 |
| Iris-CB | 0.00038 ± 0.00003 | 0.0025 ± 0.0005*** |
| Cornea | 0.00019 ± 0.00003 | 0.00076 ± 0.00029*** |
| SV-1 | ||
| Pituitary | 0.0010 ± 0.0004 | 0.0007 ± 0.0002 |
| Retina | 0.0019 ± 0.0008 | 0.0004 ± 0.0002 |
| Iris-CB | 0.00019 ± 0.00003 | 0.0005 ± 0.0001*** |
| Cornea | 0.00003 ± 0.00001 | 0.00012 ± 0.00006 |
| GHRH | ||
| Pituitary | 0.0012 ± 0.0006 | 0.0003 ± 0.0001 |
| Retina | 0.0012 ± 0.0006 | 0.0023 ± 0.0011 |
| Iris-CB | 0.00008 ± 0.00001 | 0.0005 ± 0.0001*** |
| Cornea | 0.00013 ± 0.00010 | 0.00004 ± 0.00001 |
| GH | ||
| Pituitary | 4.3079 ± 0.6512 | 6.1210 ± 0.6416 |
| Retina | 0.00013 ± 0.00004 | 0.00008 ± 0.00001 |
| Iris-CB | 0.0054 ± 0.0016 | 0.0073 ± 0.0025 |
| Cornea | 0.0039 ± 0.0011 | 0.0105 ± 0.0084 |
Data are shown as mean ± SEM. ***P < 0.01, compared with normal control (saline+saline); Mann–Whitney test; n = 9 in each group.
Expression and Localization of GHRH-R Protein in Ocular Tissues.
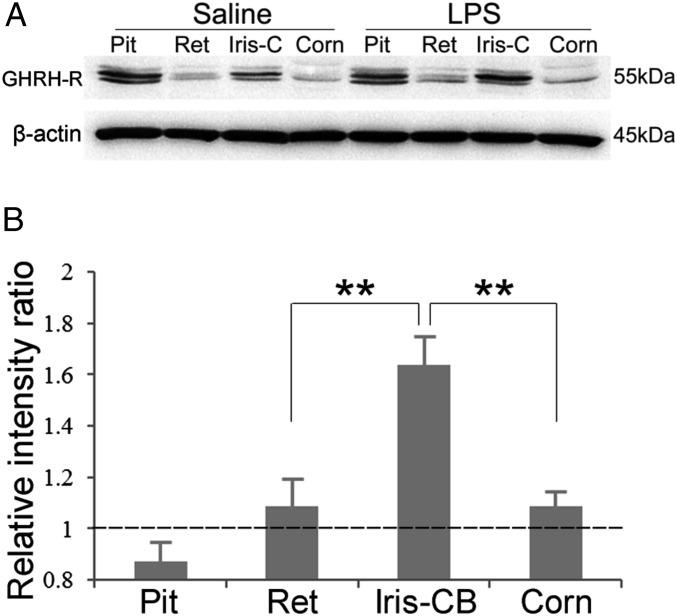
Western blot analyses revealed abundant expression of GHRH-R in the iris-CB and pituitary and moderate expression in retina and cornea (Fig. 2A). LPS induced an elevation of GHRH-R protein level in all ocular tissues examined (Fig. 2B), with the most obvious increase in iris-CB (1.64 ± 0.11-fold), which was significantly higher than the increase in retina and cornea (1.09 ± 0.10- and 1.09 ± 0.05-fold, respectively). No obvious analogous change was detected in the pituitary.
Fig. 2.
Western blot analysis of GHRH-R expression in pituitary and ocular tissues after LPS treatment. (A) GHRH-R protein was detected in the pituitary, retina, iris and ciliary body (iris-C), and cornea in normal and LPS-treated rats. Intensities of the bands corresponding to GHRH-R were normalized to that of the internal control, β-actin. (B) The relative intensity ratios were obtained from the normalized intensity values in LPS-treated rats over the corresponding values in controls. Increased GHRH-R expression was most obvious in iris and ciliary body (iris-CB). Data are shown as mean ± SEM; **P < 0.05 compared with the value in the pituitary; Mann–Whitney test; n = 3.
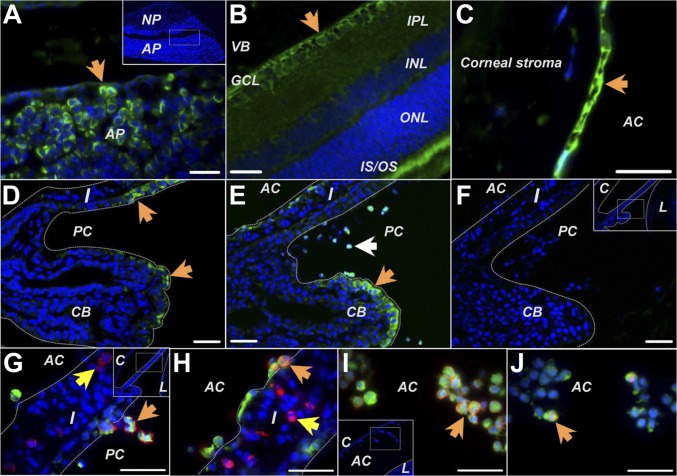
Immunocytochemistry revealed the localization of GHRH-R in the somatotrophs in the anterior lobe but not in the cells in the posterior lobe of the pituitary (Fig. 3A). GHRH-R also was found in the retinal ganglion cell layer, the photopigment layer of normal retina (Fig. 3B), cornea endothelium (Fig. 3C), and in a few epithelial cells lining the iris-CB (Fig. 3D). LPS induced obvious elevation of GHRH-R in the iris-CB. Intense staining was found on the ciliary epithelium and on infiltrating cells scattered in the anterior and posterior chambers (Fig. 3E). Double labeling showed that almost all infiltrating cells were GHRH-R–positive; some were immunopositive for CD43 (a surface antigen on leukocytes) (Fig. 3 G and I), and others were immunopositive for CD68 (a cytoplasmic antigen in monocytes and macrophages) (Fig. 3 H and J). Moreover, GHRH-R immunoreactivity was not detected on cells expressing CD43 or CD68 in the stroma of iris-CB (Fig. 3 G and H). Therefore, GHRH-R is expressed specifically on immune cells in the aqueous humor but not on those residing in the tissues.
Fig. 3.
Immunohistochemical studies of GHRH-R expression in pituitary and ocular tissues. (A) GHRH-R was found on cells (arrow) in the anterior pituitary gland. (B) In the retina, GHRH-R was stained in the ganglion cell layer (arrow) and outer and inner segments of photoreceptors. (C) GHRH-R was observed on the corneal endothelium (arrow). (D) In normal rats GHRH-R was expressed on epithelial cells in the ciliary body process and iris. (E) In LPS-treated animals GHRH-R expression was up-regulated on cells in the ciliary epithelium (orange arrow) and on cells infiltrating into the anterior and posterior chambers (white arrow). (F) Treatment without the primary antibody gave no staining on the LPS-treated eye sections. (G–J) GHRH-R (green) was intensely stained on the cells expressing the leukocyte marker CD43 (red in G and I) or the monocyte/macrophage marker CD68 (red in H and J) when they detached from the iris (orange arrows in G and H) or resided in the aqueous humor (arrows in I and J). GHRH-R was not found in the stroma cells of the iris (yellow arrows in G and H). AC, anterior chamber; AP, anterior pituitary gland; C, cornea; CB, ciliary body; GCL, ganglion cell layer; I, iris; PC, posterior chamber; IPL, inner plexiform layer; IS/OS, inner and outer segment of photoreceptors; INL, inner nuclear layer; L, lens; NP, neurohypophysis; ONL, outer nuclear layer; VB, vitreous body. (Scale bars: 35 µm.)
Total Protein and IGF1 in Aqueous Humor After LPS Insult.
Total protein concentration was increased gradually in aqueous humor (Fig. S1A), where the major GHRH-GH signaling product, IGF1, was increased dramatically at 12 and 24 h after LPS injection (Fig. S1B) but was reduced in the systemic circulation (Fig. S1C). IGF1 was increased 13-fold 24 h after LPS injection, compared with the eightfold increase in total protein in aqueous humor (Table S2), suggesting local production of IGF1 upon LPS stimulation. In the serum, IGF1 was reduced by about 50% after LPS injection, but absolute IGF1 values in serum were several times higher than in the aqueous humor.
Localization of the Signaling Molecules GH, GHR, and IGF1 in Ocular Tissues After LPS Insult.
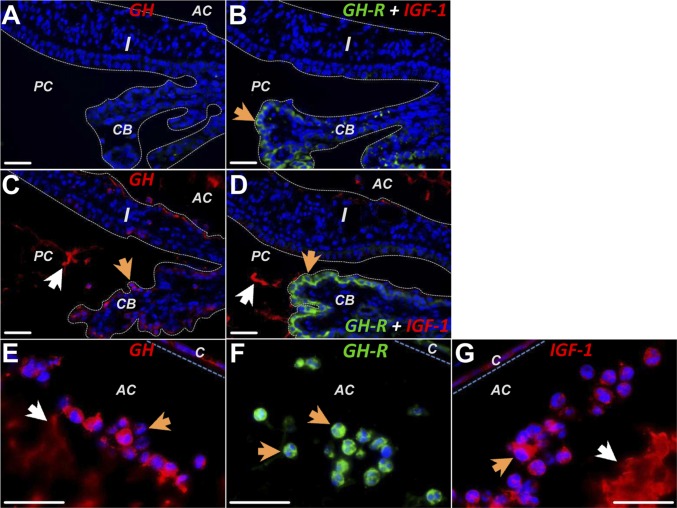
In saline controls (n = 2), GH and IGF1 immunoreactivity was not detectable in the iris-CB (Fig. 4A), whereas GHR was localized on ciliary epithelium and a few stromal cells (Fig. 4B). LPS induced obvious staining for GH in the epithelial lining of iris-CB (Fig. 4C) and for GHR in the ciliary epithelium (Fig. 4D). Moreover, GH and IGF1 immunoreactivity was localized on the amorphous substances in the aqueous humor (Fig. 4 C and D). GH, GHR, and IGF1 were all observed in the infiltrating cells (Fig. 4 E–G), suggesting that these immune cells and ciliary epithelium may be sources of these hormones.
Fig. 4.
Immunohistochemical studies of GH, GHR, and IGF1 in ocular tissues. (A) In normal rats, no GH (red) was detected in the iris and ciliary body. (B) Double-labeling in normal eye showed GHR (green) in the ciliary epithelium (arrow); IGF1 (red) was not detected. (C) Twenty-four hours after LPS injection, GH was found in the epithelial cells lining the ciliary body and iris (orange arrow) and in the protein exudates in the anterior and posterior chambers (white arrow). (D) LPS triggered up-regulation of GHR (green) on the ciliary epithelium (orange arrow) and secretion of IGF1 (white arrow) in the anterior and posterior chambers. (E–G) The infiltrating cells in the anterior chamber were strongly immunoreactive for GH (orange arrow in E), GHR (arrows in F), and IGF1 (orange arrow in G). GH and IGF1 also were found in the protein exudation in the anterior chamber. (Scale bars: 35 µm.)
GHRH-R Antagonist Reduced Inflammatory Responses Induced by LPS.
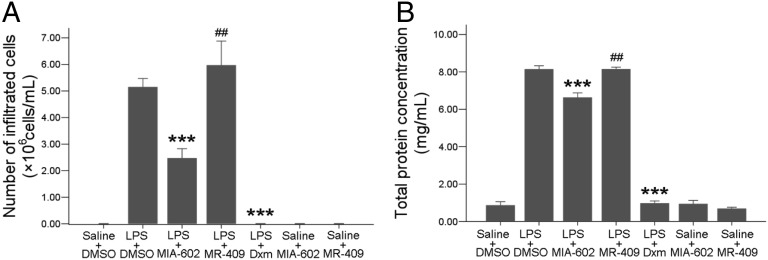
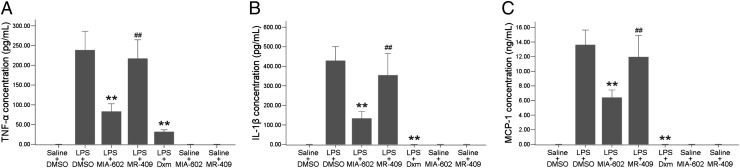
A GHRH-R antagonist (MIA-602; 20 µg per rat) or agonist (MR-409; 60 µg per rat) was injected i.v. 2 h after LPS injection. Control animals received i.v. injection of vehicle (0.2% DMSO in 5.5% mannitol sterile water). The numbers of infiltrating cells and total protein levels in aqueous humor were reduced significantly in rats injected with MIA-602 compared with those treated with LPS and vehicle only (Fig. 5). However, in animals treated with MR-409, the numbers of infiltrating cells (P = 0.52) and total protein levels (P = 0.87) were slightly higher than or were comparable to those of the LPS-plus-vehicle group. Injection of the same doses of MIA-602 or MR-409 alone without LPS did not change infiltrating cell numbers or total protein exudation. These inflammatory responses were suppressed totally by oral administration of dexamethasone (Dxm) at 1 mg/kg. ELISA of aqueous humor after treatment with GHRH agonist or antagonist showed increased secretions of proinflammatory mediators TNF-α, IL-1β, and monocyte chemotactic protein-1 (MCP-1) 24 h after LPS injection. The GHRH-R antagonist MIA-602 significantly suppressed the secretion of these factors into the aqueous humor. Such reduction was not observed in the rats treated with the agonist MR-409 (Fig. 6). MIA-602 and MR-409 alone did not generate any change in the level of proinflammatory factors.
Fig. 5.
Infiltrating cells and total protein in aqueous humor after GHRH-R antagonist treatment. LPS-induced cell infiltration (A) and total protein exudation (B) were reduced significantly by the GHRH-R antagonist MIA-602 (P = 0.004 for each) and Dxm but not by the GHRH agonist MR-409. Data are shown as mean ± SEM; ***P < 0.01; ##, no significant difference, compared with LPS+DMSO; Mann–Whitney test; n = 6 in each group.
Fig. 6.
TNF-α, IL-1β, and MCP-1 in aqueous humor after GHRH-R antagonist treatment. LPS-induced elevations of TNF-α (A), IL-1β (B), and MCP-1 (C) were reduced significantly by the GHRH-R antagonist MIA-602 and Dxm but not by the GHRH-R agonist MR-409. Data are shown as mean ± SEM; **P < 0.05; ##, no significant difference compared with LPS; Mann–Whitney test; n = 6 in each group.
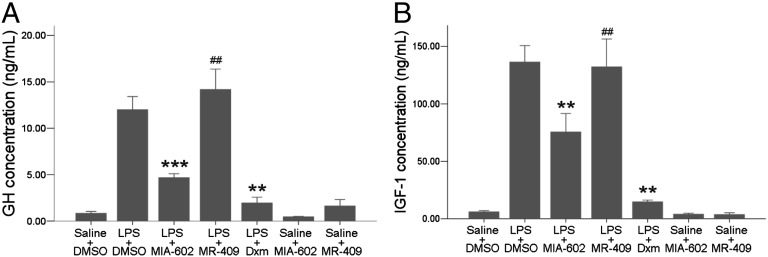
Reduction of GH and IGF1 Concentrations After GHRH Antagonist Treatment.
ELISA results showed significant suppression of LPS-induced surges of GH and IGF1 after treatment with MIA-602 but not after MR-409 injections (Fig. 7). Dxm also reduced GH and IGF1 significantly 24 h after LPS injection. MIA-602 or MR-409 alone did not change the GH or IGF1 levels, indicating an association of the anti-inflammatory actions of GHRH-R antagonist with a suppression of GH and IGF1 in the aqueous humor. In this study we have not examined overlapping or additive effects of the GHRH-R antagonist with Dxm.
Fig. 7.
IGF1 and GH in aqueous humor after treatment with the GHRH-R antagonist. LPS-induced increases in GH (A) and IGF1 (B) levels were suppressed significantly by the GHRH-R antagonist MIA-602 but not by the GHRH-R agonist MR-409. Data are shown as mean ± SEM. ***P < 0.01; **P < 0.05; ##, no significant difference compared with LPS; Mann–Whitney test; n = 6 in each group.
Effects of GHRH Antagonist on Gene Expression of GHRH and Related Signaling Molecules in the Iris-CB.
qRT-PCR showed that GHRH-R, SV-1, GHRH, and GH and their regulatory genes Pit-1 and CBP were all reduced by MIA-602. The suppression was most obvious for the Pit-1 gene (Table S3). These effects were not observed for MR-409, in which the expression of GHRH-R and SV-1 was significantly up-regulated.
Discussion
This study investigated the contributions of GHRH–GH–IGF1 axis signaling to inflammatory processes in EIU. Our major findings are (i) GHRH signaling-regulatory molecules are expressed in the iris-CB and retina; (ii) these genes are up-regulated after LPS injection, particularly in the iris-CB; (iii) GHRH-R and GHR proteins are localized on the ciliary epithelium in normal tissues, and their expression is elevated substantially after LPS injection; (iv) GHRH-R and GHR also are localized on the leukocytes and macrophages infiltrating in the aqueous humor, and these cells produce GH and IGF1; (v) the GHRH-R antagonist MIA-602, but not the agonist, ameliorates LPS-induced infiltration of immune cells, exudation of proteins, and secretion of TNF-α, IL-1β, and MCP-1 in the aqueous humor; (vi) these anti-inflammatory effects are associated with reduced production of GH and IGF1 that results from the blocking GHRH-R, suggesting a novel function of the GHRH–GH–IGF1 axis in experimentally induced uveitis.
The extrapituitary functions of GHRH are mediated through GHRH-R and its splice variants, particularly SV-1 (23–25). However, the pituitary-type GHRH-R rather than SV-1 is the predominant form of receptor expressed in normal ocular tissues (26). Moreover, the presence of Pit-1, an important activator of the GHRH-R and GH promoters (21, 27), supports the involvement of GHRH signaling in these tissues. The ocular tissues may respond to GHRH and augment synthesis of GH, which binds to GH receptor (GH-R) on the ciliary epithelium, for production of molecules downstream of GH signaling, including IGF1. It is possible that the GHRH–GH–IGF1 axis also operates in retinal cells, such as ganglion cells and photoreceptors.
LPS induces a surge in transcripts of GHRH, GHRH-R, SV-1, and Pit-1 in the ocular tissues, which are most obvious in the iris-CB. This response to LPS is confirmed by immunocytochemical and Western blot analyses, suggesting that the activation of GHRH signaling may be related to the vulnerability of the iris-CB to LPS insult. The susceptibility of the anterior uvea has been related to the higher blood flow and large fenestrae in blood vessels within these tissues, which enable more direct exposure to the circulating endotoxin (28).
The specific increase in the expression of GHRH-R and GH-R on the iris-CB epithelium and in the leukocytes and macrophages infiltrating the aqueous humor suggests that GHRH has a dual site of action after LPS stimulation, one on the ciliary epithelial cells and another on the immune cells residing in or circulating through the iris and ciliary processes. Immunohistochemical and ELISA results show that both tissues respond by producing GH and secreting it into the aqueous humor. This secreted GH then can bind to its receptor GH-R on the epithelial cells and on the infiltrating immune cells to produce IGF1. In addition to local production, the surge of GH and IGF1—the major functional proteins in the GHRH–GH–IGF1 axis—can be attributed to an increased influx of these molecules from the systemic circulation into the aqueous humor as a result of the vascular leakage caused by LPS. In aqueous humor, IGF1 is elevated 13-fold, far more than the eightfold increase in total protein content, indicating local production of this effector molecule. Moreover, the systemic level of IGF1 is decreased after LPS treatment, further supporting an autocrine and/or paracrine regulation of GHRH–GH–IGF1 signaling in the iris-CB and immune cells.
Our findings show that blocking the GHRH–GH–IGF1 signaling with the GHRH-R antagonist MIA-602, but not the agonist MR-409, substantially suppresses the inflammatory responses in LPS-induced uveitis. These results agree with earlier findings that GHRH-R antagonists suppress inflammation by reducing the production of inflammatory proteins, such as IL-β, NF-κB/p65, and COX-2, in experimental prostatic hyperplasia and breast cancer (15, 18) and alleviate inflammation-triggered oxidative stress by increasing glutathione and decreasing glutathione peroxidase activity (29). The antagonist may alleviate inflammatory responses by acting as a local modulator for the maturation and migration of immune cells, including macrophages, lymphocytes, and other leukocytes (30–32). Because GH has been shown to target macrophages to trigger various immune responses (15), we argue that the GHRH-R antagonist may block the signaling of GHRH-R on the macrophages residing in the iris-CB and so inhibit synthesis and release of GH, resulting in reduced IGF1 secretion. Reduced IGF1 inactivates PI-3/Akt/NF-κB– or Ras/Raf/MAPK/ERK-kinase downstream of the IGF1 signaling pathway (33, 34), thus decreasing the production of proinflammatory factors, including cytokines and chemokines. LPS-induced elevation of Pit-1, GHRH-R, SV-1, GHRH, and GH mRNA expression in iris-CB are significantly diminished by the GHRH-R antagonist, leading to a further reduction of GH and IGF1. The GHRH-R antagonist JMR-132 can suppress the activation of Akt-kinase and ERK-kinase and reduce production of GHRH, thus inhibiting the growth of human androgen-independent prostate cancer (35). However, IGF1 also prevents the degradation of IκB and diminishes NF-κB translocation into the nucleus (36), leaving inconclusive IGF1’s role as a pro- or anti-inflammatory agent (37). In this study we show that suppressing IGF1 production by blocking GHRH signaling alleviates inflammatory responses in ocular tissues.
Another possible action of the GHRH-R antagonist is to reduce the secretion of TNF-α, IL-1β, and MCP-1 from the inflammatory cells, thus alleviating further recruitment of leukocytes and macrophages into the aqueous humor. Earlier studies showed that, after injection, GH and GHRH up-regulate TNF-α, IL1-α/β, and IL-6 in human blood (38) and in cells incubated with GH or GHRH (36, 37). Last, the GHRH-R antagonist may inhibit the migration of immune cells into the aqueous humor, as suggested by the presence of GHRH-R only on cells in the aqueous humor but not in the stroma of the iris.
In summary, the present study provides evidence that the GHRH–GH–IGF1 signaling axis exists in the iris-CB and that its activation is associated with inflammatory responses triggered by LPS. Moreover, blocking GHRH–GH–IGF1 axis activity by the GHRH-R antagonist is effective in alleviating inflammatory responses in this model of experimentally induced uveitis. These findings demonstrate a novel activity of GHRH–GH–IGF1 axis signaling in ocular inflammation and suggest a potential therapeutic application of the GHRH-R antagonist.
Materials and Methods
EIU Model and Peptide Treatment in Rats.
All experiments were conducted according to guidelines of the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Ophthalmic and Vision Research. Ethics approval for the study was obtained from the Chinese University of Hong Kong Animal Ethics Committee. Adult Sprague–Dawley rats weighing 200–250 g, male age 6–8 wk, were obtained from the Laboratory Animal Service Center of the Chinese University of Hong Kong. The animals were housed in standard conditions, maintained at 22 ± 1 °C in 40 ± 10% humidity and a 12:12-h dark:light cycle with free access to food and water.
LPS (Salmonella typhimurium; Sigma-Aldrich) was dissolved in pyrogen-free saline. Acute uveitis was induced by footpad injection of 0.1 mL of the diluted LPS, at a dose of 1 mg/kg. Our preliminary study showed that 1 mg/kg LPS, rather than 0.5 or 2 mg/kg, was an ideal dosage to induce moderate inflammation in both eyes without producing obvious lesions in the liver or kidney.
The peptide analog GHRH-R antagonist MIA-602 and the GHRH-R agonist MR-409 were prepared in our laboratory (Endocrine, Polypeptide, and Cancer Institute, Miami Veterans Affairs Medical Center) (SI Materials and Methods). Each was dissolved in 100% DMSO (ACS grade; Sigma-Aldrich) and then was diluted 1:500 in 5.5% mannitol in sterile water. The concentration of DMSO never exceeded 0.2%. Two hours after LPS injection, 0.2 mL of MR-409 (60 μg per rat) or MIA-602 (20 μg per rat) was given by i.v. injection. Control animals were injected with 0.1 mL of pyrogen-free saline into one footpad and were injected i.v. with 0.2 mL 5.5% mannitol in sterile water containing 0.2% DMSO.
Before peptide treatment, 58 rats were divided randomly into two groups: (i) the normal control group, with footpad injection of saline followed by i.v. injection of saline 2 h after footpad injection (saline+saline, n = 29) or (ii) the LPS group, with footpad injection of LPS and i.v. injection with saline (LPS+saline, n = 29). In these animals mRNA expression of Pit-1, CBP, GHRH-R, SV-1, GHRH, and GH in the pituitary, retina, iris-CB, and cornea (n = 9 in each group), protein expression and localization of GHRH-R, GHR, GH, and IGF1 in ocular tissues (n = 2 in each group), and the secretion profiles of total protein and IGF1 in aqueous humor were determined at 4, 12, and 24 h after LPS injection (n = 18 in each group).
Another 45 rats were divided randomly into seven groups: (i) normal controls, which received footpad injection of saline followed by i.v. injection of vehicle (0.2% DMSO in 0.2 mL 5.5% mannitol sterile water) 2 h after footpad injection (saline+DMSO, n = 9); (ii) the LPS group, which received footpad injection of LPS and then i.v. injection of vehicle (LPS+DMSO, n = 9); (iii) two treatment groups in which MR-409 or MIA-602 was administered i.v. 2 h after LPS injection (LPS+MR-409, n = 9; LPS+MIA-602, n = 9); (iv) the Dxm group, which received 1 mg/kg Dxm orally 2 h after LPS injection (LPS+Dxm, n = 3); and (v) two peptide groups, which received footpad injection of saline and then i.v. injection of either MR-409 (saline+MR-409, n = 3) or MIA-602 (saline+MIA-602, n = 3). Twenty-four hours after footpad injection, the rats for sample collection were killed by i.p. injection of 4.0 mL of ketamine-xylazine mixture (1.5:1; Alfasan International B.V.).
Histological Examinations.
Under deep anesthesia, the rats were perfused with 0.01 M sterile PBS followed by 4% (wt/vol) freshly prepared paraformaldehyde. Both eyes and the pituitary were removed and immersed in 10% (wt/vol) formalin for 24 h at room temperature. The nictitating membrane was maintained in each eye to facilitate orientation. The tissues were embedded in paraffin and sectioned at 5µm thickness before immunostaining (SI Materials and Methods).
Western Blot Analysis.
Proteins from the pituitary and ocular tissues were isolated by a lysis buffer containing 1 M Tris⋅base (pH 7.4), 5 M NaCl, 10% (wt/vol) Nonidet P-40, 10% (wt/vol) sodium deoxycholate, 0.25 M EDTA, and protein inhibitor mixture (Complete Mini EDTA-free; Roche). Samples from normal rats without LPS treatment served as controls. The protein concentration was adjusted equally with a protein assay kit (Bio-Rad) before denaturing in loading buffer for 5 min at 95 °C and separation on 12.5% (wt/vol) SDS-polyacrylamide gel electrophoresis for Western blotting analysis (SI Materials and Methods).
Quantification of Pit-1, CBP, GHRH-R, SV-1, GHRH, and GH mRNA Expression.
Pituitary, retina, iris-CB, and cornea were obtained 24 h after LPS injection and washed with 0.01 M cold, sterile PBS. The tissue samples were stored in 300- to 500-μL TRIzol reagents (Invitrogen) at −80 °C until use. Similar samples were collected from the normal group as controls. Total RNA was isolated and treated with RNase-free DNase I (Qiagen) according to the manufacturer’s protocol. RNA (0.5–1 μg) was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen). PCR was performed using an iCycler PCR instrument (Bio-Rad) and lightCycle 480 II real-time PCR (Roche Applied Science) according to the manufacturer’s instructions (SI Materials and Methods). The gene-specific primers for cDNA sequences were designed with the Primer3 Input program, v. 0.4.0 (bioinfo.ut.ee/primer3-0.4.0/primer3/) (SI Materials and Methods and Table S1).
Cell Count and Protein Assay in Aqueous Humor.
Aqueous humor was collected from the anterior chambers with a 30-gauge needle. A 1-µL aliquot was diluted with 9 µL of 0.01-M PBS and suspended in 10 µL of Trypan-blue solution. The cell number was counted by an investigator blinded to the study using a hemocytometer under a light microscope. Another portion of the aqueous humor was centrifuged at 550 × g for 15 min at 4 °C. Cell-free supernatants were used for total protein assay in duplicate (Bio-Rad).
Evaluation of GH, IGF1, and Proinflammatory Cytokine Levels in Aqueous Humor.
Blood samples were collected by cardiac puncture, clotted at room temperature for 2 h, and centrifuged at 550 × g for 15 min at 4 °C. The serum and cell-free aqueous humor were determined for TNF-α (rat-ELISA kit; R&D Systems), IL-1β (rat-ELISA kit; R&D Systems), MCP-1 (rat-ELISA kit; Invitrogen), GH (rat/mouse ELISA kit; EMD Millipore), and IGF1 (mouse/rat- ELISA kit; R&D Systems) in duplicate.
Statistical Analysis.
Most data were analyzed by the nonparametric Kruskal–Wallis test. Comparisons of two groups were done by Mann–Whitney tests. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software package v. 20.0 (IBM).
Supplementary Material
Acknowledgments
We thank the Li Ka Shing Foundation for generous support. The work in Hong Kong and Shantou was supported by a block grant from the University Grants Committee Hong Kong (to C.P.P.), a Research Grant Council General Research Fund (Project CUHK461612), and a seed grant from the Lui Che Woo Institute of Innovative Medicine (Project 8303107) (to S.O.C.). The work in the United States was supported by the Medical Research Service of the Veterans Affairs Department, South Florida Veterans Affairs Foundation for Research and Education, the University of Miami’s Miller School of Medicine (A.V.S.), and the L. Austin Weeks Family Endowment for Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421815112/-/DCSupplemental.
References
- 1.Frohman LA, Kineman RD. Growth hormone-releasing hormone and pituitary development, hyperplasia and tumorigenesis. Trends Endocrinol Metab. 2002;13(7):299–303. doi: 10.1016/s1043-2760(02)00613-6. [DOI] [PubMed] [Google Scholar]
- 2.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4(1):33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 3.Lin-Su K, Wajnrajch MP. Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor. Rev Endocr Metab Disord. 2002;3(4):313–323. doi: 10.1023/a:1020949507265. [DOI] [PubMed] [Google Scholar]
- 4.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 5.Havt A, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102(48):17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korytko AI, Zeitler P, Cuttler L. Developmental regulation of pituitary growth hormone-releasing hormone receptor gene expression in the rat. Endocrinology. 1996;137(4):1326–1331. doi: 10.1210/endo.137.4.8625907. [DOI] [PubMed] [Google Scholar]
- 7.Gallego R, et al. Cellular distribution of growth hormone-releasing hormone receptor in human reproductive system and breast and prostate cancers. Histol Histopathol. 2005;20(3):697–706. doi: 10.14670/HH-20.697. [DOI] [PubMed] [Google Scholar]
- 8.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22(8):311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Siriwardana G, Bradford A, Coy D, Zeitler P. Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol. 2006;20(9):2010–2019. doi: 10.1210/me.2005-0001. [DOI] [PubMed] [Google Scholar]
- 10.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 11.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40(5):315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 12.Ikushima H, Kanaoka M, Kojima S. Cutting edge: Requirement for growth hormone-releasing hormone in the development of experimental autoimmune encephalomyelitis. J Immunol. 2003;171(6):2769–2772. doi: 10.4049/jimmunol.171.6.2769. [DOI] [PubMed] [Google Scholar]
- 13.Talhouk RS, Saadé NE, Mouneimne G, Masaad CA, Safieh-Garabedian B. Growth hormone releasing hormone reverses endotoxin-induced localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):625–631. doi: 10.1016/j.pnpbp.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108(9):3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khorram O, Yeung M, Vu L, Yen SS. Effects of [norleucine27]growth hormone-releasing hormone (GHRH) (1-29)-NH2 administration on the immune system of aging men and women. J Clin Endocrinol Metab. 1997;82(11):3590–3596. doi: 10.1210/jcem.82.11.4363. [DOI] [PubMed] [Google Scholar]
- 16.Hettinga YM, Verhagen FH, van Genderen M, de Boer JH. Characteristics of childhood uveitis leading to visual impairment and blindness in the Netherlands. Acta Ophthalmol (Copenh) 2014;92(8):798–804. doi: 10.1111/aos.12491. [DOI] [PubMed] [Google Scholar]
- 17.Tempest-Roe S, Joshi L, Dick AD, Taylor SR. Local therapies for inflammatory eye disease in translation: Past, present and future. BMC Ophthalmol. 2013;13(1):39. doi: 10.1186/1471-2415-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez R, et al. Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget. 2012;3(9):988–997. doi: 10.18632/oncotarget.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin YJ, et al. Green tea extract treatment alleviates ocular inflammation in a rat model of endotoxin-induced uveitis. PLoS ONE. 2014;9(8):e103995. doi: 10.1371/journal.pone.0103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura A, Mochizuki M. Endotoxin-induced uveitis in rats: Morphological and biochemical study. Jpn J Ophthalmol. 1988;32(4):457–465. [PubMed] [Google Scholar]
- 21.Kishimoto M, et al. Novel function of the transactivation domain of a pituitary-specific transcription factor, Pit-1. J Biol Chem. 2002;277(47):45141–45148. doi: 10.1074/jbc.M202991200. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LE, Hashimoto Y, Zanger K, Wondisford F, Radovick S. CREB-independent regulation by CBP is a novel mechanism of human growth hormone gene expression. J Clin Invest. 1999;104(8):1123–1130. doi: 10.1172/JCI7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dioufa N, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci USA. 2010;107(43):18611–18615. doi: 10.1073/pnas.1013942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83(2):303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 25.Schubert U, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci USA. 2013;110(6):2288–2293. doi: 10.1073/pnas.1221505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig B, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42(13):918–922. doi: 10.1055/s-0030-1267916. [DOI] [PubMed] [Google Scholar]
- 27.McElvaine AT, Korytko AI, Kilen SM, Cuttler L, Mayo KE. Pituitary-specific expression and Pit-1 regulation of the rat growth hormone-releasing hormone receptor gene. Mol Endocrinol. 2007;21(8):1969–1983. doi: 10.1210/me.2007-0116. [DOI] [PubMed] [Google Scholar]
- 28.Herbort CP, Chan CC, Nussenblatt RB. Endotoxin-induced uveitis in the rat: A hypothesis for preferential involvement of the anterior uvea. Curr Eye Res. 1990;9(Suppl):119–124. doi: 10.3109/02713689008999430. [DOI] [PubMed] [Google Scholar]
- 29.Brown PA, Bodles-Brakhop A, Draghia-Akli R. Plasmid growth hormone releasing hormone therapy in healthy and laminitis-afflicted horses-evaluation and pilot study. J Gene Med. 2008;10(5):564–574. doi: 10.1002/jgm.1170. [DOI] [PubMed] [Google Scholar]
- 30.Banks WA, et al. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci USA. 2010;107(51):22272–22277. doi: 10.1073/pnas.1016369107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khorram O, Garthwaite M, Golos T. The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J Clin Endocrinol Metab. 2001;86(7):3157–3161. doi: 10.1210/jcem.86.7.7652. [DOI] [PubMed] [Google Scholar]
- 32.Weigent DA. Lymphocyte GH-axis hormones in immunity. Cell Immunol. 2013;285(1-2):118–132. doi: 10.1016/j.cellimm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Jeay S, Sonenshein GE, Postel-Vinay MC, Kelly PA, Baixeras E. Growth hormone can act as a cytokine controlling survival and proliferation of immune cells: New insights into signaling pathways. Mol Cell Endocrinol. 2002;188(1-2):1–7. doi: 10.1016/s0303-7207(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 34.Panteva M, Korkaya H, Jameel S. Hepatitis viruses and the MAPK pathway: Is this a survival strategy? Virus Res. 2003;92(2):131–140. doi: 10.1016/s0168-1702(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 35.Rick FG, et al. Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci USA. 2012;109(5):1655–1660. doi: 10.1073/pnas.1120588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pons S, Torres-Aleman I. Insulin-like growth factor-I stimulates dephosphorylation of ikappa B through the serine phosphatase calcineurin (protein phosphatase 2B) J Biol Chem. 2000;275(49):38620–38625. doi: 10.1074/jbc.M004531200. [DOI] [PubMed] [Google Scholar]
- 37.Andreassen M, Frystyk J, Faber J, Kristensen LO. GH activity and markers of inflammation: A crossover study in healthy volunteers treated with GH and a GH receptor antagonist. Eur J Endocrinol. 2012;166(5):811–819. doi: 10.1530/EJE-11-1009. [DOI] [PubMed] [Google Scholar]
- 38.Bozzola M, De Amici M, Zecca M, Schimpff RM, Rapaport R. Modulating effect of human growth hormone on tumour necrosis factor-alpha and interleukin-1beta. Eur J Endocrinol. 1998;138(6):640–643. doi: 10.1530/eje.0.1380640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.