Significance
Pain is a societal burden with enormous economic and social costs. More than 50% of pain patients find current therapies unsatisfactory. A major reason for this pharmacological failure is our limited understanding of the cellular processes underlying pain. Algesic sensitization of nociceptors is a complex event in pain transduction that involves the potentiation of thermosensory channels. The molecular mechanisms remain controversial because of nociceptor heterogeneity that suggests the existence of cell-type–specific strategies. We show that algesic sensitization of transient receptor potential vanilloid 1 (TRPV1) involves the exocytotic recruitment of new receptors in peptidergic nociceptors and the modulation of channel gating in nonpeptidergic sensory neurons. Furthermore, algesic delivery of TRPV1 channels is concomitant with pro-inflammatory neuropeptide secretion, ensuring rapid modulation of pain transduction.
Keywords: pain transduction, nociception, ion channel, inflammation, sensory neuron
Abstract
Proalgesic sensitization of peripheral nociceptors in painful syndromes is a complex molecular process poorly understood that involves mobilization of thermosensory receptors to the neuronal surface. However, whether recruitment of vesicular thermoTRP channels is a general mechanism underlying sensitization of all nociceptor types or is subtype-specific remains controversial. We report that sensitization-induced Ca2+-dependent exocytotic insertion of transient receptor potential vanilloid 1 (TRPV1) receptors to the neuronal plasma membrane is a mechanism specifically used by peptidergic nociceptors to potentiate their excitability. Notably, we found that TRPV1 is present in large dense-core vesicles (LDCVs) that were mobilized to the neuronal surface in response to a sensitizing insult. Deletion or silencing of calcitonin-gene–related peptide alpha (αCGRP) gene expression drastically reduced proalgesic TRPV1 potentiation in peptidergic nociceptors by abrogating its Ca2+-dependent exocytotic recruitment. These findings uncover a context-dependent molecular mechanism of TRPV1 algesic sensitization and a previously unrecognized role of αCGRP in LDCV mobilization in peptidergic nociceptors. Furthermore, these results imply that concurrent secretion of neuropeptides and channels in peptidergic C-type nociceptors facilitates a rapid modulation of pain signaling.
Transient receptor potential vanilloid 1 (TRPV1) is a nonspecific cationic channel activated by capsaicin, noxious heat, acid pH, voltage, and membrane-derived lipids (1). TRPV1 upregulation in sensory neurons is a key element in pain development and maintenance of several pathological chronic conditions (2–5). Its contribution to thermal hyperalgesia has been well-defined through pharmacological and knockout studies (6, 7). TRPV1 channel is subject to a complex regulation by proalgesics that potentiate its activity through modulation of its gating properties (8, 9) and/or by increasing the expression of new channels into the neuronal surface (10, 11). Whether these mechanisms are a general means to sensitize TRPV1 in all nociceptor subpopulations or are cell-type–specific remains contentious.
TRPV1 is widely expressed in peptidergic and nonpeptidergic nociceptors from the neonatal and adult rat peripheral nervous system (12, 13). In mice, TRPV1 is transiently present in a wider range of sensory neurons during development, but its expression gradually becomes restricted to the peptidergic subpopulation in adult animals (14, 15). The presence of TRPV1 in C-type peptidergic nociceptors is readily evidenced by its colocalization with the main proinflammatory neuropeptides calcitonin-gene–related peptide alpha (αCGRP) and substance P (SP) (16, 17). αCGRP and SP are pivotal to develop and maintain neurogenic pain and inflammation as genetic ablation of these peptides results in pain resistance (18–21). These neuropeptides are selectively stored in large dense-core vesicles (LDCVs) and are released in response to proalgesic stimuli through a regulated, Ca2+-dependent, and SNARE-mediated secretory pathway (22). LDCVs can also serve as carriers of a plethora of signaling molecules, including ion channels and receptors that may enable fast modulation of neuronal excitability (23). This finding raises the exciting hypothesis that proalgesic recruitment of TRPV1 channels is a mechanism occurring in peptidergic nociceptors. We used ATP, a well-known proalgesic agent (24–26), to investigate the mechanisms underlying TRPV1 sensitization in sensory neurons. We report that ATP-induced TRPV1 potentiation in peptidergic nociceptors specifically involves the exocytotic mobilization of new channels to the cell surface. Notably, knockout of αCGRP expression inhibited the ATP-evoked TRPV1 delivery to the neuronal surface and prevented ATP-induced in vivo thermal hyperalgesia. Thus, algesic-induced exocytosis of TRPV1 channels in peptidergic nociceptors is a central mechanism in pain signaling.
Results
TRPV1 Sensitization in Peptidergic Nociceptors Requires Receptor Exocytosis.
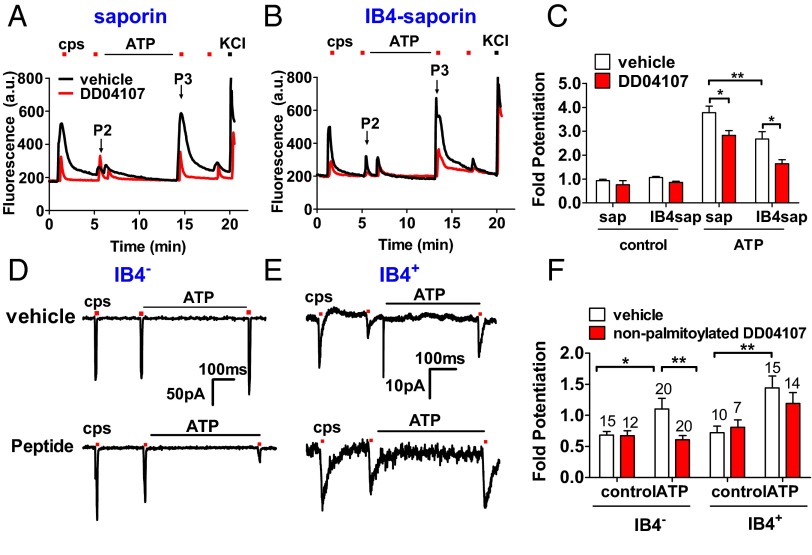
ATP-induced TRPV1 sensitization in peptidergic, IB4-negative (IB4−) nociceptors from rat dorsal root ganglia (DRG) was evaluated in neuronal cultures depleted of nonpeptidergic, IB4-positive (IB4+) with the toxin IB4-saporin. A short incubation of saporin-treated nociceptor cultures with 10 µM of ATP resulted in a 3.8-fold potentiation of desensitized capsaicin responses (Fig. 1 A–C) and a 53 ± 8% augmentation in the number of sensitized neurons (SI Appendix, Table S1). Exposure of cultures to IB4-saporin reduced ATP-induced TRPV1 potentiation by 30% (Fig. 1 B and C) and the number of sensitized neurons by 55% (SI Appendix, Table S1). Notably, incubation of nociceptors with 20 µM DD04107, a palmitoylated peptide that inhibits neuronal exocytosis (11), decreased ATP-evoked TRPV1 sensitization by 27% and 50% in saporin and IB4-saporin–treated primary cultures, respectively (Fig. 1 A–C) without affecting the number of sensitized neurons (SI Appendix, Table S1).
Fig. 1.
ATP-mediated TRPV1 sensitization in peptidergic nociceptors requires channel recruitment. (A and B) Representative microfluorometric recordings of ATP sensitization of capsaicin-evoked Ca+2 influx in saporin (A) and IB4-saporin–treated nociceptors (10 nM, 72 h) (B). (C) Fold potentiation of capsaicin responses obtained as P3/P2 peak intensities. ATP (10 µM, 8 min) applied between the second (P2) and the third (P3) capsaicin pulse (200 nM, 10 s). KCl pulse (40 mM, 10 s) was applied to distinguish viable neurons. DD04107 (20 µM, a concentration that produces maximal α-CGRP inhibition without altering neuronal morphology) preincubated 1 h at 37 °C. (D and E) Representative voltage-clamp recordings of currents elicited by capsaicin (1 μM, 10 s) in IB4− neurons (D) and IB4+ nociceptors (E). (F) Fold potentiation of TRPV1 activity. Cells held at −60 mV. TRPV1 currents were sensitized by ATP (10 µM, 8 min). Nonpalmitoylated DD04107 (100 µM) was applied through patch pipette 10 min before recording (denoted as Peptide, D, Lower). Data represent mean ± SEM, n ≥ 3 cultures, and n ≥ 100 neurons for Ca2+-imaging assays. For patch clamp, numbers above the bars represent the total registered neurons (n). Statistical analysis was made by one-way ANOVA, followed by Bonferroni post hoc test. *P < 0.05; **P < 0.01.
ATP-induced TRPV1 potentiation in IB4− and IB4+ nociceptors was also investigated by patch clamp using IB4-alexa 568 to label IB4+ nociceptors. Capsaicin-induced ionic currents in IB4− and IB4+ nociceptors were similarly potentiated by 10 µM ATP (Fig. 1 D–F). Notably, ATP-induced TRPV1 sensitization in IB4− sensory neurons was blocked by 100 µM of nonpalmitoylated DD04107 delivered to the neuronal cytosol through the pipette (Fig. 1 D, Bottom, and F). In contrast, ATP potentiation of TRPV1 in IB4+ nociceptors was insensitive to the peptide (Fig. 1 E, Bottom, and F). Taken together, these results demonstrate that ATP-induced TRPV1 sensitization in peptidergic nociceptors requires the exocytotic delivery of new channels to the cell surface.
αCGRP and SP Silencing Abrogates ATP-Induced TRPV1 Exocytotic Recruitment.
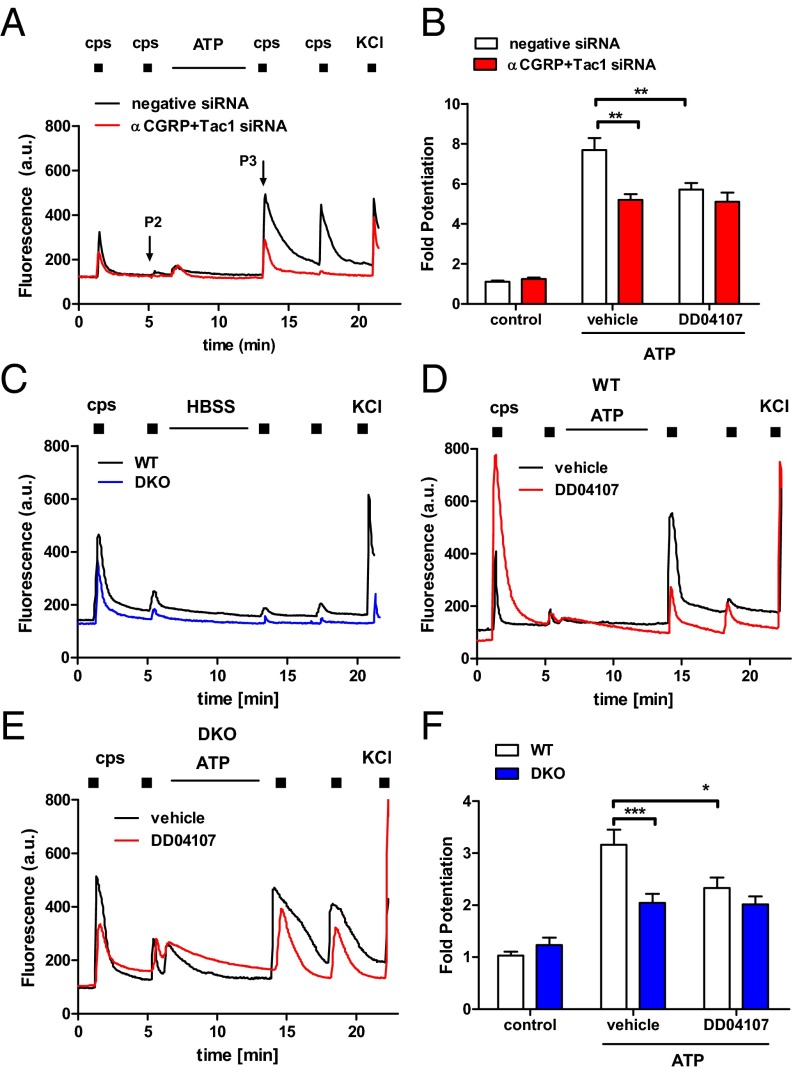
Peptidergic nociceptors express neuropeptides SP and αCGRP that are stored in LDCVs and released upon algesic sensitization (27, 28). Thus, we analyzed the impact of αCGRP and SP silencing on ATP-induced TRPV1 sensitization. Rat sensory neurons were transfected with siRNAs against Tachykinin-1 (Tac1), encoding SP and neurokinin A, and αCGRP gene expression (SI Appendix, Fig. S1A). siRNA-transfected nociceptors displayed a partial reduction of KCl-induced, Ca2+-dependent peptide secretion (SI Appendix, Fig. S1B). Silencing of both neuropeptides did not modify the number of capsaicin-responding nociceptors, their vanilloid responses, or TRPV1 total expression (SI Appendix, Fig. S2C). The percentage of nociceptors sensitized by ATP was not altered by siRNAs (SI Appendix, Table S1). However, ATP-mediated TRPV1 potentiation decreased by 40% when the expression of both neuropeptides was silenced with the siRNAs (Fig. 2 A and B). Notably, DD04107 did not affect the extent of ATP-induced TRPV1 sensitization in siRNA-transfected nociceptors (Fig. 2 A and B). Consequently, these data imply that αCGRP and/or SP expression is required for proalgesic exocytotic mobilization of TRPV1 in peptidergic nociceptors.
Fig. 2.
αCGRP and Tac1 deletion decreases ATP-induced TRPV1 sensitization in nociceptors. (A) Representative microfluorometric recordings of ATP-induced potentiation of capsaicin responses in rat nociceptors transfected with Tac1 and αCGRP siRNAs (100 nM, 48 h). (B) Fold potentiation calculated as the P3/P2 ratio. (C) Representative capsaicin-induced Ca2+ responses in nociceptors from WT and DKO mice. (D and E) Representative microfluorometric recordings illustrating ATP sensitization of TRPV1 responses in WT and DKO nociceptors in the absence (vehicle) or presence of DD04107. (F) Fold potentiation of capsaicin-induced responses. ATP (10 µM, 8 min) was added between the P2 and the P3 pulse of capsaicin (200 nM, 10 s). KCl (40 mM) pulse was used to distinguish viable neurons. DD04107 (20 µM) was preincubated for 1 h. Data represent mean ± SEM, with n = 3, and n ≥ 100 neurons. Statistical analysis was performed by two-way ANOVA, followed by Bonferroni post hoc test; *P < 0.05; **P < 0.01; ***P < 0.001.
To substantiate this finding, we generated a double-knockout mouse deficient in Tac1 and αCGRP gene expression (αCGRP−/−×Tac1−/−, referred to as DKO) by breeding the single-knockout mice (18, 20) (SI Appendix, Fig. S2A). Under physiological conditions, the distribution of nociceptor subpopulations was unaltered in DKO mice (SI Appendix, Table S2). Similarly, TRPV1 expression and its cellular location was not affected, being primarily expressed in small-to-medium peptidergic nociceptors (SI Appendix, Fig. S2 B and C). Moreover, TRPV1-expressing sensory neurons displayed similar electrical properties in wild-type (WT) and DKO mice (SI Appendix, Table S3), and TRPV1 activity was not altered as evidenced by the similar number of nociceptors responding to capsaicin (25 ± 4% WT and 23 ± 5% DKO nociceptors) and their comparable EC50 for the vanilloid (SI Appendix, Fig. S2D). Therefore, these DKO mice represent an appropriate model for examining the contribution of αCGRP and SP to ATP-induced TRPV1 sensitization in peptidergic nociceptors.
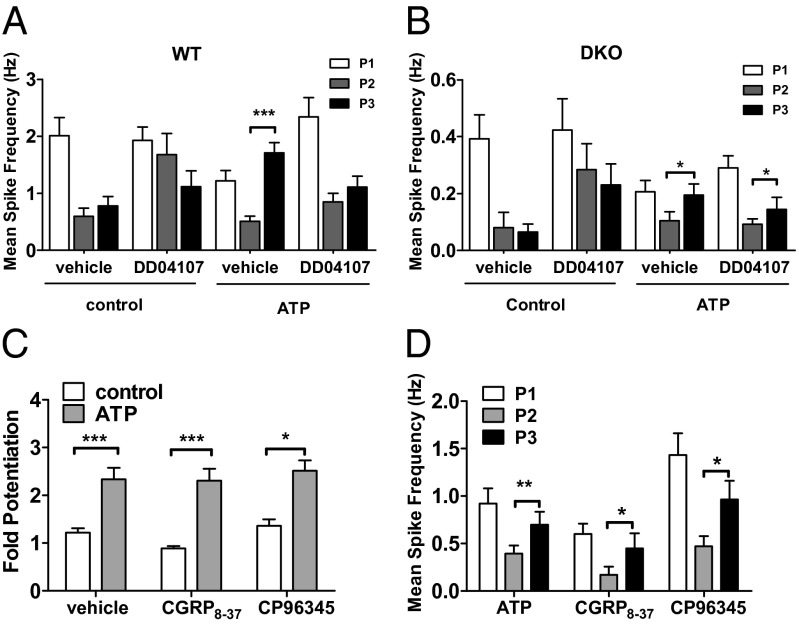
We next examined ATP-mediated TRPV1 potentiation in DKO nociceptors. As illustrated in Fig. 2 C–E, incubation of capsaicin-desensitized neuronal cultures with ATP promoted fast and significant enhancement of capsaicin responses in WT and DKO nociceptors. However, TRPV1 potentiation was ∼40% lower in DKO than in WT nociceptors (Fig. 2F). Notably, ATP-induced TRPV1 sensitization was blocked by DD04107 in WT nociceptors, but not in DKO sensory neurons (Fig. 2 D–F). A similar finding was obtained when TRPV1 sensitization was evaluated with planar multielectrode arrays (MEA) (Fig. 3). In WT nociceptors, repetitive capsaicin pulses induced a conspicuous reduction of the vanilloid-evoked electrical responses (Fig. 3A). Desensitized capsaicin responses could be recovered with ATP sensitization, and blocked by DD04107 (Fig. 3 A and C; Fig. 4A). In DKO nociceptors, capsaicin also induced neuronal activity (Fig. 3B), although with a significantly reduced mean spike frequency compared with WT neurons. Vanilloid-induced TRPV1 tachyphylaxia was partially overturned by ATP (Fig. 3B) and virtually insensitive to DD04107 (Figs. 3D and 4B).
Fig. 3.
αCGRP and Tac1 deletion decreases ATP-sensitized, TRPV1-mediated nociceptor excitability. (A) Representative MEA recordings of capsaicin-induced action potentials and desensitization (Top). Potentiation of capsaicin (500 nM, 15 s) responses was elicited by applying 10 µM of ATP between the second (P2) and third (P3) vanilloid pulse (Bottom) in WT nociceptors. (B) Representative recordings of neuronal activity of DKO nociceptors in culture using the experimental paradigm in A. (C and D) Representative recordings of ATP-induced sensitization of capsaicin-evoked neuronal excitability in WT (C) and DKO (D) nociceptors preincubated with 20 µM of DD04107 (1 h, 37 °C).
Fig. 4.
ATP-induced TRPV1 sensitization involves TRPV1 recruitment. (A) Mean spike frequency of capsaicin-induced action potential firing in WT nociceptors. (B) Mean spike frequency of vanilloid-evoked action potential firing in DKO nociceptors. (C) Fold potentiation of capsaicin-induced Ca2+ responses and (D) mean spike frequency of capsaicin-evoked action potential firing in WT nociceptors in the presence of 250 nM CGRP8–37 or 10 µM CP96345 or vehicle. Capsaicin (500 nM, 15 s) and ATP (10 µM, 8 min) were used. DD04107 (20 µM) was preincubated for 1 h at 37 °C. Mean spike frequency was calculated from recordings displayed in Fig. 3. Data are expressed as mean ± SEM; n = 3 cultures. For Ca2+ microfluorography, n ≥ 100 neurons. For mean spike frequency, n = 30–65 electrodes. Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test. For MEA, data were analyzed as paired values through comparison of the responses of each electrode in the 30-s time interval upon stimulation. ***P < 0.001; **P < 0.01; *P < 0.05. Only comparison between P2 and P3 is shown in the graphs.
It is intriguing that deletion of αCGRP and SP had such a strong impact on ATP-induced TRPV1 sensitization in peptidergic nociceptors. A possibility is that released neuropeptides could potentiate TRPV1 function by autocrinally activating their neuronal receptors CGRP1 and NK1 (27, 28). Thus, we evaluated the effect of blocking these receptors on ATP-mediated TRPV1 potentiation in WT nociceptors. Fig. 4C shows that the antagonists CGRP8–37 and CP96345 did not affect the fold potentiation of capsaicin-induced Ca2+ responses sensitized by ATP. Similarly, blockade of NK1 and CGRP1 receptors did not suppress ATP-mediated sensitization of vanilloid-evoked neuronal firing (Fig. 4D). These results demonstrate that ATP-induced sensitization of TRPV1 in peptidergic nociceptors is not due to neuronal activation of CGRP1 and NK1 receptors. Thus, the lower algesic TRPV1 sensitization in DKO nociceptors appears due to abrogation of receptor mobilization to the plasma membrane, suggesting an alteration of receptor sorting, trafficking, and/or exocytosis in these nociceptors.
ATP-Induced TRPV1 Recruitment Is Mediated by LDCV Exocytosis.
The impact of αCGRP and SP deletion on TRPV1 inflammatory mobilization suggests that the channel may be assorted into LDCVs along with the neuropeptides. Double immunofluorescence staining of TRPV1 and αCGRP showed a cortical colocalization (Fig. 5A), suggesting the presence of the thermoTRP in αCGRP-positive LDCVs. This observation was corroborated by immunoelectron microscopy images showing the presence of immunogold particles bound to TRPV1 channels present in LDCVs (Fig. 5A, Right, and B). Quantitation of these images reveals that 15 ± 1% of immunogold TRPV1 particles were at the plasma membrane, 25 ± 1% were in LDCVs, and 60 ± 1% were associated with other intracellular structures (denoted as cytoplasm) (Fig. 5C). Upon ATP sensitization, TRPV1 immunogold labeling in the plasma membrane increased up to 28 ± 1%, whereas it decreased to 16 ± 2% in LDCVs. As expected, inhibition of LDCV exocytosis with DD04107 inhibited the ATP-induced increment of TRPV1 immunogold particles at the neuronal surface (Fig. 5 B and C). Therefore, these data imply that inflammatory mobilization of TRPV1 channels to the plasma membrane in peptidergic nociceptors is by SNARE-dependent exocytosis of LDCVs.
Fig. 5.
TRPV1 is localized in CGRP-positive LDCVs, and ATP promotes exocytosis of TRPV1 secretory granules. (A) Immunofluorescence labeling of CGRP (red) and TRPV1 (green) distribution in mouse DRG neurons; double immunolabeling (merge) shows colocalization of TRPV1 and CGRP in LDCVs. (Scale bar 10 μm.) (Right) Pre-embedding immunogold labeling of TRPV1 is localized in the plasma membrane and in LDCVs in mouse DRG neurons. (B) Electron-microscopy images depicting immunogold labeling of TRPV1 in nociceptors treated by 10 µM ATP and/or 20 µM DD04107 as indicated. (C) Quantitation of immunogold TRPV1 particles. (D) Electron microscopy images illustrating immunogold labeling of TRPV1 in nociceptors from WT and DKO mice treated with 10 µM ATP or buffer. (E) Quantitation of immunogold TRPV1 particles in WT and DKO nociceptors. Data are expressed as mean ± SEM. One-way ANOVA followed by Bonferroni post hoc test was used. ***P < 0.001; **P < 0.01; *P < 0.05 versus vehicle-treated neurons; n = 3 cultures. Arrow indicates plasma membrane; arrowhead, LDCVs; and crossed arrow, cytoplasm. (Scale bar, 200 nm.)
Then we investigated if deletion of both neuropeptides affected receptor sorting into LDCVs. We observed the presence of immunogold TRPV1 particles in LDCV-like structures in DKO nociceptors (Fig. 5D), indicating that neuropeptides are not required for sorting the receptor into these granules. Nonetheless, note that DKO secretory granules had a smaller diameter than LDCVs of WT nociceptors (SI Appendix, Fig. S3). Quantitation of TRPV1 subcellular distribution revealed 11 ± 1% of immunogold particles in the plasma membrane, 28 ± 1% in LDCV-like granules, and 62 ± 2% in the cytosolic fraction (Fig. 5 D and E). Notably, ATP sensitization of DKO nociceptors did not increase the number of immunogold TRPV1 particles in the neuronal surface or decrease their presence in the LDCV-like structures (Fig. 5 D and E). Collectively, these findings indicate that suppression of αCGRP and SP expression did not affect the sorting of TRPV1 into LDCV-like granules, but abrogated the competence of these secretory granules for regulated exocytosis.
αCGRP Expression Is Required for ATP-Induced TRPV1 Sensitization.
We next evaluated the role of each neuropeptide in ATP-induced TRPV1 potentiation using nociceptors from αCGRP−/− and Tac1−/− mice (Fig. 6 A and B). Notably, vanilloid-induced TRPV1 desensitization was fully reversed by ATP in nociceptors from Tac1−/− mice (Fig. 6 A and C), but not in sensory neurons from αCGRP-null mice (Fig. 6 B and C). The number of sensitized neurons also significantly decreased in αCGRP−/− nociceptors (SI Appendix, Table S1). MEA recordings show that abrogation of αCGRP expression virtually eliminated the ATP-induced increase in mean spike frequency of capsaicin-evoked nociceptor firing (Fig. 6D). Together, these results indicate that αCGRP expression is necessary for ATP-induced TRPV1 potentiation in peptidergic nociceptors, whereas SP plays a marginal role. Note that the functional role of αCGRP is specific as αCGRP−/− nociceptors express βCGRP (29).
Fig. 6.
ATP-induced TRPV1 sensitization requires αCGRP expression in sensory neurons. (A and B) Representative microfluorometric recordings showing sensitization of capsaicin-induced Ca2+ influx (300 nM, 10 s) upon incubation with ATP (10 µM, 8 min) in WT, Tac1−/− (A), and αCGRP−/− (B) sensory neurons. (C) Fold potentiation of capsaicin-induced Ca2+ responses as P3/P2 ratio. (D) Mean spike frequency of capsaicin-induced action potential firing in WT, αCGRP−/−, and Tac1−/− nociceptors in the presence or absence of ATP. Capsaicin (500 nM, 15 s) and ATP (10 µM, 8 min) were used. Data represent mean ± SEM, n = 3 cultures, n = 30–65 electrodes for MEA experiments, or n ≥ 100 neurons for Ca2+ microfluorography. Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test. Data for MEA were analyzed as paired values through comparison of the responses of each electrode in the 30-s time interval upon stimulation. Only comparison between P2 and P3 is shown in the graphs. ***P < 0.001; *P < 0.05.
In adult mice, ATP sensitizes the TRPV1 channel mainly through activation of the G-protein–coupled receptor P2Y2 (24, 25), which extensively colocalizes with TRPV1 and αCGRP in sensory peptidergic neurons (17, 24). We questioned whether a reduced functionality of P2Y2 receptors in knockout mice could account for the decreased sensitizing capability of ATP. UTP-evoked Ca2+ release indicates that P2Y2 receptor function increased in αCGRP−/− mice, whereas the percentage of nociceptors responding to the purinergic agonist was unaltered (SI Appendix, Table S4). Therefore, the lower ATP-mediated TRPV1 sensitization in αCGRP−/− sensory neurons is not due to a decrease in purinergic signaling.
αCGRP Is Needed for ATP-Induced Thermal Hyperalgesia in Mice.
Injection of ATP causes TRPV1-dependent nocifensive responses and thermal hyperalgesia in mice. Thus, we determined if the absence of αCGRP altered ATP-induced TRPV1-mediated in vivo thermal hyperalgesia. WT, αCGRP−/−, Tac1−/−, and DKO mice were intraplantarly injected with saline or 100 nmol of ATP into the right hind paw, and time latencies to a radiant heat stimulus were measured. Local injection of the proalgesic agent promoted thermal hyperalgesia in WT and Tac1−/− animals, as evidenced by the reduction in the latency time to paw withdrawal (Fig. 7). In marked contrast, mice lacking αCGRP expression (αCGRP−/− and DKO) showed similar paw withdrawal latencies to the thermal stimulus before and after injection of ATP or saline. Therefore, these results evidence that the expression of αCGRP is essential in vivo for ATP-induced TRPV1 sensitization in peptidergic nociceptors.
Fig. 7.
αCGRP expression is needed for ATP-induced thermal hyperalgesia in mice. (Left and Right) Paw withdrawal latencies to a radiant heat stimulus before (baseline) and after a 30-min intraplantar injection of 100 nmol ATP (Left) or saline (Right) in WT, αCGRP−/−, Tac1−/−, or DKO mice. Data represent mean ± SEM; n = 8 animals per group. Statistical analysis was performed by one-way ANOVA paired values followed by Bonferroni post hoc test; *P < 0.05; **P < 0.01 compared with baseline.
Discussion
Algesic sensitization of nociceptors upon tissue or nerve damage is characterized by a significant increase of their excitability, leading to the sensory hypersensitivity that underlies thermal hyperalgesia and allodynia. A major player in the onset and maintenance of nociceptor sensitization is TRPV1, a thermoTRP receptor expressed by peptidergic and nonpeptidergic C-type nociceptors whose activity is highly potentiated by algogens. Cumulative evidence suggests that algesic agents regulate TRPV1 activity by a complex mechanism that involves the fast mobilization of new channels to the neuronal membrane through a regulated exocytotic pathway and the channel posttranslational modification (10, 11, 30). Notably, blockers of regulated exocytosis such as BoNTA or compound DD04107 reduce the algesic-induced surface expression of TRPV1 and exhibit long-lasting in vivo antinociceptive activity (31, 32). This finding indicates that mobilization of channels by algogens is a key mechanism contributing to nociceptor sensitization under pathological conditions. However, whether proalgesic recruitment of TRPV1 channels is a general mechanism of nociceptor sensitization or is cell-type–specific remains controversial, as not all algogens appear to induce regulated mobilization of TRPV1 (11). Because LDCVs are secretory granules that also can transport receptors to axonal terminals (23), we hypothesized that algesic mobilization of TRPV1 channels could be the main mechanism underlying sensitization of peptidergic nociceptors.
A salient contribution of this study is that algesic potentiation of TRPV1 activity in the subpopulation of peptidergic nociceptors primarily requires membrane insertion of channels, while in nonpeptidergic nociceptors involves modulation of channel gating. First, ATP-induced TRPV1 sensitization in peptidergic IB4− nociceptors, but not in IB4+ sensory neurons, was prevented by blocking regulated exocytosis with DD04107. Second, TRPV1 is located in LDCVs that are mobilized to the plasma membrane upon sensitization of nociceptors with ATP. Third, blockade of regulated exocytosis prevented the activity-dependent delivery of LDCVs containing TRPV1 to the neuronal surface. Finally, depletion of IB4+ nociceptors in primary cultures with IB4-saporin significantly increased the blockade efficacy of DD04107 on ATP-induced TRPV1 sensitization. Our findings also demonstrate that at least a subset of TRPV1 channels was sorted into LDCVs to be transported to the axonal terminals where it was incorporated into the neuronal surface in response to the proalgesic insult. Intriguingly, a proteomic analysis of LDCVs from dorsal spinal cord neurons did not detect the presence of TRPV1 channels among the 298 proteins identified in the membrane of these secretory vesicles (23). This controversy probably arises from the methodological limitations of the proteomic analysis. In support of this tenet, the proteomic analysis also failed to detect the δ-opioid receptor in secretory granules, although it was observed by immunological techniques (23). Thus, because our data showed the colocalization of TRPV1 and α-CGRP and the presence of the thermoTRP channel in LDCVs, they demonstrate sorting and axonal transport of TRPV1 in these secretory vesicles in peptidergic nociceptors.
The colocalization of TRPV1 with αCGRP and SP in small-diameter neurons (12, 13) and the presence of the thermoTRP in LDCVs implies the concurrent exocytosis of TRPV1 and the proinflammatory peptides under proalgesic conditions upon tissue damage. Moreover, axonal transport of TRPV1 in secretory granules suggests a potential role of both neuropeptides in TRPV1 sensitization, either by favoring channel sorting into LDCVs or by driving the regulated exocytosis of the channel. Notably, we found that silencing with siRNAs or knocking out αCGRP and Tac1 gene expression significantly reduced ATP-induced TRPV1 sensitization in nociceptors. This decrease was mainly due to abrogation of ATP-induced TRPV1 insertion into the neuronal surface as evidenced by the insensitivity of nociceptor sensitization to DD04107. Similarly, in DKO nociceptors, LDCV-like granules containing TRPV1 were not mobilized to the plasma membrane upon ATP exposure. Analysis of TRPV1 sensitization in nociceptors from the single-knockout animals (Tac1−/− and αCGRP−/−) revealed that αCGRP, but not SP, was essential for the proalgesic recruitment of the thermoTRP, consistent with the strong colocalization of both proteins in IB4− nociceptors. Accordingly, absence of αCGRP resulted in lower nociceptor sensitization and loss of ATP-induced thermal hyperalgesia in vivo, similar to blocking neuronal exocytosis with BonTA (32) or DD04107 (31).
The question that emerges is how deletion of αCGRP expression impairs inflammatory TRPV1 recruitment onto the surface of peptidergic nociceptors. A plausible explanation is that αCGRP expression is required for the formation of LDCVs in peptidergic nociceptors, thus contributing to TRPV1 transport to the axonal terminals and insertion into the plasma membrane through the regulated secretory pathway. This hypothesis seems reasonable because in the absence of αCGRP it is likely that precursors of LDCVs are not formed in the trans-Golgi network or, if formed, empty granules would be targeted to lysosomes for degradation. However, electron-microscopy images in DKO mice show the localization of TRPV1 in LDCV-like granules and that TRPV1 reaches the plasma membrane, indicating preservation of a secretory pathway for axonal transport of the channel.
LDCVs are a heterogeneous vesicular population that, in addition to neuropeptide-loaded granules, also contain Piccolo-Bassoon transport vesicles (PTVs) (33, 34). PTV vesicles have a dense-core appearance and a diameter of 50–70 nm, slightly smaller than LDCVs (33). Notably, PTV granules are used for axonal transport of synaptic proteins through a constitutive pathway, thus contributing to synaptogenesis and the homeostasis of synaptic terminals (33, 35). Accordingly, it appears reasonable to suggest that in sensory neurons TRPV1 could be assorted into both LDCVs and PTVs for trafficking to the synaptic and peripheral terminals. Indeed, TRPV1 colocalizes with Piccolo, which is consistent with its sorting into PTVs (SI Appendix, Fig. S4). In the absence αCGRP, the thermoTRP would be primarily sorted into PTVs for axonal transport. In support of this hypothesis, we observe the presence of TRPV1 in LDCV-like granules (likely PTVs) in sensory neurons from DKO mice that are unable to exocytose in response to a Ca2+ stimulus. Furthermore, secretory granules from DKO animals appear to have a smaller diameter than those from WT animals, which is consistent with the size of PTVs. Taken together, our findings indicate that inflammatory insertion of TRPV1 channels into the neuronal surface is a process mediated by αCGRP-containing secretory vesicles. Although our data unveil αCGRP as a pivotal player in the regulated exocytosis of TRPV1, and probably in other channels needed for nociceptor sensitization, additional studies are required for understanding the role of this neuropeptide in the biogenesis and Ca2+-dependent secretion of LDCVs in peptidergic nociceptors.
In conclusion, our findings demonstrate that the mechanism underlying proalgesic sensitization of TRPV1 is dependent on the cellular context, being primarily mediated by rapid mobilization of new channels to the cell membrane in peptidergic nociceptors and by modulation of channel gating in the nonpeptidergic subpopulation. These results imply that inflammatory mobilization of TRPV1 channels is concomitant to the release of proinflammatory peptides, thus providing a synergistic mechanism aimed at ensuring rapid modulation of pain signaling in the peripheral terminals. It is likely that other ion channels and membrane receptors needed for nociceptor algesic sensitization use a similar mechanism for rapid modulation of nociceptor excitability (36). Because it appears that TRPV1 may be trafficked by both LDCVs and PTVs, it would be interesting to explore the contribution of both types of secretory vesicles to the development of chronic pain where an increase in TRPV1 expression has been observed (37). Finally, our findings strongly substantiate the therapeutic potential of modulating neuronal exocytosis in peptidergic nociceptors for pain intervention and provide experimental support for the broad in vivo antinociceptive activity of BoNTA (38, 39) and DD04107 (31).
Materials and Methods
All experimental procedures were approved by the Institutional Animal and Ethical Committee of the University Miguel Hernández de Elche and in accordance with the guidelines of the Economic European Community. Neonatal Wistar rats and wild-type C57BL/6J mice (in house-bred stock originally from Harlan Laboratories). αCGRP-deficient mice (B6;129-Calca) were obtained as described (20). Tac1-deficient mice (B6.Cg-Tac1tm1Bbm/J) were purchased from The Jackson Laboratory. Double-knockout homozygous mice for αCGRP and Tac1 genes were generated in house (B6;B6;129-CalcaTac1tm1Bbm/J).
The methodology for the experiments of primary culture of sensory neurons, calcium microfluorography, patch-clamp and microelectrode array recordings, immunohistochemistry and immunocytochemistry, electron microscopy, and ATP-induced hyperalgesia, along with the chemicals used and data analysis, are described in detail in SI Appendix.
Supplementary Material
Acknowledgments
We are indebted to BCN Peptides (Barcelona) for kindly providing the peptides used in this study. This work was supported by Ministerio de Economía y Competitividad (Grants BFU2012-39092-C02-01 and CONSOLIDER-INGENIO 2010 CSD2008-00005) and by Generalitat Valenciana (Grant PROMETEO/2014/011). S.M. was a recipient of a Santiago Grisolía Fellowship from GVA.
Footnotes
Conflict of interest statement: A.F.-M. is an inventor of patent WO2010 009892, protecting the antinociceptive activity of compound DD04107.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420252111/-/DCSupplemental.
References
- 1.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 2.Ghilardi JR, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HY, et al. Differential changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;9(3):280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol. 2008;73(3):995–1004. doi: 10.1124/mol.107.041707. [DOI] [PubMed] [Google Scholar]
- 5.Szabó A, et al. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: In vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314(1):111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 7.Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4(1):1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: Implications for therapeutic intervention. Adv Exp Med Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- 9.Devesa I, et al. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J Inflamm Res. 2011;4:67–81. doi: 10.2147/JIR.S12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camprubí-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009;23(11):3722–3733. doi: 10.1096/fj.09-134346. [DOI] [PubMed] [Google Scholar]
- 12.Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 13.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8(3):263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwick M, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22(10):4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanaugh DJ, et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31(28):10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa H, Sugimoto T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 2001;890(1):184–188. doi: 10.1016/s0006-8993(00)03253-4. [DOI] [PubMed] [Google Scholar]
- 18.Cao YQ, et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 19.McCoy ES, et al. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78(1):138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmon AM, et al. Modulation of morphine analgesia in alphaCGRP mutant mice. Neuroreport. 1999;10(4):849–854. doi: 10.1097/00001756-199903170-00033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, et al. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89(2-3):265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- 22.Gustavsson N, Wu B, Han W. Calcium sensing in exocytosis. Adv Exp Med Biol. 2012;740:731–757. doi: 10.1007/978-94-007-2888-2_32. [DOI] [PubMed] [Google Scholar]
- 23.Zhao B, et al. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011;21(5):741–753. doi: 10.1038/cr.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malin SA, et al. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138(3):484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama T, et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23(14):6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98(12):6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29(15):4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: A novel pathway for heat hyperalgesia. J Neurosci. 2007;27(44):12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schütz B, Mauer D, Salmon AM, Changeux JP, Zimmer A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J Comp Neurol. 2004;476(1):32–43. doi: 10.1002/cne.20211. [DOI] [PubMed] [Google Scholar]
- 30.Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279(24):25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 31.Ponsati B, et al. An inhibitor of neuronal exocytosis (DD04107) displays long-lasting in vivo activity against chronic inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2012;341(3):634–645. doi: 10.1124/jpet.111.190678. [DOI] [PubMed] [Google Scholar]
- 32.Guo BL, et al. A closer look to botulinum neurotoxin type A-induced analgesia. Toxicon. 2013;71:134–139. doi: 10.1016/j.toxicon.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhai RG, et al. Assembling the presynaptic active zone: A characterization of an active one precursor vesicle. Neuron. 2001;29(1):131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 34.Shapira M, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38(2):237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 35.Dresbach T, et al. Assembly of active zone precursor vesicles: Obligatory trafficking of presynaptic cytomatrix proteins Bassoon and Piccolo via a trans-Golgi compartment. J Biol Chem. 2006;281(9):6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64(4):498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz Z, et al. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14(9):864–871. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Diener HC, et al. PREEMPT 2 Chronic Migraine Study Group OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 39.Ghasemi M, Ansari M, Basiri K, Shaigannejad V. The effects of intradermal botulinum toxin type a injections on pain symptoms of patients with diabetic neuropathy. J Res Med Sci. 2014;19(2):106–111. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.