Significance
Angiogenesis, or new blood vessel formation, is critical not only for normal processes such as embryonic development but also for progression of diseases such as tumor growth, metastasis, and chronic inflammatory disease. This work elucidated a molecular mechanism that is important in postnatal angiogenesis in tumor growth and ischemia–reperfusion injury in the hind limb. Specifically, we identified a posttranscriptional gene regulatory mechanism that controls the activity of a potent suppressor of gene expression, named eIF4e transporter (4E-T). Alternative splicing of 4E-T controls the level of the active form of 4E-T, which suppresses gene expression in endothelial cells. This mechanism may be targeted to control angiogenesis-dependent diseases.
Keywords: angiogenesis, RNA binding protein, eIF4e transporter, alternative splicing, tumor angiogenesis
Abstract
Posttranscriptional RNA regulation is important in determining the plasticity of cellular phenotypes. However, mechanisms of how RNA binding proteins (RBPs) influence cellular behavior are poorly understood. We show here that the RBP embryonic lethal abnormal vision like 1 (ELAVL1, also know as HuR) regulates the alternative splicing of eukaryotic translation initiation factor 4E nuclear import factor 1 (Eif4enif1), which encodes an eukaryotic translation initiation factor 4E transporter (4E-T) protein and suppresses the expression of capped mRNAs. In the absence of ELAVL1, skipping of exon 11 of Eif4enif1 forms the stable, short isoform, 4E-Ts. This alternative splicing event results in the formation of RNA processing bodies (PBs), enhanced turnover of angiogenic mRNAs, and suppressed sprouting behavior of vascular endothelial cells. Further, endothelial-specific Elavl1 knockout mice exhibited reduced revascularization after hind limb ischemia and tumor angiogenesis in oncogene-induced mammary cancer, resulting in attenuated blood flow and tumor growth, respectively. ELAVL1-regulated alternative splicing of Eif4enif1 leading to enhanced formation of PB and mRNA turnover constitutes a novel posttranscriptional mechanism critical for pathological angiogenesis.
Angiogenesis, also known as new vessel formation, is a fundamental process in embryonic development, tissue growth, and recovery from tissue injury (1). In addition, dysregulated angiogenesis is important in many conditions such as cancer growth, metastasis, age-related macular degeneration, and chronic inflammatory disease (2). Both developmental and postnatal angiogenesis are initiated by paracrine factors acting on endothelial cells to induce the formation of angiogenic sprouts, their fusion to form the primary vascular plexus and maturation processes that stabilize the newly formed blood vessels (3). However, gene expression programs in endothelial cells that drive the angiogenic process are poorly understood. Hypoxia- and flow-regulated transcriptional events have been characterized as major mechanisms that regulate gene expression during angiogenesis (4, 5). Recently, posttranscriptional gene regulation by RNA binding proteins (RBPs) and miRNAs is recognized to play important roles in the regulation of fundamental biological processes (6, 7). Indeed, miRNAs were shown to regulate of angiogenesis and expression of key regulators (8–12).
ELAVL1 (also known as Hu antigen R, HuR) is an AU-rich element (ARE) and U-rich element (URE) RBP that stabilizes mRNAs and promotes gene expression (13). Although this RBP is located primarily in the nucleus, it is translocated into the cytoplasm after cellular activation to promote gene expression. ELAVL1 binds to the 3′ UTRs of many mRNAs, often at or near miRNA binding sites (14, 15). Indeed, ELAVL1 functions in part to modulate miRNA-dependent gene regulation (9, 16). Mice deficient for Elavl1 are embryonic lethal due to defects in placental development (17). Inducible postnatal deletion of Elavl1 leads to stem/progenitor cell apoptosis leading to intestinal and hematopoietic failure and death within 10 d (18), and zebrafish elavl1 is important for regulation of gata1 expression and embryonic erythropoiesis (19). ELAVL1 stabilizes the mRNA for VEGF-A, which encodes a key angiogenic factor induced by hypoxia-inducible factor 1α (HIF-1α) (20). We and others recently showed that macrophage ELAVL1 is important in the angiogenic gene expression program (9, 21).
In this report, we investigated how posttranscriptional gene regulations via ELAVL1 control postnatal angiogenesis. This work shows that ELAVL1 regulates alternative splicing of the eukaryotic translation initiation factor 4E nuclear import factor 1 (Eif4enif1), which encodes an eIF4E transporter (4E-T) protein. The 4E-T is required for cytoplasmic RNA processing body (PB) formation and functions in mRNA translational suppression and mRNA degradation (22, 23). We hypothesize that ELAVL1-regulated alternative splicing of Eif4enif1 controls mRNA turnover, which regulates postnatal pathological angiogenesis.
Results and Discussion
ELAVL1 Regulates Alternative Splicing of Eif4enif1.
To examine the mechanisms by which ELAVL1 regulates angiogenesis, we conducted exon-microarray analysis using mouse lung endothelial cells (MLECs) isolated from endothelial cell-specific Elavl1 knockout mice (Elavl1 ECKO) (Fig. S1) as well as bone-marrow–derived macrophages (BMDMs) isolated from myeloid-specific Elavl1 knockout mice (Elavl1 MøKO) (9) and compared them with the wild-type (WT, Elavl1f/f) counterparts. Alternative splicing (AS) analysis by GeneSpring (Agilent Technologies) and AltAnalyze (24) identified four genes (Eif4enif1, Dlst, Usp1, and BC005537) to be alternatively spliced in an ELAVL1-dependent manner in both cell types (Fig. 1 and Figs. S2 and S3). Among these, the coding exon 11 of Eif4enif1 gene is spliced out in the absence of ELAVL1. This 72-nt exon encodes a 24-amino-acid domain, which is positioned between the two nuclear export signal motifs of the eukaryotic initiation factor 4E transporter (4E-T) protein (25). In contrast, the three other ELAVL1-regulated, alternatively spliced genes contain affected exons in the 5′ UTR (Usp1) or 3′ UTR (Dlst and BC005537).
Fig. 1.
ELAVL1 regulates alternative splicing of Eif4enif1. (A) Venn diagram depicting alternative splicing events in BMDMs and MLECs as determined by the microarray expression results of WT, Elavl1 ECKO MLECs, and Elavl1 MøKO BMDMs by GeneSpring (Agilent) and Altanalyze (open source) programs. Alternative exon analysis parameters are described in Fig. S2. Four genes (Eif4enif1, Dlst, Usp1, and Bc005537) were identified in both BMDMs and ECs. Scheme of the differentially expressed exons (blue box) are shown. (B) RT-PCR analysis of alternative splicing of Eif4enif1. Scheme of the alternative splicing of Eif4enif1. Primers (F1 forward; R1 reverse) are indicated in flanking exons 10 and 12. RT-PCR was done using RNA isolated from primary BMDMs, MLECs, siRNA targeting Elavl1 (siElavl1) transfected IMECs, and stable knockdown of Elavl1 in IMECs (shElavl1). (C) Eif4enif1 transcript expression and decay. The expression of Eif4enif1 transcripts was quantified using primers (F2 and R2) located in exon 10 in a qRT-PCR assay. Actinomycin D (5 µg/mL) was used to treat WT and Elavl1 ECKO for various times. Data present mean ± SEM, n = 3. (D) The 4E-T polypeptide expression in Elavl1 depleted cells. Protein extracts from WT and Elavl1 ECKO MLECs, and IMECs treated with siElavl1, were prepared and immunoblotted with anti 4E-T, ELAVL1, or β-actin antibodies. (E) IMECs expressing control shRNA (shCtl) or shElavl1 were treated with cycloheximde (100 µg/mL) for indicated times and immunoblot analysis of lysates was performed with antibodies specific to 4E-T, ELAVL1, β-actin, or cyclin D1. (F) Differential stability of 4E-TL and 4E-TS polypeptides. Endogenous Eif4enif1 was knocked down in IMECs using lentiviral shEif4enif1 construct. These cells were transduced with cDNAs encoding 4E-TL and 4E-TS polypeptides, treated with cycloheximde (100 μg/mL) for indicated times and analyzed by immunoblot analysis. Results from D–F were from a representative experiment that was repeated two to three times.
We further studied ELAVL1 regulation of alternative splicing of Eif4enif1, which encodes an important factor that is critical for cytoplasmic RNA PB formation, suppression of mRNA translation, and mRNA degradation (22, 23). Loss-of-function mutations of EIF4ENIF1 gene are associated with primary ovarian insufficiency (26) and both mouse and Drosophila homologs of 4E-T were shown to be involved in posttranscriptional gene regulation in germ cells (27). However, the role of 4E-T in angiogenesis has not been determined. RT-PCR analysis indicated that Eif4enif1 exon 11 is skipped in cells that lack ELAVL1, resulting in the reduced expression of Eif4enif1-L (4E-TL) isoform and increased expression of Eif4enif1-S (4E-Ts) isoform (Fig. 1B). ELAVL1-dependent alternative splicing of Eif4enif1 was observed in primary BMDMs, MLECs, immortalized mouse embryonic endothelial cells (IMECs) transfected with siRNA targeting Elavl1, and IMEC stably expressing the shRNA for Elavl1 (shElavl1), indicating that this event is not cell-type specific. Eif4enif1 genomic sequence shows that intron 10 contains 18 U-rich sites including a long, U-rich polypyrimidine tract (∼71 bp) immediately preceding the 3′-splice acceptor site (Fig. S4). Because ELAVL1 interaction with binding sites located close to 3′-splice junctions have been observed in the photoactivatable ribonucleoside enhanced crosslinking and immunoprecipitation analysis of human cells (14, 15), and because it was shown to regulate splicing of the FAS primary transcript in HeLa cells (28), our data suggest that nuclear function of ELAVL1 may involve the regulation of splicing of Eif4enif1 primary transcript. This event results in the predominant expression of the 4E-TL isoform.
Eif4enif1 mRNA expression was similar between WT and Elavl1 ECKO MLECs. When MLECs were treated with actinomycin D, Eif4enif1 mRNA decayed with a half-life of ∼2 h, which was similar between WT and Elavl1 ECKO cells (Fig. 1C), suggesting that ELAVL1 does not regulate the turnover of this transcript. However, immunoblot analysis using the 4E-T antibody detected a polypeptide band of ∼140 kDa, which was markedly increased in Elavl1 ECKO cells (Fig. 1D). Further, siRNA-mediated knockdown of Elavl1 in IMECs resulted in dose-dependent increase in the expression of 4E-T immunoreactive band (Fig. 1D). Because the SDS/PAGE cannot distinguish the 4E-TL and 4E-TS isoforms, we hypothesized that protein stability may be different between the two isoforms. Indeed, in shElavl1 IMEC cells, which primarily express the 4E-TS isoform, immunoreactive 4E-T is much more prominent than the control shRNA-treated counterparts and exhibits a longer half-life (Fig. 1E). To confirm the differential stability between 4E-TL and 4E-TS isoforms, we expressed each isoform in IMECs in which endogenous 4E-T was down-regulated by shRNA. As shown in Fig. 1F, the half-life of 4E-TL is much shorter than the 4E-TS isoform, suggesting that ELAVL1-induced inclusion of exon 11 destabilizes the 4E-T polypeptide. Thus, in the presence of ELAVL1, alternative splicing of Eif4enif1 gene results in the expression of the short-lived 4E-TL isoform.
4E-TS Protein in ELAVL1 Depleted Cells Induces PB Potently.
4E-T, originally identified as a nucleocytoplasmic shuttling protein, binds to eIF4E, the mRNA 5′ cap-binding protein (25). We observed that both 4E-TL and 4E-TS isoforms bind to eIF4E in a GST pull-down assay (Fig. S5). Interestingly, recent work revealed that 4E-T is required for the formation of PBs and transports the eIF4E/mRNA complex to PBs for translational repression and mRNA decay (22, 23). Thus, we examined the effect of alternatively spliced 4E-T protein isoforms in PB formation in endothelial cells. Immunofluorescence staining confirmed that 4E-T localizes predominantly within discrete foci in the cytoplasm and is colocalized with the mRNA decapping factor Dcp1a, a marker of PBs (22, 23) (Fig. 2A). Transfection of siElavl1 in IMECs to reduce ELAVL1 protein increased both the number and the area of 4E-T positive PBs (Fig. 2A). Similarly, primary endothelial cells that lack Elavl1 (Elavl1 ECKO) and Elavl1 knockdown IMECs (shElavl1) showed a similar phenotype (Fig. 2 B–D). These data suggest that expression of stable 4E-TS isoform in Elavl1 depleted cells induces the formation of abundant, large PBs. To further examine this possibility, we overexpressed 4E-TL or 4E-TS in IMECs by lentivirus transduction (Fig. S6). Dcp1a immunostaining revealed that overexpression of 4E-TL enhanced PB formation approximately twofold compared with control lentiviral transduction (Fig. 2E). In sharp contrast, 4E-TS expression was much more potent in the induction of PBs (approximately sixfold) (Fig. 2F). In Elavl1-depleted IMECs, the number and size of PBs were maximal and overexpression of 4E-TL or 4E-TS did not have an additive effect. These data indicate that formation of 4E-TS isoform, which is inhibited by ELAVL1-induced alternative splicing, is a potent inducer of PB formation in IMECs.
Fig. 2.
The 4E-TS protein in ELAVL1 depleted cells induces exaggerated PB formation. (A) The 4E-T colocalizes with Dcp1a, a marker for PBs. IMECs transfected with siCtl or siElavl1 were stained with anti–4E-T antibody (green), anti-Dcp1a (red), and DAPI (blue). Higher magnification views of boxed areas are shown in Bottom Right Insets. (B and C) MLECs from WT or Elavl1 KO mice and stable knockdown of Elavl1 in IMECs (shElavl1) were stained with anti-ELAVL1 antibody (green) and anti–4E-T antibody (red). (D) Quantitative analysis of the number and the area of PBs. The number of 4E-T positive PBs per cell are as follows: shCtl vs. shElavl1: 4.19 ± 0.56 vs. 38.06 ± 4.0. The area of 4E-T positive PBs per cell is as follows: shCtl vs. shElavl1: 67.90 ± 9.79 vs. 939.9 ± 131.6. The number of pixels per PB is as follows: shCtl vs. shElavl1: 16.20 ± 0.72 vs. 23.65 ± 1.29. n = 21 or 17, ***P < 0.0001. (E) IMECs were transfected with control siRNA or Elavl1siRNA, subsequently infected with lentivirus expressing control vector, 4E-TL, or 4E-TS and stained for Dcp1a. (F) Quantitative analysis of the number and the areas of PBs in control, 4E-TL, or 4E-TS overexpressed cells. *P < 0.05, ***P < 0.005, **P < 0.0001. (Scale bars, 10 µm.)
4E-TS Protein in ELAVL1-Depleted Endothelial Cells Promotes mRNA Turnover.
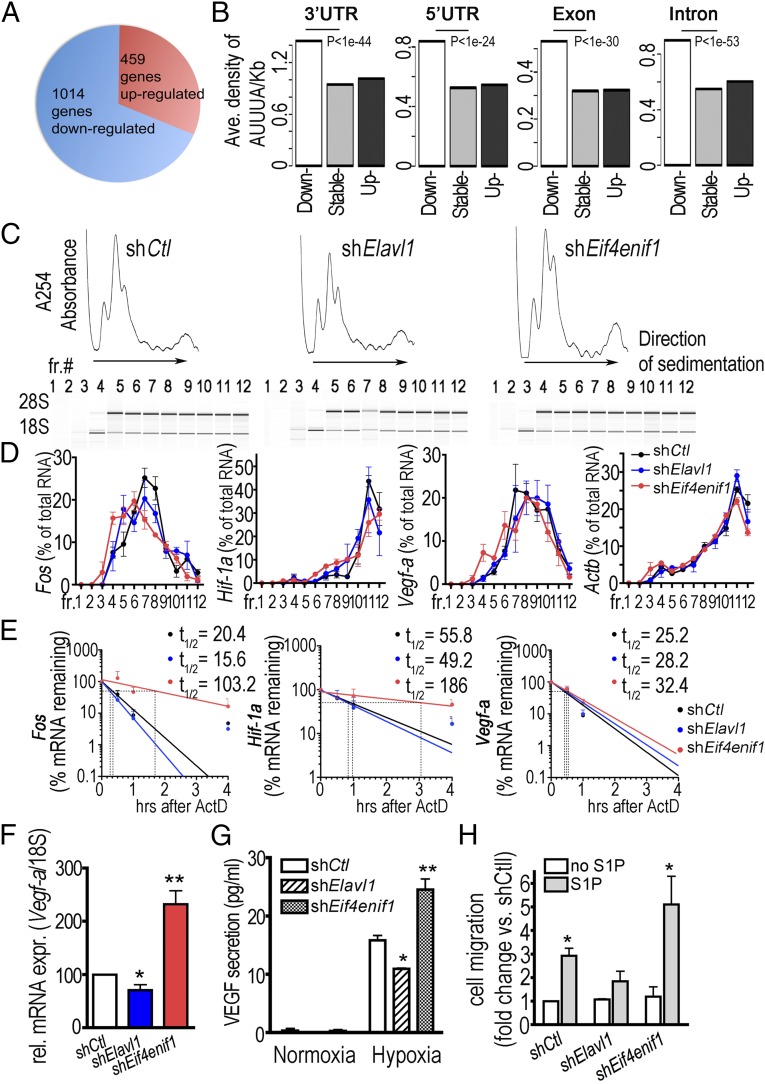
PBs are the sites at which translationally inactive mRNAs, microRNAs, RBPs, and mRNA decay machinery achieve translational repression and mRNA degradation (29). In particular, ARE-bearing mRNAs are targeted to PBs for translational silencing and rapid degradation (30). To better understand the ELAVL1-mediated gene regulation in endothelial cells, we analyzed global gene expression profiles in MLECs isolated from WT and Elavl1 ECKO mice. Among 1,473 differentially expressed genes, the majority (69%, 1,014 genes) were down-regulated in Elavl1 ECKO MLECs (fold change >1.4, n = 4/ group) (Fig. 3A). In addition, down-regulated transcripts contained higher density of both ARE and URE (14, 15) than the transcripts that are stable or up-regulated in 3′ UTR, 5′ UTR, exons, and introns (Fig. 3B and Fig. S7). These data suggest that ELAVL1 stabilizes ARE- and URE-containing transcripts (14, 15) in vascular endothelial cells.
Fig. 3.
The 4E-TS protein in ELAVL1-depleted endothelial cells promotes mRNA turnover. (A) Microarray analysis in MLECs from WT or Elavl1 KO mice. A total of 1,014 genes were down-regulated in Elavl1 KO MLECs. Fold change is >1.4, n = 4 per group. (B) Average density of AUUUA (the number of AUUUA per kilobase of transcript) in each region (3′ UTRs, 5′ UTRs, exons, and introns) of genes analyzed by microarray. (C) Polysome profiles of IMECs stably knocked down with shRNA targeted against control (Ctl), Elavl1, or Eif4enif1 mRNAs. Cell extracts were size fractionated by centrifugation through sucrose density gradients (15–45%). Arrows indicate the direction of sedimentation. Below each profile, 18S and 28S rRNA were visualized by nanogel. (D) RNA extracted from each of the 12 fractions, followed by qRT-PCR to assess the relative distribution of Fos, Hif1a, Vegfa, and β-actin mRNAs. Data represent mean ± SEM from three independent experiments. (E) Percentage of Fos, Hif1a, and Vegfa mRNA remaining in shcontrol, shElavl1, and shEif4enif1 cells upon addition of actinomycin D (5 μg/mL) for indicated times. The half-life (t1/2) of mRNA was determined from the slope and Y intercept of a best fit value in the semilogarithmic plot of mRNA abundance versus time; log10 of 50% = 10^(slope × t1/2 + Y intercept). Data represent mean ± SEM from four independent experiments. (F) Vegfa mRNA expression in shcontrol, shElavl1, and shEif4enif1 cells. Data represent mean ± SEM from five independent experiments. *P < 0.05, **P = 0.01. (G) VEGF-A secretion in the supernatant of normoxia- or hypoxia-treated shcontrol, shElavl1, and shEif4enif1 cells. Data represent mean ± SEM from three independent experiments. *P < 0.05, **P = 0.01. (H) Chemotaxis of IMECs. Migration of IMECs stably knocked down with shRNA control, Elavl1, or Eif4enif1 in response to 100 nM S1P was analyzed using a modified Boyden chamber. Data represent the mean ± SEM from three independent experiments. *P < 0.05.
To test the possible role of 4E-TS in translational repression, we measured the polysome loading of posttranscriptionally regulated angiogenesis-regulatory mRNAs, Fos, Hif1a, and Vegfa by fractionating cytoplasmic components in 15–45% sucrose gradients followed by quantitative RT-PCR (qRT-PCR) analysis (Fig. 3C). Knockdown of Elavl1 and Eif4enif1 did not alter the loading of Fos, Hif1a, and Vegfa mRNAs on light or heavy polysomes (Fig. 3D). In contrast, turnover of angiogenic regulatory mRNAs was affected by ELAVL1 and 4E-T. Fos and Hif-1a mRNA, which contain several ARE−/URE− motifs, revealed a longer half-life in Eif4enif1 knockdown cells compared with control cells (Fos: 103.2 min vs. 20.4 min and Hif1a: 186 min vs. 55.8 min), whereas a shorter half-life in Elavl1 knockdown cells (Fos: 15.6 min vs. 20.4 min and Hif1a: 49.2 min vs. 55.8 min) (Fig. 3E). These data strongly suggest that ELAVL1-regulated alternative splicing of Eif4enif1 and the formation of 4E-TS isoform control, at least in part, the delivery of specific ARE/URE-containing mRNAs to PBs and facilitate mRNA degradation. Even though Vegf-a mRNA half-life was similar in control, Elavl1 knockdown and Eif4enif1 knockdown cells (25.2 min, 28.2 min, and 32.4 min), steady-state Vegf-a mRNA expression was significantly reduced in Elavl1 knockdown cells and enhanced in Eif4enif1 knockdown cells (Fig. 3F). In addition, the secretion of VEGF is significantly reduced in the supernatant of Elavl1 knockdown cells and enhanced in the supernatant of Eif4enif1 knockdown cells under hypoxia (Fig. 3G). Accordingly, in the Boyden chamber chemotaxis assay, stimulation of IMEC migration in response to sphingosine 1-phosphate (S1P) was attenuated in Elavl1 knockdown cells but is greatly exaggerated in Eif4enif1 knockdown cells (Fig. 3H). These data suggest that the impaired angiogenic phenotype in Elavl1 knockout cells is the result of the function of 4E-TS protein.
Endothelial ELAVL1 Regulates Postnatal Pathological Angiogenesis.
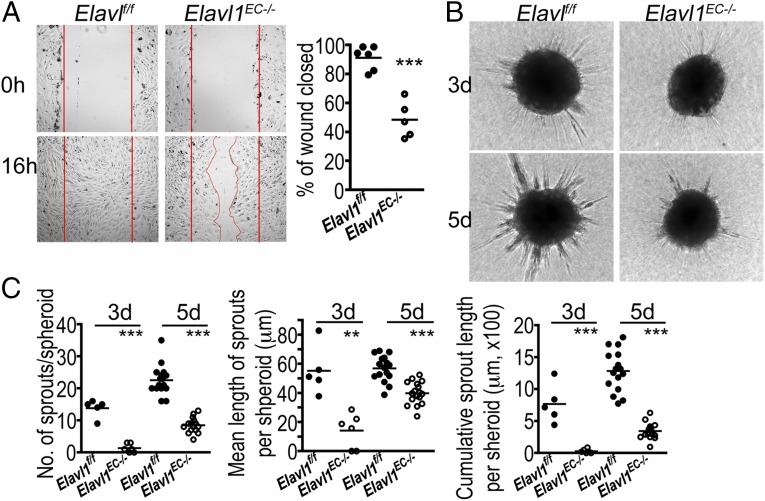
To examine the angiogenic functions of ELAVL1-mediated gene regulation in primary endothelial cells, we analyzed in vitro angiogenic phenotypes of MLECs from WT and Elavl1 ECKO mice. Loss of ELAVL1 did not affect endothelial cell proliferation in vitro (Fig. S8). However, migratory and sprouting responses of endothelial cells were greatly affected. MLECs from Elavl1 ECKO mice did not migrate as much as the WT counterparts in the scratch-induced migration assay (Fig. 4A). Similarly, 3D spheroid sprouting assay showed significantly reduced number and length of sprouts in MLECs from Elavl1 ECKO mice compared with the WT controls (Fig. 4 B and C).
Fig. 4.
Endothelial ELAVL1 regulates postnatal pathological angiogenesis. (A) Scratch wound assay and (B and C) spheroid sprouting assay using MLECs isolated from WT and Elavl1 ECKO mice. Representative images of endothelial cells in scratched area at 0 h (Top) and 16 h (Bottom). (A) Quantitative analysis of scratch closed area in WT ECs (91.18 ± 3.39, n = 6) vs. Elavl1 KO ECs (48.43 ± 5.66, n = 5). ***P < 0.0001. Data are presented as the percent of scratch covered area to the initial cell-free area. (B and C) Representative images and quantitative analysis of sprouts from spheroids at 3 d and 5 d. Number of sprouts per spheroid at 3 d (WT: 13.80 ± 1.24 vs. KO:1.33 ± 0.56) and 5 d (WT 22.56 ± 1.15 vs. 8.53 ± 0.54). Mean length of sprout per spheroid at 3 d (WT: 55.34 ± 7.40 vs. KO: 14.18 ± 4.81) and 5 d (WT: 56.86 ± 2.22 vs. KO: 39.88 ± 1.86). Cumulative sprout length of spheroids at 3 d (WT: 768.6 ± 135.5 vs. KO: 28.95 ± 13.40) and 5 d (WT: 1283 ± 79.85 vs. KO: 345 ± 29.50). Three days: n = 5 or 6 per group; 5 d: n = 16 or 17 per group, **P < 0.005, ***P < 0.0005.
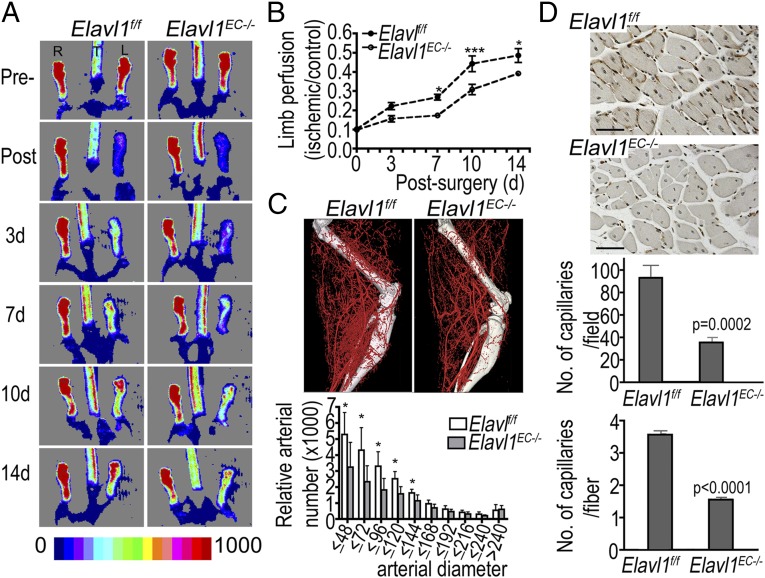
Next, the vascular phenotypes of Elavl1 ECKO mice were examined. Crosses between Elavl1f/f mice with or without VE-cadherin-Cre (31) resulted in the birth of WT and Elavl1 ECKO mice at 1:1 ratio (Fig. S9A). Both WT and Elavl1 ECKO mice appeared normal, suggesting that ELAVL1 is not required for embryonic vascular development. In addition, postnatal angiogenesis in the ear, retina, and trachea of WT and Elavl1 ECKO mice looks similar, suggesting the undisturbed angiogenesis in adult mice (Fig. S9B). However, when these mice were subjected to a model of ischemic angiogenesis in the hind limb following femoral artery ligation (32), significantly attenuated blood flow recovery was seen in Elavl1 ECKO mice (Fig. 5 A and B). Micro-CT scan of the affected limb shows attenuated angiogenesis and collateral formation (Fig. 5C) and quantitative analysis of micro-CT data confirmed that the relative number of arteries with <144-μm diameter in Elavl1 ECKO mice is significantly reduced compared with WT mice, supporting the significant reduction in blood flow recovery after femoral artery ligation in Elavl1 ECKO mice. Tissue sections were immunostained with CD31 to highlight microvessels in the ischemic gastrocnemius muscle (Fig. 5D). Quantitative analysis revealed reduced number of capillaries per field and per muscle fiber in Elavl1ECKO mice. These data indicate that the endothelial ELAVL1 is critical for optimal revascularization response after ischemic injury of the hind limb.
Fig. 5.
Endothelial ELAVL1 regulates postnatal pathological angiogenesis. Revascularization of the mouse ischemic hind limb was conducted as described in WT and Elavl1 ECKO mice. (A) Representative laser Doppler flow images of hind limb perfusion before (pre) and at different time points (post 3, 7, 10, and 14 d) after femoral artery ligation (R, control; L, ligated). (B) Changes in perfusion are shown as ratio of ischemic to control limb flow perfusion (n = 7 per group, *P < 0.05, ***P < 0.001). (C) Micro-CT reconstruction of a representative mouse hind limb 14 d after femoral artery ligation. Quantitative analysis of micro-CT angiograms in the calf is presented as total number of vascular structures of specified diameter. (n = 7 per group, *P < 0.05) (D) Representative images of CD31 immunostained sections of ligated limb muscle and quantification of CD31 positive vessels. The number of capillaries per field (20× WT: 91.1 ± 10.9 vs. Elavl1 ECKO: 35.5 ± 4.4, P = 0.0002, n = 8 images from four mice per group). The number of capillaries per fiber (WT: 3.57 ± 0.11 vs. Elavl1 ECKO 1.55 ± 0.06, P < 0.0001, n = 101 fibers from eight images per group). (Scale bar, 50 µm.)
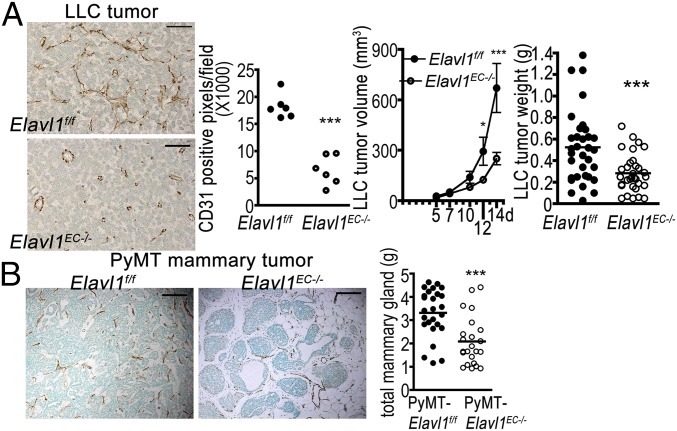
To examine the role of endothelial ELAVL1 in tumor angiogenesis, we implanted Lewis lung carcinoma (LLC) cells s.c. and examined tumor growth and associated tumor microvessels. LLC tumor implant in Elavl1ECKO mice showed significantly attenuated tumor volume and weight compared with WT mice (Fig. 6A). CD31 immunostained tumor images and quantitative analysis of CD31 positive vessels indicate that Elavl1 ECKO mice had significantly reduced vascular density and branching patterns compared with the WT counterparts (Fig. 6A). We also examined the role of endothelial ELAVL1 in a spontaneous model of mammary cancer. Elavl1 ECKO mice were crossed with transgenic mice expressing polyoma virus middle T oncogene (PyMT) under the transcriptional control of mouse mammary tumor virus promoter (MMTV-PyMT) (33). Tumors were removed at 16 wk and the total tumor burden was evaluated. PyMT:Elavl1 ECKO mice had significantly reduced tumor burden than their WT counterparts (Fig. 6B). Histological sections were stained for tissue architecture as well as immunohistochemistry to analyze the microvessels (Fig. 6B). Data revealed that mammary tumors in PyMT:Elavl1 ECKO mice showed the reduced vascular density and fewer malignant lesions compared with the WT counterparts. These data suggest that posttranscriptional gene regulation by ELAVL1 in endothelial cells is critical for tumor angiogenesis and optimal tumor growth.
Fig. 6.
Endothelial ELAVL1 regulates postnatal pathological angiogenesis. (A) LLC tumor isograft model in WT and Elavl1 ECKO mice. Representative images of CD31 immunostaining of LLC tumors. Quantitative analysis of vascular density (CD31 positive pixels per field, 20×). LLC tumor volume and LLC tumor weight of WT (0.525 ± 0.056 n = 34) and Elavl1 ECKO mice (0.285 ± 0.031, n = 32). *P < 0.05, **P < 0.005, ***P < 0.0001. (B) PyMT-induced mammary tumor model in WT and Elavl1 ECKO mice. Representative images of CD31 immunostaining of mammary tumors. Total mammary mass of PyMT:WT (3.311 ± 0.202, n = 26) and PyMT: Elavl1 ECKO mice (2.082 ± 0.219, n = 23) at the age of 16 wk. ***P < 0.0001. (Scale bar, 50 µm.)
Our results elucidate previously unidentified function of ELAVL1, which is to regulate the alternative splicing of Eif4enif1 primary transcript. In the absence of ELAVL1, exon 11 exclusion produces 4E-TS, a stable isoform that strongly induces PBs and suppresses angiogenic gene expression in endothelial cells. Our work also revealed a novel mode of regulation of angiogenic gene expression, which is to control mRNA turnover by limiting the expression of highly stable isoform of a posttranscriptional repressor. The 4E-TS promotes turnover of mRNAs for endothelial transcription factors (Fos and Hif-1a) and reduces the expression of a key growth factor (VEGF-A), suppressing chemotactic migration and sprouting behavior. This mode of gene regulation may be highly relevant in postnatal angiogenesis in the revascularization of ischemic muscle of the limb and tumor angiogenesis. Indeed, regulation of eIF4E by the mTOR/4EBP1 pathway is therapeutically targeted in oncology (34). We propose that the ELAVL1/Eif4enif1 posttranscriptional RNA regulon described here may be relevant in many pathological processes and therefore therapeutically actionable.
Materials and Methods
Animals and Cell Culture.
Tumor isograft, spontaneous mammary tumor model, and hind limb ischemic model and laser Doppler blood flow analysis were performed with institutional review board approval as described in SI Materials and Methods (Weill Cornell Medical College for tumor study, Yale University School of Medicine for hind limb ischemic mouse model). Primary mouse lung endothelial cells were isolated, cultured, and scratch wound assay and spheroid sprouting assay were performed as described in SI Materials and Methods.
RNA and Protein Analysis.
RNA isolation, mouse exon chip array, alternative splicing analysis, polysomal mRNA profiling, RT-qPCR analysis, Western blotting, and immunofluorescence experiments are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor Thomas Tuschl (The Rockefeller University) for critical comments and Dr. Jerry Pelletier (McGill University) for the gift of reagents. This work was supported by National Institutes of Health Grants HL49094, HL117798, and CA77839 (to T.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412172111/-/DCSupplemental.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16(2):180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 6.Keene JD. RNA regulons: Coordination of post-transcriptional events. Nat Rev Genet. 2007;8(7):533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 7.Moore MJ. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Olson EN. AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SH, et al. Antagonistic function of the RNA-binding protein HuR and miR-200b in post-transcriptional regulation of vascular endothelial growth factor-A expression and angiogenesis. J Biol Chem. 2013;288(7):4908–4921. doi: 10.1074/jbc.M112.423871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16(8):909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhnert F, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135(24):3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 13.Simone LE, Keene JD. Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev. 2013;23(1):35–43. doi: 10.1016/j.gde.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebedeva S, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43(3):340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee N, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43(3):327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Katsanou V, et al. The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol Cell Biol. 2009;29(10):2762–2776. doi: 10.1128/MCB.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh M, et al. Essential role of the RNA-binding protein HuR in progenitor cell survival in mice. J Clin Invest. 2009;119(12):3530–3543. doi: 10.1172/JCI38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, et al. Elavl1a regulates zebrafish erythropoiesis via posttranscriptional control of gata1. Blood. 2014;123(9):1384–1392. doi: 10.1182/blood-2013-09-526962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273(11):6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, et al. Macrophage β2 integrin-mediated, HuR-dependent stabilization of angiogenic factor-encoding mRNAs in inflammatory angiogenesis. Am J Pathol. 2012;180(4):1751–1760. doi: 10.1016/j.ajpath.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraiuolo MA, et al. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol. 2005;170(6):913–924. doi: 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamenska A, et al. Human 4E-T represses translation of bound mRNAs and enhances microRNA-mediated silencing. Nucleic Acids Res. 2013;42:3298–3313. doi: 10.1093/nar/gkt1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomonis N, et al. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proc Natl Acad Sci USA. 2010;107(23):10514–10519. doi: 10.1073/pnas.0912260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J. 2000;19(12):3142–3156. doi: 10.1093/emboj/19.12.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasippillai T, et al. Mutations in eIF4ENIF1 are associated with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98(9):E1534–E1539. doi: 10.1210/jc.2013-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villaescusa JC, et al. Clast4, the murine homologue of human eIF4E-Transporter, is highly expressed in developing oocytes and post-translationally modified at meiotic maturation. Gene. 2006;367:101–109. doi: 10.1016/j.gene.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem. 2008;283(27):19077–19084. doi: 10.1074/jbc.M800017200. [DOI] [PubMed] [Google Scholar]
- 29.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25(5):635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Franks TM, Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 2007;21(6):719–735. doi: 10.1101/gad.1494707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alva JA, et al. VE-Cadherin-Cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235(3):759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 32.Cristofaro B, et al. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140(8):1720–1729. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh AC, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17(3):249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.