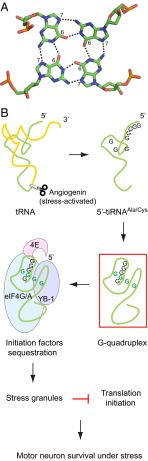
Although not generally appreciated, the first 3D visualization of a nucleic acid was provided by the crystal structures of yeast phenylalanine transfer RNA (tRNA) (1, 2). [The DNA structure determined in the 1950s was a low-resolution fiber-diffraction–based model (3).] It was interesting to see that the L-shaped tRNA molecule is maintained by both Watson–Crick and non-Watson–Crick types of base interactions. These types include Hoogsteen base pairs that use the N7-C6 face of a purine to make hydrogen-bonding interactions. The scheme of the Hoogsteen base pairing also allows the formation of a planar interaction with four guanine residues, known as G-tetrad or G-quartet (Fig. 1A). Despite the fact that the G-tetrad interaction was first proposed over 50 y ago (4), demonstration of its biological significance was much later and not until it was found in the structure of telomeres (5–7). The 3′-end overhang of vertebrate telomeres are rich in guanines and consist of many TTAGGG repeats that can spontaneously fold into four-stranded DNA structures with two or more G-tetrads stacking on top of each other. Functionally, formation of this unique structure—named G-quadruplex—plays a major regulatory role in telomere maintenance (8). Since then, G-quadruplex structures became widely noted to be present throughout the genome and the transcriptome. DNA and RNA G-quadruplexes become attractive drug targets for treating various diseases as well as novel therapeutics (9). In PNAS, Ivanov et al. suggest for the first time that G-quadruplex structures can form within a cleaved tRNA fragment, presumably requiring conformational rearrangement from its original structure in the whole tRNA (10). Moreover, these tRNA-derived G-quadruplex structures are effector molecules of a stress-response pathway that protects motor neuron, making them potential therapeutics for treating neurodegenerative diseases, especially amyotrophic lateral sclerosis (ALS).
Fig. 1.
(A) Planar interaction with four guanine nucleotides to form a G-tetrad (green, carbon; blue, nitrogen; red, oxygen; orange, phosphorus). (B) The angiogenin neuroprotective pathway. Angiogenin generates tiRNA fragments that form putative G-quadruplex structures and act to protect motor neurons under stress.
ALS, often referred to as Lou Gehrig’s Disease in the United States, is a paralyzing and ultimately fatal disease characterized by the progressive loss of motor neurons. One of the susceptibility genes for ALS is angiogenin, whose loss-of-function is strongly associated with the disease (11). Angiogenin was discovered as a secreted angiogenic factor and later demonstrated to be a tRNA-specific ribonuclease (12). Under conditions of stress, such as oxidation, secreted angiogenin re-enters the cell and cleaves tRNA at the anticodon loop, located at the tip of one arm of the L-shaped molecule (Fig. 1B). The cleavage produces two tRNA halves about 30–40 nucleotides long designated as 5′- and 3′-tiRNAs (13). In previous work of the Anderson laboratory, a subset of the 5′-tiRNAs derived from alanine and cysteine tRNAs was found to recruit translational silencer protein YB-1 and sequester eukaryotic translation initiation factor 4G/A complex to inhibit translation initiation (14) (Fig. 1B). Down-regulation of protein synthesis under stress conditions would help to conserve energy and divert the translation machinery to temporarily focus on the synthesis of stress-response genes. The specific 5′-tiRNAs also trigger the assembly of a special type of aggregate called stress granules, which function to sequester the stalled translation preinitiation complexes until the stress condition is resolved (Fig. 1B). Although escalated and prolonged production of aggregates impairs normal function and survival, especially for neuronal cells, physiological and transient stress granules can help neurons to deal with stress (15). The latter aspect, perhaps, contributes to why loss-of-function mutations of angiogenin are causatively linked with ALS. Indeed, Ivanov et al. show that treatment of angiogenin, but not its loss-of-function mutant, promotes motor neuron survival under excitotoxic and oxidative stresses (10).
Interestingly, Ivanov et al. also find that only 5′-tiRNAAla and 5′-tRNACys, but not other 5′-tiRNAs that are nonfunctional in this pathway, are assembled into putative G-quadruplex structures (10). This finding is exciting from both basic and therapeutic perspectives because G-quadruplex structures are known to be able to efficiently enter cells (9). In addition, the well-established conformational differences between DNA and RNA double helices are usually not seen in G-quadruplex structures. This consideration may have provided the authors with the rationale to test the 5′-tiDNAAla, which would be more resistant to nuclease degradation than its RNA counterpart. Indeed, Ivanov et al. find that 5′-tiDNAAla is spontaneously uptaken by human motor neurons and effectively rescues the motor neurons from stress-induced death (10).
As 5′-tiRNAs join other small RNAs—such as microRNAs and Piwi-interacting RNAs—to perform a regulatory function in eukaryotic cells, an interesting question has been whether these 5′-tiRNAs likewise also have RNA or DNA targets. An intriguing possibility has been raised from Ivanov et al.’s (10) work showing apparent interaction of 5′-tiRNAAla and 5′-tRNACys with the pathological GGGGCC RNA repeats transcribed from the C9ORF72 gene. The hexanucleotide repeat expansions are the leading genetic cause of ALS and frontotemporal dementia (FTD) (16, 17). Normal human C9ORF72 alleles have a few intronic GGGGCC repeats, whereas the ALS/FTD-associated C9ORF72 alleles contain tens to thousands of the repeats that have a length-dependent capacity to form G-quadruplex structures (18, 19). Ivanov et al. (10) find that RNA oligonucleotide (GGGGCC)23, but not (GGGGCC)4, suppresses the activity of 5′-tiRNAAla and 5′-tRNACys to induce stress granules. Therefore, the authors propose that the G-quadruplex structures formed by the extended GGGGCC repeats may interfere with the function of the endogenous tiRNAs in motor neuron maintenance, a hypothesis that may unite two distinct genetic causes of ALS. On the flip side, it would be interesting to test if the G-quadruplex–containing tiRNAs/tiDNAs could also be used to suppress the toxicity of the pathological GGGGCC repeats.
There are many remaining questions. For example, how do 5′-tiRNAAla and 5′-tRNACys form into the putative G-quadruplex structures? What do the structures look like? How does YB-1 interact with the G-quadruplexes? Does 5′-tiRNAAla and 5′-tRNACys have other physiological interaction partners in motor neurons? Answers to these questions would help to optimize these tRNA-derived G-quadruplex molecules for the treatment of motor neuron diseases.
Footnotes
The author declares no conflict of interest.
See companion article on page 18201.
References
- 1.Kim SH, et al. Three-dimensional structure of yeast phenylalanine transfer RNA: Folding of the polynucleotide chain. Science. 1973;179(4070):285–288. doi: 10.1126/science.179.4070.285. [DOI] [PubMed] [Google Scholar]
- 2.Robertus JD, et al. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- 3.Watson JD, Crick FH. Molecular structure of nucleic acids; A structure for deoxyribose nucleic acid. Nature. 1953;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 4.Gellert M, Lipsett MN, Davies DR. Helix formation by guanylic acid. Proc Natl Acad Sci USA. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- 6.Sundquist WI, Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- 7.Williamson JR, Raghuraman MK, Cech TR. Monovalent cation-induced structure of telomeric DNA: The G-quartet model. Cell. 1989;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 8.Lipps HJ, Rhodes D. G-quadruplex structures: In vivo evidence and function. Trends Cell Biol. 2009;19(8):414–422. doi: 10.1016/j.tcb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Collie GW, Parkinson GN. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem Soc Rev. 2011;40(12):5867–5892. doi: 10.1039/c1cs15067g. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov P, et al. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci USA. 2014;111:18201–18206. doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenway MJ, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38(4):411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Hu GF. Emerging role of angiogenin in stress response and cell survival under adverse conditions. J Cell Physiol. 2012;227(7):2822–2826. doi: 10.1002/jcp.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185(1):35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43(4):613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolozin B. Physiological protein aggregation run amuck: Stress granules and the genesis of neurodegenerative disease. Discov Med. 2014;17(91):47–52. [PMC free article] [PubMed] [Google Scholar]
- 16.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renton AE, et al. ITALSGEN Consortium A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy K, Zamiri B, Stanley SY, Macgregor RB, Jr, Pearson CE. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. 2013;288(14):9860–9866. doi: 10.1074/jbc.C113.452532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fratta P, et al. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep. 2012;2:1016. doi: 10.1038/srep01016. [DOI] [PMC free article] [PubMed] [Google Scholar]