Significance
The mammalian reproductive system is critically dependent upon pulsatile hormone release patterned and driven by a small population of gonadotropin-releasing hormone (GnRH) neurons. The scattered distribution of GnRH neurons within the brain has made it extremely difficult to investigate the nature and properties of these cells in any selective manner in vivo. We report here a procedure that enables selective, high-fidelity activation of the GnRH neurons that control gonadotropin secretion in ovariectomized mice. Using this approach, we have been able to define minimal parameters of activation required to generate pulsatile gonadotropin secretion in the blood. This provides critical information for understanding and manipulating the genesis of gonadotropin pulsatility in reproductive biology.
Keywords: GnRH, luteinizing hormone, pulse, optogenetics
Abstract
The mechanisms responsible for generating the pulsatile release of gonadotropins from the pituitary gland are unknown. We develop here a methodology in mice for controlling the activity of the gonadotropin-releasing hormone (GnRH) neurons in vivo to establish the minimal parameters of activation required to evoke a pulse of luteinizing hormone (LH) secretion. Injections of Cre-dependent channelrhodopsin (ChR2)-bearing adeno-associated virus into the median eminence of adult GnRH-Cre mice resulted in the selective expression of ChR2 in hypophysiotropic GnRH neurons. Acute brain slice experiments demonstrated that ChR2-expressing GnRH neurons could be driven to fire with high spike fidelity with blue-light stimulation frequencies up to 40 Hz for periods of seconds and up to 10 Hz for minutes. Anesthetized, ovariectomized mice had optical fibers implanted in the vicinity of GnRH neurons within the rostral preoptic area. Optogenetic activation of GnRH neurons for 30-s to 5-min time periods over a range of different frequencies revealed that 10 Hz stimulation for 2 min was the minimum required to generate a pulse-like increment of LH. The same result was found for optical activation of GnRH projections in the median eminence. Increases in LH secretion were compared with endogenous LH pulse parameters measured from ovariectomized mice. Driving GnRH neurons to exhibit simultaneous burst firing was ineffective at altering LH secretion. These observations provide an insight into how GnRH neurons generate pulsatile LH secretion in vivo.
Reproductive functioning in all mammals is critically dependent upon pulsatile gonadotropin secretion (1). Experiments undertaken in the 1980s clearly established that pulsatile luteinizing hormone (LH) and follicle-stimulating hormone secretion were generated by the episodic release of gonadotropin-releasing hormone (GnRH) into the pituitary portal vasculature (2–6). However, a quarter of a century since those experiments were performed, the components and mechanisms responsible for this episodic release of GnRH remain unknown and represent one of the most important unanswered questions in reproductive biology (7).
Key parameters such as the number of GnRH neurons involved in a pulse and their patterns of electrical firing are unknown. An important insight into the dynamics of a GnRH pulse has come from fast portal blood sampling in ovariectomized sheep where each GnRH pulse is reported to approximate a square wave beginning sharply over 2 min, remaining elevated for ∼5 min, and then falling to baseline over the next 3 min (8). This allowed speculation that a subgroup of GnRH neurons may fire coordinately for a period of 2–7 min to generate a pulse of GnRH (7). Disappointingly, however, direct electrical recordings of adult GnRH neurons in acute brain slices in vitro have provided no clear correlate of pulsatile hormone secretion (7, 9). Recent investigations into GnRH neuron firing in vivo in anesthetized GnRH-green fluorescent protein (GFP) mice have similarly been unable to shed light on the pulse-generating properties of these cells (10). The most promising insights into the nature of GnRH pulsatility have come from studies of embryonic GnRH neurons in vitro where episodes of burst firing, represented by calcium transients, are found to synchronize occasionally in subpopulations of GnRH neurons in a time frame similar to that of pulsatile GnRH/LH secretion (11, 12).
The best way of determining the patterns of GnRH neuron firing that generate an LH pulse would be to record the activity of hypophysiotropic GnRH neurons while simultaneously measuring LH secretion in vivo. At present this remains impossible. An alternative approach that might shed light on this issue would be to determine the minimal patterns of GnRH neuron firing that are capable of generating an LH pulse in vivo. This is now possible using optogenetic approaches, and we report here a strategy that allows hypophysiotropic GnRH neurons to be transfected with channelrhodopsins (ChR2) and subsequently activated in vivo to generate pulses of LH secretion. This reveals that GnRH neurons need only be activated at either their cell bodies or distal projections within the median eminence (ME) for 2 min at a constant 10-Hz firing rate to generate an LH pulse. Surprisingly, synchronizing burst firing among GnRH neurons is ineffective.
Results
Transfection of Hypophysiotropic GnRH Neurons with Channelrhodopsin.
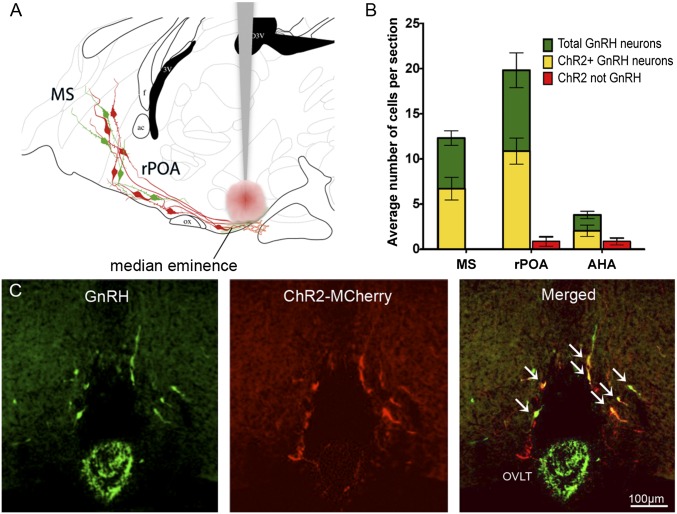
ChR2 fused with mCherry was targeted to hypophysiotropic GnRH neurons by injecting a Cre-dependent adeno-associated virus [AAV9-EF1-dflox-hChR2-(H134R)-mCherry-WPRE-hGH] bilaterally into the ME region of GnRH-Cre transgenic mice (Fig. 1A). This approach takes advantage of the retrograde transport properties of AAVs (13) and ensures that only hypophysiotropic GnRH neurons express ChR2. Three weeks after AAV injections, dual-label immunofluorescence studies showed that GnRH neurons throughout the basal forebrain were transfected (Fig. 1 A–C; Fig. S1 A–C). Approximately 50% of GnRH neurons located in the medial septum (MS), rostral preoptic area (rPOA), and anterior hypothalamus (AHA) expressed ChR2 (n = 6; Fig. 1B and Fig. S1 A–C). Within the MS, 100% of ChR2-expressing cells were GnRH neurons, 93 ± 8% in the midline rPOA, and 77 ± 3% in the AHA (n = 6). The small number of neurons that were transfected with ChR2 but negative for GnRH immunoreactivity were usually located at the very base of the brain in both the rPOA (Fig. 1C) and the AHA and may represent GnRH neurons with low levels of GnRH peptide. Although not quantified, large numbers of GnRH neuron projections adjacent to and within the ME also expressed ChR2 (Fig. S1 D–F). Outside the GnRH neuron distribution, transfected cells were detected in the lateral septum in two mice. Control injections performed in female wild-type C57BL/6 mice (n = 3) resulted in no ChR2-mCherry expression, confirming the Cre dependence of the viral vector. These results show that hypophysiotropic GnRH neurons can be targeted accurately with ChR2 using ME AAV injections.
Fig. 1.
Transfection of hypophysiotropic GnRH neurons with ChR2. (A) Indicates the strategy of injecting AAV into the region of the median eminence so that ChR2 and mCherry are expressed only in the hypophysiotropic GnRH neurons (red cells) that innervate the median eminence. MS, medial septum. rPOA, rostral preoptic area. (B) Histograms showing the mean + SEM numbers of total GnRH neurons per section (green) detected in the MS, rPOA, and anterior hypothalamic area (AHA) and also the mean numbers of GnRH neurons expressing ChR2 (yellow) overlaid on top. The numbers of ChR2-expressing cells that were not immunoreactive for GnRH are shown in red for the rPOA and AHA. No such cells were detected in the MS. (C) Photomicrographs showing GnRH immunoreactivity (green), mCherry (red), and overlay. White arrows indicate GnRH neurons expressing mCherry.
Optogenetic Activation of GnRH Neurons in Vitro.
Coronal brain slices were prepared from AAV-injected GnRH-Cre mice and cell-attached recordings made from mCherry-expressing GnRH neurons located in the rPOA. Initially, 10 GnRH neurons (from four mice) were recorded for 20–30 min to examine their firing patterns. These cells exhibited the typical firing patterns displayed by GnRH neurons of being silent (n = 6), bursting (n = 2), or firing in an irregular manner (n = 2). All 8 GnRH neurons (from three mice) tested with 10 or 40 nM kisspeptin were activated in the normal manner (Fig. S2).
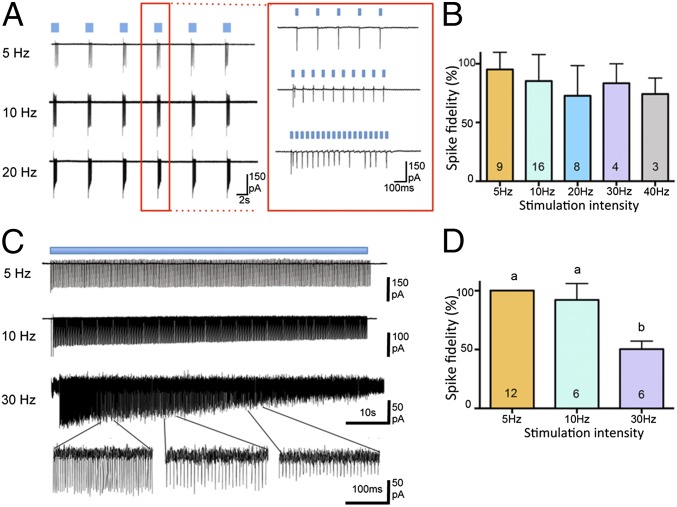
The efficacy of blue light to activate ChR2-expressing GnRH neurons in vitro was assessed in three different paradigms. In the first, 5-ms laser pulses were delivered at 5, 10, 20, 30, or 40 Hz for 1 s in a repetitive manner once every 10 s over a period of 1 min. GnRH neurons exhibited action potentials in response to blue-light activation with high spike fidelity (Fig. 2A). Laser pulses induced an action potential with 95 ± 5%, 85 ± 6%, 72 ± 9%, 83 ± 8%, and 74 ± 8% fidelity at 5 Hz (n = 9, four mice), 10 Hz (n = 16, four mice), 20 Hz (n = 8, four mice), 30 Hz (n = 4, three mice), and 40 Hz (n = 3, three mice), respectively (Fig. 2B). Although GnRH neurons occasionally failed to follow every light stimulation at higher frequencies (Fig. 2A: 20 Hz), the overall mean response fidelity rate was not significantly different across these groups. At the end of the stimulation protocols, cells went back to their normal endogenous firing pattern and were able to respond to further light stimulations.
Fig. 2.
Optogenetic activation of GnRH neurons in vitro. (A) Representative cell-attached, voltage-clamp recordings of ChR2-expressing GnRH neurons activated with 5-ms blue-light pulses given at 5, 10, and 20 Hz for repeated 1-s on (blue bar) and 9-s off time periods. (Inset Right) Expanded view of 1-s stimulations at different frequencies. (B) Histogram showing the mean + SEM evoked spike fidelities at the different stimulation frequencies. A spike fidelity of 100% means that every blue-light stimulation generates an action potential. (C) Representative cell-attached, voltage-clamp recordings of ChR2-expressing GnRH neurons activated continuously for 1 min (blue bar) at 5, 10, and 30 Hz. The expanded traces shown below the 30-Hz stimulation shows the tailing off of spike fidelity over the course of the 1-min stimulation period. (D) Histogram showing the mean + SEM evoked spike fidelities at the different continuous 1-min stimulation frequencies. Different letter superscripts indicate significant differences between the 1-min stimulations in spike fidelity (P < 0.05; parametric one-way ANOVA with post hoc Tukey’s multiple comparisons test). Numbers at base of histograms indicate number of GnRH neurons.
In the second stimulation paradigm, GnRH neurons were tested for their ability to follow 5-ms light pulses given at 5, 10, and 30 Hz of stimulation for a continuous 1-min period (Fig. 2C). Prolonged 5-Hz (n = 12, four mice) and 10-Hz (n = 6, four mice) stimulation evoked action potentials with a 100% and 92 ± 6% spike fidelity (Fig. 2 C and D). However, GnRH neurons were not able to faithfully follow prolonged 30 Hz stimulation and exhibited an overall spike fidelity of only 50 ± 3% (n = 6, three mice; P < 0.001 compared with 5 and 10 Hz). Although GnRH neurons were able to follow the 30-Hz stimulation for the first few seconds, the action potential fidelity progressively decreased over the remaining minute of activation (Fig. 2C; 30-Hz expanded traces).
In the third stimulation paradigm, we sought to entrain silent GnRH neurons to exhibit their endogenous burst firing pattern as identified recently in vivo (10). This involved giving 5-ms light pulses at 1.9 Hz for 10 s followed by a silent period of 15 s and then 1.9 Hz for 10 s and so on for 5 min. GnRH neurons were able to faithfully follow this stimulation pattern with a 100% spike fidelity (n = 9 cells, three mice). (Fig. S3).
Profile of Pulsatile LH Secretion in Adult Female Mice.
To assess the characteristics of pulsatile LH secretion in our colony of C57BL/6 mice, we used a tail blood sampling methodology (14) to take 3-min blood samples over a 2-h interval from ovariectomized (OVX) mice (n = 7). This revealed high-frequency (6.5 ± 0.6 pulses/120 min), high-amplitude (2.87 ± 0.26 ng/L) pulsatile LH secretion with pulses (n = 40) having a mean duration of 12.1 ± 0.4 min (Fig. 3). The sensitivity of the LH ELISA was 0.002 ng/mL with intra- and interassay coefficients of variation of 5 and 9%, respectively.
Fig. 3.
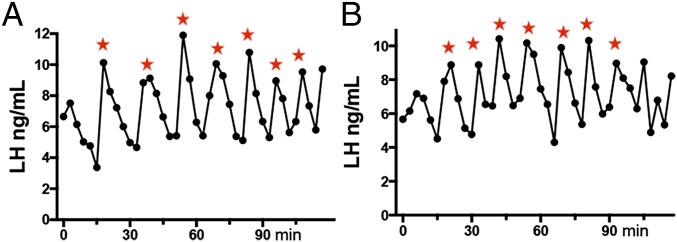
Endogenous pulsatile LH secretion in OVX mice. (A and B) Representative examples of pulsatile LH secretion measured in two ovariectomized female C57B6 mice. Stars indicate LH pulses identified by the DynPeak algorithim.
Optogenetic Activation of Pulsatile LH Secretion in Vivo.
Having established the profile of pulsatile LH secretion in OVX mice, and characterized blue-light activation of ChR2-expressing GnRH neurons in vitro, we next examined whether we could activate hypophysiotropic GnRH neurons to generate LH pulses in vivo. As the duration of an endogenous LH pulse was ∼12 min and the earlier study in OVX sheep had indicated that the GnRH pulse plateau was ∼5 min (8), we initially decided to examine the effects of different stimulation frequencies (1, 5, 10, 30, Hz) applied continuously for a 5-min period. As prior studies had suggested that the synchronization of burst firing in GnRH neurons may underlie pulsatile LH secretion (11, 12), we also used a burst-firing stimulation protocol in which the burst parameters of GnRH neurons previously recorded from the mouse in vivo (1.9 Hz for 10 s on, 15 s off) (10) were applied to rPOA GnRH neurons over a 5-min period. Prior studies in the laboratory have found that endogenous LH pulsatility is blocked in the isoflurane-anesthetized mouse.
Effects of different frequencies and burst firing on GnRH neuron cell bodies.
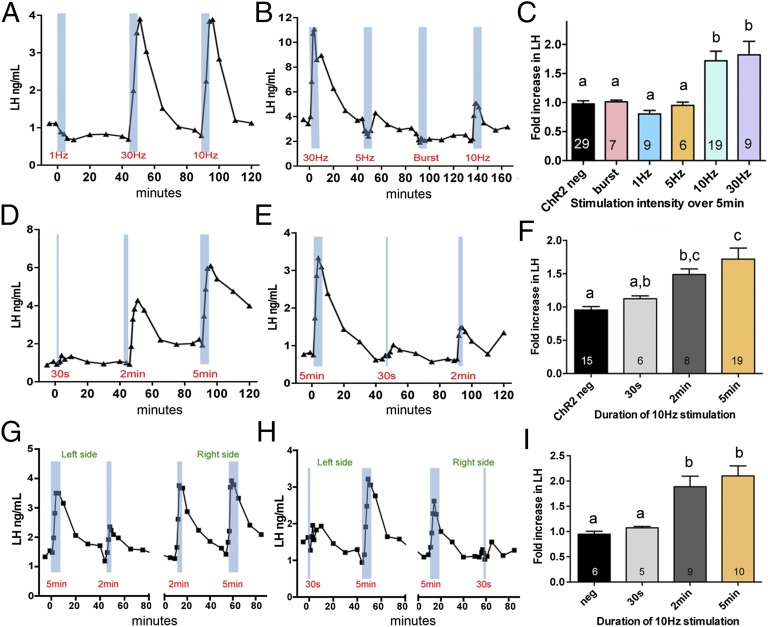
Anesthetized, OVX, AAV-injected GnRH-Cre mice were implanted with a 100-μm-diameter fiber optic probe in the rPOA, and the effects on LH secretion of 5-min continuous stimulations at different frequencies were tested. The different stimulations (three or four per mouse) were applied in a random order and randomly distributed so that no mice received the same stimulation twice. In controls (AAV-injected GnRH-Cre negative and sham-injected GnRH-Cre mice) undertaken alongside experimental animals, fiber optic activation in the rPOA had no effect on LH secretion with the fold change being 0.97 ± 0.05 (n = 24) (Fig. 4C). In AAV-injected GnRH-Cre mice, continuous stimulation for 5 min at 1 Hz (n = 10) and at 5 Hz (n = 6) did not have any significant effect on LH concentration (Fig. 4 A and B); the fold changes were 0.80 ± 0.06 and 0.95 ± 0.05, respectively, and were not significantly different from control mice (Fig. 4C). In contrast, continuous stimulation at 10 Hz (n = 19) generated a significant (P < 0.001) pulse-like increase in LH secretion (fold increase of 1.71 ± 0.16) as did stimulation at 30 Hz (fold increase of 1.82 ± 0.23, P < 0.001) (Fig. 4 A and B). No differences were detected in the LH response between 10 and 30 Hz. Evoked increments in LH consisted of a rapid, sharp increase in LH and a slower decrease taking up to 30 min to return to baseline (Fig. 4 A and B). The intermittent burst-firing stimulation pattern (n = 7) had no significant effect on LH secretion (fold change of 1.01 ± 0.02; Fig. 4 B and C). Note that the baseline levels of LH in anesthetized mice are lower than those of awake OVX females (Fig. 3).
Fig. 4.
Effects of optogenetic activation of GnRH neurons on LH secretion in vivo. (A–C) Representative examples of evoked LH secretion using different frequency blue-light stimulations of GnRH neuron cell bodies given continuously for 5 min (blue bars) in two AAV-ChR2-GnRH-Cre ovariectomized mice. (C) Histogram showing mean + SEM fold changes in LH secretion in response to different patterns and frequencies of blue-light activation of rPOA GnRH neurons. Numbers of mice stimulated with each frequency are given at the base of each histogram. (D–F) Effects of different durations of 10Hz optogenetic activation of GnRH neuron cell bodies. (D and E) Representative examples of evoked LH secretion using 10-Hz blue-light stimulations (blue bars) continuously for 30 s and 2 and 5 min in two AAV-ChR2-GnRH-Cre ovariectomized mice. (F) Histogram showing mean + SEM fold changes in LH secretion in response to different durations. Numbers of mice stimulated with each frequency are given at the base of each histogram. (G–I) Effects of different durations of 10-Hz optogenetic activation of GnRH neuron projections in the median eminence. (G and H) Representative examples of evoked LH secretion using 10-Hz blue-light stimulations (blue bars) applied continuously for 30 s and 2 and 5 min within the ME region in two AAV-ChR2-GnRH-Cre ovariectomized mice. In these experiments, both sides of the ME were stimulated in each mouse. (I) Histogram showing mean + SEM fold changes in LH secretion in response to different durations. Bars with different letter superscripts are significantly different from each other (P < 0.05; one-way ANOVA with post hoc Tukey’s multiple comparisons test). Number of stimulations with each frequency are given at the base of each histogram.
Following optogenetic stimulations, each mouse was perfused with paraformaldehyde, and the neuroanatomical relationship of the fiber optic probe to GnRH neurons was ascertained. Mice were excluded from analysis if ChR2-expressing GnRH neurons were absent or the fiber optic probe not located in the rPOA. A strong correlation (R = 0.6147, P < 0.0001) was found between the number of ChR2-expressing GnRH neurons located in proximity to the fiber optic probe and the fold-increase in LH secretion for mice receiving 10 Hz of activation (Fig. S4). The number of ChR2-expressing-GnRH neurons was determined by counting all dual-labeled cells within the two 30-μm-thick coronal rPOA sections where the fiber optic probe was visible. As a 1:3 set of brain sections was processed for immunohistochemistry, we estimated the total number of ChR2-expressing GnRH neurons to be three times our cell counts. Whereas activation of 30 GnRH neurons was ineffective, a twofold increase in LH secretion was observed when >60 GnRH neurons were in close proximity to the fiber optic probe. Studies using a range of markers of neuronal activation, including cFos and phosphorylated cAMP-response element binding protein, were found to be ineffective at identifying optogenetically activated GnRH.
Effects of different durations of 10 Hz of activation on GnRH neuron cell bodies.
Next, the duration of GnRH neuron activation required to generate an LH pulse was evaluated by applying the 10-Hz activation continuously for 30 and 2 and 5 min. In control mice, fiber optic activation in the rPOA had no effect on LH secretion with the fold change being 0.95 ± 0.05 (n = 15) (Fig. 4 D and E). Whereas the 30-s stimulation (n = 6) was ineffective, generating a 1.12 ± 0.04-fold increase in LH (Fig. 4 D and E), the 2-min stimulation (n = 8) induced a significant (P = 0.04) 1.49 ± 0.08-fold increase that was not significantly different (P = 0.06) from the 5-min duration of activation (1.71 ± 0.16-fold increase; Fig. 4F).
Effects of different durations of 10-Hz activation on the GnRH neuron projections in the ME region.
Anesthetized, OVX, AAV-injected GnRH-Cre mice were implanted with a 100-μm-diameter optic fiber probe on each side of the ME, and the effects on LH secretion of continuous 10-Hz stimulations for 30 s and 2 or 5 min tested in exactly the same manner as performed for the cell bodies. Two different stimulation times were performed on each side of the ME in random order. In control mice, fiber optic activation in the ME region had no effect on LH secretion with the fold change being 0.93 ± 0.06 (n = 6) (Fig. 4I). In AAV-injected GnRH-Cre mice, stimulation for 30 s did not have any significant effect on LH concentration (Fig. 4 H and I); the fold change was 1.07 ± 0.03 (n = 5) and was not significantly different from control mice (Fig. 4I). In contrast, stimulation for 2 min (n = 9) generated a significant (P < 0.005) pulse-like increase in LH secretion (fold increase of 1.88 ± 0.2) as did stimulation for 5 min (fold increase of 2.10 ± 0.19, P < 0.001) (Fig. 4 G–I). No differences were detected in the LH response between 2 and 5 min of stimulation.
Comparison of evoked pulse-like increments in LH secretion and endogenous LH pulses.
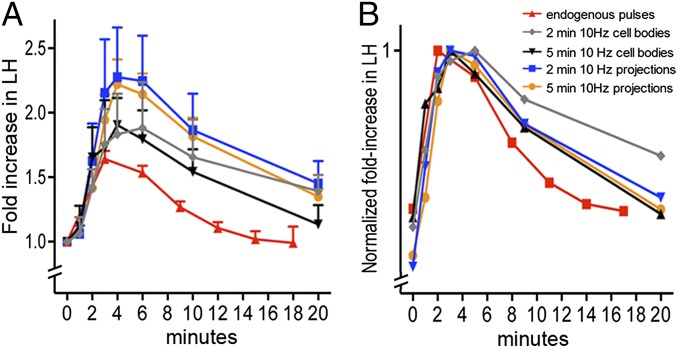
To evaluate more closely the dynamics of LH secretion evoked by optogenetic activation and compare these dynamics with endogenous LH pulses, the mean fold increase and temporal profile of endogenous LH pulses (n = 40) was compared with that evoked by a continuous 10-Hz stimulation for 2 min (cell-body stimulations, n = 8; projection stimulations, n = 9) and 5 min (cell-body stimulations, n = 19; projection stimulations, n = 10) (Fig. 5A). Profiles were also generated in which values were normalized to the peak LH concentrations (Fig. 5B). Whereas blood samples were taken every minute over the first 4 min of optogenetic activation, endogenous pulses were sampled every 3 min. The rising phases of LH secretion in endogenous and evoked pulses were found to overlap with similar peak amplitude values between all five groups although there was a tendency for GnRH neuron ME projection activations to generate higher peak levels (Fig. 5A). The decline in LH levels was sharper in endogenous pulses than in optogenetic activations (Fig. 5 A and B) although, in this case, it is important to note that blood samples were only taken at 6, 10, and 20 min postactivation compared with the regular 3-min sampling for endogenous pulses. Also, the longer temporal dynamic of evoked pulses may be an artifact of taking tail blood samples from an immobilized mouse.
Fig. 5.
Comparison of optogenetic evoked pulse-like increments in LH secretion and endogenous LH pulses. (A) Dynamics of continuous 10-Hz 2- and 5-min evoked LH increments by stimulating the cell bodies (gray, black lines) or the distal projections (yellow, blue lines) of GnRH neurons compared with endogenous LH pulses (red) overlaid upon one another. Mean + SEM values are shown. (B) Dynamics of evoked and endogenous LH pulses after normalizing values to the peak LH responses for each response.
Discussion
We have generated here a mouse model in which GnRH neurons projecting to the median eminence can be activated remotely with high temporal and spatial precision in vivo. In agreement with prior studies in rodents (15), we find that ∼50% of GnRH neurons are hypophysiotropic. Although it remains possible that expression of ChR2 in GnRH neurons may have some intrinsic action, it appears to have no untoward effects on the neurons’ spontaneous or kisspeptin-evoked electrical activity and allows them to be activated with high spike fidelity at frequencies up to 40 Hz for short periods of stimulation and up to 10 Hz for longer-minute intervals of activation. In vivo, we find that optogenetic activation of GnRH neurons at 10 Hz for 2 min are the minimal parameters required to evoke a pulse-like increment in LH. This stimulation protocol is equally effective at the GnRH neuron cell bodies/proximal dendrites and their terminal projections within the ME. Surprisingly, synchronizing GnRH neurons to fire together in their normal bursting pattern was ineffective in generating a pulse of LH.
Whereas 1 and 5 Hz activation of GnRH neurons continuously for 5 min had no effects on LH secretion, 10- and 30-Hz stimulations generated similar pulse-like increases in LH. As suggested by our in vitro studies, the similarity in LH profiles following 10 and 30 Hz likely results from the inability of individual GnRH neurons to follow prolonged optogenetic activations at 30 Hz with high fidelity. The failure of 1 and 5 Hz to stimulate significant LH release is compatible with the general concept that neuropeptide release requires relatively high-frequency activation of presynaptic terminals (16, 17). Electrical stimulation frequencies <10 Hz are also known to be ineffective at releasing GnRH from nerve terminals in mediobasal hypothalamic brain explants in vitro (18). Just how fast GnRH neurons normally fire is not well established. In the brain slice, adult GnRH neurons typically fire at around 1–2 Hz but can exhibit firing frequencies of up to 10 Hz within bursts, although these typically last only a few seconds (19–21). In vivo, mean firing rates of GnRH neurons are similarly low around 1 Hz (10). Thus, although GnRH neurons appear to be relatively slowly firing neurons, it appears that they must achieve ∼10-Hz frequencies for minute periods of time to evoke significant GnRH secretion that initiates LH release.
Much attention has focused upon understanding the genesis of burst firing in GnRH neurons (20, 22–25). Although the physiological roles of burst firing are unknown, it has been speculated that, by analogy to magnocellular neurons (26), this behavior may be related to GnRH/LH pulse generation (7, 9). This possibility has been supported by studies in embryonic cultures where the intermittent synchronization of calcium transients in GnRH neurons occurs on a time interval compatible with pulsatile LH secretion in the adult monkey and mouse (11, 12). We have addressed here directly whether synchronizing GnRH neurons to fire in a burst pattern can generate an LH pulse. To ensure that the burst firing was relevant, we activated GnRH neurons for 5 min in precisely the same manner as they are found to exhibit burst firing in adult mice in vivo (10). Against our expectations, this pattern of activation had no effect at all on LH secretion. One reason for this failure may be that more GnRH neurons (>60) need to be recruited or that they need to be activated for a longer period (>5 min). Nevertheless, the exact same subpopulation of GnRH neurons that fail to generate an LH pulse after synchronized burst activation are able to do so when stimulated to fire continuously at 10 Hz. This observation suggests that burst firing itself may not be required for pulse generation but, rather, may represent a default “idling” pattern of activity for GnRH neurons (7). It is also noteworthy that these parameters are very different from that reported for the magnocellular oxytocin neurons that do indeed use short-burst firing to evoke direct pulses of oxytocin into the bloodstream (26). We also note that one significant caveat of the present study is that investigations have been undertaken in anesthetized mice and that it will be of interest in the future to see if the same results are recapitulated in awake animals.
Recently, GnRH neuron cell filling coupled with electrophysiology demonstrated that GnRH neuron projections to the median eminence receive synaptic inputs along their complete length while also propagating action potentials (27). This “dendronic” morphology was compatible with prior evidence indicating that the GnRH fiber elements within and around the ME may be able to support episodic GnRH secretion (for review see ref. 28). Although it is not possible with a 100-μm-diameter fiber optic probe to distinguish between activation of GnRH neuron distal dendrons and axon terminals in the ME, we show here that stimulation of these distal projections is very effective in generating pulse-like increments on LH secretion.
Determining the number of GnRH neurons required to generate an LH pulse is a key question in the field. Unfortunately, we have not been able to determine the exact numbers of GnRH neurons activated in these studies using typical markers of cell activation. However, as blue light is quite inefficient at penetrating brain tissue, with a 90% loss in power density 0.5 mm from the fiber optic probe (29), we resorted to counting all ChR2-expressing GnRH neurons in preoptic sections surrounding the fiber optic probe. This suggests that ∼60 GnRH neurons need to be activated to evoke a twofold increase in LH secretion, although we recognize that this is only an approximation. It is noteworthy, nevertheless, that prior studies (30, 31) have indicated that relatively few GnRH neurons may be necessary for pulsatile LH secretion.
In summary, we report that GnRH neurons need only fire at ∼10 Hz for ∼2 min to generate a pulse-like increment in LH secretion and that this can be achieved by activating the GnRH neuron cell bodies or distal processes around the ME. Under these conditions, the dynamics of optogenetically evoked LH secretion are very similar to that of endogenous LH pulses with the rise time and increment in LH being identical. This suggests that the minimal parameters of GnRH neuron activation reported here are likely to be close to that occurring in vivo for pulsatile LH secretion. The present study provides the first major insight into the nature of GnRH neuron activation required to generate pulsatile LH secretion and provides the foundations for understanding pulsatility within the reproductive axis.
Materials and Methods
Animals.
Adult GnRH-Cre and wild-type C57BL/6 mice (6–12 wk old) were housed in a 12-h light/12-h dark cycle (lights on at 0600 hours and off at 1800 hours) with food and water available ad libitum. Where indicated, mice were bilaterally OVX and used for experiments >1 wk later. All procedures were approved by the University of Otago Animal Ethics Committee.
Stereotaxic Injections of AAV.
Adult mice were anesthetized with isoflurane, placed in a stereotaxic apparatus, and given simultaneous bilateral 1-μL injections of AAV9-EF1-dflox-hChR2-(H134R)-mCherry-WPRE-hGH (4.35 × 1013GC/mL; Penn Vector Core) into the ME at a rate of 100 nL/min. The syringes were left in situ 3 min before and 10 min after the injections. Coordinates according to the Paxinos mouse atlas (32) were 1.1 mm antero-posterior, 0.3 mm lateral to midline, and 6.0 mm in depth. Animals were used 3 wk later for experiments.
Brain Slice Electrophysiology.
Acute 250-μm-thick coronal brain slices containing the rPOA were prepared between 0900 and 1200 hours as reported previously (20). The 95%O2/5%CO2 equilibrated artificial cerebrospinal fluid (aCSF) contained the following (in mM): NaCl, 120; KCl, 3; NaHCO3, 26; NaH2PO4, 1; CaCl2,2.5; MgCl2, 1.2; and glucose, 10. Cell-attached, loose seal (10–30 MΩ) recordings were made from mCherry-fluorescent GnRH neurons viewed with a fixed-stage upright microscope (BX51WI, Olympus) and either fluorescence illumination, using the reflected light fluorescence illuminator (BX-RFA, Olympus) and filter (U-MWIG3, BA510-550, Olympus) or Nomarski differential interference contrast optics. Action currents were recorded using glass pipettes (OD: 1.5 mm; ID: 1.17; tip resistance 3–4 MΩ) filled with aCSF including 10 mM Hepes. Holding currents were set at 0 pA. Recordings of silent neurons were excluded unless firing could be evoked by 20 μM AMPA. Blue light (473 nm) was delivered using a Grass S88X Stimulator-controlled DPSS laser (Ike-Cool) coupled to a 100-μm fiber optic probe. Laser intensity at the tip of the glass pipette surrounding the fiber optic probe was 5 mW. Three different stimulation protocols were used: the first was a 1-s stimulation every 10 s repeated six times over 1 min. During the 1-s stimulation, 5-ms pulses were delivered at 5, 10, 20, 30, or 40 Hz. In the second stimulation protocol, 1 min of continuous 5-ms pulses were applied at 5, 10, or 30 Hz. The third was the burst pattern consisting of 5-ms light pulses delivered at 1.9 Hz for 10 s followed by 15 s off, repeated 12 times over 5 min. Analysis was undertaken by examining the percentage of blue-light activations that evoked an action current.
LH Pulse Profiling in OVX Mice.
Wild-type C57BL/6 adult female mice were bilaterally ovariectomized. After a 1-wk recovery period, mice were handled daily for 20 d before undertaking 3-min blood sampling (3 μL/sample) over 2 h using a recently developed tail-tip blood collection procedure (14). Blood samples for this experiment and for optogenetic stimulation experiments were analyzed for LH content by ELISA also, as reported recently (14). Pulses were detected using the DynPeak algorithm (33).
Immunohistochemistry.
Mice were killed by anesthetic overdose using sodium pentobarbital (3 mg in 100 μL) and were then transcardially perfused with 20 mL of 4% paraformaldehyde in 0.1 M phosphate buffer. Coronal brain sections were processed for free-floating GnRH immunofluorescence as reported previously (34) using a polyclonal guinea pig anti-GnRH antiserum (1:20,000; gift from Greg Anderson, University of Otago), biotinylated goat anti-guinea pig secondary immunoglobulins, and streptavidin Alexa488. Two sections at the level of the MS, rPOA, and AHA were analyzed in each mouse by counting the total numbers of single- and dual-labeled mCherry-expressing cells and GnRH-immunoreactive neurons, and mean values were combined to generate mean ± SEM values for each brain area. Sections containing the ME were also analyzed to verify the injection sites.
Optogenetic Activation in Vivo.
AAV-injected GnRH-Cre mice were bilaterally OVX and >2 wk later anesthetized with isoflurane and a 100-μm-diameter fiber optic probe connected to a laser implanted in the rPOA [coordinates: 1.0 anterior–posterior (AP), adjacent to the sagittal sinus, 4.2 depth] or on either side of the ME region (coordinates: 1.1 mm AP, 0.3 mm lateral to midline, and 6.0 mm in depth). Thirty minutes later, optical stimulation (5-ms pulses, same laser as used for in vitro experiments) protocols started, and serial 5-μL tail blood samples were taken. Ten blood samples were taken over each stimulation at −5, −1, 1, 2, 3, 4, 6, 10, 20, and 30 min, where time 0 is the start of the stimulation. Each mouse received three or four stimulation protocols given in random order for the rPOA stimulations. Each mouse received two stimulation protocols on each side of the ME given in random order for the ME region stimulations. Analysis was undertaken by calculating the area under the curve for stimulation (14 sample points) after subtraction of baseline (representing the average LH concentrations in the two samples before stimulation) and comparing the results between stimulation periods and the baseline. A ratio of stimulation over baseline was calculated to determine the fold increase in LH secretion.
Statistical Analysis.
Statistical analyses were carried out with GraphPad Prism 5 (GraphPad Software) using parametric one-way ANOVA (with post hoc Tukey’s multiple comparisons test). Differences were considered significant for P < 0.05. Values are given as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Rob Porteous and Katja Czieselsky for technical assistance and Brian Hyland, Richard Piet, and Karl Iremonger for comments on an earlier version of the manuscript. This work was supported by a University of Otago PhD Scholarship and the New Zealand Health Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415226112/-/DCSupplemental.
References
- 1.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology. 1982;111(5):1449–1455. doi: 10.1210/endo-111-5-1449. [DOI] [PubMed] [Google Scholar]
- 4.Levine JE, Duffy MT. Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology. 1988;122(5):2211–2221. doi: 10.1210/endo-122-5-2211. [DOI] [PubMed] [Google Scholar]
- 5.Caraty A, Locatelli A. Effect of time after castration on secretion of LHRH and LH in the ram. J Reprod Fertil. 1988;82(1):263–269. doi: 10.1530/jrf.0.0820263. [DOI] [PubMed] [Google Scholar]
- 6.Terasawa E, et al. Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology. 1988;123(4):1808–1816. doi: 10.1210/endo-123-4-1808. [DOI] [PubMed] [Google Scholar]
- 7.Herbison AE. Physiology of the adult GnRH neuronal network. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. Academic Press; San Diego: 2014. pp. 399–467. [Google Scholar]
- 8.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130(1):503–510. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- 9.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24(2):79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 10.Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013;33(22):9394–9401. doi: 10.1523/JNEUROSCI.0533-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantin S, Caraty A, Wray S, Duittoz AH. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology. 2009;150(7):3221–3227. doi: 10.1210/en.2008-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19(14):5898–5909. doi: 10.1523/JNEUROSCI.19-14-05898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothermel M, Brunert D, Zabawa C, Díaz-Quesada M, Wachowiak M. Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors. J Neurosci. 2013;33(38):15195–15206. doi: 10.1523/JNEUROSCI.1618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyn FJ, et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci. 1987;7(8):2312–2319. [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31(7):2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schöne C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Reports. 2014;7(3):697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer RG, Mansfield S, Yates JO. Discharge of gonadotrophin-releasing hormone from the mediobasal part of the hypothalamus: Effect of stimulation frequency and gonadal steroids. Exp Brain Res. 1980;39(4):453–460. doi: 10.1007/BF00239310. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149(7):3598–3604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30(18):6214–6224. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM. Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology. 2000;141(10):3731–3736. doi: 10.1210/endo.141.10.7690. [DOI] [PubMed] [Google Scholar]
- 22.Kuehl-Kovarik MC, et al. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22(6):2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: Possible implications for the central regulation of fertility. J Neurosci. 2006;26(46):11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasoni CL, Romanò N, Constantin S, Lee K, Herbison AE. Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2010;31(3):259–269. doi: 10.1016/j.yfrne.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29(34):10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng G, Brown D. The origins and significance of pulsatility in hormone secretion from the pituitary. J Neuroendocrinol. 1997;9(7):493–513. doi: 10.1046/j.1365-2826.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- 27.Herde MK, Iremonger KJ, Constantin S, Herbison AE. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33(31):12689–12697. doi: 10.1523/JNEUROSCI.0579-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iremonger KJ, Herbison AE. Multitasking in GnRH neuron dendrites. Neuroendocrinology 2014 doi: 10.1159/000368364. , in press. [DOI] [PubMed] [Google Scholar]
- 29.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 1988;48(1):45–52. doi: 10.1159/000124988. [DOI] [PubMed] [Google Scholar]
- 31.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2004. [Google Scholar]
- 33.Vidal A, Zhang Q, Médigue C, Fabre S, Clément F. DynPeak: An algorithm for pulse detection and frequency analysis in hormonal time series. PLoS ONE. 2012;7(7):e39001. doi: 10.1371/journal.pone.0039001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152(10):3832–3841. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.