Significance
The avian brain has numerous specializations for navigation and processing visual information, but relatively little is known about how flying birds control their position in space. To study the role of vision in controlling hovering flight, we developed a virtual reality environment where visual patterns could be displayed to a freely flying hummingbird. Normal flight could only be performed if the visual background was completely stationary. In contrast, any motion in the background image caused the birds to lose stability. In natural settings, visual motion is constantly produced when objects and observers move relative to each other. This research demonstrates that flying birds are surprisingly sensitive to movements in their visual field and direct flight to respond to those movements.
Keywords: avian flight, flight control, optic flow, visual guidance
Abstract
Relatively little is known about how sensory information is used for controlling flight in birds. A powerful method is to immerse an animal in a dynamic virtual reality environment to examine behavioral responses. Here, we investigated the role of vision during free-flight hovering in hummingbirds to determine how optic flow—image movement across the retina—is used to control body position. We filmed hummingbirds hovering in front of a projection screen with the prediction that projecting moving patterns would disrupt hovering stability but stationary patterns would allow the hummingbird to stabilize position. When hovering in the presence of moving gratings and spirals, hummingbirds lost positional stability and responded to the specific orientation of the moving visual stimulus. There was no loss of stability with stationary versions of the same stimulus patterns. When exposed to a single stimulus many times or to a weakened stimulus that combined a moving spiral with a stationary checkerboard, the response to looming motion declined. However, even minimal visual motion was sufficient to cause a loss of positional stability despite prominent stationary features. Collectively, these experiments demonstrate that hummingbirds control hovering position by stabilizing motions in their visual field. The high sensitivity and persistence of this disruptive response is surprising, given that the hummingbird brain is highly specialized for sensory processing and spatial mapping, providing other potential mechanisms for controlling position.
To precisely control their motion through the air, flying animals have evolved specialized sensory structures and associated neural architecture. Neural specializations provide hypotheses for what senses are most important to a given taxon, and although flight control has been studied extensively in insects (1), birds have until recently received limited attention. Birds have large regions of the brain dedicated to visual processing, suggesting parallels with insects, such as a leading role for optic flow in controlling flight paths (2, 3). It has recently been demonstrated that birds exhibit visually mediated position control much like bees (4, 5), even though they have complex spatial mapping in the hippocampal formation (6), and a much larger brain for interpreting visual input and dynamically integrating vision with proprioceptive and vestibular feedback (7–9).
In birds and mammals, the visual information from the eyes is divided into three separate pathways that each process a subset of motions or visual features (10). Two of these pathways, named the accessory optic system and tectofugal pathways in birds, each process a single type of motion: (i) self- or ego-motion, which is the motion produced when an observer moves relative to their environment; and (ii) object motion, when visual features move relative to the observer (2). Using the same retinal information, the visual system of a flying hummingbird must separate motions arising from the bird moving through foliage toward a flower from the motion caused by an approaching competitor or predator. During hovering, the hummingbird similarly must determine if visual motion is caused by positional instability, causing the observer to move relative to a stationary background feature, or by background motion independent of hovering stability. In natural settings, hummingbirds are able to precisely hover in place, even though natural settings are rarely devoid of visual motion in the background. Hummingbirds could hold a stable position using a variety of sensory information, including referencing stationary visual features in their environment. Here, we examine the role of vision in avian flight control by testing two predictions: hovering hummingbirds will (A) be destabilized by a moving visual background if the stimulus is sufficiently large, and (B) maintain stability with a disruptive visual background if stationary visual landmarks are present.
Results
The free-flight responses of Anna’s hummingbirds (Calypte anna) to different optic flow cues were studied in a circular chamber that included a projection screen with a feeder in the center, and a 3D tracking system to measure head position (Fig. 1A). When at the feeder, the visual stimulus occupied ∼180° horizontally and 102° vertically of the visual field. Feeding bouts were composed of two intermittent phases, docked feeding and undocked look-ups, during which the bird withdrew from the feeder and hovered in front of it. The two hovering phases are normally characterized by the bird holding the position of its head stable in space. We introduced motion with moving gratings for lateral or vertical visual flow and with rotating spiral patterns for constant looming (expanding) or receding (contracting) visual flow. We predicted that a hovering hummingbird, much like a standing pigeon (11), will respond to directional visual motion stimuli with a matched, direction-specific behavioral response.
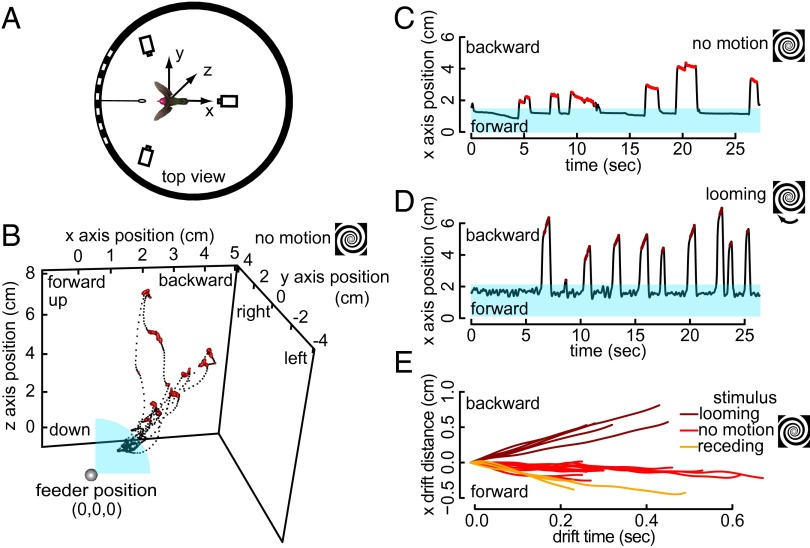
Fig. 1.
Anna’s hummingbirds respond to moving visual patterns during free-flight hovering. A back-projection screen allows images to be projected onto the wall of a cylindrical flight arena with a feeder at the center of the screen. A three-camera tracking system determines the head position of a freely flying hummingbird (A). The 3D traces of head position (B) are then separated into motion along three axes: forward-backward (x), left-right (y), and down-up (z). With a stationary background, the bird is stationary during docked feeding (blue region) and undocked look-ups (red) near the feeder (C), but a looming spiral pattern disrupts the bird’s stability during both hovering phases (D). By isolating the responses during the look-up portions (red and maroon in C and D, respectively) of the free-flight recording, we show that visual motion produces a matched destabilization response in an individual hummingbird (E).
When there was a nonmoving pattern on the screen, the head position was stable during both docked and undocked phases (Fig. 1 B and C). A looming stimulus elicited oscillations in head position during docked feeding, and elicited nonoscillatory backward drifts during look-up phases (Fig. 1D). Both responses are consistent with the prediction that looming produces backward avoidance response. Rotating the same spiral in the opposite direction causes receding visual motion. In this case, the feeder physically blocked forward motion during docked feeding, so we focus on the undocked look-ups for this experiment. We provide all of the raw traces of drifts during look-ups in the x axis for one representative bird in response to looming, receding, and stationary spirals (Fig. 1E).
In addition to looming and receding visual motion, we also present the flight responses of hummingbirds to left, right, down, and up motion, caused by vertical and horizontal linear gratings. Raw traces of responses to all motion types for one individual are provided in Figs. S1–S3. The complete set of look-up phases that were extracted from all eight individuals and all stimulus treatments is provided in Fig. 2 A–D. In all treatments without pattern motion, the magnitude of drifts during look-ups was relatively small, and exhibited a forward tendency. The average look-up response to treatments with pattern motion (Fig. 2 E and F) illustrates that hovering hummingbirds respond to large, moving visual patterns along all three linear axes, with a flight response that matches the stimulus motion.
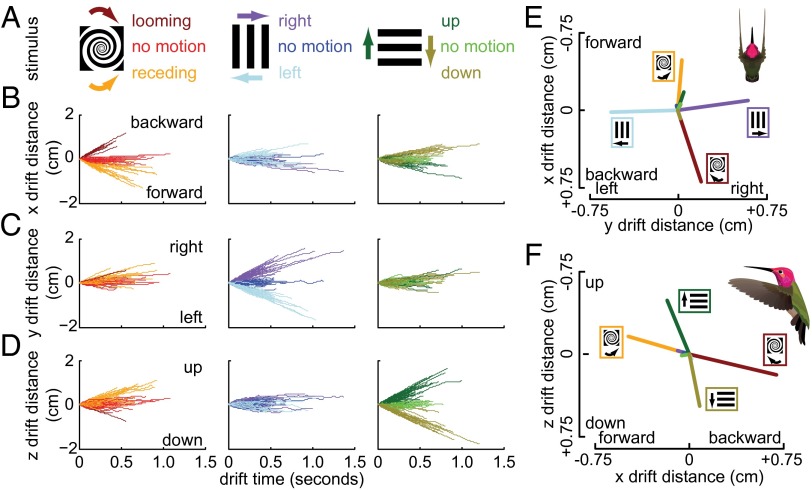
Fig. 2.
Rotating spirals and moving gratings caused hummingbirds to lose positional stability in the orientation of the motion but stationary patterns did not affect hovering. Two motion treatments and one no-motion treatment were conducted for each of three black-and-white patterns (A): spiral (red), vertical grating (blue), and horizontal grating (green). The look-up drifts for all hummingbirds (n = 8) are plotted by stimulus pattern with drift distance along the x, y, and z axes shown in rows B–D, respectively. Means of all drifts for a single stimulus treatment are shown in both a top view (E) and a side view (F) to illustrate the directional matching of response to stimulus motion. Almost no directional drifting occurs with no-motion treatments.
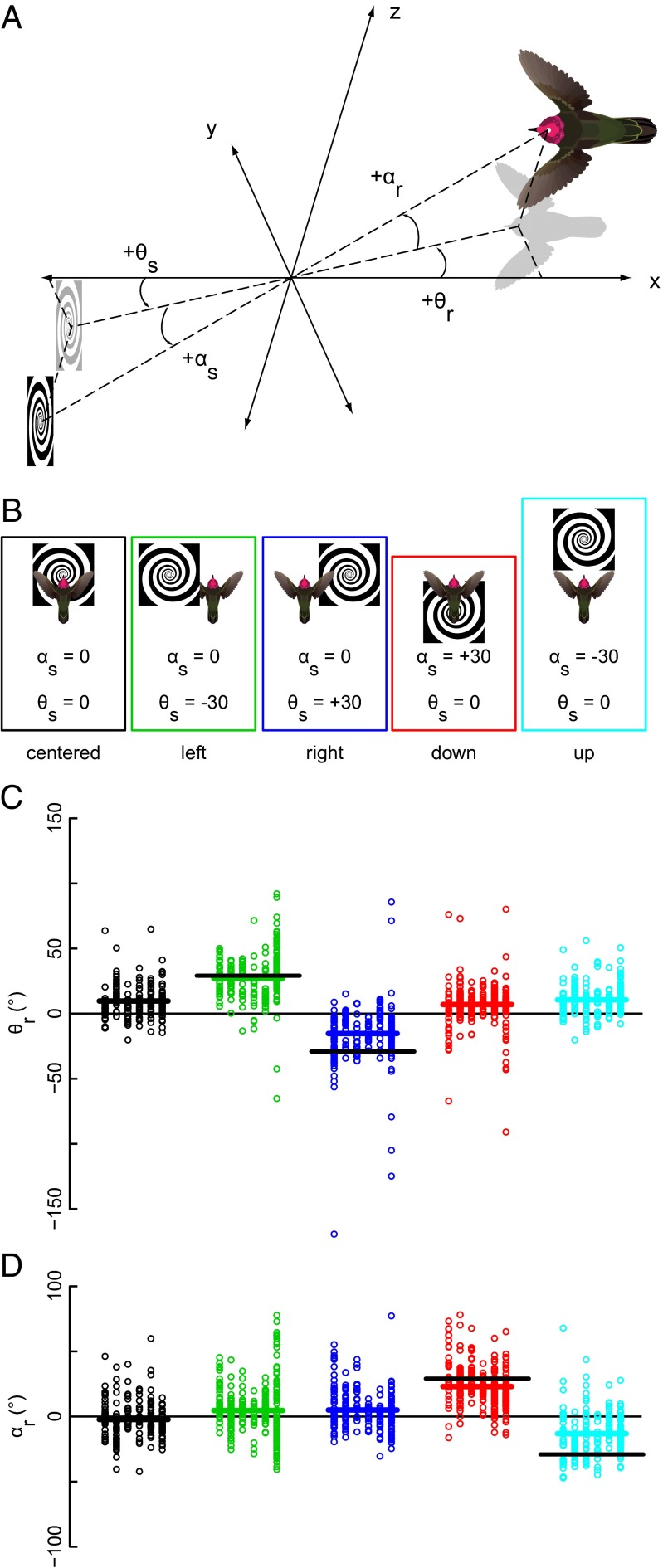
As the hummingbirds attempt to stabilize disruptive visual motion, how precisely do they match specific properties of the stimulus? To address this question, we performed the next experiment with just looming spirals because the flight response can be observed during both feeding and look-up phases. The center of the spiral was shifted left, right, up, and down with respect to the feeder to determine if the angular orientation of the flight response was coupled to the center of expansion in the looming stimulus (Fig. 3 A and B). Shifts in pattern position to the left and right caused matched shifts in the azimuth (θ) angle of flight drifts and no change in the elevation (α) angle (Fig. 3C) (F4, 1,197 = 170.7, P < 0.0001). Moving the looming spiral up and down caused matched shifts in elevation, but not azimuth (Fig. 3D) (F4, 1,197 = 213.4, P < 0.0001). The results of the first two experiments demonstrate that hummingbirds respond to visual motion with a high level of directional sensitivity.
Fig. 3.
Hummingbirds shift the orientation of their backward flight to match offsets in the position of the looming spiral. The angular positions of the stimulus (s) and response (r) were defined using two mirrored spherical coordinate systems (A). We moved the center of the spiral by 30° in either the horizontal (azimuthal angle, θs) or vertical (elevation angle, αs) plane (B). Left and right spiral positions elicited matched changes in backward flight in the horizontal plane (θr) and no change in the vertical plane (αr) (C and D). The opposite is true for spiral center offsets above or below the center. The thick black lines in the offset conditions indicate the magnitude of a perfectly matched response at 30°. Each column of points within the treatments represents the backward drifts for a single individual with the overall mean shown by the horizontal bar. Individual identifications in order from left to right are 22–27.
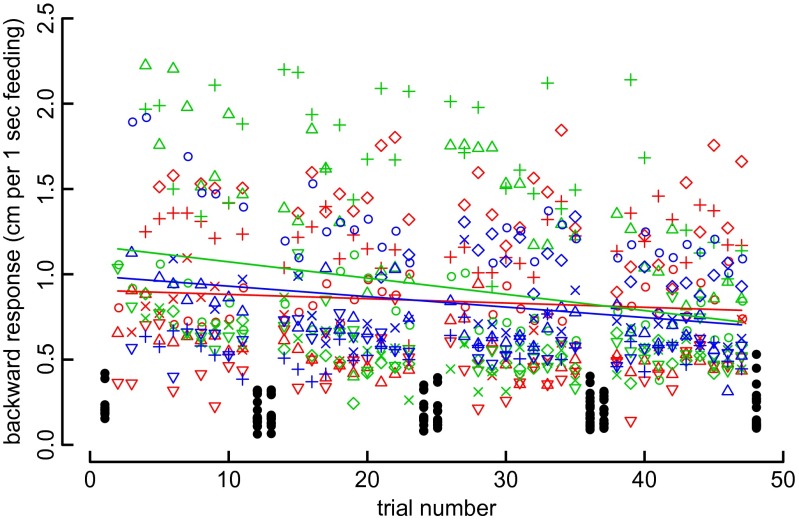
The hummingbirds’ sensitivity to looming background motion is sufficient to disrupt feeding because they fly backward far enough to lose contact with the feeder and completely undock. Does repeated exposure to a disruptive visual motion change or decrease the hummingbird flight response? We addressed this question by presenting a looming spiral to feeding hummingbirds in 40 sequential trials, spaced 15-min apart and spread over 2 days. An unbiased and relevant measurement is the backward response, defined as the total distance traveled in the backward direction, normalized for different feeding durations. Because spiral rotation frequency affects the perceived time to collision (τ), we tested the spiral rotating at several speeds. A pilot study with different individuals indicated that hummingbirds exhibited a strong response at 0.5 Hz (τ = 1.9 s), so we also tested equally spaced slow and fast rotation frequencies of 0.1 Hz (τ = 9.3 s) and 0.9 Hz (τ = 1.0 s), respectively. We used 18 unique birds for this experiment, with 6 individual birds per rotation-frequency treatment. Rotation frequency did not significantly affect the backward response (P = 0.659), but repeated exposure did cause a significant decline in the backward response over time (P = 0.020). However, the looming spirals were significantly disruptive relative to controls over the entire 2 days of exposure (P < 0.0001) (Fig. 4).
Fig. 4.
Looming spiral patterns with different rotation frequencies produce a similar and strong backward avoidance response for 40 consecutive trials over 2 days. Hummingbirds oscillate in the forward-backward axis during feeding in front a looming spiral, from which we calculate the total distance traveled backward normalized to 1 s of flight time. Blocks of 10 experimental trials were bracketed by a stable spiral control (solid black). There were three rotation frequency treatments (red = 0.1 Hz, green = 0.5 Hz, blue = 0.9 Hz), with six individuals per treatment (n = 18, symbols).
We have demonstrated that hovering hummingbirds consistently lose position in response to moving visual patterns when the stimulus covers the front half of their visual field (prediction A). In natural visual landscapes, however, hummingbirds can hover in place presumably because they do not encounter large moving patterns. Therefore, our second prediction is that hummingbirds will maintain stable position—even in the presence of disruptive visual motion—if there are prominent stationary features in their visual field. We tested this prediction by shrinking the visual stimulus to 120° horizontally and 100° vertically in the visual field and combining looming visual motion from the spiral with a prominent stationary pattern, a black and white checkerboard. Because a moving pattern may be more disruptive in specific regions of the visual field, we tested two configurations: (i) the spiral obscuring the center of the checkerboard, and (ii) the checkerboard obscuring the center of the spiral. For each configuration, the backward response was measured over a range of relative spiral and checkerboard sizes.
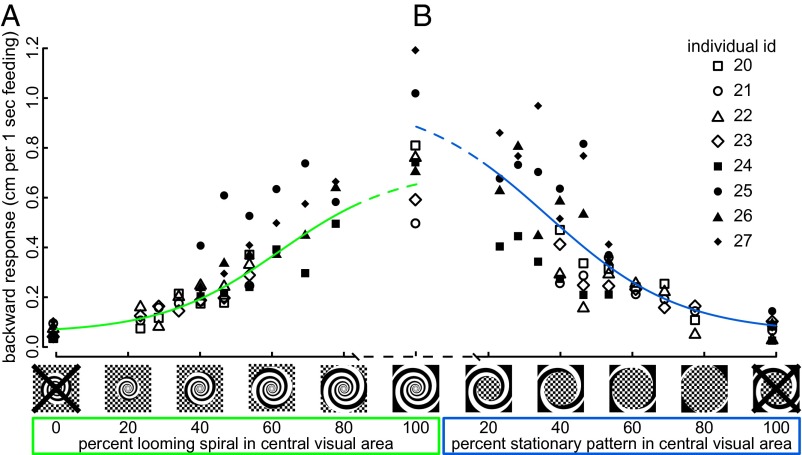
When the looming spiral obscured the center of a stationary checkerboard, the backward response increased monotonically with the increase in looming area (Fig. 5A). Surprisingly, even though hummingbirds respond to the position of the center of a spiral (Fig. 3), the same backward response trend was observed when the checkerboard obscured the center of the spiral (Fig. 5B). We conclude that opposite to our second prediction, hummingbirds respond to even small regions of looming spiral in the presence of stationary features. The relationship between the backward response and the relative amount of looming motion was analyzed using a nonlinear mixed model that included trial order. From the docked portion of the experiment described in Figs. 1 and 2, we know that the average backward response to a looming spiral entirely covering 180° of the horizontal visual field is 0.79 cm for every second of feeding. The model based on an experiment with a stimulus that only covers 120° of the visual field still predicts this saturated response (Fig. 5).
Fig. 5.
Even with a prominent stationary pattern, a looming spiral produces the characteristic avoidance response, which increases with an increased proportion of looming spiral in the projected image. The backward responses to looming motion, when it is centrally embedded in a checkerboard background, are on the left (A, green). Responses to a stationary image, a checkerboard in the middle of a looming spiral, are on the right (B, blue). The dashed portions of the green and blue lines are the fitted sigmoidal model projection for images with >80% looming spiral pattern and 100% spiral response is shown for reference using data from previous experiments (with a larger projected image). The two controls (0% in A and 100% in B) were performed with an image that included a stationary checkerboard and a nonrotating spiral. Symbols indicate individual birds.
Discussion
Collectively, these results indicate that hovering hummingbirds are highly sensitive to the direction (Figs. 1 and 2) and orientation (Fig. 3) of visual pattern motion when presented with large coherent stimuli (prediction A). Behavioral responses to optic flow, called optomotor responses, are common across animal taxa, and we demonstrate that hovering hummingbirds will respond in all directions by changing their body position. We further demonstrate that the response to moving patterns is maintained after repeated exposure to a single stimulus (Fig. 4) and also when the motion stimulus is weakened by decreasing its size and adding prominent stationary features (Fig. 5). Hummingbird sensitivity to even minimal motion in the background was unexpected (prediction B), given their ability to precisely hover in place in complex and dynamic natural environments.
Stationary objects are salient features for guiding flight. Tethered flies in a closed loop virtual environment will “steer” to fixate a bar in front of them (12). Stabilizing a stationary feature in the fovea or another portion of the visual field also offers a potential mechanism for holding station during hovering. Our flight arena was not fully immersive and included multiple stationary features, including a projected image with distinct edges in the lateral field, an open ceiling with cameras, a perch, and a single feeder. During docked feeding, the clear plastic feeder (filled with clear liquid) could subtend an angle up to 26°, although this feature occupies much less of the visual field during look-ups. The experiment that combined visual motion with the stable checkerboard was designed to further emphasize stationary features. Despite the presence of numerous stationary features—intentional and otherwise—and sharp reduction of the moving stimulus, visual motion was consistently disruptive. This finding suggests that, unlike in other studies where visually guided behavior was similar with grating and natural scene manipulations (13), the spiral produces a stronger signal than would naturally occur during hovering flight.
The brains of hovering birds exhibit specialization for processing unidirectional visual motion. The accessory optic system of birds encodes optic flow produced when an observer moves relative to their environment. Its neurons have wide receptive fields and are directionally biased (14). One of the key accessory optic system nuclei is the lentiformis mesencephali, and it is hypertrophied in all birds capable of hovering, including transient hovering (15). Hummingbirds have the largest lentiformis mesencephali relative to all other birds, which led Iwaniuk and Wylie to propose that directionally selective responses are a key adaptation for controlling hovering flight (15). Our behavioral experiments lend further support to this hypothesis, and hypertrophy of the lentiformis mesencephali also offers a potential mechanism for the heightened motion sensitivity we observed.
The use of directionally selective neurons in hovering position control represents a subset of the neural mechanisms required for the flight repertoire of hummingbirds. Visual processing and integration with other sensory information has not been studied during flight in a hummingbird or in any other bird. However, evidence from fictive flight preparations of other birds and insects indicates that they can change how senses are integrated and that different neuron populations are emphasized in different behaviors. An example of variable sensory integration comes from restrained pigeons that exhibit different head stabilization reflexes and tail responses to visual and vestibular perturbations during simulated flight than during resting conditions (16, 17). At the level of sensory neurons, cells of the avian accessory optic system are divided into populations that differ in maximum sensitivity to either fast or slow motion (14, 18, 19). The functional roles and the relative abundance and distribution of fast and slow cells have not been described in any bird. This finding is in contrast to the directionally selective visual neurons of insects, which have been studied in several species and behavioral contexts (1, 20). A comparative study with 10 species of insects revealed that the lobula plate neurons of hovering insects are maximally sensitive to low temporal frequencies of sine wave gratings, whereas the equivalent cells in fast-forward flying insects are maximally sensitive to high speeds (21). Combining free-flight experiments that manipulate sensory information with neuroscience approaches to understand the underlying cell populations, and their responses represents an exciting new direction for avian research.
Development of virtual reality approaches with both tethered preparations and in free-flight arenas has been essential for decades of research on the visual guidance of insect flight (1, 20, 22). Similar to the hummingbirds, hovering insects have previously been shown to exhibit sensitivity to the direction (23, 24) and orientation (25) of visual motion during flight behavior. Our study joins a handful of recent avian studies that highlight the convergence between visual guidance strategies for flight in insects and birds (4, 5, 26, 27) and also suggest potential similarities in neural specialization to match flight styles. The ability to study the visual motion detection in flying hummingbirds now provides an opportunity to examine how large populations of visual neurons are used to guide behavior. We suggest that future research on the properties of the additional cells in the hummingbird accessory optic system may yield novel insight into the evolution of flight in birds.
Materials and Methods
Animal Model.
All experimental subjects were male Anna’s hummingbirds, Calypte anna, that were caught on the campuses of either the University of California, Riverside (individuals numbered 1–15, caught March 5 to June 15, 2011) or the University of British Columbia (individuals numbered 16–27, caught October 11, 2011 to October 29, 2013). Hummingbirds were individually housed in 0.61 × 0.61 × 0.91-m cages and fed ad libitum sugar [15 g/100 ml (wt/vol)] or Nektar-Plus [Nekton, 13 g/100 ml (wt/vol)] solution. Individuals were allowed to acclimate to captivity for 3 days and were then trained in the experimental chamber with a feeding schedule. The feeder, filled with sugar solution, was closed to prevent feeding between experimental trials and opened at intervals of either 15 or 20 min, depending on the experiment. When the feeder was opened, birds were allowed to feed until they departed. Restricting food between feeding was important to increase the time birds spent at the feeder. All experiments were performed with approval of either the University of California, Riverside Institutional Animal Care and Use Committee or the University of British Columbia Animal Care Committee.
Experimental Rig.
The experimental chamber was a large clear acrylic cylinder (Fig. 1A) (0.70-m diameter, 0.61-m height). A 0.91-m section of the wall (41.4% of circumference) was covered on the outside by a frosted window coating (wallpaperforwindows.com) allowing back projection onto the cylinder. We used two different Liquid Crystal on Silicon (LCoS) projectors for these experiments: a Canon REALiS SX80 Mark II projector (3,000-lm lamp, 1400 × 1050 SXGA, 60 Hz) was used for the three different experiments with mixed stimuli, and an Aaxa Technologies P2 pico projector (33-lm LED lamp, 800 × 600 SVGA, 60 Hz) was used for the 2-day response-change experiment with a single stimulus. Visual stimuli were generated and controlled using custom scripts in VisionEgg (28).
At the center of the projection screen was a small hole where a clear plastic feeder was attached so that it extended 0.175 m into the chamber. The ceiling of the chamber was nylon mesh with holes for the lenses (Computar H2Z0414C-MP) of up to three cameras (Prosilica GE680; Allied Vision Technologies). These three cameras were used to autotrack a painted white spot on the top of the bird’s head using Flydra 3D tracking software (29). Filming was conducted at 100 frames per second for a three-camera setup (mixed stimuli) and 132 frames per second with only a single camera (single, repeated spiral stimulus). The tracked flight trajectories were converted into 3D (x, y, z) coordinates and exported for further analysis using custom scripts in Matlab (Mathworks R2012a). All of the 3D trajectories that were not discarded (see below) were deposited in the Dryad repository, datadryad.org, doi:10.5061/dryad.65f2k.
Stimulus Description.
The spiral pattern was produced using Matlab to draw a four-armed 10° logarithmic spiral. Areas between the four spiraling lines were filled with black and white in alternating fashion. The same spiral pattern was rotated in either a clockwise or counterclockwise direction at rotation frequencies of 0.1, 0.5, or 0.9 Hz. For clockwise rotation (looming), these values correspond to time-to-collision (τ) values of 9.31, 1.86, and 1.03 s, respectively. For a counterclockwise (receding) spiral, the analog of τ is the time to double distance and has the same values (11). We tested the luminance of the spiral projected with the Canon LCoS projector. White spiral segments ranged from 316 to 160 cd/m2 from the center of the spiral to the periphery. Black segments measured 87 cd/m2 in the center and 35 cd/m2 at the periphery.
The linear gratings used in these experiments were black and white bars moving at temporal frequency 0.5 Hz (cycles per second) either left and right for vertical bars, or up and down for horizontal bars. The spatial frequency of these linear gratings was 0.044 cycles per degree. We selected this spatial frequency because it produced gratings with the same number of cycles as encountered when moving radially outward from the center of the spiral pattern. For pattern combinations, a stationary black and white checkerboard pattern with 0.5-cm squares was used.
Experimental Protocol for Mixed Stimuli.
Three sets of experiments relied on filming during a prolonged feeding bout and trials were conducted every 20 min. Each experiment lasted a single day. Any feeding flight where the hummingbird consumed <0.25 mL was discarded and the trial was repeated. Access to food was restricted by removing the feeder between trials and any moving stimulus patterns were stopped. Trials stopped when a bird finished feeding (left the camera views) or after 2 min without a bird approaching the feeder. Restriction of feeding bouts, combined with a 12 g/100 ml (wt/vol) sucrose solution, increased the feeding duration relative to feeding flights when hummingbirds were given ad libitum access to higher sugar concentrations.
Each of the mixed stimulus experiments had a different set of treatments. For the experiment testing the response to forward-backward, lateral, and vertical visual motion, three background black-and-white patterns were used: a spiral, vertical grating, and horizontal grating. The spiral was either rotated clockwise or counterclockwise to produce constant looming or receding motion, respectively. The vertical gratings were moved either left or right and the horizontal version was moved up or down. The temporal frequency of stimulus motion in all experiments with mixed stimuli was 0.5 Hz. In addition, each pattern had a stationary (no-motion) treatment for a total of nine treatments. Hummingbird subjects were exposed to a randomized series of these treatments with every stimulus shown twice (18 trials per individual, 8 individuals total).
The experiment testing the response to an offset spiral center had five treatments where the center of a looming spiral was moved left, right, up, down or remained centered in the background. The spiral center was moved by ∼0.105 m or 30° (from the docked feeding position of the bird’s head) in all directions. Again each stimulus was repeated twice and the overall sequence was randomized for six individuals in total.
The experiment testing the response to stationary pattern combined with a looming spiral used a square projected image (288 × 288 pixels) partitioned into a background image (either checkerboard or spiral) and a circular image with variable radius overlaid in the center (the opposite pattern). The spiral could be stationary or rotated to produce looming. By changing the size of the central circular image, we tested how sensitive the birds were to different amounts of visible looming. By alternating the pattern in the middle, we changed the location of the looming stimulus, either in the center of the image or at the periphery. Preliminary experiments suggested that birds exhibited less response at small amounts of visible spiral so the first four birds were tested with small central spirals and large central checkerboards (limited spiral visible at the periphery). Six different radius treatments were used for the central looming spiral: 80, 88, 96, 104, 112, 120 pixels, corresponding to 24.24%, 29.33%, 34.91%, 40.97%, 47.51%, and 54.54% of the projected image, respectively. A large series of radii were used for the central checkerboard to obscure most of the spiral as well, with central checkerboards with radius 144, 136, 128, 120, 112, and 104 pixels yielding spiral percentages of 21.46%, 29.94%, 37.94%, 45.46%, 52.49%, and 59.03%, respectively. No-motion trials were also conducted for the 96-pixel radius circle with both a central spiral and a central checkerboard. In the first phase of data collection, 14 randomly ordered trials were conducted for each individual and no trials were duplicated.
We later decided to conduct the treatments with greater percentages of visible looming spiral and tested an additional four hummingbirds with the large radii (listed above) used for central looming spirals (144–104 pixels: 78.54%, 70.06%, 62.06%, 54.54%, 47.51%, 40.97%) and the small radii used for central checkerboard patterns (80–120 pixels: 75.76%, 70.67%, 65.09%, 59.03%, 52.49%, 45.46%). Again, no-motion trials were conducted for a total of 14 different stimulus treatments during the second phase of data collection. Thus, there were 26 different stimulus treatments for this experiment.
Experimental Protocol for Response Change Over Time.
To determine the effect of repeated trials on the flight response of individual hummingbirds, feeding flights were filmed over the course of 2 consecutive days, with two blocks of trials per day. Each block of trials consisted of a nonrotating spiral control, then 10 rotating spiral trials, and finally another control. Trials were spaced every 15 min and stimulus and food [∼22 g/100 ml (wt/vol) sucrose solution] were removed between trials. Within a single day, blocks of trials were 3-h long and were separated by a 2-h period where the subject was returned to his home cage and given ad libitum food. Each individual was exposed to a single spiral rotation frequency (three groups of six birds each, three rotation frequencies: 0.1 Hz, 0.5 Hz, and 0.9 Hz) and was a wild-caught bird that had not previously been used in laboratory experiments. Therefore, the first time the bird experienced a rotating spiral was the first noncontrol trial of the first block.
Statistical Analysis.
Flight-response measurements generated by custom Matlab (Mathworks R2012a) analysis scripts were further analyzed using linear and nonlinear mixed models in R (30, 31). Detailed descriptions of the analysis with supporting results is available in SI Results, Figs. S1–S8, and Tables S1 and S2.
Supplementary Material
Acknowledgments
We thank D. Kress, D. Lentink, I. Schiffner, and M. Srinivasan for constructive feedback on this research; and F. Goller, D. Irwin, and two anonymous reviewers for comments that greatly improved the manuscript. This work was supported by National Science Foundation Grant IOS 0923849, Natural Sciences and Engineering Research Council of Canada Grant 402677, and Human Frontier Science Program Grant RGP0003/2013.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All of the 3D trajectories that were not discarded were deposited in the Dryad repository, datadryad.org, doi:10.5061/dryad.65f2k.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415975111/-/DCSupplemental.
References
- 1.Taylor GK, Krapp HG. Insect Mechanics and Control. In: Casas J, Simpson SJ, editors. Advances in Insect Physiology. Academic Press; New York: 2007. pp. 231–316. [Google Scholar]
- 2.Frost BJ. A taxonomy of different forms of visual motion detection and their underlying neural mechanisms. Brain Behav Evol. 2010;75(3):218–235. doi: 10.1159/000314284. [DOI] [PubMed] [Google Scholar]
- 3.Mronz M, Lehmann FO. The free-flight response of Drosophila to motion of the visual environment. J Exp Biol. 2008;211(Pt 13):2026–2045. doi: 10.1242/jeb.008268. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan MV, Lehrer M, Kirchner WH, Zhang SW. Range perception through apparent image speed in freely flying honeybees. Vis Neurosci. 1991;6(5):519–535. doi: 10.1017/s095252380000136x. [DOI] [PubMed] [Google Scholar]
- 5.Bhagavatula PS, Claudianos C, Ibbotson MR, Srinivasan MV. Optic flow cues guide flight in birds. Curr Biol. 2011;21(21):1794–1799. doi: 10.1016/j.cub.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Ward BJ, et al. Hummingbirds have a greatly enlarged hippocampal formation. Biol Lett. 2012;8(4):657–659. doi: 10.1098/rsbl.2011.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelah A, Barlow HB. Visual illusion from running. Nature. 1996;381(6580):283. doi: 10.1038/381283a0. [DOI] [PubMed] [Google Scholar]
- 8.Lappe M, Bremmer F, Van den Berg AV. van den Berg AV Perception of self-motion from visual flow. Trends Cogn Sci. 1999;3(9):329–336. doi: 10.1016/s1364-6613(99)01364-9. [DOI] [PubMed] [Google Scholar]
- 9.Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29(49):15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wylie DRW, Gutierrez-Ibanez C, Pakan JMP, Iwaniuk AN. The optic tectum of birds: Mapping our way to understanding visual processing. Can J Exp Psychol. 2009;63(4):328–338. doi: 10.1037/a0016826. [DOI] [PubMed] [Google Scholar]
- 11.Martinoya C, Delius JD. Perception of rotating spiral patterns by pigeons. Biol Cybern. 1990;63(2):127–134. [Google Scholar]
- 12.Reichardt W, Wenking H. Optical detection and fixation of objects by fixed flying flies. Naturwissenschaften. 1969;56(8):424–425. doi: 10.1007/BF00593644. [DOI] [PubMed] [Google Scholar]
- 13.Baird E, Dacke M. Visual flight control in naturalistic and artificial environments. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198(12):869–876. doi: 10.1007/s00359-012-0757-7. [DOI] [PubMed] [Google Scholar]
- 14.Winterson BJ, Brauth SE. Direction-selective single units in the nucleus lentiformis mesencephali of the pigeon (Columba livia) Exp Brain Res. 1985;60(2):215–226. doi: 10.1007/BF00235916. [DOI] [PubMed] [Google Scholar]
- 15.Iwaniuk AN, Wylie DRW. Neural specialization for hovering in hummingbirds: Hypertrophy of the pretectal nucleus lentiformis mesencephali. J Comp Neurol. 2007;500(2):211–221. doi: 10.1002/cne.21098. [DOI] [PubMed] [Google Scholar]
- 16.Bilo D, Bilo A. Wind stimuli control vestibular and optokinetic reflexes in the pigeon. Naturwissenschaften. 1978;65(3):161–162. [Google Scholar]
- 17.McArthur KL, Dickman JD. State-dependent sensorimotor processing: Gaze and posture stability during simulated flight in birds. J Neurophysiol. 2011;105(4):1689–1700. doi: 10.1152/jn.00981.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wylie DRW, Crowder NA. Spatiotemporal properties of fast and slow neurons in the pretectal nucleus lentiformis mesencephali in pigeons. J Neurophysiol. 2000;84(5):2529–2540. doi: 10.1152/jn.2000.84.5.2529. [DOI] [PubMed] [Google Scholar]
- 19.Winship IR, Hurd PL, Wylie DRW. Spatiotemporal tuning of optic flow inputs to the vestibulocerebellum in pigeons: Differences between mossy and climbing fiber pathways. J Neurophysiol. 2005;93(3):1266–1277. doi: 10.1152/jn.00815.2004. [DOI] [PubMed] [Google Scholar]
- 20.Borst A, Haag J, Reiff DF. Fly motion vision. Annu Rev Neurosci. 2010;33:49–70. doi: 10.1146/annurev-neuro-060909-153155. [DOI] [PubMed] [Google Scholar]
- 21.O’Carroll DC, Bidwell NJ, Laughlin SB, Warrant EJ. Insect motion detectors matched to visual ecology. Nature. 1996;382(6586):63–66. doi: 10.1038/382063a0. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan MV, Poteser M, Kral K. Motion detection in insect orientation and navigation. Vision Res. 1999;39(16):2749–2766. doi: 10.1016/s0042-6989(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 23.Kelber A, Zeil J. Tetragonisca guard bees interpret expanding and contracting patterns as unintended displacement in space. J Comp Phys A. 1997;181(3):257–265. [Google Scholar]
- 24.Kern R, Varjú D. Visual position stabilization in the hummingbird hawk moth, Macroglossum stellatarum L. I. Behavioural analysis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1998;182(2):225–237. doi: 10.1007/s003590050173. [DOI] [PubMed] [Google Scholar]
- 25.Tammero LF, Dickinson MH. Collision-avoidance and landing responses are mediated by separate pathways in the fruit fly, Drosophila melanogaster. J Exp Biol. 2002;205(Pt 18):2785–2798. doi: 10.1242/jeb.205.18.2785. [DOI] [PubMed] [Google Scholar]
- 26.Collett TS, Land MF. Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J Comput Phys. 1975;99(1):1–66. [Google Scholar]
- 27.Eckmeier D, et al. Gaze strategy in the free flying zebra finch (Taeniopygia guttata) PLoS ONE. 2008;3(12):e3956. doi: 10.1371/journal.pone.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straw AD. Vision egg: An open-source library for realtime visual stimulus generation. Front Neuroinform. 2008;2:4. doi: 10.3389/neuro.11.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straw AD, Branson K, Neumann TR, Dickinson MH. Multi-camera real-time three-dimensional tracking of multiple flying animals. J R Soc Interface. 2011;8(56):395–409. doi: 10.1098/rsif.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinheiro J, Bates D, DebRoy S, Sarkar D; R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-109. Available at CRAN.R-project.org/package=nlme. Accessed February 1, 2014.
- 31.R Development Core Team 2014 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna) Available at www.R-project.org/. Accessed February 1, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.