Significance
The present study provides evidence for a role of glutamate as a neuromodulator of the afferent nociceptive information. Our results show that the nociceptive impulse generated by an inflammatory event in the peripheral tissue is regulated in the dorsal root ganglia (DRG) by a system that involves satellite glial cells and glutamatergic NMDA receptors. To our knowledge, this work is one of the first demonstrations of the involvement of glutamate in a modulatory process in the DRG, a site where there are no synapses, in addition to its classical role as a neurotransmitter.
Keywords: hyperalgesia, dorsal root ganglion, NMDA receptor, inflammatory pain, satellite cells
Abstract
The present study evaluated the role of N-methyl-d-aspartate receptors (NMDARs) expressed in the dorsal root ganglia (DRG) in the inflammatory sensitization of peripheral nociceptor terminals to mechanical stimulation. Injection of NMDA into the fifth lumbar (L5)-DRG induced hyperalgesia in the rat hind paw with a profile similar to that of intraplantar injection of prostaglandin E2 (PGE2), which was significantly attenuated by injection of the NMDAR antagonist d(-)-2-amino-5-phosphonopentanoic acid (d-AP-5) in the L5-DRG. Moreover, blockade of DRG AMPA receptors by the antagonist 6,7-dinitroquinoxaline-2,3-dione had no effect in the PGE2-induced hyperalgesia in thepaw, showing specific involvement of NMDARs in this modulatory effect and suggesting that activation of NMDAR in the DRG plays an important role in the peripheral inflammatory hyperalgesia. In following experiments we observed attenuation of PGE2-induced hyperalgesia in the paw by the knockdown of NMDAR subunits NR1, NR2B, NR2D, and NR3A with antisense-oligodeoxynucleotide treatment in the DRG. Also, in vitro experiments showed that the NMDA-induced sensitization of cultured DRG neurons depends on satellite cell activation and on those same NMDAR subunits, suggesting their importance for the PGE2-induced hyperalgesia. In addition, fluorescent calcium imaging experiments in cultures of DRG cells showed induction of calcium transients by glutamate or NMDA only in satellite cells, but not in neurons. Together, the present results suggest that the mechanical inflammatory nociceptor sensitization is dependent on glutamate release at the DRG and subsequent NMDAR activation in satellite glial cells, supporting the idea that the peripheral hyperalgesia is an event modulated by a glutamatergic system in the DRG.
The involvement of excitatory amino acids in the transmission of the nociceptive information from the primary afferent neurons to the spinal cord is, at present, supported by an immense number of studies (1–3). In fact, a role of the amino acid glutamate (GLU) as a synaptic mediator has been demonstrated by electrophysiological experiments that showed its release by stimulation and consequent increase in the probability that the target cell will fire an action potential (3, 4). In addition, glutamatergic receptors, especially the N-methyl-d-aspartate receptor (NMDAR), detected throughout the entire nervous system (5, 6), have been associated with the development and maintenance of spinal cord neuron sensitization (7, 8). Stimulation of spinal neurons by GLU through NMDARs was also associated to inflammatory processes (3, 9). For instance, sensitization of spinal nerves has been frequently related to the wind-up phenomenon, an increase in the electrical activity of spinal cord neurons and pain sensation independent of primary nociceptor input (10).
The detection of glutamatergic receptors in the presynaptic membrane of afferent fibers associated with nociception and hyperalgesia raised the hypothesis that GLU released into the synaptic cleft could also activate receptors expressed in the central terminals of primary afferent neurons (11, 12). Moreover, the possibility that GLU is involved in nociceptor sensitization was strengthened by reports of the expression of NMDARs in nociceptors (13) and their role in acute and persistent inflammatory mechanical hyperalgesia in the rat paw (13, 14). In addition, the glutamatergic role in the sensitization of the primary sensory nociceptive neuron is indirectly supported by GLU-induced neuronal depolarization (4).
Previous studies from our group have stressed the importance of GLU in the sensitization of the primary sensory neuron with the introduction of the concept of glutamate retrograde sensitization (15–17), focusing especially on the role of NMDARs in this process. In those studies, we demonstrated that the intrathecal (i.t.) injection of GLU, NMDA, or AMPA (another ionotropic glutamatergic receptor agonist) induced mechanical hyperalgesia in the rat hind paws in a dose-dependent manner. However, because the intraplantar (i.pl.) injection of either morphine or the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP) [previously demonstrated, along with dipyrone and diclofenac, to cause local analgesia (18–20)], ipsilaterally antagonized hyperalgesia induced by i.t. NMDA, but not AMPA, we have proposed that the primary nociceptive neuron is the main site of action of the i.t.-injected NMDA. Moreover, i.t. administration of the NMDA antagonists d(-)-2-amino-5-phosphonopentanoic acid (d-AP-5) or MK801, but not of the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione, inhibited the hyperalgesia induced by i.pl. injection of prostaglandin E2 (PGE2) or carrageenan. These results suggested that the maintenance of nociceptor sensitization by inflammatory stimuli depends on a continuous spinal release of GLU that acts on presynaptic NMDA-type receptors (15, 17). Furthermore, the proposal of a GLU-dependent retrograde sensitization of the primary sensory neuron was supported by the additional demonstration that the selective knockdown of NaV1.8 (SNS/PN3) sodium channels [described to be expressed only in nociceptors and associated with inflammatory sensitization (21)] in the dorsal root ganglion (DRG) by i.t. treatment with antisense oligodeoxynucleotides (AS-ODNs) abolished the mechanical hyperalgesia induced by i.t. administration of NMDA (16).
Those data, and the fact that the cerebrospinal fluid, in addition to being in contact with the spinal cord, also bathes part of the DRG (22, 23), led to the suggestion that i.t.-injected drugs could directly reach the DRGs of the primary sensory neurons (24). Thus, considering the methodology used to demonstrate the retrograde sensitization (15, 17), we raised the possibility that i.t-administered drugs actually targeted receptors located at the DRG and not at the presynaptic membrane in those experiments.
In the present study, we investigated whether NMDARs expressed at the DRG participate in the modulation of the excitability of primary afferent neurons during inflammatory nociceptive sensitization. We tested whether the intraganglionar (i.gl.) injection of NMDA, into the fifth lumbar (L5)-DRG, had a direct hyperalgesic effect and whether i.gl. injection of the NMDAR antagonist d-AP-5 could inhibit the nociceptor sensitization induced by i.t. NMDA or i.pl. PGE2 administration. The specificity of ganglionar and spinal GLU receptors was tested by comparing the effects of selective antagonists for NMDA and AMPA receptors. We also evaluated the functional expression of NMDARs in DRG primary cultures in experiments using a fluorescent calcium indicator. Finally, we investigated the individual relevance of each subunit of NMDAR for the development of i.pl. PGE2-induced hyperalgesia and in the activation of NMDARs in DRG cultures.
Results and Discussion
Mechanical Hyperalgesia Induced by i.t. or i.gl. Injection of NMDA.
Currently, drugs administered via the i.t. route are assumed to act mainly in the spinal cord (25, 26). However, the cerebrospinal fluid also bathes the proximal processes of the primary sensory neurons, as well as a portion of the DRG (22, 23, 27). Considering that (i) the direct effect of AS-ODN, which decreases the mRNA expression of different molecules in the DRG of peripheral neurons following i.t. injections, is well documented (16, 28), and (ii) behavioral tests have shown that i.t. injection of PGE2 or NMDA exerts a predominantly hyperalgesic action on primary sensory neurons (15, 17), we compared the magnitude and time course of the mechanical hyperalgesia induced by NMDA administered by two different routes—either i.t. or i.gl. Of note, it is important to highlight that, for the i.gl. administration of the compounds in the present study, small volumes were used, allowing their injection to be restricted to the ganglia (24).
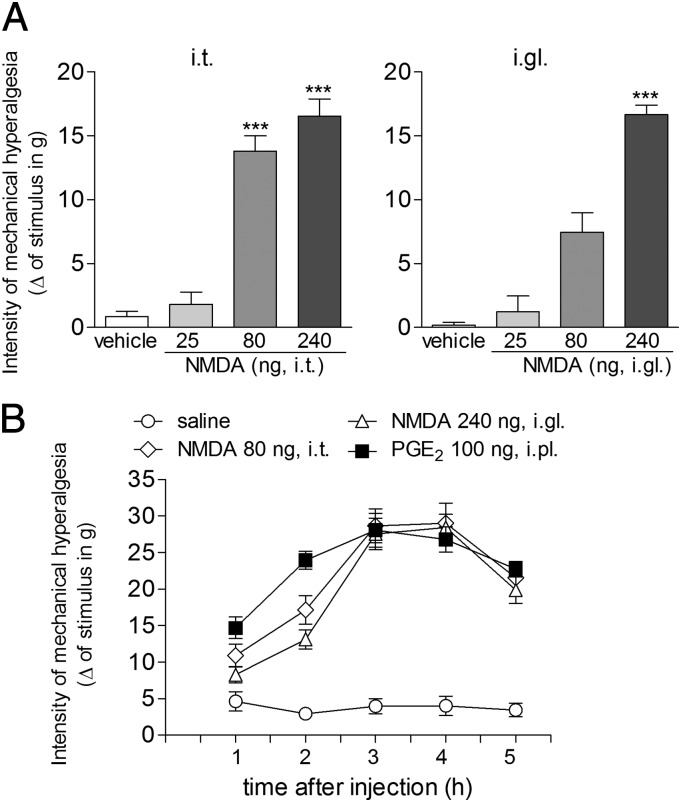
Fig. 1A shows the hyperalgesic effect of three doses of NMDA (25, 80, and 240 ng) administered by the i.t. (Fig. 1 A, Left) or i.gl. (Fig. 1 A, Right) route. The lack of significant difference in the magnitude of the hyperalgesia induced by the injection of 80 and 240 ng of NMDA by the i.t. route may indicate that i.t.-injected drugs reach several DRGs. Thus, a small i.t. dose produces a stronger effect than when it is injected into a single ganglion. Also, this apparent contradiction may reflect the diffusion of the i.t. injection of NMDA through the spinal fluid, affecting other ganglia involved in the mechanical hyperalgesia in the hind paw, in addition to the L5-DRG. Based on these results, we selected the doses of 80 and 240 ng of NMDA for the i.t and i.gl. injections, respectively, in the other experiments of this study. These doses were also used to compare the time course of the NMDA-induced hyperalgesia with the hyperalgesia induced by i.pl. injection of PGE2, shown in Fig. 1B. We observed that the mechanical hyperalgesia induced by the i.pl. injection of PGE2 and by NMDA, injected by the i.t. or i.gl. route, displayed a very similar profile, with the peak of sensitization at the third hour after administration. This time point was thus chosen for the analysis of further experiments.
Fig. 1.
Mechanical hyperalgesia induced by i.t. or i.gl. injection of NMDA. (A) NMDA was injected by the i.t. (Left) or i.gl. (Right) route, and the mechanical threshold was evaluated 3 h later by the electronic von Frey test. Results represent the means ± SEM of five paws per group. ***P < 0.001 compared with the saline and the 25-ng groups (Left), and to the saline and the 25- and 80-ng groups (Right) (one-way ANOVA followed by Bonferroni posttest). (B) Time course of the mechanical hyperalgesia induced by i.pl. injection of PGE2 (■) and i.t. (♢) or i.gl. (△) administration of NMDA at the indicated doses, evaluated by the electronic von Frey test. Results represent the means ± SEM of five paws per group [no significant difference was found between the ■, ♢, and △ groups; P < 0.05 when those groups are compared with the saline group (one-way ANOVA followed by Bonferroni posttest)].
Mechanical Hyperalgesia in the Hind Paw Induced by i.gl. Injection of NMDA Is Not Dependent on Release of PGE2.
Because we observed similarities between the hyperalgesia induced by NMDA and PGE2, we considered the possibility that the effect of NMDA could result from release of endogenous prostanoids. In this case, its effect would be indirect and prevented by the treatment with the nonselective ciclooxygenase inhibitor indomethacin (29). Fig. S1 shows that neither the i.t. (100 μg per 10 μL) nor the systemic [intraperitoneal (i.p.); 5 mg/kg] administration of indomethacin affected the hyperalgesia induced by the i.gl. administration of NMDA, indicating a direct effect of NMDA on DRG cells, not dependent on ciclooxygenase activation and further prostaglandin release.
Blockade of NMDARs in the DRG Attenuates the Mechanical Hyperalgesia Induced by i.t. NMDA or i.pl. PGE2.
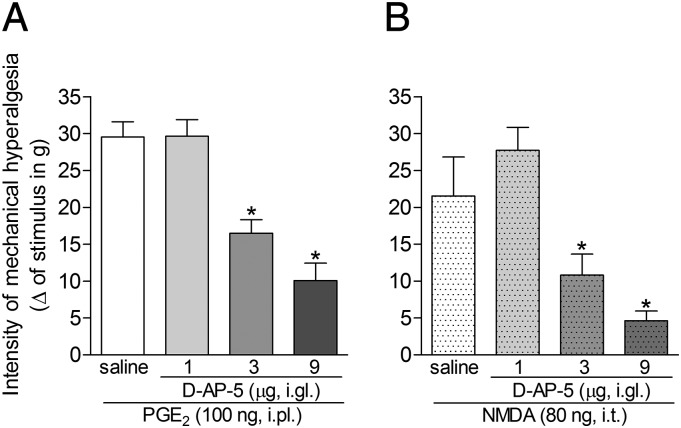
Fig. 2 shows that i.gl. administration of 3 or 9 μg of the selective NMDAR antagonist d-AP-5 equally attenuated the mechanical hyperalgesia induced by i.pl. PGE2 (Fig. 2A) or i.t. NMDA (Fig. 2B) only in the ipsilateral paw, with no effect on the contralateral paw.
Fig. 2.
Effect of the i.gl. injection of d-AP-5 on the mechanical hyperalgesia induced by NMDA or PGE2. Mechanical hyperalgesia in the hind paw was evaluated 3 h after the injection of PGE2 (100 ng, i.pl.; A) or NMDA (80 ng, i.t.; B), in the presence of 1, 3, or 9 µg of the NMDAR antagonist d-AP-5, injected into the DRG (i.gl.) 60 min before the measurements. In both cases, the doses of 3 and 9 μg of d-AP-5 significantly attenuated the hyperalgesia. *P < 0.05 compared with the saline groups; results represent the means ± SEM of five paws per group (ANOVA followed by Bonferroni posttest).
We also evaluated the effect of the NMDAR antagonist on the time course of the i.pl. PGE2-induced mechanical hyperalgesia. The results shown in Fig. S2 strongly suggest that the hyperalgesia induced by injection of PGE2 in the hind paw is dependent on the activation of NMDARs. When d-AP-5 was injected by the i.t. route (Fig. S2A), a short-lasting but significant inhibition of the PGE2 hyperalgesia was observed, but this effect was no longer present by the third-hour time point, when the mechanical sensitization reached values similar to those detected in the control group (PGE2 plus saline). This result contrasts with the marked inhibition observed in the group treated with i.gl. injection of d-AP-5 (Fig. S2B), in which the mechanical hyperalgesia induced by PGE2 remained attenuated until the third hour after PGE2, then increasing until it reached values comparable with those observed in the control group (PGE2 plus saline). The groups that received only d-AP-5 injected by either route did not exhibit significant changes in mechanical threshold. This difference in the effect of d-AP-5 on the PGE2-induced hyperalgesia when injected by different routes may reflect the possibility that drugs injected i.t. diffuse promptly into the cerebrospinal fluid. However, in the DRG, the diffusion rate might be reduced because the drug will be confined in a smaller compartment, slowing its diffusion rate.
Because d-AP-5 caused a strong inhibition of the hyperalgesia in the ipsilateral, with no effect on the contralateral, paw when administered into the DRG and in both paws when injected in the spinal cord, it is safe to infer that drugs administered into the DRG have a major effect at this site. In addition, these results suggest that the modulation of the hyperalgesia induced by peripheral injection of PGE2 is dependent on the activation of NMDARs present at the DRG.
AMPA Receptors Involved in the i.pl. PGE2-Induced Hyperalgesia Are Not Located at the DRG.
Fig. S3A shows that the administration of AMPA by the i.t., but not by the i.gl., route induced mechanical hyperalgesia. This hyperalgesic effect of AMPA was, as depicted in Fig. S3 B and C, significantly inhibited by i.t. (Fig. S3B), but not by i.gl. (Fig. S3C), injection of the AMPA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX; 40 ng). Moreover, the i.pl. injection of PGE2 (Fig. S3D) induced hyperalgesia that was significantly attenuated by i.t., but not i.gl., injection of DNQX. Together, these results indicate that the activation of AMPA receptors located in the spinal cord is necessary for the hyperalgesia induced by i.pl. PGE2.
All three ionotropic glutamatergic receptors—i.e., AMPA, kainate and NMDA—have been shown to be expressed in rat DRG neurons (30, 31). However, the ganglionar AMPA receptors, in contrast to those expressed postsynaptically in the dorsal horn, do not appear to be involved in the sensitization of the primary sensory neuron, as suggested herein and in our previous study (15). Conversely, it has been suggested that presynaptic AMPA receptors on the central terminals of primary afferent neurons play a role in the modulation of nociceptors involved primarily in neuropathic pain (31). Thus, based on the present model of nociceptor sensitization, AMPA receptors are mainly involved in the sensitization of second-order neurons in the dorsal horn of the spinal cord.
Activation of NMDARs in Primary DRG Cultures.
Functional responses of NMDARs were observed through confocal microscopy as calcium transients in DRG cultures loaded with the calcium-sensitive fluorescent probe Fluo-3 AM (Figs. 3 and 4 and Fig. S4).
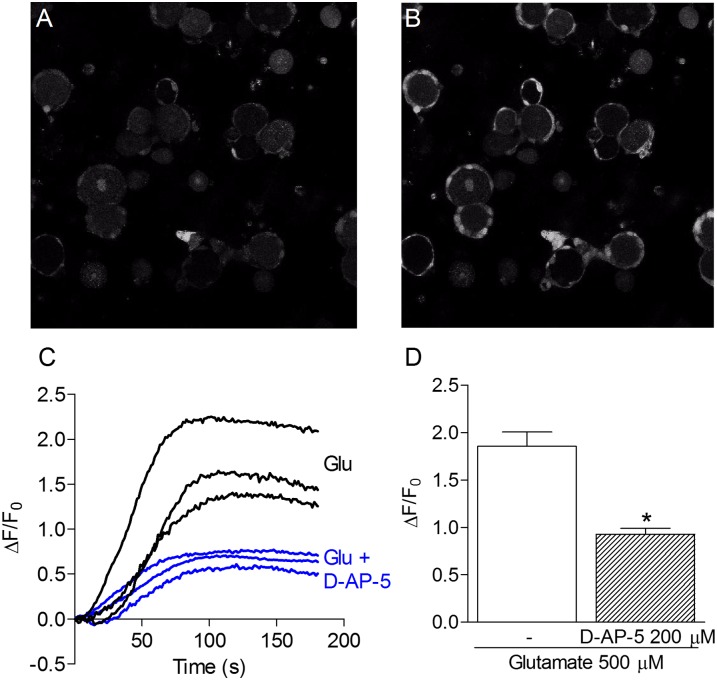
Fig. 3.
GLU-induced calcium transients in glial satellite cells. A and B show confocal images of primary DRG cells cultured for 24 h, in which satellite cells are found attached to the primary sensory neuron soma. (A) Basal fluorescence of DRG cells. (B) Fluorescence changes immediately after GLU (500 μM) administration. (C) Representative curves of the fluorescence variation over time induced by GLU (500 μM) in the presence or absence of d-AP-5 (200 μM). Each curve represents the response of one satellite cell. (D) Maximum fluorescence increase induced by GLU (500 μM) alone or in the presence of d-AP-5 (200 μM). Results represent the means ± SEM of 16–26 cells in three different experiments. Experiments were performed in the presence of glycine (10 μM) in Mg2+-free buffer. *P < 0.05 compared with the effect of GLU without d-AP-5 (ANOVA followed by Bonferroni posttest).
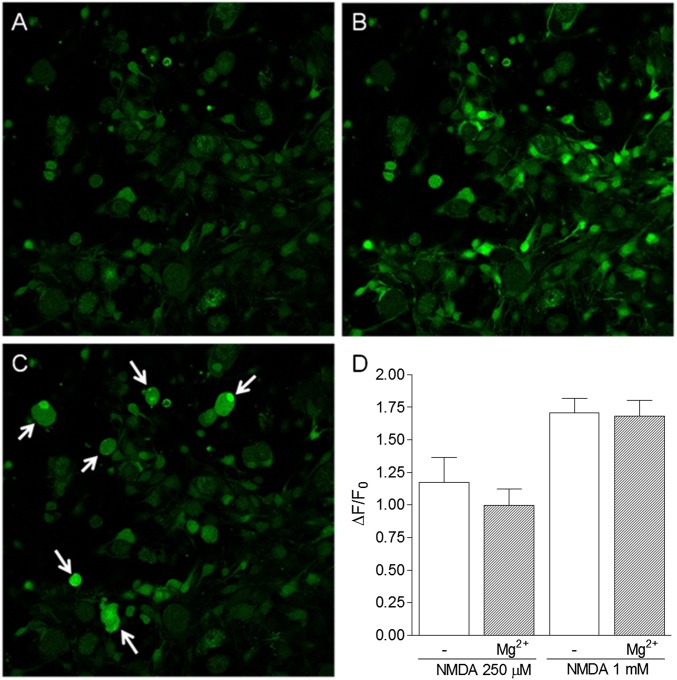
Fig. 4.
NMDA-induced calcium transients in satellite cells in the presence or absence of Mg2+. A–C show confocal images of Fluo-3 AM-loaded primary DRG cells cultured for 36 h, in which the satellite cells are observed to proliferate and migrate to the bottom of the dish. (A) Basal fluorescence of DRG cells. (B) Increase in fluorescence observed immediately after NMDA (250 μM) administration. (C) Increase in fluorescence observed in neurons after the administration of capsaicin (1 μM), used to verify the viability of nociceptive neurons. (D) Maximal fluorescence increase after NMDA (250 μM or 1 mM) administration in the presence or absence of Mg2+ (0.9 mM) in buffer. Results represent the means ± SEM of 17–22 cells in three different experiments. Statistical analysis, performed by using ANOVA followed by Bonferroni posttest, failed to detect significant differences between the groups.
Primary cultures of DRG contain mainly primary sensory neurons and satellite glial cells. Upon dissociation of DRG cells, satellite cells remained attached to neurons. After 24 h in culture, those cells still surrounded neurons and could be clearly visualized in the fluorescent confocal images (Fig. 3 A and B and Fig. S4 A–C). At this step of the protocol, calcium experiments with neurons were difficult to perform because most of the calcium sensor (Fluo-3 AM) was localized in the satellite cells and did not reach neurons. However, as shown in Fig. S4 A–C, after 36 h in culture, the satellite cells migrated to the bottom of the dish and proliferated, assuming a fibroblast-like shape.
As shown in Fig. 3, the administration of GLU (500 μM)—in the presence of glycine (10 μM) in buffer solution without Mg2+—to the DRG cultures after 24 h, induced calcium transients only in satellite cells, even though nociceptive neurons were present and functional, as confirmed by their response to administration of the transient receptor potential vanilloid 1 (TRPV1) agonist capsaicin. Moreover, the effect of GLU was significantly attenuated by prior administration of the NMDAR antagonist d-AP-5 (200 μM; Fig. 3D). Similarly, the administration of NMDA (250 μM; Fig. 4) under the same conditions described for GLU also induced calcium transients in satellite cells, but not in neurons.
The GLU- or NMDA-induced calcium transients in neurons were very rare, although functional nociceptive neurons were present, as confirmed by the treatment with capsaicin (Fig. 4C). In fact, we did observe neuronal responses to capsaicin in a 24-h culture; however, such responses were more easily observed in 36-h cultures because of the easier loading of neurons with the fluorescent indicator in this case. Therefore, our experiments indicate that the release of GLU in the DRG activates NMDARs on the satellite cells and suggest a glutamatergic role at the DRG as a communicating system between neurons and these glial cells. Because there are no synapses in the DRG, this cross-talk between neurons and satellite cells may well have physiological relevance because GLU cannot diffuse from one neuron to another. Also, this possibility is strengthened by the expression of GLU transporters and glutamine synthetase in satellite cells, as reported (32, 33).
In resting state, NMDARs have been described to be blocked by a voltage-sensitive “plug” of Mg2+ (34). To allow the entrance of calcium through the activated receptor pore, the Mg2+ plug must be removed by a previous depolarizing stimulus (34–36). However, we found that the NMDARs expressed in satellite cells are not sensitive to the Mg2+ blockade (Fig. 4D), as observed in other glial cells such as astrocytes (37, 38). Thus, these receptors in the satellite cells can be promptly activated regardless of the negative membrane resting potential of these cells.
Release of GLU in the DRG as a regulatory mechanism of the nociceptive information has also been suggested in a recent study from Kung et al. (39), who observed an increase in GLU in cell bodies and satellite cells after depolarization and consequent activation of AMPA, kainate, and metabotropic receptors, in addition to NMDARs. However, those results contrast with ours, because we have observed activation of NMDARs only in satellite cells. Differences in experimental protocols used in these studies could explain such discrepancies. While those authors used the calcium-sensitive fluorescent dye Fura-2 AM, which requires excitation at two wavelengths, and a CCD camera, which provides limited resolution of images to register neuronal activation in cultures, we used Fluo-3 AM, which requires excitation at only one wavelength, and confocal microscopy. In this regard, in our experience, without the use of confocal microscopy, it is very difficult to differentiate the effects of stimulation from satellite cells and neurons in 24-h cultures. Indeed, at this time point, satellite cells usually cover neuronal cell bodies, and in this configuration, most of the fluorescent indicator is loaded in these cells, not reaching neurons, as clearly shown in Fig. 3B. Importantly, it is also of note that our results show a role of NMDA, but not AMPA, receptors in the DRG in the development of inflammatory hyperalgesia, whereas those authors have shown the involvement of other types of GLU receptors in a model of neuropathic pain [i.e., chronic constriction injury (40, 41)]. These findings suggest the involvement of different types of GLU receptors in the DRG during inflammatory and neuropathic hyperalgesia. Nevertheless, both reports stress the importance of the glutamatergic receptors in DRG satellite cells in the regulation of the afferent nociceptive information as a new concept idea in terms of neuronal physiology.
Role of NMDARs Subtypes in the Mechanical Hyperalgesia Induced by PGE2.
Three main families of NMDAR subunits have been identified: NR1, NR2 (NR2A, -2B, -2C, and -2D), and NR3 (NR3A and NR3B) (42, 43). Recent studies have provided an understanding on how the expression of individual subunits may alter the mechanisms underlying the signaling properties of NMDARs (44, 45). However, only a few brief studies address the functional properties of NMDARs in primary sensory neurons (4, 46).
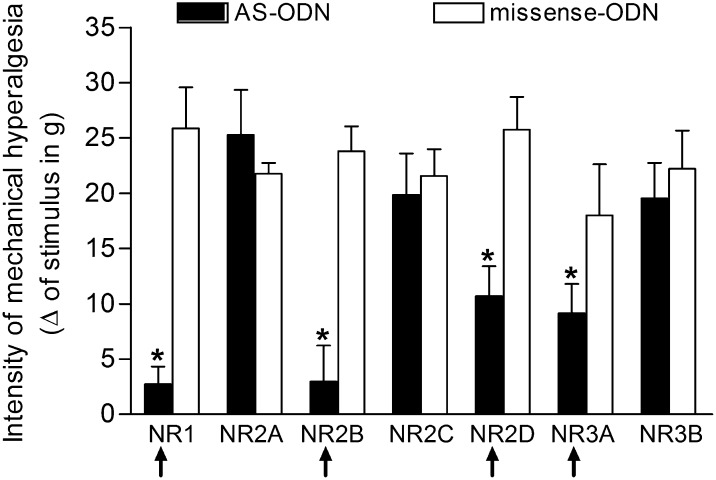
In the last series of experiments, the contribution of each NMDAR subunit in the mechanical hyperalgesia induced by i.pl. injection of PGE2 (100 ng; Fig. 5) was investigated. The selective knockdown of each subunit was produced by the i.gl. treatment with AS-ODN (20 μg in 5 μL) for five consecutive days. On the fifth day, PGE2 was injected in the hind paw, and, 3 h later, the mechanical hyperalgesia was evaluated. Importantly, none of the AS-ODNs caused change in the mechanical threshold in the hind paw per se. Fig. 5 shows that the mechanical hyperalgesia induced by PGE2 was significantly attenuated by the knockdown of the subunits NR1, NR2B, NR2D, or NR3A, whereas no changes were observed following the knockdown of NR2A, NR2C, or NR3B. Of note, this attenuation of hyperalgesia was stronger in the NR1- and NR2B-depleted animals than in the NR2D- and NR3A-depleted animals, although the depletion of all of these subunits significantly attenuated the PGE2-induced hyperalgesia compared with the missense-ODN–treated groups. To confirm the effect of the AS-ODN on the expression of the different NMDAR subunits in the DRG, after the behavioral tests, the L5-DRGs of the animals were surgically harvested for mRNA analysis by real-time PCR. We found that, in all tested groups, there was a significant decrease in the mRNA expression of the respective NMDAR subunits (Fig. S5).
Fig. 5.
Effect of the selective knockdown of NMDAR subunits in the DRG on the mechanical hyperalgesia induced by i.pl. administration of PGE2. AS-ODNs specific for each NMDAR subunit were administered, in different groups of rats, for five consecutive days into the L5-DRG (20 μg/5 μL). On the fifth day, after the injection of the ODNs, PGE2 (100 ng) was administered s.c. in the plantar surface of the rat hind paw, and, 3 h later, the mechanical threshold was evaluated by the electronic von Frey test. Significant attenuation of the PGE2-induced hyperalgesia was observed in the groups treated with AS-ODN (filled bars) for the subunits NR1, NR2B, NR2D, and NR3A. Results represent the means ± SEM of five paws per group. *P < 0.001 compared with respective missense-ODN groups (open bars) (one-way ANOVA followed by Bonferroni posttest).
We also evaluated in vitro the role of the subtypes of NMDARs that were shown to be relevant for the hyperalgesic effect of PGE2 in the hind paw, in the functional responses to NMDA administration observed in satellite cells. Primary cultures were prepared from rats treated, for four consecutive days, with AS-ODN against the mRNAs of the subunits NR1, NR2B, NR2D, or NR3A. As shown in Fig. S6, the AS-ODN treatment resulted in a reduction of calcium transients induced by NMDA, in a similar way to the attenuation of PGE2 hyperalgesia observed in vivo. The inhibition of expression of the NR1 or NR2B subunit caused a marked reduction in the calcium transients. However, inhibition of the expression of the NR2D or NR3A subunit caused a smaller, but still significant, reduction, compared with those caused by NR1 or NR2B knockdown. In both in vitro and in vivo experiments, the smaller effect produced by the NR3A AS-ODN treatment probably reflects the reduction in calcium permeability caused by the NR3A in the NMDAR channel (45, 47). Thus, even if the total number of receptors is reduced by blockade of the NR3A subunit expression, the calcium influx can be partially compensated by the increased permeability of other NMDAR subunits.
The present knockdown experiment showed the role of the NR1, NR2B, NR2D, and NR3A subunits in the mechanical hyperalgesia induced by i.pl. PGE2 (Fig. 5). The observation that NR1 and NR2B depletion caused a decrease in the intensity of the mechanical hyperalgesia was not surprising, because NR1 subunits are essential for the function of the NMDARs (48), and the involvement of NR2B in pain processing has been demonstrated (49). In this context, it has been described that functional NMDARs are formed only when the NR1 subunit is coexpressed with the NR2 subunits (50). Of note, the presence of NR3A subunits has been proposed to decrease the sensitivity of NMDARs to extracellular Mg2+ (45). This result is in line with our finding that NMDARs in the satellite cells were not sensitive to Mg2+ blockade (Fig. 4D), as observed in other glial cells such as astrocytes (37, 38). Therefore, we suggest that these receptors can be activated regardless of the negative membrane resting potential of these cells. In fact, we did find expression of the NR3A subunit only in satellite cells (Fig. S7), reinforcing this idea. Together, our in vivo and in vitro experiments using AS-ODN indicate that NMDARs activated during the PGE2-induced hyperalgesia are most likely present in glial cells rather than in neurons.
Conclusion
The present study shows compelling evidence in support of a glutamatergic-dependent signaling mechanism in the DRG and proposes a physiological role of DRG NMDARs in the expression of peripheral inflammatory mechanical hyperalgesia. The mechanisms by which satellite cells activated by GLU interfere with the neuronal excitability and thus contribute to the maintenance of sensitization to mechanical stimulation remain to be determined. Importantly, the chemical communication between neurons and satellite cells in the DRG has been described (51). In that study, ATP released by neurons was shown to activate P2X7 receptors in satellite cells in vitro. We may speculate that such neuron–glia communication might be involved in the cross-depolarization that is known to affect neurons neighboring excited neurons in the DRG (52).
The retrograde sensitization of the primary sensory neuron was proposed as an essential mechanism for induction and maintenance of peripheral inflammatory hyperalgesia. It was suggested that this phenomenon was due to the release of GLU in the spinal cord, which acted retrogradely on NMDARs present at the presynaptic terminals of the primary sensory neuron (15, 17). In the present investigation, we performed a series of experiments that altogether change this view and suggest that the retrograde sensitization occurs predominantly in the DRG itself. This suggestion is supported by the finding that GLU is present in the cell bodies of the DRG (53). Satellite glial cells are found wrapping the neuronal somata, isolating the ganglionar cell bodies. Thus, GLU released by sensory neurons in the DRG basically activates NMDARs in satellite cells. The mechanism by which GLU-activated satellite cells influence neuronal excitability remains to be elucidated. Devor (27) has highlighted several peculiarities of the DRG, including the finding that it lacks a blood–nerve barrier. This observation, along with our conclusion that the communication between glial cells and neurons is a determinant in the maintenance of mechanical hyperalgesia, opens the possibility of designing NMDAR antagonists with primarily peripheral activity.
Materials and Methods
For complete details, see SI Materials and Methods.
Animals.
The experiments were performed on male Wistar rats (100 g for in vitro tests; 180–200 g for behavioral tests).
ODNs.
AS-ODNs specific for each NMDAR subunit were used to induce knockdown of the NR1, NR2A, NR2B, NR2C, NR2D, NR3A, or NR3B subunits in rat L5-DRG neurons. I.gl. injections were performed for four (Fig. S6) or five (Fig. 5 and Fig. S5) consecutive days. For the experiment shown in Fig. 5 and Fig. S5, on the fifth day, after the behavioral tests with PGE2 (100 ng), the L5-DRGs of the ipsilateral side of PGE2 injection were harvested and prepared for evaluation of the expression of NMDAR subunits by real-time PCR.
Drug Administration.
I.t. injections were performed as described (25). The i.gl. drug administration was carried out in accordance with refs. 24 and 54. Briefly, rats were anesthetized by inhalation of 2% (vol/vol) isoflurane. The injecting needle was inserted through the punctured skin, toward the intervertebral space between L5 and L6, until the tip touched the lateral region of the vertebrae. To reach the space between the transverse processes of the L5 and L6 vertebrae, smooth movements of the needle were made until the bone resistance was diminished and an ipsilateral paw flinch reflex was observed.
Mechanical Hyperalgesia Evaluation: Electronic von Frey Test.
Mechanical hyperalgesia was evaluated in rats as reported (55). The test consisted of evoking a hind-paw flexion reflex in rats, placed in acrylic cages with wire grid floors, with a hand-held force transducer (electronic anesthesiometer; IITC Life Science) adapted with a 0.7-mm2 polypropylene tip, which was applied by the investigator perpendicularly to the central area of the hind paw with a gradual increase in pressure.
Primary DRG Cultures.
Rats were euthanized by decapitation under anesthesia. DRGs were collected and processed as described (56). Cells were dissociated and plated in six-well plastic plates coated with Matrigel (BD). The cultures were maintained in a humid 5% CO2 atmosphere at 37 °C for 24–36 h.
Intracellular Calcium Images.
Calcium influx was used as a parameter to indicate neuronal activation. Intracellular calcium was monitored by using the fluorescent calcium indicator Fluo-3 AM (Molecular Probes). Cells were loaded with 5 μM Fluo-3 AM for 1 h at room temperature, washed three times, and allowed to de-esterify for ∼15 min in Hank’s buffered saline solution containing 10 mM Hepes. Fluorescent images were acquired by using a confocal microscope (Leica SP5) with an Argon laser (488-nm wavelength for Fluo-3 excitation). All drugs were directly administered during image acquisition using a micropipette. Data are presented as ΔF/F0 to normalize for differences in cell loading.
Data Analysis.
Results are presented as the means ± SEM of groups containing five animals in the in vivo experiments and of three different experiments in the in vitro essays. The statistical analysis was performed by using one-way ANOVA followed by the Bonferroni test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Sergio R. Rosa and Ieda R. Schivo dos Santos for technical assistance. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo, Fundação de Amparo à Pesquisa do Estado de Minas Gerais, and the Conselho Nacional de Pesquisa.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420601111/-/DCSupplemental.
References
- 1.Carpenter KJ, Dickenson AH. Amino acids are still as exciting as ever. Curr Opin Pharmacol. 2001;1(1):57–61. doi: 10.1016/s1471-4892(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 2.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28(5):633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovinger DM, Weight FF. Glutamate induces a depolarization of adult rat dorsal root ganglion neurons that is mediated predominantly by NMDA receptors. Neurosci Lett. 1988;94(3):314–320. doi: 10.1016/0304-3940(88)90037-7. [DOI] [PubMed] [Google Scholar]
- 5.Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 7.Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12(9):3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127(3):253–262. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 11.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- 12.Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101(3):699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 13.Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109(3):443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7(4):895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira SH, Lorenzetti BB. Glutamate spinal retrograde sensitization of primary sensory neurons associated with nociception. Neuropharmacology. 1994;33(11):1479–1485. doi: 10.1016/0028-3908(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 16.Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci USA. 2003;100(5):2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira SH, Lorenzetti BB. Intrathecal administration of prostaglandin E2 causes sensitization of the primary afferent neuron via the spinal release of glutamate. Inflamm Res. 1996;45(10):499–502. doi: 10.1007/BF02311085. [DOI] [PubMed] [Google Scholar]
- 18.Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;217(2-3):225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzetti BB, Ferreira SH. Mode of analgesic action of dipyrone: Direct antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 1985;114(3):375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- 20.Tonussi CR, Ferreira SH. Rat knee-joint carrageenin incapacitation test: An objective screen for central and peripheral analgesics. Pain. 1992;48(3):421–427. doi: 10.1016/0304-3959(92)90095-S. [DOI] [PubMed] [Google Scholar]
- 21.Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256(1):17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- 22.Michael GJ, et al. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porreca F, et al. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci USA. 1999;96(14):7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari LF, Cunha FQ, Parada CA, Ferreira SH. A novel technique to perform direct intraganglionar injections in rats. J Neurosci Methods. 2007;159(2):236–243. doi: 10.1016/j.jneumeth.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32(4):197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 26.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17(6):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 27.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;6(Suppl 6):S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- 28.Lai J, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95(1-2):143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 29.Park YH, Shin CY, Lee TS, Huh IH, Sohn UD. The role of nitric oxide and prostaglandin E2 on the hyperalgesia induced by excitatory amino acids in rats. J Pharm Pharmacol. 2000;52(4):431–436. doi: 10.1211/0022357001774039. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Willcockson H, Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell Tissue Res. 2008;334(1):17–23. doi: 10.1007/s00441-008-0662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger UV, Hediger MA. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J Comp Neurol. 2000;421(3):385–399. doi: 10.1002/(sici)1096-9861(20000605)421:3<385::aid-cne7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Miller KE, Richards BA, Kriebel RM. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002;945(2):202–211. doi: 10.1016/s0006-8993(02)02802-0. [DOI] [PubMed] [Google Scholar]
- 34.Mayer ML, Miller RJ. Excitatory amino acid receptors, second messengers and regulation of intracellular Ca2+ in mammalian neurons. Trends Pharmacol Sci. 1990;11(6):254–260. doi: 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990;57(5):1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59(3):279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 37.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26(10):2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziak D, Chvátal A, Syková E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res. 1998;47(5):365–375. [PubMed] [Google Scholar]
- 39.Kung LH, et al. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS ONE. 2013;8(7):e68312. doi: 10.1371/journal.pone.0068312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 41.Kernisant M, Gear RW, Jasmin L, Vit JP, Ohara PT. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. J Neurosci Methods. 2008;172(1):43–47. doi: 10.1016/j.jneumeth.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33(8):1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 43.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 44.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: Diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki YF, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol. 2002;87(4):2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- 46.Si JQ, Li ZW. Inhibition by baclofen of NMDA-activated current in rat dorsal root ganglion neurons. Zhongguo Yao Li Xue Bao. 1999;20(4):324–328. [PubMed] [Google Scholar]
- 47.Cavara NA, Hollmann M. Shuffling the deck anew: How NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38(1):16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- 48.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 49.Boyce S, et al. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: Correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38(5):611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 50.Hawkins LM, et al. Export from the endoplasmic reticulum of assembled N-methyl-d-aspartic acid receptors is controlled by a motif in the c terminus of the NR2 subunit. J Biol Chem. 2004;279(28):28903–28910. doi: 10.1074/jbc.M402599200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104(23):9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16(15):4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tracey DJ, De Biasi S, Phend K, Rustioni A. Aspartate-like immunoreactivity in primary afferent neurons. Neuroscience. 1991;40(3):673–686. doi: 10.1016/0306-4522(91)90004-8. [DOI] [PubMed] [Google Scholar]
- 54.Araldi D, et al. Peripheral inflammatory hyperalgesia depends on the COX increase in the dorsal root ganglion. Proc Natl Acad Sci USA. 2013;110(9):3603–3608. doi: 10.1073/pnas.1220668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vivancos GG, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37(3):391–399. doi: 10.1590/s0100-879x2004000300017. [DOI] [PubMed] [Google Scholar]
- 56.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118(1):69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.