Abstract
Cytomegalovirus (CMV) is increasingly recognized as an accomplished modulator of cell-signaling pathways, both directly via interaction between viral and cellular proteins, and indirectly by activating metabolic/energy states of infected cells. Viral genes, as well as captured cellular genes, enable CMV to modify these pathways upon binding to cellular receptors, up until generation of virus progeny. Deregulation of cell-signaling pathways appears to be a well-developed tightly balanced virus strategy to achieve the desired consequences in each infected cell type. Importantly and perhaps surprisingly, identification of new signaling pathways in cancer cells positioned CMV as a sophisticated user and abuser of many such pathways, creating opportunities to develop novel therapeutic strategies for inhibiting CMV replication (in addition to standard of care CMV DNA polymerase inhibitors). Advances in genomics and proteomics allow the identification of CMV products interacting with the cellular machinery. Ultimately, clinical implementation of candidate drugs capable of disrupting the delicate balance between CMV and cell-signaling will depend on the specificity and selectivity index of newly identified targets.
Keywords: AMPK, ATM, CMV, mTOR, signal transduction, Wnt
Introduction
Infection with human cytomegalovirus (CMV), a member of the herpesvirus family, is common in humans. Seroprevalence rates increase with age, reaching 90% in individuals older than 80 years. The virus establishes lifelong persistence in infected individuals, without causing apparent sequelae. In immunocompromised hosts, transplant recipients and patients with AIDS, CMV infection is associated with significant morbidity and mortality (1).CMV is the most common congenital infection causing mental retardation and deafness in infected children. While in normal hosts latency does not cause overt damage, in populations at risk for CMV disease, the additional stress imposed by chemotherapy, radiation or organ development allows CMV to disrupt the fine stalemate with the cell, reactivate and cause disease.
Other syndromes have been associated with detection of CMV in endothelial cells, including coronary artery disease, stroke, atherosclerosis in transplanted hearts and rates of graft rejection (1). CMV reactivation may also affect the outcome of sepsis and pulmonary complications in patients in intensive care units. Thus, virus reactivation may have a wider role in health outcomes.
To accomplish infection of multiple cell types, during its evolution with the host CMV developed multiple tactics to either cause lytic replication, or remain latent depending on the target cell. In vitro studies of lytic replication are usually performed in human fibroblasts, while latency is studied in endothelial/epithelial cells and monocytes. Although CMV-glycoprotein B (gB) is abundant in all virus strains and induces cell-signaling pathways, during adaptation to tissue culture, the laboratory-adapted strains (AD169, Towne) lost certain genetic regions. These include 19 genes in the UL/b′ boundary (encoding for cytokine and chemokine homologs) and the gH/gL/UL128–131 complex (required for virus endocytic entry [or endocytosis] into endothelial/epithelial cells)—which allows clinical isolates (TR, TB40/E and others) to enter endothelial/epithelial cells (2,3). These genetic changes could result in differential modulation of cell-signaling pathways.
This review provides an update on newly identified human cell-signaling pathways modulated by CMV and their potential relevance to CMV therapeutics. Previously reported pathways are briefly reviewed. Table 1 summarizes CMV-associated cell-signaling, virus facilitators and potential therapeutics.
Table 1.
Cell-signaling pathways controlled by cytomegalovirus
| Primary pathway | Virus-derived facilitator (s) | Drug | Reference |
|---|---|---|---|
| AMPK | Unknown | Compound C, AICAR, ST0–609 | (4,5) |
| mTOR | IE72, IE86, UL38 | Sirolimus, everolimus, rapamycin, torin | (9–12) |
| CDKs | Unknown | Roscovitine | (21) |
| PDGFR-α | gB (?) | Imatinib mesylate, IMC-3G3, | (2) |
| PI3K | gB | LY294002 | (23) |
| NF-κB | gB/gH, UL76, UL144, IE86 | BAY-11–7082, dexamethasone, lactacystin | (25–28,35,36) |
| RAS/RAF/MEK1/2 | IE86, IE72 | Sorafenib, U0126, PD98059, FHPI | (38–41) |
| UPR/proteasome degradation | US2, US11, pp71, UL38 | Clotrimazole | (51) |
| WNT | Unknown | Monensin, nigericin, salinomycin | (56) |
AMPK, AMP-activated protein kinase; CDKs, cyclin-dependent kinases; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa beta; PDGFR-α, platelet-derived growth factor receptor-α; PI3K, phosphatidylinositol-3-kinase; UPR, unfolded protein response.
Signaling pathways operate at the protein level, beginning with an extracellular signal triggering a receptor, leading to a chain of events whereby specific proteins transduce the signal to the nucleus, activating or deactivating transcription of specific genes. CMV binding to cellular receptors initiates the first wave of signal modulation, followed rapidly by effects imposed by components of the virion and subsequently to effects of viral gene products.
Platelet-Derived Growth Factor Receptor
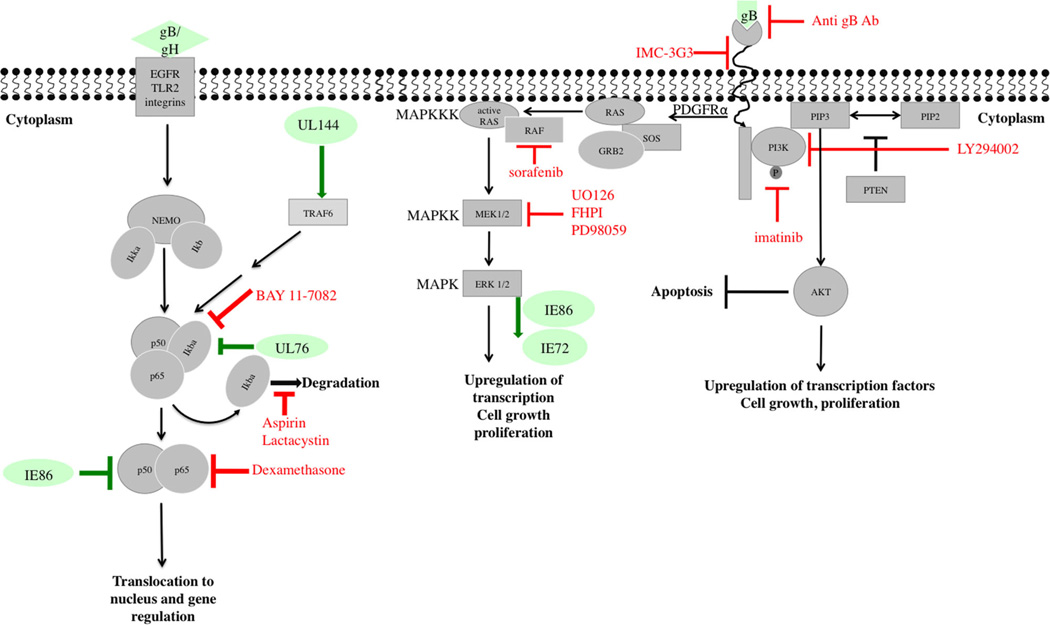
Platelet-derived growth factor receptors (PDGFRs) are tyrosine kinase receptors, minimally expressed in normal tissues, but over-expressed in multiple malignancies. There are two types of PDGFR, α and β, and their ligands, PDGFs A and B, are important for cell migration and proliferation. Physiologically, they participate in wound healing, inflammation and angiogenesis. Blocking PDGFR-α with an anti-PDGFR-α antibody or imatinib mesylate (Gleevec), an inhibitor of several tyrosine kinases used for treatment of chronic myeloid leukemia and solid gastrointestinal tumors, inhibited entry of several CMV strains and viral gene expression in fibroblasts and epithelial/endothelial cells (Figure 1). Neutralizing antibodies against CMV-gB prevented PDGFR-α activation, suggesting a potential interaction between gB and PDGFR-α (4). However, in another study (5) CMV entry was not blocked by anti-PDGFR-α antibody or by short hairpin silencing of PDGFR-α in epithelial cells. Since PDGFR-α transduction enhanced entry of TR (a clinical isolate) and AD169 (a laboratory-adapted strain) into epithelial/endothelial cells, it was suggested that CMV may not interact with PDGFR-α, but rather PDGFR-α induces a novel entry pathway for CMV (5). Additional studies are required to elucidate how PDGFR-α promotes CMV entry, which could lead to identification of specific inhibitors of CMV entry.
Figure 1. Modulation of PDGFR, MAPK and NF-κB pathways by CMV.
Depicted are schematics of PDGFR-α, MAPK and NF-κB signaling pathways as well as downstream effects and resulting cell activities. Viral proteins that modulate or interact with these pathways at various stages are shown in green. Compounds or antibodies that were reported to inhibit CMV replication by targeting specific steps in a given pathway are shown in red. Cellular proteins appear in gray. CMV, cytomegalovirus; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa bet; PDGFR, platelet-derived growth factor receptor.
AMP-Activated Protein Kinase
AMP-activated protein kinase (AMPK) consists of three subunits (α, β, γ) and plays a central role in numerous metabolic processes. Through adenylate kinase action, low adenosine triphosphate (ATP) levels result in increased concentrations of adenosine monophosphate (AMP), which, in turn, induce AMP binding to AMPK and subsequent stimulation of AMPK activity, primarily through liver kinase B1 or Ca2+-calmodulin-dependent kinase kinase (CaMKK)-dependent phosphorylation. Upon activation, AMPK restores ATP pools by activating ATP-producing pathways and inhibiting ATP-consuming pathways.
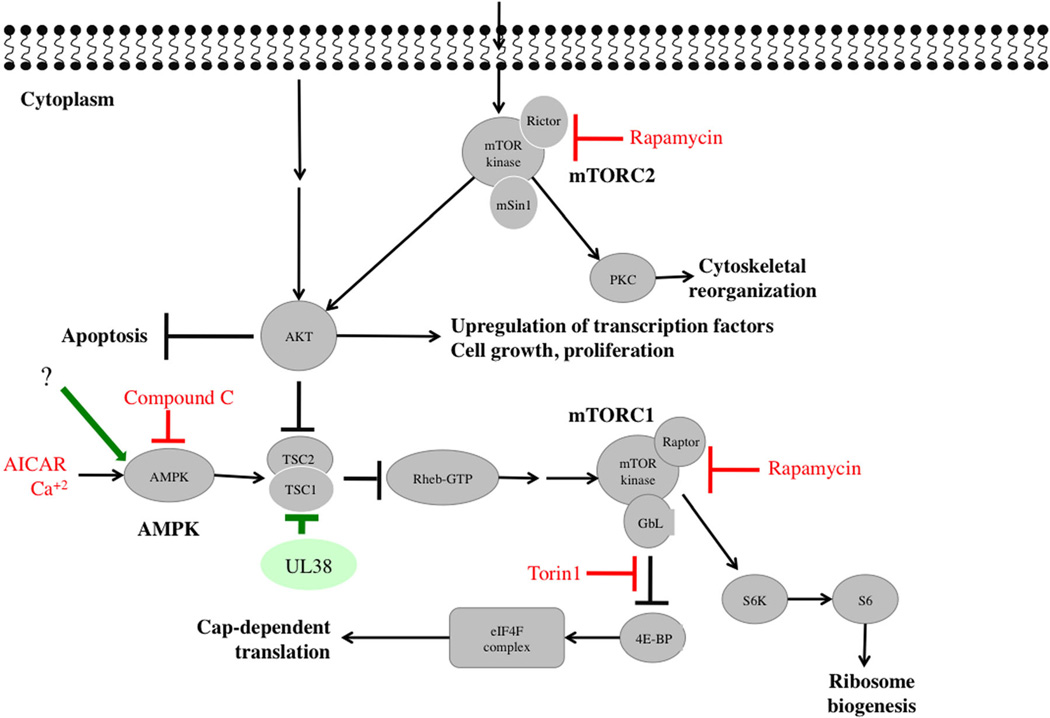
RNA inhibition screen identified the AMPK pathway as a key kinase in CMV replication (6,7). An AMPK inhibitor (compound C) reduced CMV-induced metabolic changes and infectious progeny (6,7). AMPK inhibition attenuated early and late CMV gene expression and viral DNA synthesis, but had no impact on CMV immediate early (IE) gene expression, suggesting the effects of AMPK occur at the IE to early transition of viral gene expression. Inhibition of CaMKK with a small-molecule ST0–609 blocked CMV-mediated AMPK activation and virus production. Interestingly, specific levels of AMPK activity are required to create a favorable environment for CMV replication, since both AICAR (an AMPK activator) and compound C abrogated CMV replication (Figure 2). The fine, tightly regulated balance of this pathway is also supported by: (a) Akt activation reduces AMPK activity (8). Since CMV induces Akt early during infection (4), it must activate AMPK using alternative pathways; (b) AMPK activates tuberous sclerosis complex 1/2 (TSC1/2) to decrease mammalian target of rapamycin (mTOR) signaling, which would not be beneficial to CMV. However, CMV-encoded UL38 binds to TSC1 and prevents it from responding to AMPK phosphorylation (9). The net effect of protein function changes during infection, resulting in productive or abortive replication.
Figure 2. Modulation of AMPK and mTOR pathways by CMV.
The AMPK and mTOR pathways with viral effectors are shown. Extracellular signals and upstream proteins resulting in the activation of Akt are shown in greater details in Figure 1. Viral proteins that modulate or interact with these pathways at various stages are shown in green. Compounds or antibodies that were reported to inhibit CMV replication by targeting specific steps in a given pathway are shown in red. Cellular proteins appear in gray. AMPK, AMP-activated protein kinase; CMV, cytomegalovirus; mTOR, mammalian target of rapamycin.
A novel role of AMPK was discovered in cancer cells, sensing genomic stress and activating the DNA damage response (DDR) pathway. Chemotherapy and radiation activated AMPK to mediate signal transduction downstream of ataxia telangiectasia mutated (ATM) (10). Similar interactions in CMV-infected cells have not been studied.
Mammalian Target of Rapamycin
mTOR, a member of the phosphatidylinositol 3-kinase (PI3K) related kinase superfamily, is found in two complexes that have distinct functions and different sensitivities to rapamycin. mTORC 1 (consisting of mTOR, raptor and mLST8) regulates translation and cell growth via phosphorylation of S6 kinase (S6K) and eukaryotic initiation factor eIF4E binding protein (4E-BP), and is inhibited by rapamycin. mTORC2 (consisting of mTOR, rapamycin-insensitive rictor and mSIN1) regulates a diverse set of substrates, including AKT S473, glucocorticoid-regulated kinase, and protein kinase C-α, and is resistant to acute (but not chronic) administration of rapamycin; chronic administration inhibits both mTORC1 and mTORC2 (Figure 2).
Clinical and basic studies demonstrate the importance of this pathway in CMV replication. Retrospective and prospective studies identified the benefit of mTOR inhibitors, sirolimus (Rapamune) and everolimus (Afinitor), in reducing the risk of CMV infection compared to other immunosuppressive regimens (11). The underlying mechanisms for decreased episodes of CMV are not well understood, but may involve an improvement in CMV-specific CD8+/CD4+ T cell responses (12). Everolimus add-on therapy against CMV disease in comparison to valganciclovir/ganciclovir alone is in phase II clinical trial for CMV disease in renal transplant recipients (ClinicalTrials.gov NCT00828503). These studies are important for directing immunosuppressive therapy in CMV-positive solid organ recipients and in supporting implementation of anti-CMV agents that target cell-signaling pathways (rather than directly inhibiting a virus protein).
In vitro studies have elegantly shown a complex and dynamic relationship between CMV and components of mTOR, leading to its activation at different time points during infection and altering its expected sensitivity to rapamycin. CMV IE proteins activate PI3K/Akt, resulting in mTOR activation and maintaining cap-dependent translation (13,14). Although this pathway is inhibited by rapamycin, the function of eIF4F is maintained in CMV-infected cells (13). CMV also activates mTORC2 via increased phosphorylation of Akt S473. mTORC2 is important in CMV replication, since CMV inhibition by rapamycin is rictor-, not mTORC1-, dependent, and both raptor- and rictor-containing complexes mediate the phosphorylation of 4E-BP and S6K (13). Use of Torin1, which inhibits protein synthesis by disrupting the formation of the eIF4F complex, revealed that rapamycin-resistant mTORC1 activity is required for CMV DNA accumulation (Figure 2) (14). Torin1 activities were mTORC2-independent because they occurred in cells lacking rictor. Thus, inhibition of eIF4F-dependent translation by Torin1 may result in decreased expression of cellular protein(s) necessary for viral DNA replication.
CMV induction of mTOR indicates that it overcomes AMPK inhibition of mTOR, the latter mediated through phosphorylation of TSC1/2. CMV-encoded UL38 binds to TSC1 and prevents it from responding to AMPK phosphorylation (9). While early AMPK activation (by AICAR) inhibited CMV-induced phosphorylation of 4E-BP and S6K, likely secondary to virus inhibition (15), AICAR treatment at 12 h post-infection did not inhibit CMV or the activation of 4E-BP/S6K. Taken together, CMV controls components of mTOR and its upstream effectors. While both AMPK activation and inhibition constrain CMV replication, these activities occur at different stages of virus replication resulting in differential effects on other signaling pathways (6).
Ataxia Telangiectasia Mutated and the DNA Damage Response
ATM is a central protein kinase in DDR. It is activated in response to DNA double-strand breaks and phosphorylates downstream proteins to initiate the DNA damage checkpoint, leading to cell cycle arrest and DNA repair, or, if damage is too severe, to apoptosis. A human protein microarray identified approximately 100 shared substrates of all herpesvirus-conserved kinases (16). DDR proteins were enriched, and the histone acetyltransferase TIP60 (an upstream regulator of DDR) was required for replication of all tested herpesviruses. Knockdown of TIP60 in CMV-infected cells reduced extracellular viral progeny.
CMV deregulates the DDR pathway, initially by activating ATM and ataxia telangiectasia and rad-3 related kinases (ATR) (17–19) followed by blockage at the checkpoint kinase 2 (Chk2) (19).ATM and Chk2, which normally localize to the nucleus, instead migrate later during infection to the cytoplasm where they colocalize with virion structural proteins. Despite localizing to viral replication centers, proteins required for nonhomologous end joining, which might rejoin viral replicating DNA ends, are excluded from the replication centers (17). Thus, the host DDR (targeted toward viral inhibition) seems to become dysfunctional (17,19).
The exact requirement of ATM for CMV replication is debated: originally, CMV was reported to replicate in cells lacking ATM (17), but a recent report suggests inhibition of virus replication in these cells (20). The difference in these findings may not be adequately explained by strain differences, but could originate from the use of cells at different stages of cell cycle. The E2F1 transcription factor may play a role in mediating DDR and CMV replication (20). Since ATM and ATR control multiple pathways, additional studies are required to elucidate how CMV targets the DDR, and which specific components are regulated by CMV. The CMV protein kinase, UL97, phosphorylates and inactivates Rb tumor suppressor gene (21), releasing E2F1, which regulates transcription of many genes, including those required for S-phase progression and DNA repair. AMPK may therefore control DDR through Rb in CMV-infected cells.
Cyclin-Dependent Kinases
Cyclin-dependent kinases (CDKs) control cell cycle progression. The human genome encodes for 13 putative CDKs and 25 cyclins. Many viruses modify CDK activity to regulate their own replication cycle and synchronize it to the host cell so as to avail of the required enzymes and biomolecules. The host cell in turn, has evolved multiple checkpoints that prevent a cell from replicating when damaged and/or infected. CMV infection modifies cell cycle regulation and CDKs. Several steps of CMV replication require CDK1, CDK2, CDK7 and CDK9 along with their respective cyclins (reviewed in (22)). Roscovitine (Seliciclib), a CDK2 inhibitor used in clinical trials for B cell malignancies and lung cancer, disrupts IE gene expression and halts virus replication (23).
CDKs are induced at a very early stage of CMV infection (24). During the S/G2 phase of the cell cycle, CMV cannot initiate IE gene expression. When cellular CDK is inhibited by roscovitine, CMV overcomes this limitation and S/G2 cells become fully permissive for CMV. Moreover, in undifferentiated teratocarcinoma cells, in which CMV establishes latency, CDK inhibition relieves the block of IE gene expression and reactivates CMV, suggesting a broader role for CDK activity in generating a nonpermissive host environment for viral gene expression (24). Thus, the mobilization of CDKs and progression of CMV replication are also delicately balanced, dependent not only on the relative levels of host and viral proteins, but also on the stage of viral replication relative to the host cell cycle.
Phosphatidylinositol-3-Kinase
Binding of CMV envelope glycoproteins to host cell receptors causes an immediate activation of mitogenic cell-signaling pathways required for virus replication, such as the cellular PI3K (Figure 1). LY294002, a specific PI3K inhibitor, reduced viral titers in fibroblasts; it did not inhibit CMV entry, but down-regulated viral IE gene expression (25). Adding downstream molecules of the PI3K pathway restored virus replication, confirming the specificity of LY294002 for PI3K (25).
Phosphorylation of PI3K in CMV-infected cells results in subsequent activation of Akt, p70 S6K and nuclear factor kappa beta (NF-κB). Activation of these targets suppresses apoptosis, yet activates host gene expression and protein synthesis (25,26). The following main pathways downstream of the signaling pathways mentioned above are dysregulated by CMV.
Nuclear factor kappa beta
This transcription factor family is a central hub of signaling events and is rapidly activated by multiple triggers, including viruses, many of which harbor binding sites for NF-κB in their promoter elements to ensure a strong and programmed cellular response. CMV-gB and gH interact with cellular receptors (epidermal growth factor receptor, integrins, Toll-like receptor 2), resulting in activation of NF-κB and specificity protein 1; (Figure 1) (27–30). Signaling activation through gB/gH was reported in fibroblasts and monocytes, indicating this to be an important strategy for CMV. In monocytes NF-κB maintains the balance between latency and reactivation (31).
Following virus entry, NF-κB activates viral IE genes, mainly through binding to the CMV major IE promoter (MIEP) (32). Since NF-κB activation, albeit an important strategy for efficient CMV replication (27,33), will ultimately trigger the synthesis of anti-viral cytokines, CMV must balance these deleterious effects. This is corroborated by the lack of effect of NF-κB binding site deletion in MIEP on CMV replication (34,35) and IE86-mediated suppression of NF-κB-dependent cytokine/chemokine gene expression (36). Although NF-κB inhibitors (aspirin and MG-132) blocked CMV MIEP activation (37), off-target effects of these compounds should be considered.
Specific viral components that modulate NF-κB activity were recently identified. UL76 (a highly conserved virion-associated protein) induced IL-8 expression through the NF-κB pathway (38) and UL144 (a truncated tumor necrosis factor (TNF)α-receptor homolog), present in clinical isolates but deleted in laboratory-adapted strains, up-regulated NF-κB activity via TNF receptor-associated factor 6 (35).
NF-κB activation, an integral response of both host and virus, is central to pro- and anti-viral responses. The resolution of the apparently conflicting outcomes would require the separation of these two contrary facets of NF-κB activation. Given the complex effects of CMV on NF-κB signaling, there is no focal point at which the NF-κB network can be specifically targeted to contain viral infection or improve host response. Compounds such as artesunate were reported to inhibit CMV replication (in addition to their anti-malarial activities) and down-regulate NF-κB (39). However, their mechanism of CMV inhibition is currently unknown and NF-κB inhibition likely represents a final readout of upstream effects.
RAS/RAF/ERK pathway
Mitogen-activated protein kinase (MAPK) pathways are kinase modules that coordinate cellular response to extracellular signals resulting in growth, proliferation, differentiation, migration and apoptosis. Five different groups of MAPKs have been identified in mammals. Of these, CMV activates ERK1/2 and p38 MAPK pathways for its productive replication (Figure 1). The kinase inhibitor, sorafenib (Nexavar, used for treatment of solid tumors), inhibited replication of different CMV strains. IE expression was decreased through inhibition of Raf, and depletion of Raf reduced IE expression (40). MEK1/2 inhibitors, U0126 and PD98059, inhibited IE72 and IE86 phosphorylation in infected cells without altering their protein expression (41,42). At the transcriptional level, the UL4 promoter is MAPK-responsive. MEK1/2 inhibitor, U0126, and the p38 MAPK inhibitor, FHPI, reduced UL4 promoter activity in vitro. However, endogenous UL4 RNA expression was not as low, suggesting an MEK/MAPK independent component to UL4 activation (43).
Recently identified pathways with or without requirement for protein kinases are as follows.
Unfolded Protein Response and Ubiquitin-Mediated Protein Degradation
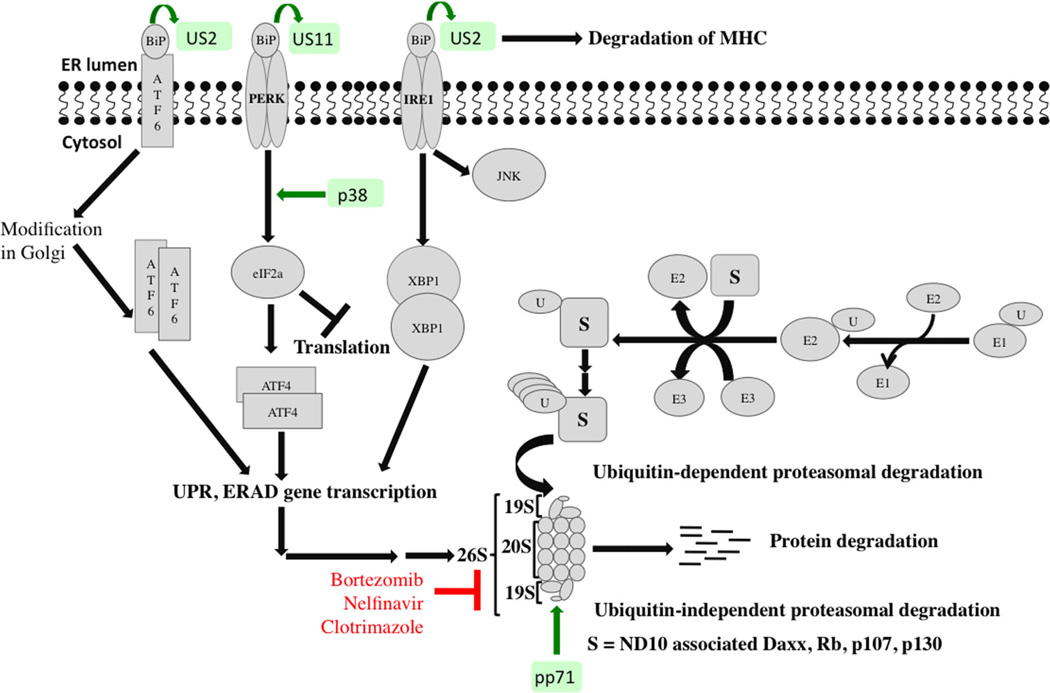
The proteasome is indispensable for selective degradation of cellular proteins. Its major function is to prevent accumulation of damaged, misfolded and mutant proteins. Proteins degradation is an ATP-dependent multistage process that requires ubiquitination of the target protein prior to its degradation by the 26S proteasome. Ubiquitination is achieved via an enzymatic cascade involving E1 (ubiquitin-activating), E2 (ubiquitin-conjugating) and E3 (ubiquitin ligase) enzymes. Generally, proteasome activity increases during the course of CMV infection, and proteasome inhibition have negative effects on CMV replication (44,45), including reduction of expression of early and late CMV proteins (46). The 19S and other proteasome subunits relocalize to the periphery of replication centers. The viral tegument protein, pp71, promotes ubiquitin-independent, proteasome-mediated degradation of nuclear domain 10-associated Daxx, Rb, p107 and p130 proteins in favor of virus replication (47,48).
Unfolded protein response (UPR), functionally related to the proteasome, is a mechanism that counters endoplasmic reticulum (ER) stress resulting from unfolded polypeptide chains exceeding the protein folding capacity of the ER. CMV modulates multiple ER stress response pathways to its advantage, since prolonged ER stress (leading to apoptosis) would be detrimental to virus replication (Figure 3).
Figure 3. Modulation of UPR and proteasome-mediated degradation by CMV.
Simplified schematic of the crosstalk between UPR and proteasome-mediated protein degradation is shown. “S” represents substrate proteins being targeted to the 26S proteasome. Viral proteins that modulate or interact with these pathways at various stages are shown in green. Compounds or antibodies that were reported to inhibit CMV replication by targeting specific steps in a given pathway are shown in red. Cellular proteins appear in gray. CMV, cytomegalovirus; UPR, unfolded protein response.
Under normal conditions, ER chaperone BiP is bound to inositol-requiring enzyme 1 (IRE-1) and PKR-like ER kinase (PERK), rendering them inactive. In response to ER stress, BiP is sequestered away to bind to unfolded proteins, releasing and activating PERK and IRE-1. PERK phosphorylates initiation factor 2 (eIF2) leading to a general attenuation of translation (inhibitory to viral growth). However, it increases translation of activating transcription factor 4 (ATF4), which, in turn, transcribes genes encoding metabolism and redox regulatory factors to help the cell recover from ER stress. CMV activates the UPR to benefit viral replication. Regulation of PERK, ATF6, and IRE-1 by CMV results in a replication-favorable environment. Although CMV infection activated eIF2, the phosphorylation of eIF2 was limited such that global translation was barely inhibited. Thus, the positive effects of ATF4 activation were maintained while virus damage from PERK activation was limited (49). CMV-UL38 modulates ER stress and prevents ER stress-induced cell death independent of mTORC1 activation (50). UL38-deficient CMV fails to up-regulate PERK and eIF2 phosphorylation resulting in loss of robust ATF4 accumulation (Figure 3). Although ATF6 activation was suppressed in CMV-infected cells, genes that are normally activated by ATF6 were activated via an ATF6-independent mechanism. CMV infection also activated the IRE-1 pathway, indicated by splicing of Xbp-1 mRNA. However, transcriptional activation of the XBP-1 target gene was inhibited.
Another marker of CMV-induced stress response is the accumulation of BiP. A BiP-specific subtilase cytotoxin SubAB blocks CMV assembly. CMV proteins US2 and US11 bind to BiP to degrade MHC class I proteins, preventing viral recognition by the host immune response (51,52). Induction of ER stress and terminal UPR as a strategy for virus control was tested using Clotrimazole, which induces ER stress by disrupting ER calcium homeostasis. Clotrimazole inhibited production of CMV virions in vitro primarily because of an inhibition in global translation (53). Protease inhibitors induce ER stress and cause accumulation of misfolded proteins. Although the HIV-protease inhibitor, Nelfinavir, was reported to inhibit herpesviruses including CMV, whether this inhibition is UPR-dependent is unknown (54).
Wnt Signaling
Signaling by the Wnt family of secreted glycoproteins is a fundamental mechanism directing cell proliferation, cell polarity and cell fate determination during embryonic development and tissue homeostasis. The most studied Wnt pathway is the canonical Wnt signaling, which regulates the amount of the transcriptional co-activator bcatenin and controls key developmental gene expression programs. It is a tightly regulated pathway and deregulated frequently in cancers. Because of the role Wnt signaling plays in embryonic development, a time in which CMV infects multiple cells and causes injury to major organs, it is not surprising that CMV would control this pathway. Recent reports from cancer chemotherapy suggest that cancer cure depends on targeting stem cells within the tumor environment that are resistant to available chemotherapeutic agents (55). Compounds that inhibit cancer stem cell growth via modulation of Wnt were recently reported (56). One of these compounds, salinomycin, was toxic to cancer cells but had no toxic effects on normal cells. While Wnt signaling is targeted and induced by γ-herpesviruses, EBV and KSHV, the interaction of CMV with this pathway appears to be unique and finely balanced. CMV infection of human fibroblasts and extravillous cytotrophoblasts results in degradation of β-catenin and a decrease in its transcriptional activity in response to Wnt ligand stimulation (57).We investigated the effects of CMV infection on several components of the Wnt pathway (58). CMV infection resulted in significant decrease in the expression of Wnt 5a/b and β-catenin, an effect that was augmented as infection proceeded. Phosphorylated and total lipoprotein receptor related protein 6 levels were also reduced significantly in CMV-infected cells. CMV infection resulted in enhanced and sustained expression of the negative Wnt regulator, axin 1. Interestingly, Wnt modulating agents, monensin, nigericin and salinomycin, inhibited CMV replication, resulting in decreased expression of axin 1 and β-catenin, demonstrating again the concept of “right amount at the best timing” for efficient CMV replication, and supporting further research to disrupt this fine balance. Wnt modulators were suggested as potential biomarkers for CMV disease (59).
Summary
CMV uses multiple cell-signaling pathways to achieve efficient replication. There is growing interest in identifying compounds that can modulate cellular pathways used by CMV. Such “anti-viral anti-cellular” agents may not be under direct viral control and therefore are less likely to select for resistant mutants. Compounds with specific effects on cellular pathways have been reported to inhibit CMV replication. Broadly, these can be grouped into early inhibitors of CMV replication and others active at multiple steps of virus replication. An ongoing concern with these compounds is their potential cellular toxicity especially during prolonged course of therapy. Although host protein kinases and the downstream pathways they control play critical roles in CMV replication, studies are needed to identify kinases that have a good selectivity against CMV replication; that is the therapeutic window between cellular toxicity and virus inhibition must be demonstrated. Recently discovered anti-CMV agents that modulate cell-signaling pathways have shown differential toxicity; that is they were toxic to cancer cells while nontoxic to primary cells. Dissection of CMV interaction with specific components of cell-signaling pathways could pave the way to developing novel anti-viral agents.
Acknowledgments
This work was supported by the National Institutes of Health grant 1R01AI093701 and March of Dimes #6-FY11–268 (RA-B). We apologize to CMV investigators for not being able to cite all their important contributions in this review.
Abbreviations
- 4E-BP
eukaryotic initiation factor eIF4E binding protein
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ATF4
activating transcription factor 4
- ATM
ataxia telangiectasia mutated
- ATP
adenosine triphosphate
- CaMKK
Ca2+-calmodulin-dependent kinase kinase
- CDK
cyclin-dependent kinase
- Chk2
checkpoint kinase 2
- CMV
cytomegalovirus
- DDR
DNA damage response
- ER
endoplasmic reticulum
- ERK
extracellular signal-related kinase
- gB
glycoprotein B
- IE
immediate early
- IRE-1
inositol-requiring enzyme 1
- MAPK
mitogen-activated protein kinase
- MIEP
major IE promoter
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa beta
- PDGFR
platelet-derived growth factor receptor
- PERK
PKR-like ER kinase
- PI3K
phosphatidylinositol-3-kinase
- S6K
S6 kinase
- TNF
tumor necrosis factor
- TSC 1/2
tuberous sclerosis complex 1/2
- UPR
unfolded protein response
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Mocarski ES, Shenk T, Griffiths PD, Pass RF. Chapter 6: Cytomegaloviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; Health; 2013. pp. 1961–2014. [Google Scholar]
- 2.Cha TA, Tom E, Kemble GW, Duke GM, Mocarski ES, Spaete RR. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL 131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 5.Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC. PDGF receptor-alpha does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog. 2012;8:e1002905. doi: 10.1371/journal.ppat.1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terry LJ, Vastag L, Rabinowitz JD, Shenk T. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2012;109:3071–3076. doi: 10.1073/pnas.1200494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McArdle J, Moorman NJ, Munger J. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8:e1002502. doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 9.Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe. 2008;3:253–262. doi: 10.1016/j.chom.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15:156–169. doi: 10.4161/cbt.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nashan B, Gaston R, Emery V, et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation. 2012;93:1075–1085. doi: 10.1097/TP.0b013e31824810e6. [DOI] [PubMed] [Google Scholar]
- 12.Havenith SH, Yong SL, van Donselaar-van derPant KA, et al. Everolimus-treated renal transplant recipients have a more robust CMV-specific CD8+T-cell response compared with cyclosporine-or mycophenolate-treated patients. Transplantation. 2013;95:184–191. doi: 10.1097/TP.0b013e318276a1ef. [DOI] [PubMed] [Google Scholar]
- 13.Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc Natl Acad Sci U S A. 2006;103:14182–14187. doi: 10.1073/pnas.0605825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol. 2010;84:5260–5269. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol. 2007;81:3649–3651. doi: 10.1128/JVI.02079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Zhu J, Xie Z, et al. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe. 2011;10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo MH, Rosenke K, Czornak K, Fortunato EA. Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)-and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection. J Virol. 2007;81:1934–1950. doi: 10.1128/JVI.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo JP, Frame FM, Rogoff HA, Pickering MT, Yurochko AD, Kowalik TF. Human cytomegalovirus IE 1–72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J Virol. 2005;79:11467–11475. doi: 10.1128/JVI.79.17.11467-11475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspar M, Shenk T. Human cytomegalovirus inhibits a DNA damage response by mislocalizing checkpoint proteins. Proc Natl Acad Sci U S A. 2006;103:2821–2826. doi: 10.1073/pnas.0511148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering EX, Debatis MT, Castillo M, et al. An E2F1-mediatedDNA damage response contributes to the replication of human cytomegalovirus. PLoS Pathog. 2011;7:e1001342. doi: 10.1371/journal.ppat.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hume AJ, Finkel JS, Kamil JP, Coen DM, Culbertson MR, Kalejta RF. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320:797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez V, Spector DH. Subversion of cell cycle regulatory pathways. Curr Top Microbiol Immunol. 2008;325:243–262. doi: 10.1007/978-3-540-77349-8_14. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez V, McElroy AK, Yen J, et al. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL 122–123 and UL37 immediate-early transcripts and at later times for virus production. J Virol. 2004;78:11219–11232. doi: 10.1128/JVI.78.20.11219-11232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zydek M, Hagemeier C, Wiebusch L. Cyclin-dependent kinase activity controls the onset of the HCMV lytic cycle. PLoS Pathog. 2010;6:e1001096. doi: 10.1371/journal.ppat.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, Alwine JC. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3’-OH kinase pathway and the cellular kinase Akt. J Virol. 2002;76:3731–3738. doi: 10.1128/JVI.76.8.3731-3738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yurochko AD, Mayo MW, Poma EE, Baldwin AS, Jr, Huang ES. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-kappaB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 29.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soderberg-Naucler C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest. 1997;100:3154–3163. doi: 10.1172/JCI119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambucetti LC, Cherrington JM, Wilkinson GW, Mocarski ES. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalik TF, Wing B, Haskill JS, Azizkhan JC, Baldwin AS, Jr, Huang ES. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc Natl Acad Sci U S A. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustems M, Borst E, Benedict CA, et al. Regulation of the transcription and replication cycle of human cytomegalovirus is insensitive to genetic elimination of the cognate NF-kappaB binding sites in the enhancer. J Virol. 2006;80:9899–9904. doi: 10.1128/JVI.00640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benedict CA, Angulo A, Patterson G, et al. Neutrality of the canonical NF-kappaB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro. J Virol. 2004;78:741–750. doi: 10.1128/JVI.78.2.741-750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor RT, Bresnahan WA. Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NFkappaB-dependent gene expression. J Virol. 2006;80:10763–10771. doi: 10.1128/JVI.01195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMeritt IB, Milford LE, Yurochko AD. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol. 2004;78:4498–4507. doi: 10.1128/JVI.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa H, Nascimento R, Sinclair J, Parkhouse RM. Human cytomegalovirus gene UL76 induces IL-8 expression through activation of the DNA damage response. PLoS Pathog. 2013;9:e1003609. doi: 10.1371/journal.ppat.1003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efferth T, Marschall M, Wang X, et al. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med. 2002;80:233–242. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- 40.Michaelis M, Paulus C, Loschmann N, et al. The multi-targeted kinase inhibitor sorafenib inhibits human cytomegalovirus replication. Cell Mol Life Sci. 2011;68:1079–1090. doi: 10.1007/s00018-010-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodems SM, Spector DH. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J Virol. 1998;72:9173–9180. doi: 10.1128/jvi.72.11.9173-9180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson RA, Ma XL, Yurochko AD, Huang ES. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J Gen Virol. 2001;82:493–497. doi: 10.1099/0022-1317-82-3-493. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Stinski MF. Activation of transcription of the human cytomegalovirus early UL4 promoter by the Ets transcription factor binding element. J Virol. 2000;74:9845–9857. doi: 10.1128/jvi.74.21.9845-9857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaspari M, Tavalai N, Stamminger T, Zimmermann A, Schilf R, Bogner E. Proteasome inhibitor MG132 blocks viral DNA replication and assembly of human cytomegalovirus. FEBS Lett. 2008;582:666–672. doi: 10.1016/j.febslet.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Prosch S, Priemer C, Hoflich C, et al. Proteasome inhibitors: A novel tool to suppress human cytomegalovirus replication and virus-induced immune modulation. Antivir Ther. 2003;8:555–567. [PubMed] [Google Scholar]
- 46.Tran K, Mahr JA, Spector DH. Proteasome subunits relocalize during human cytomegalovirus infection, and proteasome activity is necessary for efficient viral gene transcription. J Virol. 2010;84:3079–3093. doi: 10.1128/JVI.02236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalejta RF, Shenk T. The human cytomegalovirus UL82 gene product (pp71) accelerates progression through the G1 phase of the cell cycle. J Virol. 2003;77:3451–3459. doi: 10.1128/JVI.77.6.3451-3459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalejta RF, Bechtel JT, Shenk T. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol Cell Biol. 2003;23:1885–1895. doi: 10.1128/MCB.23.6.1885-1895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian Z, Xuan B, Gualberto N, Yu D. The human cytomegalovirus protein pUL38 suppresses endoplasmic reticulum stress-mediated cell death independently of its ability to induce mTORC1 activation. J Virol. 2011;85:9103–9113. doi: 10.1128/JVI.00572-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegde NR, Chevalier MS, Wisner TW, et al. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J Biol Chem. 2006;281:20910–20919. doi: 10.1074/jbc.M602989200. [DOI] [PubMed] [Google Scholar]
- 52.Buchkovich NJ, Maguire TG, Yu Y, Paton AW, Paton JC, Alwine JC. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J Virol. 2008;82:31–39. doi: 10.1128/JVI.01881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isler JA, Maguire TG, Alwine JC. Production of infectious human cytomegalovirus virions is inhibited by drugs that disrupt calcium homeostasis in the endoplasmic reticulum. J Virol. 2005;79:15388–15397. doi: 10.1128/JVI.79.24.15388-15397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gantt S, Carlsson J, Ikoma M, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrob Agents Chemother. 2011;55:2696–2703. doi: 10.1128/AAC.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu D, Carson DA. Inhibition of Wnt signaling and cancer stem cells. Oncotarget. 2011;2:587. doi: 10.18632/oncotarget.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelova M, Zwezdaryk K, Ferris M, Shan B, Morris CA, Sullivan DE. Human cytomegalovirus infection dysregulates the canonical Wnt/beta-catenin signaling pathway. PLoS Pathog. 2012;8:e1002959. doi: 10.1371/journal.ppat.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapoor A, He R, Venkatadri R, Forman M, Arav-Boger R. Wnt modulating agents inhibit human cytomegalovirus replication. Antimicrobial Agents Chemother. 2013;57:2761–2767. doi: 10.1128/AAC.00029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ueland T, Rollag H, Hartmann A, et al. Secreted Wnt antagonists during eradication of cytomegalovirus infection in solid organ transplant recipients. Am J Transplant. 2014;14:210–215. doi: 10.1111/ajt.12506. [DOI] [PubMed] [Google Scholar]