Abstract
The close association of Epstein–Barr virus (EBV) infection with non-keratinizing nasopharyngeal carcinomas and a subset of gastric carcinomas suggests that EBV infection is a crucial event in these cancers. The difficulties encountered in infecting and transforming primary epithelial cells in experimental systems suggest that the role of EBV in epithelial malignancies is complex and multifactorial in nature. Genetic alterations in the premalignant epithelium may support the establishment of latent EBV infection, which is believed to be an initiation event. Oncogenic properties have been reported in multiple EBV latent genes. The BamH1 A rightwards transcripts (BARTs) and the BART-encoded microRNAs (miR-BARTs) are highly expressed in EBV-associated epithelial malignancies and may induce malignant transformation. However, enhanced proliferation may not be the crucial function of EBV infection in epithelial malignancies, at least in the early stages of cancer development. EBV-encoded gene products may confer anti-apoptotic properties and promote the survival of infected premalignant epithelial cells harbouring genetic alterations. Multiple EBV-encoded microRNAs have been reported to have immune evasion functions. Genetic alterations in host cells, as well as inflammatory stroma, could modulate the expression of EBV genes and alter the growth properties of infected premalignant epithelial cells, encouraging their selection during carcinogenesis.
Keywords: Epstein–Barr virus, nasopharyngeal carcinoma, gastric carcinoma, lymphoeptithelioma-like carcinomas, BARTs, LMP1
Introduction
Epstein–Barr virus (EBV) is a human cancer-associated virus that infects >90% of the global population 1. Despite its close association with a range of lymphoid and epithelial malignancies, the virus does not cause major symptoms in the majority of lifelong carriers with EBV-infected B memory lymphocytes 2,3. The role of EBV in transformation in human malignancies remains unclear, particularly in epithelial cancers (but see a recent review elsewhere in this Issue for the role of EBV in lymphomas 3). For the past two decades, increasing interest has focused on the EBV-associated epithelial cancers that represent 80% of all EBV-associated malignancies 1. Among these, nasopharyngeal carcinoma (NPC) and EBV-associated gastric cancers (EBVaGCs) are the most common, with 78 000 and 84 000 new cases, respectively, reported annually worldwide 1. Clonal EBV genome and the expression of a subset of viral latent gene products are consistently detected in practically every cell in these cancers 4,5. Therefore, a crucial role of EBV in the pathogenesis of these cancers has been postulated.
NPC is a distinctive histological subtype of head and neck cancer arising from the nasopharynx. The incidence and mortality rates of NPC are remarkably high in southern China and South-East Asia, but NPC is rarely seen in Western countries 6. According to the recent World Health Organization (WHO) classification, NPC is classified into two major histological subtypes: non-keratinizing carcinoma (either differentiated or undifferentiated) and keratinizing squamous cell carcinoma 7. Non-keratinizing NPC is consistently associated with EBV infection and accounts for the majority of NPCs in endemic regions. It is commonly described as lympho-epithelioma of the nasopharynx because of its prominent lymphocytic infiltration (Figure 1). EBV latent infection is also seen in keratinizing NPCs from endemic regions, but not in non-endemic regions. In summary, almost 98% of all NPCs are EBV-associated.
Figure 1.
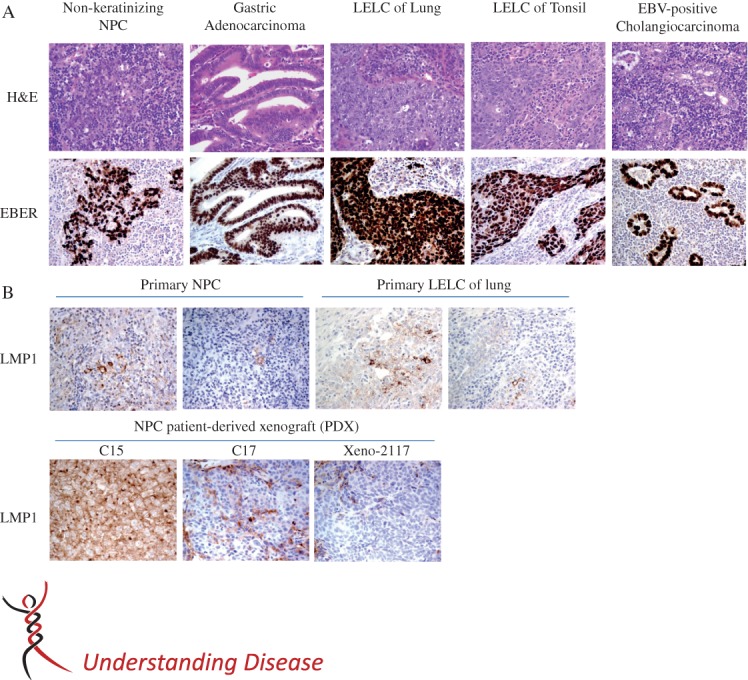
Epstein–Barr virus (EBV) latent infection in various epithelial malignancies. (A) Histopathology of Epstein–Barr virus (EBV)-positive carcinomas (upper panel) and their corresponding EBER in situ hybridization (lower panel). Nasopharyngeal carcinomas (NPC) most commonly form syncytial sheets or scattered undifferentiated carcinoma cells among dense lymphoplasmacytic infiltrate, and hence display features of lympho-epithelioma-like carcinoma (LELC). A subset of gastric carcinomas which harbour EBV show morphological features of LELC or, more commonly, resemble the usual gastric adenocarcinoma but with variable amounts of lymphoplasmacytic infiltrate. EBV-positive carcinomas in lung and other head and neck regions (e.g. tonsil) have the morphological features of LELC. Rarely, cholangiocarcinoma can harbour EBV. EBV-positive cholangiocarcinoma usually displays morphology of adenocarcinoma with small tubular glands among dense lymphoplasmacytic infiltrate. A representative case of EBV-associated gastric adenocarcinoma, LELC of lung and tonsil and EBV-positive cholangiocarcinoma, are illustrated. (Upper panel) Haematoxylin and eosin (H&E) stain, original magnification = ×400; (lower panel) EBER in situ hybridization, original magnification = ×400. (B) Detection of LMP1 expression in NPC and LELC of lung by immunohistochemical (IHC) staining: (upper panel) LMP1 staining pattern in representative samples of NPC and LELC of lung; LMP is typically expressed in only a small population of scattered carcinoma cells: (lower panel) LMP1 expression patterns in three NPC xenografts; in Xeno-2117 and C17, LMP1 is also expresssed in a small population of scattered carcinoma cells; however, in C15, the IHC staining signal of LMP1 exhibits diffuse positivity; original magnification = ×400.
EBV infection is also detected in two types of gastric cancer; in 16% of conventional gastric adenocarcinomas and 89% of lympho-epithelioma-like gastric carcinomas. In summary, EBVaGCs represent approximately 10% of all gastric cancers and are not an endemic disease 8,9. Lymphoeptithelioma-like carcinoma (LELC) is defined as a poorly differentiated carcinoma with dense lymphocytic infiltration and has similar histological features to undifferentiated NPC. In addition to NPC and EBVaGC, EBV is also consistently detected in LELCs of the salivary gland, lung and intrahepatic biliary epithelium (Figure 1), which are rare tumour subtypes found in these regions 10,11. The close association of EBV infection with LELC implies that the poorly differentiated properties of epithelial cells and an inflammatory environment are involved in viral oncogenesis 12, which may also be true for EBV-associated lymphomas 3. The selective expression of EBV genes (type II latency) is believed to contribute to the malignant transformation of epithelial cells by disrupting various cellular processes and signalling pathways. The distinct mutation signature and methylation pattern identified in EBVaGC illustrate that EBV infection facilitates a unique and alternate tumourigenic process in epithelial malignancies 13,14.
EBV infection in epithelial cells
EBV readily infects and transforms primary B cells in vitro into proliferating lymphoblastoid cell lines, which strongly supports its role in B cell malignancies. Lymphoblastoid transformation of B cells by EBV in vivo is the major cause of infectious mononucleosis, a self-limiting lymphoproliferative disease in immunocompetent individuals 2. Primary infection in humans is believed to be initiated by the virus crossing the epithelium of the oropharynx, infecting the naïve B cells present in the Waldeyer's tonsillar ring circumscribing the entrance to the nasopharynx and oropharynx. Through a series of viral latency transcription programmes, the EBV-infected B cells are eventually driven into resting memory B cells and life-long infection is established. The differentiation of memory B cells into plasma cells triggers lytic infection and releases EBV particles that infect the oropharyngeal epithelial cells for viral replication and transmission 15. Persistent EBV infection is maintained by a check-and-balance of the immune system in the body to eliminate EBV-infected cells. In immunocompromized persons, including organ-transplantation recipients and human immunodeficiency virus patients, this intricate balance is tipped, resulting in an uncontrolled proliferation of EBV-infected lymphocytes that can be life-threatening.
Distinct EBV entry mechanisms in epithelial cells
EBV infects B cells and epithelial cells with differential tropism. EBV readily infects B lymphocytes by binding its envelope protein, gp350, to the CR2 (CD21) present on the B cell surface. Binding of viral gp42 with the human leukocyte antigen (HLA) class II protein on the B cell surface activates the core fusion machinery of EBV, which involves the viral envelope proteins gB and gHgL 16,17. EBV infection of epithelial cells is much more inefficient; neither the CR2 nor the HLA class II protein are expressed on the epithelial cell surface. A recent study reported that viral gHgL interacts with the integrin complex, αvβ6 and αvβ8, of epithelial cells to trigger the fusion of the EBV envelope protein with the cell membrane and facilitate entry 17. Gp42 impedes the entry of EBV into epithelial cells by interfering with binding to the gHgL complex. Interestingly, EBV particles released from epithelial cells are rich in gp42, facilitating their infection of B cells but not of epithelial cells, whereas those released from B cells are lacking in gp42, facilitating their infection of epithelial cells. This dual cell tropism suggests that EBV shuttles continuously between B cells and epithelial cells during its infection cycle, which may be crucial for the establishment of persistent infection in humans 16,17. Another EBV glycoprotein, BMRF2, is involved in the infection of polarized epithelial cells at the basolateral surface by cell-free virus through an interaction with α5β1 18.
Notably, EBV infection of nasopharyngeal epithelial cells is greatly enhanced by transforming growth factor-β1 (TGF-β1), which is known to regulate integrin assembly and actin dynamics 19. The downstream events after infection and the intracellular trafficking of EBV to the nucleus for gene transcription have yet to be defined. Latent EBV infection is rarely detected in normal pharyngeal epithelium, but is consistently detected in precancerous lesions and invasive NPC 20,21. The establishment of latent EBV infection in premalignant epithelial cells may represent an essential initiation step in the development of epithelial malignancies.
Host factors modulate persistence EBV latent infection
EBV infection in epithelial cells exhibits an expression programme that is distinct from that of B cells (Table1). EBV infection of primary B cells initiates a robust growth and proliferation programme in which type III latency genes are expressed, including non-coding RNAs (EBERs), six nuclear proteins (EBNA1, EBNA-LP, EBNA2, EBNA3A, EBNA3B and EBNA3C) and three membrane proteins (LMP1, LMP2A and LMP2B) 22. In contrast, EBV infection does not induce clonal expansion in primary epithelial cells 23. EBNA-LP, EBNA2 and EBNA3C, which play a crucial role in B cell immortalization and cell cycle progression, are not expressed in infected epithelial cells. A more restricted group of latent genes (type II latency) are expressed, including EBNA1, LMP1, LMP2A and EBERs 2,19,23,24. Notably, high levels of BamHI A rightward transcripts (BARTs) are expressed in both NPC and EBVaGC, suggesting their involvement in epithelial malignancies 24–26.
Viral gene expression patterns in different Epstein–Barr virus (EBV) latency types
| EBV latency | EBV gene transcription* | Examples |
|---|---|---|
| Type 0 | EBERs | Resting memory B cells |
| Type I | EBERs, EBNA1, BARTs | Burkitt's lymphoma |
| Type II | EBER, EBNA1, LMPs, BARTs | Hodgkin's disease, T/natural killer cell lymphoma, nasopharyngeal carcinoma, gastric carcinoma, other lympho-epithelioma-like carcinomas (?) |
| Type III | EBERs, EBNA1, EBNA-LP, EBNA2, EBNA3A, EBNA3B, EBNA3C, LMPs | Transformed B cells (lymphoblastoid cell lines); human immunodeficiency virus patients, post-transplant lymphoproliferative disorders |
BARTs, BamH1 A transcripts; EBERs, non-coding RNA; EBNA, EBV nuclear antigen; LMP, genes for latent membrane proteins.
Host cell factors and genetic alterations have a profound influence on the gene expression and growth properties of EBV in infected cells. EBV infection was shown not to transform or induce the proliferation of primary or immortalized nasopharyngeal epithelial cells 19. EBV-infected cells arrest or enter into senescence, with increased expression of p16 and p21 27. The inactivation of p16 and/or over-expression of cyclin D1 over-ride the growth-inhibitory effects of EBV infection in these cells, resulting in stably infected cells that express type II latent genes 26. Both p16 inactivation and cyclin D1 over-expression are commonly present in premalignant nasopharyngeal epithelium 6,27,28. The polycomb complex protein, Bmi-1, which is over-expressed in 39% of NPCs, efficiently immortalizes primary nasopharyngeal epithelial cells and supports latent EBV infection 29,30. Hence, an intricate interplay of host cell factors and viral gene expression is probably involved in the regulation of the growth and transformation properties of EBV-infected epithelial cells.
In EBV-associated epithelial malignancies, the undifferentiated properties of the epithelial cells may be a prerequisite for establishing latent EBV infection and activation of the viral lytic programme may be induced by differentiation. In immunocompromized patients, lytic infection with EBV occurs in oral hairy leukoplakia at the lateral sides of the tongue. The expression of the immediate early lytic gene, BZLF1, was only detected in the upper differentiated layers of the EBV-infected epidermis but not in the basal undifferentiated epithelial layer 31. Notably, differentiation-responsive elements were found to be present in the promoter (Zp) of the BZLF1 gene 32. In EBV-infected epithelial cell models, induction of cell differentiation by TGF-β1 resulted in the expression of BZLF1 and the lytic reactivation of EBV 33. These findings indicate the importance of cell differentiation in persistent latent EBV infection in epithelial cells.
ΔNp63, an isoform of the p53 family protein p63, is highly expressed in undifferentiated cells at the basal layers of stratified epithelium and plays an important role in squamous differentiation. In EBV-associated NPC, ΔNp63 is commonly over-expressed and may contribute to the maintenance of EBV latent infection 34; ΔNp63 knockdown in EBV-infected telomerase-immortalized normal oral keratinocytes can induce lytic gene expression 33,35. On the other hand, the genetic alterations in premalignant epithelial cells can also perturb cellular differentiation to support latent EBV infection 6,27,36. Notably, over-expression of cyclin D1 dampened the differentiation response in immortalized nasopharyngeal epithelial cells treated with high levels of serum and calcium and suppressed lytic EBV gene expression in infected cells 27. All these studies support a crucial role of epithelial differentiation in the regulation of lytic and latent infection of EBV in epithelial cells, an area that warrants further investigation (see also a review elsewhere in this Issue, that discusses the mechanisms of evading immunity during latency and of inducing lytic cycle as potential therapeutic approaches 37).
Contribution of EBV latent infection to oncogenesis
The presence of a clonal episomal genome suggests that EBV infection is an early event in the oncogenic transformation process in EBV-associated epithelial malignancies. Many studies have demonstrated that both lytic and latent EBV genes may be involved in the tumourigenesis of human malignancies 38,39. However, the role of lytic EBV infection in epithelial malignancies is unclear. Recurrent lytic activation of EBV promotes genome instability and drives the progression of NPC cells to acquire a more malignant phenotype 40, suggesting an interplay between lytic and latent EBV genes in the pathogenesis of epithelial malignancies. Lytic EBV genes may induce genomic stability in infected cells and latent viral genes may provide survival signals to genetically altered cells.
In EBV-associated epithelial malignancies, EBV may provide only a subset of the oncogenic hits and additional events are required to complete malignant transformation. Recent comprehensive molecular characterization of EBVaGC revealed a distinct genomic signature that featured genome-wide hypermethylation, frequent p16/CDKN2A silencing and PIK3CA mutations, and recurrent amplification of JAK2, PDL-1 and PD-L2 13,14. Notably, several molecular characteristics, including extreme DNA hypermethylation, frequent p16 inactivation, recurrent alterations in the PI3K–AKT pathway and a rarity of p53 mutations, were also found in NPC 6,36,41. Our pilot study has also detected frequent over-expression of PDL-1 and PD-L2 in both NPC tumour lines and primary tumours (unpublished data), suggesting a unique oncogenic process for EBV-associated epithelial malignancies. Among the genetic changes identified, inactivation of the p16/CDKN2A gene is consistently detected in almost all of these EBV-associated epithelial cancers 6,14,36. As shown in our in vitro study, p16 silencing is essential for persistent EBV infection in the epithelial cells 27. It is believed that p16 inactivation is an early event prior to clonal expansion of EBV-infected cells and is the most crucial genetic change in the development of EBV-associated epithelial malignancies. The discovery of PD-L1 and PD-L2 over-expression as common events in EBV-associated NPC and EBVaGC indicates the importance of immune evasion in the tumorigenic process 14. The up-regulation of these immune editing proteins may help EBV-infected cells to survive in response to the host immune response. Notably, the consistent PIK3CA mutation found in EBVaGC suggest a role for PI3K–AKT pathway activation. Aside from these reported events, the contribution of chromatin remodelling in the development of EBV-associated epithelial malignancies is pinpointed by the high frequency of ARID1A mutations 13,14,41. Although p53 mutation is common in most epithelial malignancies, including non-EBV-associated gastric cancers, it occurs in <10% of primary EBV-associated NPCs and EBVaGCs 6,14,36,41. In NPCs, p53 mutation is always found in advanced disease and metastasis 6,41. A significant difference in the mutational rate of p53 between EBVaGCs and other gastric cancer subtypes strongly supports the possible role of EBV infection in interference of p53 function during cancer development 14. Lastly, the relatively low mutation rate reported in the whole-exome sequencing study of NPCs provides new evidence for the role of EBV in tumourigenesis. In EBV-associated epithelial cancers, a number of oncogenic properties and aberrant signalling pathways may be triggered by the EBV-encoded gene products; thus, only a few additional acquired genetic changes are required for the transformation process.
In EBV-associated epithelial malignancies, the latent genes, including EBNA1, EBERs and BARTs, including miR-BARTs, are consistently expressed in all tumour cells. However, the heterogeneous expression of LMP1 and LMP2A has also been commonly detected in these cancers. These viral genes may contribute to tumourigenesis by targeting various hallmarks of cancer described by Hanahan and Weinberg 42 (Figure 2). The transformation properties of these EBV genes and their encoded products commonly expressed in EBV-associated epithelial malignancies are highlighted below.
Figure 2.
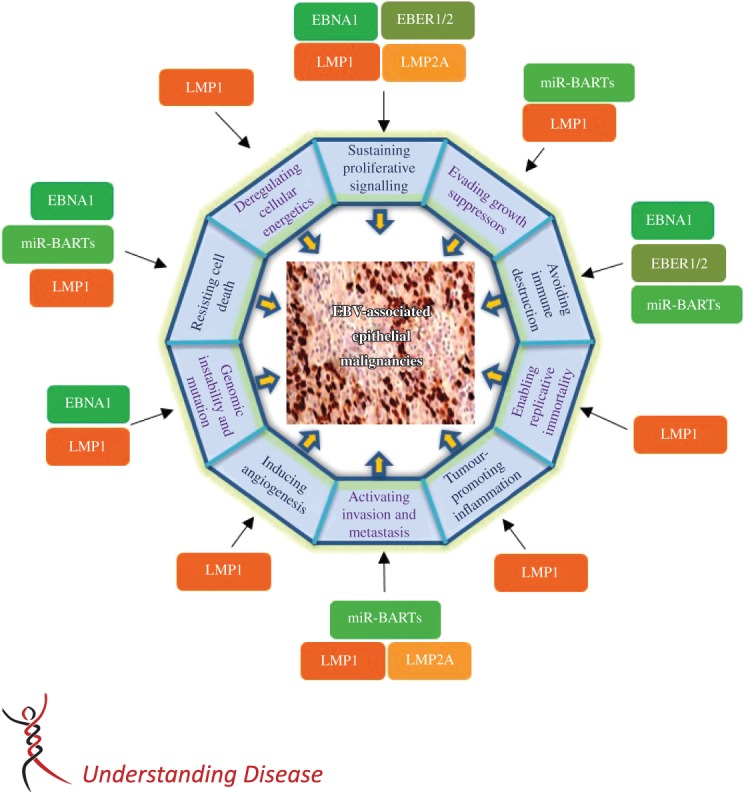
Epstein–Barr virus (EBV) latent genes target cancer hallmarks of epithelial malignancies. EBV contributes multiple cancer hallmarks of epithelial malignancies by expressing type II latent genes. These EBV latent genes induce oncogenic properties by disrupting various cellular and signalling machineries, as described in this review. The constitutively expressed latent genes (EBNA1, EBER1/2 and miR-BARTs) are shown in the green boxes. They mainly contribute to the resistance of cell death, the counteraction of the host immune responses and the induction of genomic instability. The heterogeneously expressed LMPs (orange boxes) are viral oncogenes and play roles in almost all described cancer hallmarks. The expression of high levels of LMPs in a subset of tumour cells may help them to acquire stemness properties and drive tumour progression in invasive epithelial cancers.
Epstein–Barr virus nuclear antigen 1 (EBNA1)
EBNA1 is an important latent viral protein that is required for the persistence of EBV genomes in all EBV-associated malignancies. It governs the replication and mitotic segregation of EBV episomes to maintain a stable number of EBV genome in daughter cells after cell division 22. In EBV-infected epithelial cells, EBNA1 plays an emerging role in promoting cell survival upon DNA damage and inducing genetic instability 43. In both NPCs and EBVaGCs, EBNA1 can disrupt the promyelocytic leukaemia (PML) nuclear bodies that contain many cellular proteins for the regulation of processes such as cell survival, DNA repair and p53 activation 44. It can induce the loss of PML nuclear bodies by binding and regulating CK2 kinase or ubiquitin-specific protease 7, which degrades p53 44. Thus, EBNA1 may contribute predominantly by reducing p53 levels to promote the survival of cells upon DNA damage. Moreover, EBNA1 has been reported to promote DNA damage in NPC cells by inducing reactive oxygen species and up-regulating the oxidative stress response proteins SOD1 and Prx1 45. The enhanced survival of these EBNA1-expressing cells harbouring DNA damage may enhance the genetic instability of the EBV-infected epithelial cells and promote oncogenesis. EBNA1 may also contribute to oncogenesis by modulating various signalling pathways, including suppressing TGF-β1 signalling 46 and enhancing nuclear accumulation of the distinct NF-κB complex p50–p50–bcl3, which inhibits the phosphorylation of IKKα/β and nuclear translocation of p65/RelA 47. This unique NF-κB signal was shown to be important in modulating the tumour microenvironment and enhancing the survival of NPC cells.
EBV-encoded small RNA 1/2 (EBER1/2)
In EBV-infected cells, two EBV small non-polyadenylated RNAs, EBER1 and EBER2, are abundantly expressed. They are 167 and 172 nucleotides long, respectively, and form double-stranded RNA-like structures. These transcripts may promote cellular growth and modulate innate immunity in EBV-associated cancers 48. Their double-stranded RNA-like structures allow them to interact with the retinoic acid-inducible gene 1 (RIG-1) and Toll-like receptor 3 (TLR3), which then induce the phosphorylation of a downstream effector molecule, IRF-3, and the release of insulin-like growth factor 1 (IGF-1) 48,49, which stimulates autocrine growth of infected cells. Nevertheless, the role of EBERs in EBV oncogenesis is still unclear. On the one hand, EBERs were reported to be responsible for innate immune activation by EBV, which results in the production of antiviral and antiproliferative cytokines, such as type 1 interferons (IFNs). On the other hand, EBERs counteract the effects of IFNs by inhibiting their major downstream signalling events 50–52. EBERs inhibit phosphorylation of the cellular substrate of PKR, eIF-2α, which signals the translational block of protein synthesis. By inactivating PKR signalling, EBV-infected cells are also protected from the Fas-mediated apoptosis induced by IFNs 52.
BamH1 A rightwards transcripts (BARTs)
BARTs are a family of multispliced rightward transcripts from the BamH1 A region of the EBV genome 53. The BARTs are abundantly expressed at extremely high levels only in EBV-infected epithelial cancers, but not in EBV-transformed lymphocytes 14,25,53, and it has been postulated that BARTs play a crucial role in EBV-associated epithelial malignancies. The protein products produced by in vitro translation of several open reading frames in the spliced transcripts, such as RPMS1 and A73, were shown to function as negative regulators of the NOTCH and RACK1 signalling pathways, respectively 54,55. However, evidence for the endogenous expression of potential BART-encoded proteins is still lacking. Another possibility is that BARTs may act as long non-coding RNAs, which are involved in repressive complexes to regulate cellular gene expression 25. Notably, the expression of BARTs is regulated by interferon regulatory factors (IRF5 and IRF7) and possibly NF-κB signals 56. This highlights the potential importance of local inflammation and the role of inflammatory cytokines in the expression of BARTs. The functional roles of BARTs in contributing to EBV-associated tumourigenesis have yet to be defined.
EBV encodes a number of microRNAs located within the BARTs (miR-BARTs) 26,53, all of which are transcribed from the same BART transcript and derived from intron processing. miR-BARTs are approximately 8–13-fold higher in epithelial than B cells 57. In NPC and EBVaGC, miR-BARTs are expressed at various levels, due to different biogenesis and processing 26,58,59. These abundantly expressed miR-BARTs are believed to play a key role in tumourigenesis by targeting multiple viral and cellular genes. Prevention of apoptosis is a major function of miR-BARTs in epithelial cancers. Three BART cluster-1 miRNAs (miR-BART1-5p, -16 and -17-5p) can down-regulate the expression of EBV-encoded LMP1 to avoid the growth inhibition effect and alter the balance of the growth-promoting and pro-apoptotic actions of LMP1 by fine-tuning its expression 60. Importantly, expression of miR-BART5, miR-BART16 and multiple miR-BARTs in cluster 1 directly impairs apoptosis by targeting the pro-apoptotic proteins PUMA, TOM22 and BIM, respectively 61–63. In addition to these intrinsic effects, miR-BARTs may protect EBV-infected premalignant or malignant epithelial cells by impairment of the host immune response. miR-BART2-5p suppresses the expression of major histocompatibility complex class I-related chain B (MICB), involved in the initiation of immune responses that eliminate infected cells by activating the NKG2D type II receptor in natural killer cells, CD8 αβT cells and γδT cells 64. In contrast, miR-BART3 targets a nuclear importer receptor, importin 7 (IPO7), for immune evasion 63. It is believed that miR-BART3 may be transported to neighbouring immune cells via exosomes and thereby inhibits IPO7 expression, impairing their cytotoxic function. Notably, we recently showed that miR-BART22 suppressed expression of the immunogenic viral antigen LMP2A to protect NPC cells from immunological attack 65. miR-BARTs are also involved in various other oncogenic processes. miR-BART3-5p promotes cellular growth by targeting the DICE1 tumour-suppressor gene and miR-BART9 targets E-cadherin to enhance invasiveness and metastatic ability of NPC cells 66,67. Furthermore, the miR-BARTs facilitate EBV latency by limiting the expression of multiple lytic genes (e.g. BZLF1, BRLF1 and BALF5) in infected epithelial cells 68,69. The identification of target genes that mediate the functions ascribed to miR-BARTs may unveil the role of EBV in the oncogenesis of epithelial cancers.
BamH1-A fragment rightward reading frame 1 (BARF1)
BARF1 is a homologue of human colony stimulating factor 1 receptor (CSF1R), encoded in the BamH1 A region, and is highly expressed in NPC and EBVaGC 70,71. The expression of BARF1 can immortalize monkey kidney primary epithelial cells and transform immortalized nasopharyngeal epithelial cells that express H-ras 72,73. At present, its oncogenic role remains controversial and awaits further investigation 70.
Latent membrane proteins (LMP1 and LMP2)
LMP1 and LMP2A exert multiple oncogenic properties and have transformation potential in epithelial cells by activating multiple signalling pathways and modulating the expression of various oncogenes and tumour-suppressor genes. As viral oncogenes, they may drive the transformation of epithelial cells by the induction and maintenance of tumour phenotypes, including cell proliferation, resistance to apoptosis, invasion and motility and angiogenesis 74,75.
LMP1 is a transmembrane protein that acts as a constitutively activated tumour necrosis factor receptor 1. It activates multiple signalling pathways, including NF-κB, JNK–p-38, PI3K–AKT, ERK–MAPK and JAK–STAT 74,75. LMP1 can stimulate the growth of NPC cells by up-regulating growth factor receptors (e.g. EGFR, c-Met) and suppressing cell cycle regulators (e.g. p16, p21) 76–78. To enhance cell survival, LMP1 can promote the expression of anti-apoptotic proteins (e.g. survivin and Mcl-1) or inactivate pro-apoptotic proteins (e.g. Bad and Foxo3a) 74,75. LMP1 enables epithelial cells to resist the growth-suppressive effect of TGF-β1 by inducing inhibitor of differentiation-1 (Id-1) 79. It also contributes to angiogenesis by reducing degradation of hypoxia inducible factor-1α (HIF-1α) and inducing the expression of vascular endothelial growth factor (VEGF) expression 80. Recently, we found that LMP1 inhibits the LKB–AMPK pathway to alter cellular metabolism 81. In addition to inducing epithelial–mesenchymal transition (EMT), LMP1 was reported to induce cancer stem/progenitor-like cells in NPC cells, possibly by activating the Hedgehog signalling pathway 82–85. Notably, LMP1 may modulate the tumour microenvironment and induce tumour-promoting inflammation via activating NF-κB pathways 74,75. Most importantly, LMP1 can induce promoter hypermethylation and epigenetic silencing of cellular genes through the activation of DNA methyltransferase 1 (DNMT1) and the polycomb group protein, Bmi-1 86,87, contributing to the global methylation and inactivation of multiple cancer genes in EBV-associated epithelial cancers.
LMP2A and LMP2B are transcribed from two distinct forms of mRNA that share the same exons 2–9. Only LMP2A has the N-terminal cytoplasmic domain that contains multiple signalling domains that contribute to the modulation of several signalling pathways, including PI3K–AKT, RhoA and MAPK–ERK 75. To date, few studies have addressed the function of LMP2B in epithelial cells. Through activation of the PI3K–AKT pathway and phosphorylation of GSK3, LMP2A induces remarkable phenotypic changes, including anchorage-independent growth in soft agar and promotes β-catenin signalling in epithelial cells 88–90. It also inhibits cellular differentiation and promotes cell survival through the PI3K–Akt-mediated stabilization of ΔNp63 91. Other roles of LMP2A include counteraction of the growth inhibitory and pro-apoptotic effects of TGF-β1 during epithelial carcinogenesis and the promotion of proliferation and protein synthesis in cells by the activation of the mTOR pathway 92,93. LMP2A promotes the invasive and migratory properties of epithelial cells, which may relate to the metastatic phenotype 94,95. Similar to LMP1, LMP2A can also activate the Hedgehog signalling pathway that induces cancer stem-like properties 85. Exogenous expression of LMP2A induces EMT, stimulates stem cell marker expression and enhances the acquisition of side-populations in NPC cells 96. Interestingly, LMP2A was reported to be localized at the tumour-invasive front 96. These findings support a role of LMP2A in the induction of cancer stem cells in EBV-associated epithelial malignancies.
The possible role of heterogeneous LMP expression in tumourigenesis
The expression of LMP1 and LMP2A in NPC and EBVaGC is heterogeneous in terms of prevalence and distribution within the tumours 75. Although LMP1 protein was previously reported to be expressed in 20–40% of NPCs, recent sensitive approaches using immunohistochemical staining have revealed LMP1 expression in almost 100% of primary NPC specimens 74,75,97. By high-coverage transcriptome sequencing, low to high levels of LMP1 expression were found in primary EBVaGCs, despite the absence in previous reports 14,25. To define its precise role in oncogenesis, LMP1 expression patterns need to be evaluated comprehensively in these cancers, using sensitive methods. Nevertheless, the most important concept is that LMP1 expression is highly heterogeneous among the malignant cells in NPCs or other EBV-related epithelial cancers.
LMP1 is always expressed in rare individual cells and small clusters in the primary tumours of NPC, as well as in well-established xenografts (Figure 1). Notably, LMP1 is detected in premalignant lesions and is especially concentrated in the basal layers 27,97. These findings imply that LMP1 participates in tumour initiation, although it is only constitutively expressed in a subpopulation of malignant cells. As high-level LMP1 expression inhibits cell growth and induces apoptosis in epithelial cells, it may be suppressed by various mechanisms in the majority of malignant cells in epithelial cancers, while only a subset of cells sustain a high level 74. As mentioned above, LMP1 might be involved in the maintenance of stem cell properties and LMP-expressing epithelial cells may exhibit a cancer stem/progenitor-like cell phenotype that is resistant to induced apoptosis. The significant up-regulation of LMP1 in NPC sphere-forming cells supports this hypothesis 98.
Based on the cumulative evidence, we hypothesize that LMP1 may play different roles in the early and late stages of cancer development. The tumourigenesis process may start after the establishment of a persistent latent EBV infection in epithelial cells with stem-like properties, probably the basal stem cells over-expressing anti-apoptotic BCL2 99. The expression of EBV latent genes may protect the infected cells from the host immune response and inhibit apoptosis during clonal expansion. In precancerous lesions, the majority of dysplastic cells may express stemness properties and allow a high level of LMP1 expression to trigger its oncogenic functions, inducing genetic instability and epigenetic changes. A large variety of genetic and epigenetic changes may be induced and accumulated in these LMP1-expressing cells and persist in their progenies. Cells that contain acquired genetic/epigenetic alterations that can substitute for LMP1 functions may then become the dominant population during cancer progression. LMP1 is down-regulated to avoid its cytotoxic effects in the majority of advanced malignant cells, except for the rare cancer stem/progenitor cells present in invasive tumours. The constitutive activation of multiple signalling pathways (e.g. NF-κB and PI3K–AKT) and the epigenetic silencing of E-cadherin in NPC are consistent with this hypothesis, which would also explain the genome-wide hypermethylation and relatively greater number of mutations identified in EBVaGC. Similar to LMP1, the expression of LMP2A is also confined to a subset of malignant cells, because of its immunogenicity. More studies are needed to define these speculative roles of LMP proteins in the development of EBV-associated epithelial cancers.
Contribution of chronic inflammation in EBV-infected epithelial malignancies
Both NPCs and EBVaGCs arise from a special type of mucosal epithelium that is heavily infiltrated with lymphoid elements, often referred to as MALTs (mucosa-associated lymphoid tissues). Chronic inflammation may contribute to the malignant transformation of premalignant epithelial cells 100. Reactive oxygen species induced by, or secreted from, activated inflammatory cells may enhance DNA damage and genomic instability in nearby epithelial cells, which may generate clones of genetically altered precursors susceptible to latent EBV infection. Inflammation-mediated mutagenesis may enhance the development of cancer through the subsequent clonal expansion of EBV-infected premalignant cells.
The role of inflammatory stroma and the rich cytokine milieu will also have a major impact on growth promotion and the expression of EBV latent or lytic genes in infected epithelial cells. Cytokines released from inflammatory cells may activate the NF-κB and STAT3 signalling pathways in EBV-infected epithelial cells, stimulating their growth and survival. In NPC, the expression of LMP1 or the occurrence of somatic changes constitutively activates NF-κB signalling and up-regulate inflammatory cytokines, thereby further recruiting more inflammatory lymphocytes 47. In our study, we also found that the IL-6–STAT3 signalling axis is potentiated in EBV-infected nasopharyngeal epithelial cells, promoting a positive feedback loop of LMP1 expression 101. These data suggest that EBV may co-evolve with infected host cells to modulate latent EBV gene expression and cell signalling pathways for NPC development. Defining the impact of chronic inflammation on EBV-infected epithelial cells will contribute to our understanding of its role in pathogenesis in undifferentiated NPCs and EBVaGCs.
Concluding remarks
EBV infection is listed as a Group I carcinogen category by the International Agency for Research on Cancer (IARC). Although the oncogenic properties of multiple EBV latent genes, notably LMP1 and LMP2A, have been demonstrated, their concerted actions and interplay with host genetic alterations in the transformation of premalignant epithelial cells into cancer cells remains elusive. The abundant expression of BARTs and miR-BARTs strongly implicates their roles in the pathogenesis of these cancers. EBV infection may offer immune evasion and survival advantages to infected tumour cells for their selective growth in vivo. The impact of chronic inflammation, revealed by the characteristically heavy infiltration of lymphocytic cells, to the growth and survival of EBV-infected cells during tumour initiation and progression remains to be further elaborated. Defining the role of inflammatory stroma will be important for understanding the pathogenic role(s) of EBV infection in epithelial malignancies and may provide effective therapeutic targets for clinical management of these diseases. Recently, whole EBV genome sequencing studies have revealed the NPC-derived EBV strains from endemic region, which show significant difference from the reported EBV genomes from non-endemic populations 102–106. The findings suggest the existence of disease specific viral variations which may possess higher oncogenic properties, propensity for infection of epithelial cells and persistence of the latent programme, or less efficiency in inducing host immune response, especially in the NPC endemic population. The hypothesis can be confirmed by comparing these tumour-derived EBV strains with viral strains from the blood and saliva samples of patients and healthy individuals in the endemic region. Comprehensive characterization of the properties of these putative pathogenic strains might provide new insights to the role of EBV in epithelial malignancies.
Author contributions
SWT, CMT, KFT and KWL were involved in data analysis and writing the paper. All authors had final approval of the submitted manuscript.
Acknowledgments
The authors acknowledge the generous funding sources for the above study: the Health and Medical Research Fund (Grant Nos HMRF: 12110942 and 13120872), a CRCG Grant from the University of Hong Kong, Focus Scheme A from the Chinese University of Hong Kong, the Hong Kong Research Grant Council – GRF and CRF (Grant No. CUHK8/CRF/11R), AoE NPC (Grant No. AoE/M-06/08) and the Theme-Based Research Scheme (Grant No. T12-401/13-R), Hong Kong.
References
- Cohen JI, Fauci AS, Varmus H, et al. Epstein–Barr virus: an important vaccine target for cancer prevention. Sci Transl Med. 2011;3:107fs7. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Vockerodt M, Yap L-F, Shannon-Lowe C, et al. The Epstein–Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235:312–322. doi: 10.1002/path.4459. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Flynn K. The structure of the termini of the Epstein–Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Pittaluga S, Loke SL, So KC, et al. Clonal Epstein–Barr virus in lymphoepithelioma-like carcinoma of the stomach: demonstration of viral genome by in situ hybridization and Southern blot analysis. Mod Pathol. 1992;5:661–664. [PubMed] [Google Scholar]
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- Chan JK, Bray F,McCarron. Nasopharyngeal carcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and Genetics. Head and Neck Tumors. Lyon: IARC Press; 2005. pp. 85–97. [Google Scholar]
- Shibata D, Tokunaga M, Uemura Y, et al. Association of Epstein–Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469–474. [PMC free article] [PubMed] [Google Scholar]
- Shibata D, Weiss LM. Epstein–Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein–Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308–315. doi: 10.1093/ajcp/103.3.308. [DOI] [PubMed] [Google Scholar]
- Chan AW, Tong JH, Sung MY, et al. Epstein–Barr virus associated lymphoepithelioma-like cholangiocarcinoma: a rare variant of intrahepatic cholangiocarcinoma with favorable outcome. Histopathology. 2014;65:674–683. doi: 10.1111/his.12455. [DOI] [PubMed] [Google Scholar]
- Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol. 2014;26:99–115. doi: 10.1016/j.semcancer.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Hawkins JB, Tracy SI, et al. The pathogenesis of Epstein–Barr virus persistent infection. Curr Opin Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher LM. Epstein–Barr virus entry. J Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein–Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Tsang CM, Zhang G, Seto E, et al. Epstein–Barr virus infection in immortalized nasopharyngeal epithelial cells: regulation of infection and phenotypic characterization. Int J Cancer. 2010;127:1570–1583. doi: 10.1002/ijc.25173. [DOI] [PubMed] [Google Scholar]
- Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein–Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- Chan AS, To KF, Lo KW, et al. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res. 2000;60:5365–5370. [PubMed] [Google Scholar]
- Longnecker RM, Kieff E, Cohen JI. Epstein–Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1898–1959. [Google Scholar]
- Tsao SW, Tsang CM, Pang PS, et al. The biology of EBV infection in human epithelial cells. Semin Cancer Biol. 2012;22:137–143. doi: 10.1016/j.semcancer.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Chugh PE, et al. Expression profile of microRNAs in Epstein–Barr virus-infected AGS gastric carcinoma cells. J Virol. 2014;88:1389–1393. doi: 10.1128/JVI.02662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Xu G, Coco J, et al. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: implications for possible immune adjuvant therapy. PLoS Pathog. 2013;9:e1003341. doi: 10.1371/journal.ppat.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung RW, Tong JH, To KF. Emerging roles of small Epstein–Barr virus-derived non-coding RNAs in epithelial malignancy. Int J Mol Sci. 2013;14:17378–17409. doi: 10.3390/ijms140917378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CM, Yip YL, Lo KW, et al. Cyclin D1 overexpression supports stable EBV infection in nasopharyngeal epithelial cells. Proc Natl Acad Sci USA. 2012;109:E3473–3482. doi: 10.1073/pnas.1202637109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AB, Or YY, Takano H, et al. Array-based comparative genomic hybridization analysis identified cyclin D1 as a target oncogene at 11q13.3 in nasopharyngeal carcinoma. Cancer Res. 2005;65:8125–8133. doi: 10.1158/0008-5472.CAN-05-0648. [DOI] [PubMed] [Google Scholar]
- Yip YL, Pang PS, Deng W, et al. Efficient immortalization of primary nasopharyngeal epithelial cells for EBV infection study. PLoS One. 2013;8:e78395. doi: 10.1371/journal.pone.0078395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- Young LS, Lau R, Rowe M, et al. Differentiation-associated expression of the Epstein–Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65:2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi L, Crawford DH, Speck S, et al. Identification of an epithelial cell differentiation responsive region within the BZLF1 promoter of the Epstein–Barr virus. J Gen Virol. 1995;76:759–765. doi: 10.1099/0022-1317-76-4-759. [DOI] [PubMed] [Google Scholar]
- Kenney SC, Mertz JE. Regulation of the latent-lytic switch in Epstein–Barr virus. Semin Cancer Biol. 2014;26:60–68. doi: 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T, Nicholls JM, Brooks L, et al. High level expression of δN-p63: a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene. 2000;19:3439–3444. doi: 10.1038/sj.onc.1203656. [DOI] [PubMed] [Google Scholar]
- Wille CK, Nawandar DM, Panfil AR, et al. Viral genome methylation differentially affects the ability of BZLF1 versus BRLF1 to activate Epstein–Barr virus lytic gene expression and viral replication. J Virol. 2013;87:935–950. doi: 10.1128/JVI.01790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Daskalogianni C, Pyndiah S, Apcher S, et al. Epstein–Barr virus-encoded EBNA1 and ZEBRA; targets for therapeutic strategies against EBV-carrying cancers. J Pathol. 2015;235:334–341. doi: 10.1002/path.4431. [DOI] [PubMed] [Google Scholar]
- Ma SD, Yu X, Mertz JE, et al. An Epstein–Barr virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol. 2012;86:7976–7987. doi: 10.1128/JVI.00770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Chen JY, Wang JT, et al. Epstein–Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J Virol. 2007;81:5166–5180. doi: 10.1128/JVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CY, Lee CH, Wu CC, et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int J Cancer. 2009;124:2016–2025. doi: 10.1002/ijc.24179. [DOI] [PubMed] [Google Scholar]
- Lin DC, Meng X, Hazawa M, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46:866–871. doi: 10.1038/ng.3006. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Frappier L. Role of EBNA1 in NPC tumourigenesis. Semin Cancer Biol. 2012;22:154–161. doi: 10.1016/j.semcancer.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Sivachandran N, Sarkari F, Frappier L. Epstein–Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathog. 2008;4:e1000170. doi: 10.1371/journal.ppat.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JY, Mansouri S, Frappier L. Changes in the nasopharyngeal carcinoma nuclear proteome induced by the EBNA1 protein of Epstein–Barr virus reveal potential roles for EBNA1 in metastasis and oxidative stress responses. J Virol. 2012;86:382–394. doi: 10.1128/JVI.05648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood VH, O'Neil JD, Wei W, et al. Epstein–Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFβ signaling pathways. Oncogene. 2007;26:4135–4147. doi: 10.1038/sj.onc.1210496. [DOI] [PubMed] [Google Scholar]
- Chung GT, Lou WP, Chow C, et al. Constitutive activation of distinct NF-κB signals in EBV-associated nasopharyngeal carcinoma. J Pathol. 2013;231:311–322. doi: 10.1002/path.4239. [DOI] [PubMed] [Google Scholar]
- Takada K, Nanbo A. The role of EBERs in oncogenesis. Semin Cancer Biol. 2001;11:461–467. doi: 10.1006/scbi.2001.0413. [DOI] [PubMed] [Google Scholar]
- Samanta M, Iwakiri D, Takada K. Epstein–Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene. 2008;27:4150–4160. doi: 10.1038/onc.2008.75. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Takizawa T, Iwanaga Y, et al. Malignant transformation of B lymphoma cell line BJAB by Epstein–Barr virus-encoded small RNAs. FEBS Lett. 2000;484:153–158. doi: 10.1016/s0014-5793(00)02145-1. [DOI] [PubMed] [Google Scholar]
- Nanbo A, Inoue K, Adachi-Takasawa K, et al. Epstein–Barr virus RNA confers resistance to interferon-α-induced apoptosis in Burkitt's lymphoma. EMBO J. 2002;21:954–965. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbo A, Yoshiyama H, Takada K. Epstein–Barr virus-encoded poly(A)-RNA confers resistance to apoptosis mediated through Fas by blocking the PKR pathway in human epithelial intestine 407 cells. J Virol. 2005;79:12280–12285. doi: 10.1128/JVI.79.19.12280-12285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Raab-Traub N. The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol. 2012;22:166–172. doi: 10.1016/j.semcancer.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mozaini M, Bodelon G, Karstegl CE, et al. Epstein–Barr virus BART gene expression. J Gen Virol. 2009;90:307–316. doi: 10.1099/vir.0.006551-0. [DOI] [PubMed] [Google Scholar]
- Smith PR, de Jesus O, Turner D, et al. Structure and coding content of CST (BART) family RNAs of Epstein–Barr virus. J Virol. 2000;74:3082–3092. doi: 10.1128/jvi.74.7.3082-3092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Huang J, Wu FY, et al. Regulation of expression of the Epstein–Barr virus BamHI-A rightward transcripts. J Virol. 2005;79:1724–1733. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cosmopoulos K, Pegtel M, et al. A novel persistence associated EBV miRNA expression profile is disrupted in neoplasia. PLoS Pathog. 2011;7:e1002193. doi: 10.1371/journal.ppat.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Pfuhl T, Motsch N, et al. Identification of novel Epstein–Barr virus microRNA genes from nasopharyngeal carcinomas. J Virol. 2009;83:3333–3341. doi: 10.1128/JVI.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Chen GH, Chen YH, et al. Characterization of Epstein–Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PloS One. 2010;5:e12745. doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, To KF, Lo KW, et al. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EY, Siu KL, Kok KH, et al. An Epstein–Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Nam CS, et al. The Epstein–Barr virus BART microRNAs target the pro-apoptotic protein Bim. Virology. 2011;412:392–400. doi: 10.1016/j.virol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölken L, Malterer G, Erhard F, et al. Systematic analysis of viral and cellular microRNA targets in cells latently infected with human γ-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe. 2010;7:324–334. doi: 10.1016/j.chom.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Nachmani D, Stern-Ginossar N, Sarid R, et al. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Lung RW, Tong JH, Sung YM, et al. Modulation of LMP2A expression by a newly identified Epstein–Barr virus-encoded microRNA miR-BART22. Neoplasia. 2009;11:1174–1184. doi: 10.1593/neo.09888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Yuen KS, Xu R, et al. Targeting of DICE1 tumor suppressor by Epstein–Barr virus-encoded miR-BART3* microRNA in nasopharyngeal carcinoma. Int J Cancer. 2013;133:79–87. doi: 10.1002/ijc.28007. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Yi YH, Chang KP, et al. The Epstein–Barr virus-encoded microRNA MiR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog. 2014;10:e1003974. doi: 10.1371/journal.ppat.1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Choi H, Kim H, et al. MicroRNA miR-BART20-5p stabilizes Epstein–Barr virus latency by directly targeting BZLF1 and BRLF1. J Virol. 2014;88:9027–9037. doi: 10.1128/JVI.00721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Pfuhl T, Mamiani A, et al. Epstein–Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 2008;36:666–675. doi: 10.1093/nar/gkm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe EK, Le Large TY, Greijer AE, et al. BamHI-A rightward frame 1, an Epstein–Barr virus-encoded oncogene and immune modulator. Rev Med Virol. 2013;23:367–383. doi: 10.1002/rmv.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houali K, Wang X, Shimizu Y, et al. A new diagnostic marker for secreted Epstein–Barr virus-encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- Wei MX, de Turenne-Tessier M, et al. Establishment of a monkey kidney epithelial cell line with the BARF1 open reading frame from Epstein–Barr virus. Oncogene. 1997;14:3073–3081. doi: 10.1038/sj.onc.1201128. [DOI] [PubMed] [Google Scholar]
- Jiang R, Cabras G, Sheng W, et al. Synergism of BARF1 with Ras induces malignant transformation in primary primate epithelial cells and human nasopharyngeal epithelial cells. Neoplasia. 2009;11:964–973. doi: 10.1593/neo.09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao SW, Tramoutanis G, Dawson CW, et al. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:473–487. doi: 10.1016/s1044579x02000901. [DOI] [PubMed] [Google Scholar]
- Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22:144–153. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Miller WE, Earp HS, Raab-Traub N. The Epstein–Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa T, Sheen TS, Takeshita H, et al. Induction of c-Met proto-oncogene by Epstein–Barr virus latent membrane protein-1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am J Pathol. 2001;159:27–33. doi: 10.1016/S0002-9440(10)61669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, Huang DP, Lo KW, et al. Phenotypic alterations induced by the Hong Kong-prevalent Epstein–Barr virus-encoded LMP1 variant (2117-LMP1) in nasopharyngeal epithelial cells. Int J Cancer. 2004;109:919–925. doi: 10.1002/ijc.20051. [DOI] [PubMed] [Google Scholar]
- Lo AK, Dawson CW, Lo KW, et al. Upregulation of Id1 by Epstein–Barr virus-encoded LMP1 confers resistance to TGFβ-mediated growth inhibition. Mol Cancer. 2010;9:155. doi: 10.1186/1476-4598-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka N, Kondo S, Yoshizaki T, et al. Epstein–Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1α. Mol Cell Biol. 2004;24:5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, Lo KW, Ko CW, et al. Inhibition of the LKB1-AMPK pathway by the Epstein–Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol. 2013;230:336–346. doi: 10.1002/path.4201. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Yang J, Kondo S, et al. Twist and epithelial–mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res. 2007;67:1970–1978. doi: 10.1158/0008-5472.CAN-06-3933. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Yoshizaki T, Kondo S, et al. Epstein–Barr virus latent membrane protein 1 induces snail and epithelial–mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer. 2011;104:1160–1167. doi: 10.1038/bjc.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Wakisaka N, Muramatsu M, et al. Epstein–Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol. 2011;85:11255–11264. doi: 10.1128/JVI.00188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RJ, Pinheiro-Maia S, Hu C, et al. Epstein–Barr virus induction of the Hedgehog signalling pathway imposes a stem cell phenotype on human epithelial cells. J Pathol. 2013;231:367–377. doi: 10.1002/path.4245. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Li HP, Lu YJ, et al. Activation of DNA methyltransferase 1 by EBV LMP1 Involves c-Jun NH(2)-terminal kinase signaling. Cancer Res. 2006;66:11668–11676. doi: 10.1158/0008-5472.CAN-06-2194. [DOI] [PubMed] [Google Scholar]
- Dutton A, Woodman CB, Chukwuma MB, et al. Bmi-1 is induced by the Epstein–Barr virus oncogene LMP1 and regulates the expression of viral target genes in Hodgkin lymphoma cells. Blood. 2007;109:2597–2603. doi: 10.1182/blood-2006-05-020545. [DOI] [PubMed] [Google Scholar]
- Scholle F, Bendt KM, Raab-Traub N. Epstein–Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Longnecker R. Epstein–Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt pathway. J Virol. 2007;81:9299–9306. doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein–Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J Virol. 2005;79:2375–2382. doi: 10.1128/JVI.79.4.2375-2382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham JA, Mazzucca S, Raab-Traub N. Epstein–Barr virus latent membrane protein-2A-induced ΔNp63α expression is associated with impaired epithelial cell differentiation. Oncogene. 2010;29:4287–4296. doi: 10.1038/onc.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Longnecker R. Latent membrane protein 2A inhibits transforming growth factor-β1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J Virol. 2004;78:1697–1705. doi: 10.1128/JVI.78.4.1697-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Scott RS, Amirghahari N, et al. Modulation of the cell growth regulator mTOR by Epstein–Barr virus-encoded LMP2A. J Virol. 2005;79:5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Subramanian A, Sheen TS, et al. Epstein–Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: possible role in nasopharyngeal carcinoma metastasis. J Virol. 2005;79:15430–15442. doi: 10.1128/JVI.79.24.15430-15442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lin WH, Chen SY, et al. Syk tyrosine kinase mediates Epstein–Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281:8806–8814. doi: 10.1074/jbc.M507305200. [DOI] [PubMed] [Google Scholar]
- Kong QL, Hu LJ, Cao JY, et al. Epstein–Barr virus-encoded LMP2A induces an epithelial–mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6:e1000940. doi: 10.1371/journal.ppat.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benders AA, Tang W, Middeldorp JM, et al. Epstein–Barr virus latent membrane protein 1 is not associated with vessel density nor with hypoxia inducible factor-1α expression in nasopharyngeal carcinoma tissue. Head Neck Pathol. 2009;3:276–282. doi: 10.1007/s12105-009-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun SW, Cheung ST, Cheung PF, et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS One. 2012;7:e52426. doi: 10.1371/journal.pone.0052426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Elia G, Lucas S, et al. Bcl-2 proto-oncogene expression in Epstein–Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer. 1993;53:29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol. 2014;26:99–115. doi: 10.1016/j.semcancer.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang G, Tsang CM, Deng W, et al. Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS One. 2013;8:e62284. doi: 10.1371/journal.pone.0062284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Fang X, Feng Z, et al. Direct sequencing and characterization of a clinical isolate of Epstein–Barr virus from nasopharyngeal carcinoma tissue by using next-generation sequencing technology. J Virol. 2011;85:11291–11299. doi: 10.1128/JVI.00823-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok H, Tong AH, Lin CH, et al. Genomic sequencing and comparative analysis of Epstein–Barr virus genome isolated from primary nasopharyngeal carcinoma biopsy. PLoS One. 2012;7:e36939. doi: 10.1371/journal.pone.0036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso KK, Yip KY, Mak CK, et al. Complete genomic sequence of Epstein–Barr virus in nasopharyngeal carcinoma cell line C666-1. Infect Agent Cancer. 2013;8:29. doi: 10.1186/1750-9378-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok H, Wu CW, Palser AL, et al. Genomic diversity of Epstein–Barr virus genomes isolated from primary nasopharyngeal carcinoma biopsy samples. J Virol. 2014;88:10662–10672. doi: 10.1128/JVI.01665-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MH, Raykova A, Klinke O, et al. Spontaneous lytic replication and epitheliotropism define an Epstein–Barr virus strain found in carcinomas. Cell Rep. 2013;5:458–470. doi: 10.1016/j.celrep.2013.09.012. [DOI] [PubMed] [Google Scholar]


