Abstract
The present study examined whether functional MRI (fMRI) can identify changes in the neural substrates of language in young children following traumatic brain injury (TBI).
Eight children with TBI (F/M = 3/5, age (Mean ± SD) = 7.98 ± 1 years, range = 6–9 years) and a comparison group of nine children with orthopedic injuries (OI) (F/M = 4/5, age (Mean ± SD) = 7.4 ± 1 years, range = 6–9 years) participated in an fMRI study of covert verb generation (VG). Results revealed significantly different BOLD signal activation in perisylvian language areas between the groups, after accounting for potential confounders such as verbal fluency and executive function. We also found significant associations between the BOLD signal activation and performance on language-specific neuropsychological tests (NEPSY verbal fluency score, Verbal IQ) and Glasgow Coma Scale (GCS) score.
This study suggests that children with TBI have significantly different brain activation patterns in language circuitry compared to children with orthopedic injuries. Although we found clear differences in brain activation between the two groups, conventional MR images showed no evidence of structural abnormalities in five of eight children with TBI. Our study demonstrates the feasibility and potential utility of fMRI as a means of quantifying changes associated with language deficits in future pediatric TBI studies.
1. Introduction
The consequences of TBI in children include deficits in intelligence, memory, attention, learning, and social judgment [1-4]. Academic achievement, school performance, and adaptive abilities may also be impaired [4,5]. These problems may persist for years, adversely impacting both the child and his or her family [6,7].
Although language skills have received relatively less attention than other aspects of behavioral and neuropsychological recovery following TBI, they may underlie observed problems in academic performance, social competence, and peer integration. Previous investigators have documented deficits in expressive and receptive language skills [8,9], naming ability, and written language [10,11] following TBI in children. Studies of narrative discourse have also revealed difficulties with higher-order language abilities following TBI, with language characterized by fewer complex statements and more incomplete or simplistic utterances [9] and a reduced ability to transform or synthesize information [12].
Although there have been many systematic and carefully designed studies that have examined the cognitive consequences of TBI in school-age children, less is known about the consequences for infants, toddlers and preschool children. However, existing data suggest that TBI in a young child results in different and potentially more severe sequelae than is the case for older children [8,13]. Certain skills, such as language, executive function/self control, and social competence, emerge during the preschool years and provide the foundation for subsequent academic achievement and social adjustment. Because these skills are undergoing rapid change during early childhood, they may be particularly vulnerable to disruption by brain trauma. For example, expressive language abilities such as word fluency, naming, and narrative discourse appear to be more significantly impaired after TBI in young children than in children injured at an older age [10,12,14]. Thus, the emphasis in the present study is on the neural substrates of language skills in children following TBI that occurred during early childhood. These skills are both likely to be disrupted or delayed and may have important implications for subsequent normal development.
Although researchers are increasingly using fMRI to both identify the neural substrates of complex human behaviors, such as language [15,16], memory [17-21] and attention [22,23] and to understand the underlying processing differences between individuals with specific diagnoses and healthy comparison groups, there have been virtually no functional imaging studies following TBI in children.
Previous studies of working-memory in adults with moderate to severe TBI have documented differences in neural processing relative to controls that corresponded to working-memory deficits [24,25]. However, in adults with mild TBI, research revealed differences in activation patterns during working-memory tasks suggesting inefficient processing compared to normal controls, even when objective performance was unaffected [26,27]. Specifically, adults with mild TBI demonstrated over-activation in the neural circuitry mediating working-memory shortly after injury relative to healthy controls on tasks of moderate difficulty (2-back) [26, 27]. These findings suggest that TBI in children may also result in either neural reorganization (i.e., activation in parts of the brain not typically involved with that type of task) or less efficient allocation of neural resources (i.e., greater neural activation to achieve average performance) depending on the severity of the injury. Since the relationship between underlying brain/neural changes and observed deficits following pediatric TBI is poorly understood, fMRI may be helpful in understanding the effects of TBI on the pathophysiology of brain function and, more importantly, the relationship between neural processing and recovery. Ultimately, fMRI may aid evaluation of intervention efficacy in enhancing recovery.
The present investigation had three goals: 1) to demonstrate the feasibility of conducting functional imaging in young children (ages 6-9) with TBI; 2) to examine differences in neural activation during a language task in children with TBI relative to a matched comparison group of children with orthopedic injuries (OI); and 3) to characterize the relationship between performance on standardized language tasks and activation patterns in language circuitry during language tasks. We hypothesized that children with both TBI and OI would activate similar networks of brain regions during the task as identified in the previous studies of similar language tasks, but that the children with TBI would demonstrate an over-activation of certain brain regions supporting the task compared to the orthopedic controls. This hypothesis is based in part of the lack of anatomical findings in the MRI images of our subjects coupled with performance differences in standardized language measures. Additionally, previous fMRI studies have demonstrated compensatory enhancement of brain activation in children with deficits struggling to perform language task such as reading [28]. We further hypothesized that children with greater deficits on neuropsychological indices would show greater differences in the areas of fMRI activation. Given that the children in our TBI cohort had relatively mild injuries (Mean Glasgow Coma Scale [GCS] = 11) and were closely matched with our OI comparison group on performance, we hypothesized that our findings would be similar to those of McAllister and colleagues suggesting that mild TBI in adults is associated with increased activation of the relevant neural circuitry.
2. Methods
This project was part of a larger study of recovery from TBI in young children. The parent project employed a concurrent cohort/prospective research design involving repeated assessments (soon after the injury and at 6, 12, and 18 months post-injury) of young children with TBI and young children with orthopedic injuries and their families. Children in the imaging study reported here were seen for a single additional assessment at greater than 12 months post injury to complete the fMRI task. Inclusion of a comparison group of children with orthopedic injuries allowed us to examine the consequences of brain injury relative to the functioning of a group of children likely to be similar in pre-injury behavior and family characteristics, and provided us with a point of reference for the practice effects associated with repeated administration of cognitive measures. Specifically, impulsive child behavior and social environmental characteristics such as the degree of parental supervision and monitoring have been shown to contribute to the risk for injury (both TBI and OI) and may also reflect premorbid neurological differences. Previous research suggests that children with orthopedic injuries provide a more comparable comparison group in this regard than typically developing children or convenience samples [29]. Both the parent project and the imaging study reported here were approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
2.1. Recruitment criteria
Consecutive admissions of children with mild, moderate and severe TBI or with orthopedic injuries not involving the CNS were recruited for the parent project. Eligibility requirements included: age between 36 to 84 months at the time of injury, no documentation in the medical chart or in parent interview of child abuse as a cause of the injury, and English as the primary spoken language in the home. Children in the TBI group must have had a TBI requiring overnight admission to the hospital and either evidence of altered neurological status clinically or on the Glasgow Coma Scale (GCS) or abnormalities on imaging (MRI or CT scan). The lowest GCS score recorded at the time of hospitalization provided a measure of injury severity in the TBI group. GCS scores are generated by combining ratings of eye opening, best verbal response, and best motor response at the time of evaluation. Consistent with previous investigations, severe TBI was defined as one resulting in a GCS score of 8 or less and moderate TBI was defined as a GCS score of 9–12 or a score of 13–15 accompanied by evidence of brain insult (on CT scan or MRI at the time of injury). Mild TBI was defined as a GCS score of 13–15 with no imaging abnormalities. (However, based on the recruitment criteria described above, children with a GCS score of 15 at the time of injury and no evidence of brain insult on initial imaging were not enrolled in the present study.) Children with both TBI and accompanying orthopedic injuries were included in the TBI group. Children who sustained non-blunt head trauma (e.g., projectile wounds, strokes, drowning) were excluded. Inclusion in the orthopedic group required a documented bone fracture (other than the skull) requiring an overnight hospital stay and the absence of any evidence of loss of consciousness or other findings suggestive of brain injury (e.g., symptoms of concussion). We contacted all children in the parent study who had sustained a TBI and who were six years of age (or older) and at least 12 months post-injury regarding participation in the neuroimaging study. Potentially eligible children with orthopedic injuries were matched on time since injury, age, gender, ethnicity, and handedness with the TBI group.
2.2. Participants
A total of 14 children with TBI and 17 children with orthopedic injuries were identified from the parent project investigating TBI in young children based on age and time since injury. Because of the preliminary nature of the current study, the original sample size was not determined by a power analysis. Of these, 23 (74%) consented to participate in the imaging study. Among the consented subjects, 8 children with TBI and 12 children with orthopedic injuries successfully completed MRI/fMRI scanning and were retained for subsequent data analysis. Those who consented but were not included in the analysis had unusable data due to excessive head motion: n = 3 with orthopedic injuries. The demographic information of subjects included in the current report is listed in Table 1. The TBI group and OI group did not differ significantly in age, time since injury, gender, ethnicity, handedness, verbal IQ or maternal education level. Based on the criteria for injury severity outlined above, one child in the TBI group had a severe TBI and seven had injuries of moderate severity.
Table 1.
Demographic information of subjects included in the current study. In the TBI group, one had severe TBI and seven had injuries of moderate severity
| ID | Time since injury (yrs) |
Age (yrs) |
Sex | Injury mechanism |
GCS | NEPSY | VIQ | BRIEF GEC |
Maternal education |
Focal abnormality score |
Neuroimaging findings* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TBI_1 | 3.33 | 9.04 | F | Fall | 15 | 10 | 96 | 57 | 2 | 0 | negative |
| TBI_2 | 3.08 | 6.72 | M | Ped Vs MVC | 9 | 9 | 92 | 55 | 1 | 0 | negative |
| TBI_3 | 2.83 | 9.01 | M | Bicycle | 13 | 6 | 77 | 60 | 2 | 0 | negative |
| TBI_4 | 2.75 | 9.10 | M | Fall | 15 | 11 | 105 | 60 | 2 | 0 | negative |
| TBI_5 | 2.5 | 6.93 | M | Fall | 14 | 17 | 118 | 55 | 2 | 0 | negative |
| TBI_6 | 2.17 | 7.82 | M | Ped Vs MVC | 10 | 11 | 87 | 78 | 5 | 3 | Small cyst bilaterally frontal |
| TBI_7 | 1.75 | 8.33 | F | Fall | 9 | 16 | 115 | 46 | 2 | 2 | Heterotopic gray mat- ter (L frontal) |
| TBI_8 | 1.25 | 6.90 | F | Fall | 3 | 9 | 88 | 61 | 2 | 45 | volume loss with mild, moderate and severe encephalomalacia & tiny hemorrhage |
| OI_1 | control | 7.34 | M | Fall | NA | 7 | 103 | 67 | 2 | 0 | negative |
| OI_2 | control | 6.55 | M | Rough housing | NA | 16 | 90 | 43 | 4 | 0 | negative |
| OI_3 | control | 6.47 | M | Fall | NA | 14 | 115 | 52 | 4 | 0 | Chiari I & small syrinx |
| OI_4 | control | 9.10 | F | Sledding | NA | 10 | 94 | 47 | 2 | 1 | Abn signal R par wm; mildly prominent perivascular |
| OI_5 | control | 8.62 | F | Fall | NA | 10 | 75 | 65 | 4 | 1 | Small head, big ade- noids & tonsils |
| OI_6 | control | 6.63 | F | Playground | NA | 10 | 110 | 36 | 5 | 0 | negative |
| OI_7 | control | 8.19 | F | Trampoline | NA | 11 | 144 | 59 | 5 | 0 | negative |
| OI_8 | control | 6.46 | M | Ped Vs MVC | NA | 12 | 93 | 43 | 2 | 0 | negative |
| OI_9 | control | 7.32 | F | Furniture fell on leg |
NA | 8 | 80 | 66 | 3 | 0 | negative |
Note 1: TBI = traumatic brain injury; OI = orthopedic injury; Ped. = pedestrian; MVC = motor vehicle collision; GCS = Glasgow coma scale; VIQ = verbal IQ; BRIEF = behavior rating inventory of executive function.
Note 2: maternal education level: 1 = 2 yrs high school; 2 = high school degree; 3 = 2 yrs college; 4 = 4 yrs college; 5 = graduate degree.
Note 3: Images were evaluated for volume loss, abnormal gray matter signal, abnormal white matter signal and evidence of hemorrhage. Each focal abnormality score was ranked as 0 none; 1 mild; 2 moderate; 3 severe. For the four lobes, each hemisphere was evaluated separately.
Neuroimaging obtained > 12 months post injury.
2.3. Clinical MRI findings
A board certified pediatric neuroradiologist quantitatively evaluated the structural MRI scans of all participants with TBI or OI for focal abnormalities in the brain. The evaluation included evidence of volume loss, abnormal signal, or evidence of hemorrhage in eleven regions and sub-structures. The severity scores ranged from 0 for none (normal), 1 – mild, 2 – moderate and 3 – severe for a given region. The sum of the individual regional scores for each feature was compiled for a global rating of chronic brain injury and tabulated in Table 1. Eight of the regions considered were classified by hemisphere (right and left) and lobe (frontal, temporal, parietal, and occipital). The remaining three regions included the brainstem, cerebellum (vermis and hemispheres) and deep nuclei. Any other cranial abnormalities, if present, were also noted. Among participants retained for final analysis, two (one in the TBI group, one in the control group) had abnormal findings of significant pathology and were referred for a clinical MRI. Additionally, one participant with severe TBI scored a value of 45 due to severity scores of 3 in multiple brain regions. In three other participants (two in the TBI group and one in the control group) radiology reports reflected either incidental findings or likely stable TBI sequelae. The one OI control had imaging findings demonstrating a Chiari I malformation scaled as a 1. Five out of eight participants in the TBI group had normal imaging results according to this evaluation schema. Table 1 details the imaging results for the cohorts.
2.4. fMRI paradigm
The Verb Generation paradigm (VG) is based on a task initially developed for PET imaging [30] that involves the auditory presentation of a series of concrete nouns in a 30-second on-off block design. The paradigm has previously been used successfully with young participants [31]. All stimuli were presented using MacStim (White Ant Software, Melbourne, Australia). Stimuli were presented at a rate of one every 5 s for a total of 6 stimuli during each active epoch. During the active epochs, the subjects silently generated appropriate verbs, such as “throw” or “kick”, to aurally presented nouns such as “ball”. During the control epochs, subjects tapped their fingers when they heard a warble tone of 1 s duration at random frequencies (400– 2500 Hz) and intervals (1–3 sec). This was designed to control for sublexical auditory processing, as well as providing a task to distract the subjects (so they would not continue generating verbs into the control epoch). Previous studies have shown that the finger tapping task activates distinct parts of the brain (motor strip) from the language task, indicating minimal interference between tasks. It also provided a reference area of activation within the motor strip as an independent means of validating subject compliance. Participants were also monitored inside the scanner to ensure that they engaged in the finger-tapping exercise during appropriate intervals of the paradigm. This performance assessment is critical to ensure the participation of young children in an otherwise passive language paradigm.
2.5. Neuropsychological battery
The NEPSY Verbal Fluency Subtest (ages 3–12 years) [32,33] requires the child to generate a list of different types of animals and foods/drinks as quickly as he or she can. It is a measure of both verbal fluency and mental flexibility or the ability to shift from one conceptual set to another. This standardized verbal fluency test is widely used in pediatric clinical neuropychological evaluations to identify language and executive function deficits in school age children.
The Differential Ability Scales (DAS) was used to assess global verbal intelligence. The DAS is a battery of cognitive tests for ages 2.5 through 17 years [34]. Internal consistency indexes are 0.89 or higher across the target age range, and test-retest reliability is 0.90 over a 4-week interval. This measure of intelligence was chosen for its excellent psychometric properties and because it has norms extending into the preschool age range, and thus can be administered across the age range of the parent study population.
The Behavior Rating Inventory of Executive Function (BRIEF) Global Executive Composite (GEC) provided a parent-report measure of deficits in attention, impulse control, and executive function, factors that are often affected following TBI [35]. The GEC is comprised of two indexes, Behavioral Regulation and Meta-cognition. The BRIEF has demonstrated high internal consistency, test-retest reliability, as well as discriminant validity in a number of clinical populations (Learning Disabilities, ADHD Inattentive and Combined Types). We examined the BRIEF GEC in the current analyses to control for the effects of attention and organization during the imaging task.
2.6. Image acquisition
All images were acquired on a 3.0 Tesla Siemens Trio MRI scanner in the Department of Radiology at Cincinnati Children’s Hospital. Gradient-echo EPI scans were acquired in the axial plane with the following parameters: TR/TE = 3000/38 msec, FOV = 25.6 × 25.6 cm, matrix = 64 × 64 and 36 slices with 4 mm slice thickness. The duration of the firm sequence was 5 minutes 30 seconds. A whole brain T1-weighted 3D MP-RAGE anatomical image was also acquired with the following parameters: TR/TE = 2000/2.93 ms, FOV = 21.9 × 21.9 cm, matrix = 256 × 205, scan time = 3 minutes and 50 seconds at the end of the functional imaging session. This series of images was used for anatomical co-registration of the fMRI results. Details about the image acquisition can be found in the appendix.
2.7. Data processing
Image reconstruction, post-processing, and the group statistical analysis were performed using Cincinnati Children’s Hospital Imaging Processing Software (CCHIPS) written in Interactive Data Language (IDL) (Research Systems Inc., Boulder, CO). The EPI images were corrected for Nyquist ghosting and geometrical distortion (due to B0 field inhomogeneity) at the same time, based on a multi-echo phase reference scan [36]. The reconstructed EPI-fMR images were corrected for drift using a quadratic baseline correction on a pixel-by-pixel basis. Artifacts due to subject motion were also corrected using a pyramid iterative co-registration algorithm [37]. All data sets met the criterion of median voxel displacement at the center of the brain < 0.7 voxel. The images were then transformed into Talairach coordinates [38] using a linear affine transformation, previously validated for the age range 5–18 years [39, 40].
3. Statistical analysis
The fMRI data was post-processed using a general linear model (GLM) [41] and implemented in CCHIPS/IDL on a pixel-by-pixel basis to identify the activated brain regions in a given experiment. For each subject, a pixel-by-pixel Pearson’s correlation coefficient (between BOLD signal and the HRF-convoluted time course) was calculated and converted to the corresponding z-score maps using the Fisher’s z-transformation. The composite group activation map was calculated from individual z maps based on a one-sample t-test (for each pixel across all subjects) for the significance of a pixel in the group. A statistical parametric map was generated based on these t-statistics. The composite t-score map can be used to identify brain regions for the entire group with the most significant contrast between the active (language) and the control (finger tapping) tasks. This methodology accounts for both intra-subject and inter-subject variance and is commonly referred to as a random-effects analysis. A cluster method was used to improve the specificity by adjusting the inflated alpha for multiple comparisons [42]. In addition, Monte-Carlo simulation was used to determine the p-value corresponding to a certain combination of cluster size and z-threshold.
We also performed an analysis similar to previous studies [43] to test the difference in BOLD signal activation between the TBI and OI groups. This analysis involved the following steps: (1) The difference in brain activation between the two groups in response to the verb generation task was first studied with a pixel-wise simple linear regression analysis. (2) To investigate whether any of the group differences in brain activation were influenced by other potential confounders, the regression model was repeatedly tested and expanded in the following manner. Each variable was pre-tested separately for its potential influence on the overall and the group difference in BOLD activation in relation to the verb generation task. After adding a putative variable (one at a time) into the otherwise simple linear regression between BOLD signal and the GROUP variable, we calculated the change of regression coefficient (BETA value) on a pixel-by-pixel basis to evaluate the influence of the confounding variable. We restricted these analyses to the cortical areas that had been found to correlate significantly with the GROUP variable in the previous univariate analyses. A variable was included in the subsequent final multivariate analysis as a covariate if it caused more than 20% of the pixels within the predetermined regions of interest to have a change greater than 10% in the BETA value. In this study we tested NEPSY verbal fluency, BRIEF GEC and DAS verbal IQ for their potential confounding effects. The above procedure resulted in including NEPSY score and BRIEF GEC as covariates in the final regression model.
Finally, by combining both the TBI and OI groups a correlation analysis was performed to test the significance of the relationship between brain activation and NEPSY verbal fluency score and DAS verbal IQ score, respectively. Similarly, a within group correlation analysis between the GCS score and the BOLD signal activation was also performed for the TBI group.
4. Results
Figure 1a presents the statistical parametric map (composite Z-score map) for significant activation (nominal z = 5, cluster = 30, corrected p < 0.05) for language processing across the entire OI group after correcting for multiple comparisons in our study. Figure 1b presents the statistical parametric map (nominal z = 4.5, cluster = 15, corrected p < 0.05) corrected for multiple comparisons for the entire TBI group. Similar to previous reports [31], the functional areas in the inferior frontal gyrus (IFG) and superior temporal gyrus (STG) for the verb generation task are activated in our OI control cohort (Fig. 1a): the left inferior frontal gyrus including Broca’s area (Brodmann Area [BA] areas 44, 45 and 46), the left superior temporal gyrus and the left middle temporal gyrus including Wernicke’s area (BA 21 and BA 22), as well as some homologues that are located contralaterally in the right hemisphere.
Fig. 1.

(a) Composite Z-score map showing areas of significant activation for language processing across the entire OI control group (nominal z = 5, cluster size = 30, with p < 0.05 and corrected for multiple comparisons). Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation. 1 left inferior frontal gyrus and 2 left and right superior/middle temporal gyrus. (b) Composite Z-score map showing areas of significant activation for language processing across the entire TBI cohort (nominal z = 4.5, cluster size = 15, with p < 0.05 and corrected for multiple comparisons). Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation.1left inferior frontal gyrus and 2 left superior/middle temporal gyrus.
A strong left hemispheric dominance of frontal and temporal language activation consistent with previous studies [31,44], is observed in the composite maps for the two groups. Although the TBI cohort (Fig. 1b) shows similar left hemispheric activation in Broca’s area, the right superior temporal gyrus (BA 22) and the right middle temporal gyrus (BA 21) show significantly different activation patterns. The activation pattern is strongly concentrated in a relatively few language-related areas compared to the OI cohort. This finding provides support for the hypothesis that neural activation during language tasks is altered following moderate-to-severe TBI in young children.
Figure 2 is a correlation map (with corresponding z-scores) showing differences between the TBI and OI groups. Figure 2a shows regions where the OI group activation is greater than in the TBI group and highlights the following language-related brain regions: superior/medial temporal gyrus and angular gyrus, both bilaterally (nominal z = 5.5, cluster = 15, corrected p < 0.001). The corresponding brain regions are tabulated in Table 2a. Figure 2b and Table 2b show the language-related brain regions with greater activation intensity in the TBI group (although not as strong as the previous OI group activation) as compared with the OI group (nominal z = 4, cluster = 25, corrected p < 0.02). Notable differences are highlighted in this comparison in the regions of right parietal, occipital and temporal junction regions, left middle temporal gyrus, left inferior frontal gyrus and the dorsolateral prefrontal cortex.
Fig. 2.

(a) Group difference correlation map showing regions where activation intensity in the OI group is significantly greater than in the TBI patients (nominal z = 5.5, cluster size = 15, corrected p < 0.001). Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation. Corresponding brain areas are tabulated in Table 2a. (b) Group difference correlation map showing the language-related brain regions with significantly greater activation intensity in the TBI group compared with the OI control group (nominal z = 4, cluster size = 25, corrected p < 0.02). Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation. Corresponding brain areas are tabulated in Table 2b.
Table 2.
| a. Activation foci (Talairach coordinates) for the brain areas shown in Group Difference activation map – Fig. 2a | |||
|
| |||
| Number | Broadmann’s Area | Brain Region | X, Y, Z |
|
| |||
| 1 | 21, 22 | L. Middle temporal gyrus and superior temporal gyrus | −38, −25, 19 |
| R. Middle temporal gyrus and superior temporal gyrus | 58, −45, 15 | ||
| 2 | 19, 39, 40 | L. Middle occipital lobule, angular gyrus and inferior parietal lobule | −38, −45, 35 |
| R. Middle occipital lobule, angular gyrus and inferior parietal lobule | 46, −29, 23 | ||
|
| |||
| b. Activation foci (Talairach coordinates) for the brain areas shown in Group Difference activation map – Fig. 2b | |||
|
| |||
| Number | Broadmann’s Area | Brain Region | X, Y, Z |
|
| |||
| 3 | 18, 19, 37 | R. Posterior parietal lobe, temporo-parietal-occipital area | 22, −81, −9 |
| 4 | 21, 22 | L. Middle temporal gyrus and superior temporal gyrus | −54, −41, 3 |
| 5 | 44, 45, 46, 47 | L. Inferior frontal gyrus and dorsolateral prefrontal area | −34, 55, −7 |
|
| |||
5. Correlation between BOLD activation and neuropsychological test scores: Voxel-based within- and between-group analyses
To test the significance of the relationship between NEPSY verbal fluency score and the language-related BOLD signal activation in the combined population (both OI and TBI), we conducted a simple correlation analysis. Several key cortical areas demonstrate significant associations in this analysis. Figure 3a shows the correlation coefficient color-coded and overlaid on the structural images (nominal z = 5, cluster = 25, corrected p < 0.05). The Talairach coordinates of the foci for these regions are tabulated in Table 3. Figure 3b shows the linear regression between the average z-score of BOLD activation (in brain regions shown in Fig. 3a) and the corresponding NEPSY score (R = −0.5, p = 0.036) for all OI and TBI subjects. Notably no significant positive correlations between BOLD signal and NEPSY score were found.
Fig. 3.

(a) Correlation map showing areas where correlation between BOLD signal and NEPSY score is significant (nominal z = 5, cluster size = 25, corrected p < 0.05) for the combined group of OI and TBI subjects. Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation. Corresponding brain regions are tabulated in Table 3. (b) Linear regression of average z-score BOLD activation against NEPSY score for the combined group of OI and TBI subjects for brain regions highlighted in Fig. 3a.
Table 3.
Activation foci (Talairach coordinates) for the brain areas shown in Fig. 3
| Number | Broadmann’s Area | Brain Region | X, Y, Z |
|---|---|---|---|
| 1 | 45, 47 | L. Inferior frontal gyrus | −30, 27, −1 |
| R. Inferior frontal gyrus | 46, 11, 7 | ||
| 2 | 44, 46 | L. Inferior frontal gyrus | −46, 15, 11 |
| R. Inferior frontal gyrus | 42, 39, 23 | ||
| 3 | 41, 42, 21, 22 | L. Middle temporal gyrus, superior temporal gyrus, primary auditory cortex | −54, −25, −1 |
| R. Middle temporal gyrus, superior temporal gyrus, primary auditory cortex | 42, −25, 19 | ||
| 4 | 8, 9, 32 | L. Precuneus/posterior cingulate | −14, 31, 35 10, 31, 35 |
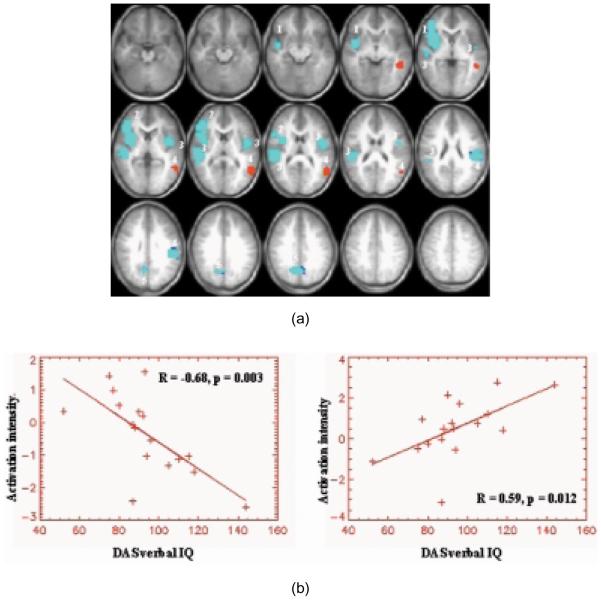
Similarly, a correlation analysis between the DAS verbal IQ (DAS-VIQ) score and the z-score of the BOLD signal activation in the combined population was performed. Figure 4a shows both positive and negative correlation coefficients coded in color and overlaid on the structural images (nominal z = 5, cluster = 25, corrected p < 0.01). The colors light blue and red represent negative and positive correlations, respectively. The corresponding brain regions are tabulated in Table 4. DAS-VIQ was negatively correlated with brain activation in many of the same brain regions as the NEPSY. This was expected as NEPSY and DAS-VIQ scores are significantly correlated (R = 0.46, p = 0.03) for the 23 subjects recruited for the study. However, activation in the left angular gyrus showed a positive correlation with the DAS-VIQ. Figure 4b shows the linear regression of the voxels with positive and negative correlation between the average BOLD z-score in brain regions shown in Fig. 4a for each subject (R = 0.6, p = 0.01 and R = −0.67, p = 0.003 respectively).
Fig. 4.
(a) Correlation (red represents positive and light blue represents negative) map showing areas where correlation (positive and negative) between BOLD signal and DAS verbal IQ score is significant (nominal z = 5, cluster size = 25, corrected p < 0.01) for the combined group of OI and TBI subjects. Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation. Corresponding brain regions are tabulated in Table 4. (b) Linear regression (both positive and negative) of average z-score BOLD activation against DAS verbal IQ score for the combined group of OI and TBI subjects for brain regions highlighted in Fig. 4a.
Table 4.
Activation foci (Talairach coordinates) for the brain areas shown in Fig. 4 (VERBAL IQ)
| Number | Broadmann’s Area | Brain Region | X, Y, Z |
|---|---|---|---|
| 1 | 45, 47 | R. Inferior frontal gyrus | 46, 7, −5 |
| 2 | 44, 46 | R. Inferior frontal gyrus | 50, 27, 11 |
| 3 | 41, 42, 21, 22 | L. Middle temporal gyrus, superior temporal gyrus, primary auditory cortex | −42, −9, 15 |
| R. Middle temporal gyrus, superior temporal gyrus, primary auditory cortex | 50, −17, 7 | ||
| 4 | 39, 40 | L. Angular gyrus, Inferior parietal lobule | −46, −29, 23 |
| 5 | 19, 7, 31 | R. Precuneus/posterior cingulate | 10, −57, 35 |
Finally, we conducted a within-group correlation analysis between the GCS score and the BOLD signal z-score (language-related areas) in the TBI group. (This scale was not available or relevant in the OI group.) Figure 5 shows the negative correlation coefficients coded in light blue and overlaid on the structural images (nominal z = 5, cluster = 25, corrected p < 0.005). The correlation between the average BOLD z-score in brain regions shown in Fig. 5 and the corresponding GCS score was highly significant at R = −0.82 with a p value of 0.013. Bilateral brain regions, including the inferior frontal gyri, middle and superior temporal gyri, angular gyri and posterior cingulate, showed a negative correlation with GCS scores, indicating that more severe injuries (i.e., lower GCS scores) were associated with greater activation in these regions.
Fig. 5.

Correlation map showing areas where correlation between BOLD signal and GCS score is significant (nominal z = 5, cluster size = 25, corrected p < 0.005) for TBI subjects. Slice range −65 to +75 mm (Talairach coordinates). Images are in radiological orientation.
6. Discussion
Structural and functional imaging studies of the effects of TBI on the developing brain are still in their infancy. Heterogeneity of injury foci and severity and the complications of conducting imaging studies on an immature brain have contributed to this dearth of information [45]. The current findings are important in several respects and represent the first investigation of brain activation patterns in language in children following traumatic brain injury. First, the present study demonstrates the feasibility of using fMRI to study brain and behavior relationships in this population; this is by no means an easy task considering the difficulties associated with performing fMRI in children [46]. Second, the findings support our hypotheses that fMRI would reveal greater activation of involved brain regions in children with moderate to severe TBI relative to the OI group and that children with greater deficits on neuropsychological indices would show greater differences in the areas of fMRI activation. The findings are consistent with previous fMRI studies by our group showing reorganization of brain function associated with the verb generation task in children with severe brain injury due to perinatal stroke [47]. Although only a limited number of studies have shown language reorganization in children [48], studies of adults [48– 52] overwhelmingly support functional reorganization after brain injury. Finally, we were able to find correlations between performance on neuropsychological tests of target language functions with neuroimaging indices of brain activation. Taken together, these findings provide the first evidence that children’s brains function differently following a traumatic brain injury and that in language tasks differences in activation may be reflected as differences in neuropsychological task performance. The current results provide a critical first step in understanding the neural underpinnings of the cognitive and behavioral deficits commonly observed following TBI in children.
These young children with TBI typically showed BOLD activation in and around the expected brain areas for this language based task, suggesting that the severity and nature of the injuries were not sufficient to require total neural reallocation. However, we did find significant differences in activation between the children with TBI and our comparison group (OI) in specific areas of the language circuitry of the brain. Consistent with our hypothesis and previous research on neural activation following mild TBI in adults [26], we found increased activation in certain areas of the language circuitry, particularly the left inferior frontal gyrus (BA 44/45/46, i.e., Broca’s Area). This brain region is implicated in expressive language processing [44,53,54]. Several studies have implicated Broca’s area in syntactic processing at the sentence level [55–58]. The findings of these studies are in line with recent studies showing left hemispheric specialization for sentence comprehension and language processing [59, 60]. Furthermore, some studies have also shown the involvement of Broca’s area in verbal fluency experiments [31,61]. However, its appearance in a task involving semantic processing of silent verb generation indicates a role beyond expressive language or syntactic/semantic processing [62]. The notion that Broca’s area subserves broader cognitive tasks than previously believed is further supported by a recent diffusion tensor imaging (DTI) study [63]. The study postulated that Broca’s area could extend beyond its classical boundaries to incorporate a part of the middle frontal gyrus and the inferior precentral gyrus involved in higherorder language processing.
The VG task also requires that children manipulate the target noun and possible verb matches in working memory. Thus, for the current task, activation in the anterior left inferior frontal gyrus (BA 46) was likely associated with monitoring and manipulation in working memory [62,64]. Consistent with previous neuropsychological studies [12,65,66], these findings provide evidence that children with TBI are unable to process semantic/syntactic transcoding, semantic association, and working memory demands as efficiently as uninjured children. As a consequence, children with TBI may need to draw upon parts of the language circuitry not usually required to perform the task to a greater extent than typically developing children. This is the probable explanation for the greater activation in dorsolateral prefrontal cortex in the TBI group than in the OI control group shown in Fig. 2b and tabulated in Table 2b as area 5.
We found less activation in the TBI group in the left/right middle temporal gyrus (BA21), left/right superior temporal gyrus (BA 22/STG) and the left/right angular gyrus relative to the orthopedic injury comparison group. These findings are consistent with previous research on pediatric neurological conditions indicating that brain trauma may result in less activation in damaged brain regions that typically subserve the task. Activation in STG has previously been reported in studies of processing of semantic anomalies [67-69]. However, the idea that word meaning is primarily processed by the classical Wernicke’s area has been challenged [70]. The alternative hypothesis implicates STG in spectral and temporal processing of the auditory input with the information then projected to higherorder association cortex [70,71]. Therefore, our results may support the notion that children with TBI have deficiencies in spectral/temporal processing and the associated projection mechanisms. On the other hand, the angular gyrus is mainly thought to be involved in higher order semantic processing [53]. The involvement of angular gyrus, in terms of higher order semantic processing, may be debatable for VG given the simplicity of the task. However, there is evidence from lesional [72] and neuroimaging [73,74] studies to support the involvement of angular gyrus in semantic processing.
Activation in the angular gyrus has also been detected in studies involving: 1) rehearsal of things heard to keep them in memory [75-77] and 2) attentional focus on verbal material [78]. Although some studies have implicated only the left angular gyrus [79] in semantic processing, it is generally assumed that this structure is recruited bilaterally [74]. In the context of VG, both rehearsal and attentional focus may be relevant and adversely affected by TBI as shown by activation differences (bilateral) in the angular gyrus [80]. Thus, our data supports the notion that both attentional and language processing deficiencies may directly and indirectly contribute to observed language deficits following pediatric TBI. Unfortunately, clinical imaging at the time of injury (primarily CT scans) was inadequate to shed light on the areas of the brain that may have sustained initial subtle damage in these children. Further research is necessary to more fully understand how TBI actually affects the language circuitry of the brain and the relationship between these changes and observed language deficits over time, as well as modifiability of these circuits to intervention.
In the sample as a whole, activation levels in most of the language circuitry as reflected in the GLM z-scores were negatively correlated with test performance on measures of verbal intelligence and fluency. This reflected increased brain activity in these regions in children with poor performance scores. One exception is the activation in the left angular gyrus which was found to be positively correlated with the DAS-VIQ. Although the mechanism of involvement of the left angular gyrus in DAS-VIQ is still a matter of debate, it may have some bearing on attentional focus and higher-order language processing. These findings suggest that additional neural recruitment directed at performing the task translated into poorer rather than better performance. Put differently, children with greater neural resources needed less mental reserves to achieve superior performance. Likewise, within-group analyses of the relationship between GCS scores and brain activation in the TBI group revealed a negative correlation (i.e., children with more severe injuries/lower GCS scores had greater activation) in many areas of the language circuitry. These findings suggest that the brains of children with more severe injuries are exerting greater effort during the task. Taken together, these correlation analyses suggest that increased neural effort reflects processing inefficiencies that correspond to injury severity as well as diminished performance.
Several limitations of the current investigation should be noted. First, the sample size was small, limiting both statistical power and the ability to control for confounding variables. However, this limitation is tempered by the fact that the TBI and comparison cohort were within a fairly limited age range (6–9 years) and well-matched on key demographic factors (such as socioeconomic status) which may be associated with neural activation. The fact that two of the children in the OI comparison group had abnormalities on the structural scans may have reduced our ability to detect significant group differences, thus making the finding of multiple differences more compelling. Another limitation of the current study was the inconsistency of imaging data at the time of injury. The majority of the children in the current sample had CT scans rather than MRI at the time of injury, making it difficult to characterize the nature and location of the white matter lesions at the time of injury. Additionally, we did not have fMRI studies from the acute injury period, making it impossible to examine changes in neural activation patterns over the initial year following pediatric TBI. Previous imaging studies suggest that depending on severity of lesion and location (cortical vs sub-cortical) etc., cortical reorganization and associated changes in motor behavior changes over time may vary [81,82]. All but one of the children in the current TBI sample had moderate rather than severe injuries. Thus, it is possible that findings for children with severe injuries would reflect a greater degree of neural re-allocation than observed in the current study. This fact may be relevant to the current study given the two groups are not completely homogenous (5 out of 8 subjects in the TBI group showed normal imaging results). Newer, more sophisticated fMRI analysis techniques such as Independent Component Analysis (ICA) [71,83,84], Structural Equation Modeling [85,86], Path Analysis [87] and Dynamical Causal Modeling [88-91] may further help resolve unanswered questions regarding the information flow abnormalities in TBI children compared to a normative group. In addition, structural changes in brain connectivity could be explained using MRI diffusion tensor imaging (DTI) [63] to reveal a more direct view of the influences of TBI on white matter pathways connecting task-related cortical areas.
Because these findings represent neural activation at a single point in time during the chronic recovery phase (greater than 12 months post injury), they do not provide information about acute changes in neural allocation or activation levels, nor do they shed light on the process of neural reorganization over time. It’s possible that treatments given to children following their TBI altered their neural recovery and subsequent development. Of the 8 children with TBI, three had no followup treatment for their injuries. Of the others, two were treated with stimulant medication for secondary attention deficit hyperactivity disorder and three received speech therapy, with two of these children also receiving occupational therapy and physical therapy. Thus, it is possible that these therapies played an important role in the child’s neural recovery, which repeat scans over time would reveal.
Finally, we would like to mention a design limitation of the current study, namely the inability to take in-scanner performance data (although the finger tapping task provides a gross assessment of task performance). Therefore, confounding effects due to performance have not been eliminated completely in the current analysis although the task was specifically designed to be easily performed by children as young as 5 years and the groups did not differ on DAS-VIQ or NEPSY scores. Performance may also be correlated with age, which in turn has been shown to be positively correlated with fMRI BOLD signal [92]. However, the impact due to this correlation may be less important in the current study because all groups were carefully matched on age and sex. Since we expect the task performance to be correlated with at least some of the neuropsychological measures, it is reasonable to assume that isolating performance as a confound may not be possible in the current study. However, future TBI studies will utilize a newly developed version of the current VG task which incorporates a completely silent scanner interval, during which an overt response can be made and recorded, allowing better evaluation of in-scanner performance data [93,94].
7. Conclusions
The current findings suggest that fMRI is a feasible, noninvasive means of examining changes in neural allocation and processing following TBI in young children. Moreover, our results indicate that fMRI can shed light on changes in brain function in children with mild-moderate as well as severe injuries. Future investigations that follow larger cohorts over time will be necessary to characterize changes in cortical reorganization over time following TBI in children and their relationship to specific therapies and language outcomes. In this regard, it will be particularly important to have sufficiently large cohorts of children with mild, moderate, and severe TBI to evaluate how changes in brain activation may vary depending on the nature and severity of the injury. Nonetheless, the current findings underscore the promise of fMRI for understanding the neural underpinnings of cognitive and behavioral deficits associated with pediatric TBI.
Acknowledgements
This study is supported in part by 1) NIH grant RO1-HD044279 from the National Council on Medical Rehabilitation Research in the National Institute of Child Health and Human Development; 2) UPHS GCRC Grant # M01 RR 08084 from the National Center for Research Resources, NIH; 3) NIH grant RO1HD044279 from the U.S. National Institute of Child Health and Human Development; and 4) NIH grant RO1-HD38578 from the US National Institute of Child Health and Human Development. Funding for the fMRI scans was provided by the Association of Volunteers of the Convalescent Hospital for Children, Cincinnati Children’s Hospital Medical Center.
Appendix
At the beginning of each child’s imaging series we acquired a 3-plane localizer image using a fast gradient echo method (31 second acquisition time) as well as a lower resolution MP-RAGE whole brain image with the same TR and TE but with a lower resolution and a shorter scan time: FOV = 21.9 × 21.9 cm, matrix = 256 × 128, scan time = 2 minutes and 30 seconds. The low-resolution anatomical image was acquired at the conclusion of the fMRI to be used in data analysis in case the high resolution anatomical image acquisition was unsuccessful due to failing compliance of the young subjects. In addition to anatomical, functional and alignment scans, a phase reference scan was also acquired for each subject. This scan required 3 minutes and 12 seconds and was used during reconstruction of the EPI scans to correct for ghost artifacts and geometrical distortions [36]. This correction is imperative at field strengths of 3 Tesla and higher where distortions of the EPI scans relative to the higher bandwidth T1 images of brain anatomy can lead to misleading activation maps.
References
- [1].Fay GC, et al. Outcome of pediatric traumatic brain injury at three years: a cohort study. Arch Phys Med Rehabil. 1994;75(7):733–741. [PubMed] [Google Scholar]
- [2].Fletcher JM, et al. Behavioral changes after closed head injury in children. J Consult Clin Psychol. 1990;58(1):93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- [3].Janusz JA, et al. Social problem-solving skills in children with traumatic brain injury: long-term outcomes and prediction of social competence. Child Neuropsychol. 2002;8(3):179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- [4].Taylor HG, et al. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- [5].Stancin T, et al. Health-related quality of life of children and adolescents after traumatic brain injury. Pediatrics. 2002;109(2):E34. doi: 10.1542/peds.109.2.e34. [DOI] [PubMed] [Google Scholar]
- [6].Rivara JM, et al. Predictors of family functioning and change 3 years after traumatic brain injury in children. Arch Phys Med Rehabil. 1996;77(8):754–764. doi: 10.1016/s0003-9993(96)90253-1. [DOI] [PubMed] [Google Scholar]
- [7].Wade SL, et al. A prospective study of long-term caregiver and family adaptation following brain injury in children. J Head Trauma Rehabil. 2002;17(2):96–111. doi: 10.1097/00001199-200204000-00003. [DOI] [PubMed] [Google Scholar]
- [8].Anderson VA, et al. Predicting recovery from head injury in young children: a prospective analysis. J Int Neuropsychol Soc. 1997;3(6):568–580. [PubMed] [Google Scholar]
- [9].Morse S, et al. Early effects of traumatic brain injury on young children’s language performance: a preliminary linguistic analysis. Pediatr Rehabil. 1999;3(4):139–148. doi: 10.1080/136384999289405. [DOI] [PubMed] [Google Scholar]
- [10].Ewing-Cobbs L, et al. Language functions following closed-head injury in children and adolescents. J Clin Exp Neuropsychol. 1987;9(5):575–592. doi: 10.1080/01688638708410770. [DOI] [PubMed] [Google Scholar]
- [11].Hallett TL. Linguistic competence in paediatric closed head injury. Pediatr Rehabil. 1997;1(4):219–228. doi: 10.3109/17518429709167362. [DOI] [PubMed] [Google Scholar]
- [12].Chapman SB, et al. Discourse macrolevel processing after severe pediatric traumatic brain injury. Dev Neuropsychol. 2004;25(1–2):37–60. doi: 10.1080/87565641.2004.9651921. [DOI] [PubMed] [Google Scholar]
- [13].Ewing-Cobbs L, et al. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc. 1997;3(6):581–591. [PubMed] [Google Scholar]
- [14].Catroppa C, Anderson V. Recovery and predictors of language skills two years following pediatric traumatic brain injury. Brain Lang. 2004;88(1):68–78. doi: 10.1016/s0093-934x(03)00159-7. [DOI] [PubMed] [Google Scholar]
- [15].Kingstone R.C.a.A. Handbook of Functional Neuroimaging of Cognition. MIT Press; Cambridge, Massachusetts: 2001. [Google Scholar]
- [16].Cabeza R, Kingston A. Handbook of Functional Neuroimaging of Cognition. The MIT press; Cambridge, Massachusetts: 2001. [Google Scholar]
- [17].Szaflarski JP, et al. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5(2):244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [18].Gabrieli JD, et al. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276(5310):264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- [19].Cabeza R, Nyberg L. Neural bases of learning and memory: functional neuroimaging evidence. Curr Opin Neurol. 2000;13(4):415–421. doi: 10.1097/00019052-200008000-00008. [DOI] [PubMed] [Google Scholar]
- [20].Detre JA, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50(4):926–932. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- [21].Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol. 1993;50(8):812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- [22].Pugh KR, et al. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4(3):159–173. doi: 10.1006/nimg.1996.0067. Pt 1. [DOI] [PubMed] [Google Scholar]
- [23].Poner M. Editor’s note: Attention as a cognitive neurosystem. J Cogn Neurosci. 1991;3:303. doi: 10.1162/jocn.1991.3.4.303. [DOI] [PubMed] [Google Scholar]
- [24].Christodoulou C, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;71(2):161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McAllister TW, et al. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- [26].McAllister TW, et al. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53(6):1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- [27].McAllister TW, et al. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol. 2001;23(6):775–791. doi: 10.1076/jcen.23.6.775.1026. [DOI] [PubMed] [Google Scholar]
- [28].Shaywitz BA, et al. Age-related changes in reading systems of dyslexic children. Ann Neurol. 2007;61(4):363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- [29].Taylor HG, et al. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- [30].Petersen SE, et al. Positron emission tomography studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–586. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- [31].Holland SK, et al. Normal fMRI Brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- [32].Korkman M, Kirk U. NEPSY – A Developmental Neuropsychological Assessment. Psychological Corporation; San Antonio: 1998. K. S.L. [Google Scholar]
- [33].Korkman M, Kemp SL, Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev Neuropsychol. 2001;20(1):331–354. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- [34].Elliot C. Differential Ability Scales: Introductory and Technical Handbook. The Psychological Corporation; San Antonio: 1990. [Google Scholar]
- [35].Gioia GA, et al. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- [36].Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20(6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thevenaz P, Unser M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Transactions on Image Processing. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- [38].Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. p. 122. [Google Scholar]
- [39].Muzik O, et al. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- [40].Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17(1):48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Worsley KJ, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- [42].Xiong J, Gao J-H. Cluster pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- [43].Yuan W, et al. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Szaflarski JP, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59(5):796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bigler ED. Neuroimaging in pediatric traumatic head injury: diagnostic considerations and relationships to neurobehavioral outcome. J Head Trauma Rehabil. 1999;14(4):406–423. doi: 10.1097/00001199-199908000-00009. [DOI] [PubMed] [Google Scholar]
- [46].Byars AW, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17(12):885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jacola LM, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37(1):46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Staudt M, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57(1):122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- [49].Booth JR, et al. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol. 2000;18(2):139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- [50].Brizzolara D, et al. Timing and type of congenital brain lesion determine different patterns of language lateralization in hemiplegic children. Neuropsychologia. 2002;40(6):620–632. doi: 10.1016/s0028-3932(01)00158-0. [DOI] [PubMed] [Google Scholar]
- [51].Muller RA, et al. Differential patterns of language and motor reorganization following early left hemisphere lesion: a PET study. Arch Neurol. 1998;55(8):1113–1119. doi: 10.1001/archneur.55.8.1113. [DOI] [PubMed] [Google Scholar]
- [52].Papanicolaou AC, et al. Brain plasticity for sensory and linguistic functions: a functional imaging study using magnetoencephalography with children and young adults. J Child Neurol. 2001;16(4):241–252. doi: 10.1177/088307380101600403. [DOI] [PubMed] [Google Scholar]
- [53].Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Szaflarski JP, et al. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2005;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Just MA, et al. Brain activation modulated by sentence comprehension. Science. 1996;274(5284):114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- [56].Stromswold K, et al. Localization of syntactic comprehension by positron emission tomography. Brain Lang. 1996;52(3):452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- [57].Inui T, et al. A functional MRI analysis of comprehension processes of Japanese sentences. Neuroreport. 1998;9(14):3325–3328. doi: 10.1097/00001756-199810050-00032. [DOI] [PubMed] [Google Scholar]
- [58].Caplan D, et al. Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp. 2000;9(2):65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hashimoto R, Sakai KL. Specialization in the left prefrontal cortex for sentence comprehension. Neuron. 2002;35(3):589–597. doi: 10.1016/s0896-6273(02)00788-2. [DOI] [PubMed] [Google Scholar]
- [60].Sakai KL, et al. Selective priming of syntactic processing by event-related transcranial magnetic stimulation of Broca’s area. Neuron. 2002;35(6):1177–1182. doi: 10.1016/s0896-6273(02)00873-5. [DOI] [PubMed] [Google Scholar]
- [61].Gaillard WD, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- [62].Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. Neuroimage. 2006;31(3):1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [63].Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- [64].Braver TS, et al. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14(1):48–59. doi: 10.1006/nimg.2001.0791. Pt 1. [DOI] [PubMed] [Google Scholar]
- [65].Levin HS, et al. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18(2):240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- [66].Ewing-Cobbs L, et al. Executive functions following traumatic brain injury in young children: a preliminary analysis. Dev Neuropsychol. 2004;26(1):487–512. doi: 10.1207/s15326942dn2601_7. [DOI] [PubMed] [Google Scholar]
- [67].Friederici AD, et al. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- [68].Kuperberg GR, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12(2):321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- [69].Ni W, et al. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12(1):120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- [70].Scott SK, et al. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. Pt 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: A functional magnetic resonance imaging study. Neuroimage. 2005;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- [73].Nakai T, et al. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla-comprehension level and activation of the language areas. Neurosci Lett. 1999;263(1):33–36. doi: 10.1016/s0304-3940(99)00103-2. [DOI] [PubMed] [Google Scholar]
- [74].Newman AJ, et al. An event-related fMRI study of syntactic and semantic violations. J Psycholinguist Res. 2001;30(3):339–364. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- [75].Awh E, et al. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychological Science. 1996;7(1):25–31. [Google Scholar]
- [76].Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- [77].Ravizza SM, et al. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- [78].Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16:315–339. [Google Scholar]
- [79].Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95(15):8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Behrman M, Geng JJ, Shomstein S. Parietal cortex and attention. Current Opinion in Neurobiology. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [81].Jang SH, et al. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter-blind preliminary study. Arch Phys Med Rehabil. 2005;86(11):2218–2223. doi: 10.1016/j.apmr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- [82].Liepert J, et al. Motor strokes: the lesion location determines motor excitability changes. Stroke. 2005;36(12):2648–2653. doi: 10.1161/01.STR.0000189629.10603.02. [DOI] [PubMed] [Google Scholar]
- [83].McKeown MJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Calhoun VD, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Solodkin A, et al. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14(11):1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- [86].Karunanayaka PR, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2006;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- [87].Bullmore E, et al. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11(4):289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- [88].Penny WD, et al. Comparing dynamic causal models. Neuroimage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- [89].Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- [90].Friston KJ. Bayesian estimation of dynamical systems: an application to fMRI. Neuroimage. 2002;16(2):513–530. doi: 10.1006/nimg.2001.1044. [DOI] [PubMed] [Google Scholar]
- [91].Mechelli A, et al. A dynamic causal modeling study on category effects: bottom-up or top-down mediation? J Cogn Neurosci. 2003;15(7):925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- [92].Schapiro MB, et al. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15(17):2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chiu CY, et al. Sound blending in the brain: a functional magnetic resonance imaging investigation. Neuroreport. 2005;16(9):883–886. doi: 10.1097/00001756-200506210-00002. [DOI] [PubMed] [Google Scholar]
- [94].Schmithorst VJ, Holland SK. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn Reson Med. 2004;51(2):399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]