Abstract
Background
Knowledge of hemodynamic factors accounting for the development of hypertension should help to tailor therapeutic approaches and improves blood pressure control. Few data exist regarding sex differences of hemodynamic factors contributing to hypertension progression among untreated non-diabetic prehypertensive (PreHyp), Stage I and II subjects as define by the Joint National Commission-7 guidelines (JNC-7).
Methods
We utilized non-invasive impedance cardiography, applanation tonometry, and plasma measures of angiotensin II, angiotensin-(1-7), serum aldosterone, hs-C reactive protein, and cytokine biomarkers of inflammation to characterize the hemodynamic and hormonal profile of 100 untreated hypertensive subjects (39 females).
Results
Despite there being no differences between females and males in terms of office blood pressure, heart rate, and body mass index, males demonstrated lower values of pulse pressure, systemic vascular resistance, brachial artery pulse wave velocity, and augmentation index. In each of the three hypertension categories, the increased blood pressure in males was associated with significant augmentations in stroke volume and cardiac output when compared to females. Sex related hemodynamic differences were associated in females with higher plasma levels of leptin, hs-C-reactive protein, plasma angiotensin II, and serum aldosterone and no differences in the serum concentrations of cytokinins. In women but not men, hs-C reactive protein correlated with plasma concentrations of TGF-β1 and body weight, in addition, plasma TGF-β1 correlated with levels of serum VCAM-1.
Conclusions
The impact of sex differences in the hemodynamic factors accounting for the elevation in arterial pressure in essential hypertensive subjects has been poorly characterized or this information is not available. We suggest that this gap in knowledge may adversely influence choices of drug-treatment since our study shows for the first time significant differences in the hemodynamic and hormonal mechanisms accounting for the increased blood pressure in women compared to men.
Keywords: angiotensin II, blood pressure, cardiac output, central aortic pressure, essential hypertension, inflammation, vascular disease
Introduction
Cardiovascular disease (CVD) in women is a major public health issue, ranking first among all disease categories in hospital discharges, and surpassing men in terms of the absolute number of deaths due to diseases of the heart and the blood vessels (Mosca et al., 2010; Mosca et al., 2011; Mosca et al., 2013). Although 70% of deaths in women are attributable to modifiable risk factors such as hypertension, the question of whether antihypertensive therapy should take into consideration potential differences in mechanisms between sexes has not been answered. Women have lower blood pressure control rates (Abuful et al., 2005), they are less likely to be appropriately treated (Ferrario et al., 2013; Joyner et al., 2012; Lloyd-Jones et al., 2005a; Lloyd-Jones et al., 2005b), and data suggest that treatment efficacies differ between the two sexes (Turnbull et al., 2007; Turnbull et al., 2008a; Turnbull et al., 2008b). In an investigation of the response rates to different drug regimens, Thoenes et al. (Thoenes et al., 2010) found a higher use of thiazides and beta-blockers in women. Another study reported a greater efficacy of aldosterone antagonists in reversing endothelial dysfunction in postmenopausal women (Rossi et al., 2011). A post-hoc analysis of the data obtained in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study showed less regression of electrocardiographic indices of left ventricular hypertrophy in women even after correction of co-factors such as treatment effects and blood pressure changes (Okin et al., 2008).
Consistent with our previous studies that underscored the importance of tailoring antihypertensive therapy based on the hemodynamic characteristics derived from non-invasive assessments (Abdelhammed et al., 2005; Ferrario et al., 2007; Ferrario and Smith, 2006; Smith et al., 2006a), we evaluated the characteristics of untreated hypertensive men and women in terms of the hemodynamic mechanism contributing the hypertension together with a direct assessment of renin angiotensin system components and inflammatory cytokines previously reported to be biomarkers of vasoconstriction, salt retention, and vascular inflammatory response. In accomplishing these objectives, we agree with Safar and Smulyan (Safar and Smulyan, 2004) opinion, who stated proposed that an understanding of the diverse mechanisms participating in the blood pressure elevation could “reduce the therapeutic trial and error now necessary for the selection of an individual patient’s antihypertensive regimen.”
Methods
The study included 100 non-diabetic, essential hypertensive subjects clinically free of overt atherosclerosis, other cardio-vascular-renal disease, or other major diseases. General chemistries, urinary sodium, non-invasive hemodynamic measurements, and plasma/serum biomarkers were obtained in subjects fasting for 24 h. Basic demographic data and the effects of a 12 month treatment with either an atenolol-based or an olmesartan-based therapy on vascular hypertrophy are published (Smith et al., 2008). The previous publication did not include any of the data reported here.
Patient eligibility was based upon the absence of the following exclusion criteria: seated diastolic blood pressure < 90 mm Hg or > 109 mm Hg or systolic pressure < 140 mm Hg or > 179 mm Hg; a secondary cause of hypertension; diabetes mellitus; a history of myocardial infarction, transient ischemic attack, or cerebrovascular accident within the prior 3 months; congestive heart failure, ejection fraction <50%, or other significant heart disease; active autoimmune disease; a body mass index (BMI) ≥ 35 kg/m2; malignancy; azotemia (serum creatinine >3.0 mg/dL); serum potassium < 3.3 mEq/L; significant hematologic or hepatic test abnormality; alcohol or substance abuse; or known hypersensitivity to all blood pressure medications. The study was approved by the Institutional Review Board of Wake Forest University School of Medicine as a component of the trial that investigated the effects of an atenolol-based therapy versus an olmesartan-based therapy on blood pressure control and peripheral vascular remodeling (Smith et al., 2006a; Smith et al., 2006b). All patients signed an informed consent.
Hemodynamic Measures
Non-invasive hemodynamic measures were obtained using the BioZ impedance cardiography (ICG) hemodynamic Monitor (CardioDynamics, San Diego, CA) by a technician after 5–10 min of rest in the supine position. As validated in a number of publications from our laboratory (Abdelhammed et al., 2005; Ferrario et al., 2007; Ferrario and Smith, 2006; Smith et al., 2006a), the device measures changes in impedance by injecting a high frequency (60 kHz Minimum), low amplitude (4.0 mA rms Maximum) alternating electrical current through the subject’s thorax between a pair of sensors placed on the neck and another pair placed on the mid-axillary line at the xiphoid process level. Changes in thoracic impedance as a function of time allow the non-invasive determination of stroke volume, cardiac output and thoracic fluid volume. Concurrent oscillometric measures of arterial pressure allow for the calculation of vascular resistance and the secondary determination of systemic vascular resistance index.
Brachial artery pulse wave velocity (baPWV) was obtained using an automatic waveform analyzer (VP-2000, Colin Medical Technology, Komaki, Japan) while the patient was lying in a supine position 5–10 min after occlusion cuffs were placed snugly around each arm and ankles. Brachial-tibial arteries pressure waveforms were then recorded simultaneously. The central arterial pressure and the derived augmentation index were determined with the technique of applanation tonometry (SphymoCor Pulse Wave Analysis System, PVW Medical Pty Ltd, Sidney, Australia). We (Abdelhammed et al., 2005) and others (Asmar et al., 1995; Asmar et al., 2001; Chemaly et al., 2002) have documented the inter- and intra-variability indexes for these measures previously.
Biochemical Assays
Laboratory measures were assessed at the completion of the washout period in the fasting state. The measures included determinations of plasma glucose, insulin, serum creatinine and electrolytes (sodium, potassium, and bicarbonates). Plasma concentrations of angiotensin peptides [angiotensin II (Ang II) and angiotensin-(1-7) [Ang-(1-7)] were determined by radioimmunoassay (RIA) from venous blood collected in ethylenediaminetetraacetic acid (EDTA) tubes containing a cocktail of protease inhibitors including 0.44 mM 1,20 ortho-phenanthrolene monohydrate (Sigma, St. Louis MO.), 0.12 mM pepstatin (Peninsula Labs, Belmont CA), and 1 mM Na p-hydroxymercuribenzoate (Sigma, St. Louis MO), as described by us elsewhere (Ferrario et al., 1998; Ferrario et al., 2002; Kohara et al., 1991). Recoveries of radiolabeled angiotensin added to the sample and followed through the extraction were 92% (n=23). The minimum detectable levels of the assays were 0.9 fmol (0.8 pg/tube). The intra-assay coefficient of variation is 12% for Ang II. RIA measures of serum aldosterone were determined from venous blood collected in antibody-coated tubes. The assay sensitivity was 1 ng/dL and the intra-assay precision was 5% CV at a mean of 25 ng/dL; the coefficient of variation (CV) of the inter-assay precision was 6.6% at a mean of 25 ng/dL.
Serum concentrations of the inflammatory markers: intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), P-selectin, high sensitivity (Hs-) C reactive protein and the cytokines transforming growth factor-B (TGF-β1) and tumor necrosis factor-alpha(TNF-α) were measured by Enzyme Linked-Immuno-Sorbent Assays (ELISA) using reagents from R&D Systems (Minneapolis, MN, 55413). The assay sensitivity for ICAM-1 is 0.35 ng/mL and the intraassay precision is 7.8% CV at a mean of 125.6 ng/mL. Interassay precision is 10.2% CV at a mean of 254 ng/mL. The assay sensitivity for VCAM-1 is 2.0 ng/mL. Intra-assay precision (CV) is 10.7% at a mean of 212 ng/mL. Inter-assay precision is 9.5% CV at a mean of 1,236.4 ng/mL. The assay sensitivity for P-selectin is 0.5 ng/mL. Intraassay precision is 5.0% CV at a mean of 20.2 ng/mL. Inter-assay precision is 9.9% CV at a mean of 94 ng/mL. The sensitivity of the ELISA TGF-β1 is 7 pg/mL. The intra-assay CV is 4.9 % and the inter-assay CV is 7.7 %. TNF-α′ assay sensitivity is 3 pg/mL. The intra-assay CV is 5.2% and the inter-assay CV is 8.0%.
Statistical Analysis
Unless noted otherwise, all data are expressed as mean ± 1 errors of the mean. Comparisons between sex groups were performed by analysis of variance (ANOVA) and post hoc multiple comparisons were made by the unpaired Student’s t test with appropriate correction of the significance level for multiple comparisons. Statistical significance was set to a P value <0.05.
Results
The study population comprised 100 untreated hypertensive non-diabetic patients (39% females) age 53 ± 1 year. Grouped average office seating systolic and diastolic blood pressures were 147 ± 1 mm Hg and 93 ± 1 mm Hg, respectively. No differences were observed in baseline office arterial pressure and heart rates between the sexes. While on an average female subjects had lower body weights than males, their body mass index was not significantly different (Table 1). Females had lower serum creatinine values and higher plasma leptin concentrations compared to males (Table 1) while no sex differences were demonstrated in plasma glucose and insulin concentrations (Table 1).
TABLE 1.
BASELINE VALUES
| VARIABLE | ALL PATIENTS (N =100) | FEMALES (N =39) | MALES (N =61) | P value |
|---|---|---|---|---|
|
| ||||
| Age, yrs. | 53 ± 1 | 53 ± 1 | 53 ± 1 | n.s. |
| Body weight, lb | 192 ± 3 | 168 ± 5 | 207 ± 3 | 0.001 |
| Body Mass Index, Kg x m2 | 28.62 ± 0.39 | 27.96 ± 0.78 | 29.04 ± 0.39 | n.s. |
| Systolic Blood Pressure, mm Hg | 147 ± 1 | 148 ± 1 | 146 ± 2 | n.s. |
| Diastolic Blood Pressure, mm Hg | 93 ± 1 | 91 ± 1 | 94 ± 1 | n.s. |
| Heart Rate, beats x min−1 | 74 ± 1 | 75 ± 1 | 73 ± 1 | n.s. |
| Fasting Blood glucose, mg x dL−1 | 97.19 ± 0.89 | 96.05 ± 1.36 | 97.92 ± 1.18 | n.s. |
| Serum Creatinine, mg x dL−1 | 0.87 ± 0.02 | 0.75 ± 0.03 | 0.92 ± 0.02 | 0.001 |
| Plasma Glucose, mg x dL−1 | 97.19 ± 0.89 | 96.05 ± 1.36 | 97.92 ± 1.17 | n.s. |
| Plasma Insulin, μU x mL−1 | 8.46 ± 0.97 | 7.57 ± 1.71 | 9.02 ± 1.17 | n.s. |
| Plasma Leptin, μg x L−1 | 12.64 ± 1.01 | 20.11 ± 1.88 | 7.87 ± 0.53 | 0.001 |
| Plasma Angiotensin II, fmol x mL−1 | 42.35 ± 1.65 | 48.96 ± 2.95 | 38.13 ± 1.74 | 0.001 |
| Plasma Angiotensin-(1-7), fmol x mL−1 | 13.08 ± 2.10 | 11.47 ± 1.16 | 14.10 ± 3.36 | n.s. |
| Serum Aldosterone, ng x dL−1 | 11.29 ± 0.56 | 13.17 ± 1.07 | 10.08 ± 0.56 | 0.006 |
| Hs-C Reactive Protein, mg x dL−1 | 0.30 ± 0.03 | 0.45 ± 0.06 | 0.21 ± 0.03 | 0.0005 |
| Serum ICAM, ng x dL−1 | 200 ± 6 | 199 ± 8 | 200 ± 9 | n.s. |
| Serum VCAM, ng x dL−1 | 569 ± 19 | 550 ± 28 | 580 ± 25 | n.s. |
| Serum P-selectin, ng x dL−1 | 100 ± 3 | 101 ± 4 | 100 ± 4 | n.s. |
| Serum TGF-β, ng x mL−1 | 17.08 ± 0.61 | 18.40 ± 0.87 | 16.25 ± 0.82 | n.s. |
| Serum TNF-α, pg x mL−1 | 25.32 ± 1.50 | 24.26 ± 2.57 | 25.98 ± 1.84 | n.s. |
Values are means ± SEM of variables obtained at week -1. P value within Table denotes statistical differences between female and males.
Hemodynamic Differences between Female and Male Untreated Hypertensives
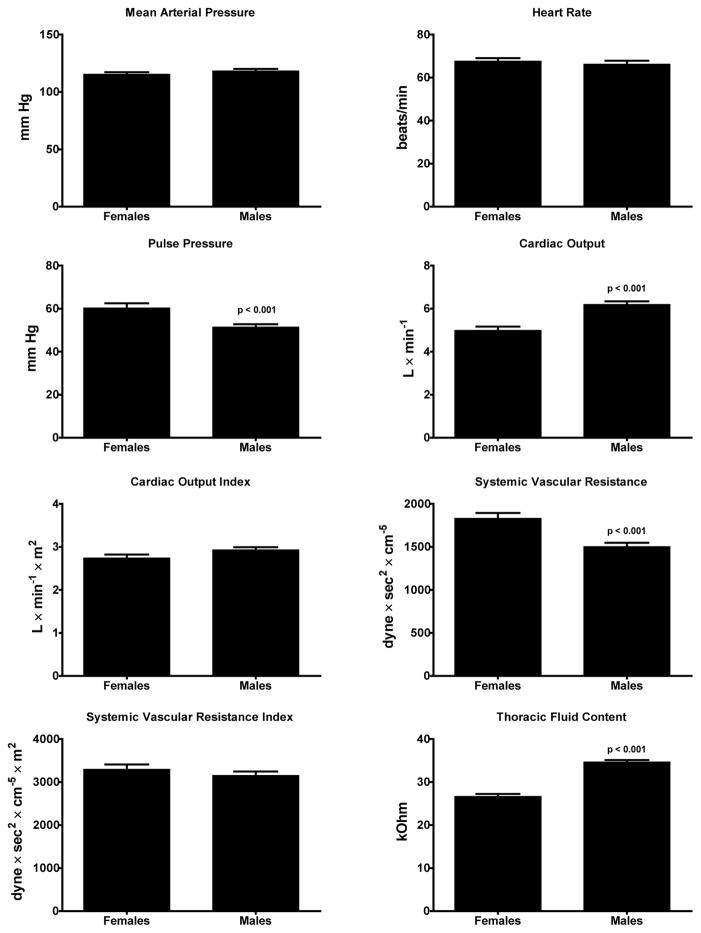
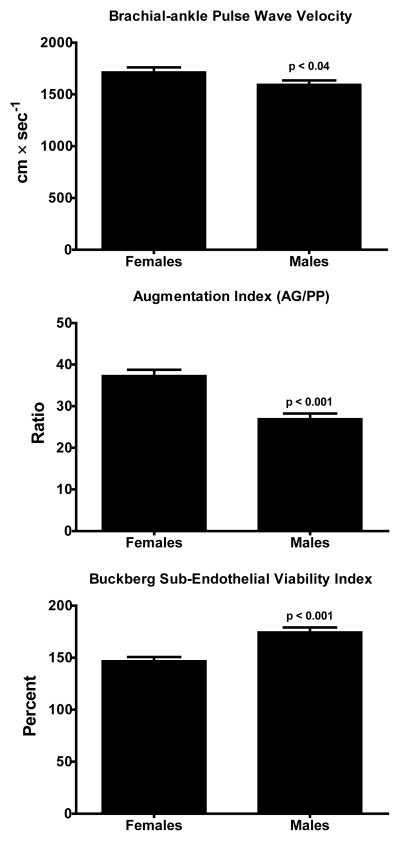
Figure 1 illustrates the hemodynamic patterns found in the untreated 61 male and 39 female subjects at the time of non-invasive hemodynamic measures. Although mean arterial pressure and heart rate values were similar in both groups of subjects, hypertensive males showed lower values of pulse pressure and systemic vascular resistance while their cardiac output and thoracic fluid content is higher compared to female hypertensive subjects. Figure 2 shows that male hypertensive subjects had lower values of both brachial-ankle pulse wave velocity (baPWV) and augmentation index (AG/PP) associated with a higher Buckberg index (diastolic pressure-time index/systolic pressure-time index) (Saito and Kasuya, 2003; Sharma et al., 1999; Siebenhofer et al., 1999) (Figure 2). The higher pulse pressure measured in female subjects (60 ± 14 mm Hg) compared to males (51 ± 12, p < 0.0001) was higher in females and was correlated with age (r = 0.52, p <0.05). Pulse pressure did not correlate with age in males.
Figure 1.
Group average hemodynamic values determined in 39 female and 61 male untreated hypertensive subjects. Values are means ± SEM.
Figure 2.
Brachial-ankle pulse wave velocity values in 39 female and 61 male untreated hypertensive subjects. Values are means ± SEM.
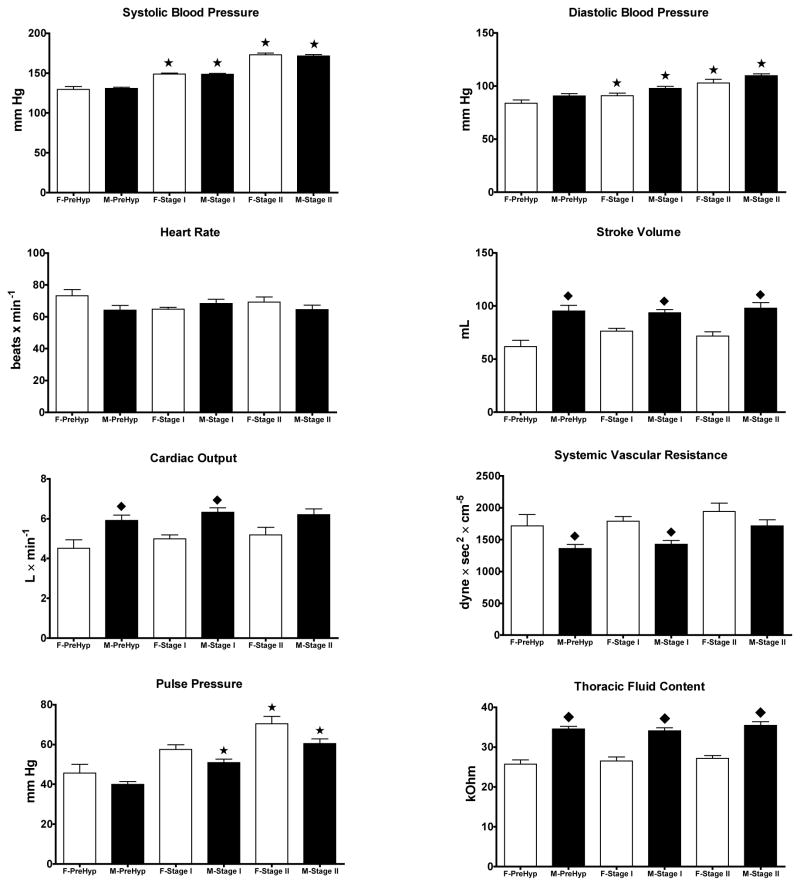
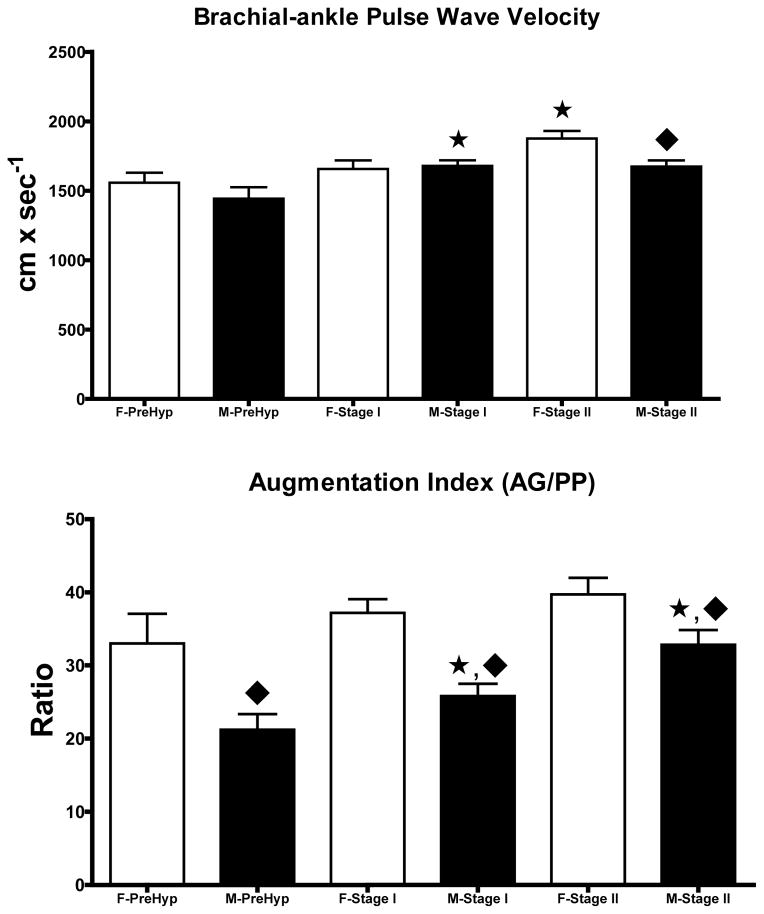
Because hemodynamic differences found in the two groups could be influenced by the magnitude of the blood pressure elevation, we proceeded to analyze the data according to the JNC-7 blood pressure categories (Figure 3). Six females and 15 males were defined as prehypertensives based on blood pressure average values of 130 ± 4/84 ± 3 mm Hg and 131 ± 2/91 ± 2 mm Hg, respectively (p > 0.05) at the time of hemodynamic measures. Blood pressure in stage I hypertensive subject (20 females and 35 males) was 149 ± 1/91 ± 2 mm Hg and 149 ± 1/98 ± 2 mm Hg, respectively. An additional 14 female and 20 males fell into Stage II hypertension with average blood pressure values of 173 ± 2/103 ± 3 mm Hg and 172 ± 2/110 ± 2 mm Hg, respectively. Throughout the hypertension stages, sex differences in cardiac output pulse pressure, stroke volume, cardiac output, systemic vascular resistance, and thoracic fluid volume are still present. When the data were broken down in terms of hypertension stages, stage I and II females were heavier than prehypertensive ones (78 ± 3 vs. 65 ± 6 Kg) and this was associated with increased pulse pressure (63 ± 2 vs. 46 ± 4 mm Hg) and higher systemic vascular resistance index (3,390 ± 123 dynes x sec2 x/cm−5 x m2 vs. 2,739 ± 227 dynes x sec2 x/cm−5 x m2). There were no differences in either cardiac output or cardiac index among hypertension stages in females (Figure 3). Similarly, male Stage I and II hypertensives differed from male prehypertensive subjects in being heavier (94 ± 2 kg vs. 90 ± 3 Kg) and manifesting higher values for pulse pressure (55 ± 2 mm Hg vs. 40 ± 1 mm Hg) and systemic vascular resistance index (3,262 ± 112 dynes x sec2 x cm−5 x m2 vs. 2,793 ± 98 dynes x sec2 x cm−5 x m2). At all stages of blood pressure, males had higher stroke volumes and cardiac output, lower values of systemic vascular resistance, pulse pressure and increased thoracic fluid content (Figure 3). baPWV was higher in Stage II hypertensive females while it was increased in males with either Stage I or Stage II hypertension (Figure 4). Male hypertensive subjects had lower values of the augmentation index throughout all stages of hypertension compared to females (Figure 4).
Figure 3.
Hemodynamic characteristics by sex and blood pressure stages between female (F-) and male (M-) subjects. Data are means ± SE for 21 prehypertensive subjects (6 females and 15 males), 55 Stage I hypertensives (20 females and 35 males), and 28 Stage II hypertensive subjects (14 females and 14 males). *, p < 0.05 compared to prehypertensive subjects of the same sex; ◆, p < 0.05 compared to male subjects.
Figure 4.
Data are means ± SE of brachial-ankle pulse wave velocity and augmentation index measurements in female and male untreated hypertensives by stages of high blood pressure. *, p < 0.05 compared to prehypertensive subjects of the same sex; ◆, p < 0.05 compared to male subjects.
Sex Differences in Biomarkers between Female and Male Hypertensives
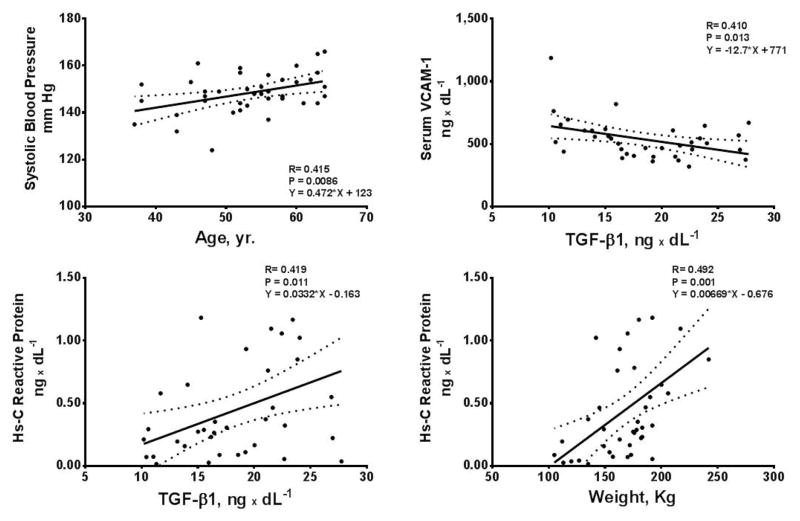
Hypertensive females had higher plasma concentrations of Ang II, serum aldosterone, and Hs-CRP compared to male hypertensive subjects. Baseline values for serum ICAM-1, VCAM-1, and P selectin were similar in both males and females (Table 1). In female, but not males, significant correlations were found between systolic blood pressure and age, serum concentrations of TGF-β1 with VCAM-1 and Hs-C Reactive Protein, and Hs-C Reactive Protein and weight (Figure 5). Serum aldosterone correlated with stroke volume (r = 0.43, p = 0.006), cardiac output (r = 0.44, p = 0.004), and ICAM-1 (r = 0.40, p = 0.01) in females, but not in males.
Figure 5.
Scatterplots and 95% confidence limits of the relations in untreated hypertensive women between systolic blood pressure and age, serum concentrations of VCAM-1, Hs-C Reactive Protein and TGF-β1 and body weight.
Table 2 shows the presence of statistical gender differences in plasma concentrations of Ang II, serum aldosterone, and Hs-CRP in the subjects with both prehypertension and Stage I hypertension. Baseline values for serum glucose, serum insulin, serum ICAM-1, VCAM-1, and P selectin were not different among female and male subjects with different degrees of hypertension. Similarly, there were no differences in serum levels of TNF-α and TGF-β1 between women and men at the various stages of hypertension.
TABLE 2.
Profile of Humoral Biomarkers in Female and Male Subjects
| Prehypertension | Stage I Hypertension | Stage II Hypertension | ||||
|---|---|---|---|---|---|---|
| VARIABLE | Females (n= 5) | Males (n= 15) | Females (n= 30) | Males (n= 37) | Females (n= 4) | Male (n= 9)s |
| Plasma Angiotensin II, fmol x mL−1 P value |
58.8 ± 9.07 | 39.59 ± 3.75 0.03 |
47.99 ± 3.39 | 39.26 ± 2.12 0.02 |
43.94 ± 7.38 | 31.08 ± 4.74 |
| Plasma Angiotensin-(1-7), fmol x mL−1 P value |
7.78 ± 0.65 | 9.45 ± 1.67 | 11.33 ± 1.06 | 17.63 ± 5.45 | 17.15 ± 8.22 | 7.34 ± 0.59 |
| Serum Aldosterone, ng x dL−1 P value |
13.92 ± 2.21 | 8.50 ± 0.62 0.003 |
12.27 ± 0.93 | 10.33 ± 0.62 0.077 |
18.95 ± 7.68 | 11.72 ± 2.63 |
| Hs C-Reactive Protein, mg x dL−1 P value |
0.57 ± 0.28 | 0.11 ± 0.02 0.009 |
0.43 ± 0.07 | 0.21 ± 0.05 0.008 |
0.42 ± 0.25 | 0.34 ± 0.10 |
| Plasma Leptin, μg x L−1 P value |
20.78 ± 5.09 | 7.31 ± 1.05 0.001 |
20.28 ± 2.35 | 7.68 ± 0.70 0.0001 |
18.07 ± 1.17 | 9.50 ± 1.31 0.002 |
Values are means ± SEM from 100 patients at baseline. P values within Table denote statistical difference between females and males for each of the three blood pressure categories.
Discussion
Seldom, if ever, approaches to hypertension therapy consider sex as an element in the selection of antihypertensives agents or base the choice of a specific antihypertensive class in terms of the hemodynamic factors accounting for the blood pressure elevation. The present study provides a comprehensive noninvasive hemodynamic and hormonal assessment of hypertension in untreated men and women. The study documents important sex-driven differences in the contribution that cardiac output and peripheral vascular resistance make to the elevation of arterial pressure. Our study demonstrates that for equivalent levels of systolic and diastolic blood pressure elevations, untreated hypertensive women had higher pulse pressures, lower cardiac output values, higher systemic vascular resistance, and reduced thoracic fluid content compared to men. This hemodynamic pattern in women was associated with higher baPWV and augmentation index. Since the hemodynamic characteristics found in hypertensive women did not change as function of the hypertensive stages, these data suggest that these phenotypes are sex-specific. On the other hand, differences in cardiac output and vascular resistance between hypertensive men and women were no longer significant after adjustment for body weight as the average weight of the male population was significantly higher. Since body mass index in female and male hypertensives were not different, these findings suggest that normalization by weight is masking the higher degree of vascular constriction shown in hypertensive women. The differential hemodynamics of the two sexes extended to hormonal biomarkers of the hypertensive process since women displayed lower values of serum creatinine and higher circulating concentrations of leptin, Ang II, serum aldosterone and Hs-C Reactive protein.
ICG is a simple accurate noninvasive method for measurements of stroke volume, cardiac output and thoracic fluid volume (Smith et al., 2006a). Coupled with oscillometric measures of arterial pressure, peripheral vascular resistance is calculated from the cardiac output values determined by ICG. A number of studies have validated the sensitivity and reproducibility of this technique (Abdelhammed et al., 2005; Demarzo, 2009; Ferrario et al., 2007; Ferrario and Smith, 2006; Siebenhofer et al., 1999; Smith et al., 2006a). In a comparative study of healthy nonhypertensive and hypertensive subjects (Abdelhammed et al., 2005), we validated the differences in the hemodynamic factors accounting for the blood pressure elevation and showed the prospective value of this noninvasive technique in therapeutic decision-making in hypertensive subjects (Smith et al., 2006a). DeMarzo (Demarzo, 2009) reported on the value of ICG in detecting subclinical vascular disease in women while we showed that impedance cardiography guided therapy was more effective than standard care in achieving hypertension control in subjects on more than one antihypertensive medication (Smith et al., 2006a). Pulse wave velocity and the augmentation index are now accepted methods for assessment of vascular stiffness (Hitsumoto, 2012; Rabkin et al., 2012; Salmi et al., 2012; Simova et al., 2011; Tomiyama and Yamashina, 2011; Yang et al., 2011; Zhang et al., 2013). The strength of our current research is based in having combined hemodynamic measures with non-invasive indexes of vascular compliance in concert with concomitant measures of hypertension-related biomarkers. This approach provided a comprehensive picture of vascular disease in men and women and a glance at disease progression based on hypertensive stages.
Among the hypertensive participants, more males than females met the JNC-7 criteria for prehypertension and stage 2 hypertension. Despite the numerical differences in the sampled population, stroke volume and cardiac output were consistently lower in prehypertensive and Stage 1 hypertensive women when compared to men. The higher degree of vasoconstriction found in women was no longer present in those women with Stage 2 hypertension. At these levels of arterial pressure, increased in peripheral vascular resistance became the main mechanism accounting for hypertension. While it may be argued that the lower values of stroke volume and cardiac output found in women are an expression of the fact that their weight was less than that found in men, no statistical differences were found in body mass index between men and women. In keeping with the suggestion that a higher vascular resistance contributes to the early stages of hypertension in women, baPWV and the augmentation index were higher in women compared to men. Reanalysis of these indices as a function of the hypertensive stages showed persistence of higher pulse wave velocity in women compared to men throughout the JNC-7 classification of hypertension. Differences were most notable in the augmentation index which was markedly less in men compared to women. Pulse pressures were higher in Stage 1 and 2 hypertensive women. Altogether, these findings demonstrate a higher compromise of vascular elasticity in the early stages of hypertension in women compared to men. While our findings in general agree with previous studies of hemodynamic changes in prehypertensive and hypertensive subjects (Drukteinis et al., 2007), partitioning of hemodynamic variables as a function of sex and hypertensive stages are not reported in the previous literature. Igashi et al. (Drukteinis et al., 2007; Higashi et al., 2013) found a stronger association of the augmentation index with parameters of diastolic function in normotensive women compared to men. While the mechanisms for this association remain to be investigated, a structural differences in the aortic tree in women may contribute to an earlier reflected pressure wave return and account for augmentation of the first forward wave during late systole (Hayward and Kelly, 1997). In an earlier, well-conducted study, Messerli and colleagues (Messerli et al., 1987) assessed the hemodynamic profile of essential hypertensive men and women in whom antihypertensive medications were discontinued 4 weeks beforehand. In their study, women were characterized as displaying higher cardiac index, left ventricular ejection time and pulse pressure, and a lower total peripheral resistance when compared to men (Messerli et al., 1987). The difference between our findings and those reported by Messerli et al. (Messerli et al., 1987) may be explained in part by 1)- the fact that our study was carried out in a patient population who were an average of 10 years older than those reported by them; and 2)- and their hypertension was less severe. This interpretation agrees with our findings of a significant correlation between systolic blood pressure and age in women (Figure 5), and the disappearance of the differential in cardiac output values between men and women with Stage 2 hypertension (Figure 3).
The hemodynamic pattern found in essential hypertensive women suggests a higher more severe degree of vascular remodeling when compared to men for the same levels of arterial pressure. This interpretation is supported by the demonstration of a pattern of hormonal biomarkers reflective of increased vascular inflammation and injury. The presence of significantly higher plasma Ang II concentrations, serum aldosterone, and Hs-C reactive protein in women parallel a hemodynamic profile of higher vascular resistance and reduced vessel elasticity. The observation that circulating TGF-β1, a cytokine stimulating the production of various extracellular matrix proteins, correlated in women, but not men with serum VCAM-1 and Hs-C reactive protein is a novel finding that further reinforces the interpretation of significant loss of vascular elasticity as reflected by the increased pulse wave velocity and augmentation index. Hs-C reactive protein is an accepted biomarker of both acute and chronic inflammation (Tsimikas et al., 2006). Although elevated levels of Hs-C reactive protein appear to be more predictive of mortality hazards in men compared to women (Doran et al., 2013), these findings do not negate its value as a marker of existent cardiovascular injury or inflammatory processes.
In this non-diabetic population of men and women with normal levels of fasting glucose and plasma insulin, we confirmed the existence of a large sex difference in plasma leptin values. This adipocytokine has an important role in energy homeostasis and circulating levels are reported to be higher in men than in women (Jeppesen and Asferg, 2010; Ugrinska et al., 2013). In addition, recent studies showed that leptin is a biomarker of the amount of body fat and an important determinant of hypertension risk (Jeppesen and Asferg, 2010; Shankar et al., 2012). In our untreated hypertensives plasma leptin values were 2.5-fold higher in women than in men. In addition, plasma leptin correlated with both weight (r = 0.48, p <0.05) and plasma insulin (r = 0.46, p < 0.05) in women, but only with weight (r = 0.56, p < 0.05) in men. Although a correlation between plasma leptin and body mass index has been reported in healthy overweight women (Ugrinska et al., 2013), our findings of an additional sex difference in terms of its relation with plasma insulin is a new observation, the mechanism of which will require further investigation. Because our study showed that the higher leptin values were associated in women with other biomarkers of inflammation, our findings agree with Shankar et al. (Shankar et al., 2012) observations that high plasma leptin values are associated with a higher risk of hypertension.
Although differences on the prevalence, clinical presentation, and development of hypertension-related complications in women are documented in numerous epidemiological and observational studies (Doumas et al., 2013), the applicability of this knowledge has not been fully translated in specific therapeutic strategies. Inclusion of women in antihypertensive trials is limited, while morbidity and mortality outcomes suggest that they receive less benefit from antihypertensive therapies (Kaplan, 1995). Hypertension and impaired diastolic function with preserved left ventricular ejection fraction are seen more often in women, particularly after estrogen loss during menopause. Women develop diastolic heart failure twice as often as men and trials suggest ACE inhibitors may be less effective in women than men receiving treatment for hypertension and heart failure. The differential hemodynamic and hormonal characteristics of untreated hypertension in the men and women included in our study suggest a need to better understand the female sex-specific underpinnings of the hypertensive processes to tailor optimal interventions for this vulnerable, yet expanding population.
Our hemodynamic and biochemical findings document a consistent pattern of a higher degree of vascular compromise and vasoconstriction in women compared to men for the same level of arterial pressure most notably in prehypertensive and Stage I hypertension. These data lead us to hypothesize that the increased risk of cardiovascular events associated with the post-menopause stage may be anteceded or be accompanied by functional and structural vascular changes that may in part be a result of the differences in body habitus, body fat composition, and shorter, narrower blood vessels compared to men.
Acknowledgments
Funding Acknowledgment
We acknowledge partial support of these studies through an unrestricted grant provided by Daiichi Sankyo, Inc. (Parsippany, NJ); from the National Heart, Blood, Lung Institute of the NIH through grant number HL-051952; and the former CardioDynamics Corporation (San Diego, CA) for their generous gift the BioZ com equipment to CMF.
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest in preparing this article. CM Ferrario has received funding from Daiichi Sankyo Inc., and has served as a member of their Speaker’s bureau.
References
- 1.Abdelhammed AI, Smith RD, Levy P, Smits GJ, Ferrario CM. Noninvasive hemodynamic profiles in hypertensive subjects. Am J Hypertens. 2005;18:51S–59S. doi: 10.1016/j.amjhyper.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Abuful A, Gidron Y, Henkin Y. Physicians’ attitudes toward preventive therapy for coronary artery disease: is there a gender bias? Clin Cardiol. 2005;28:389–393. doi: 10.1002/clc.4960280809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 4.Asmar R, Topouchian J, Pannier B, Benetos A, Safar M. Pulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior Study. J Hypertens. 2001;19:813–818. doi: 10.1097/00004872-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Chemaly E, London G, Benetos A, Darne B, Asmar R. Comparison of central pulse pressure estimated from pulse wave propagation velocity and carotid pulse pressure measured by applantation tonometry. Arch Mal Coeur Vaiss. 2002;95:637–640. [PubMed] [Google Scholar]
- 6.Demarzo AP. Using impedance cardiography to detect subclinical cardiovascular disease in women with multiple risk factors: a pilot study. Prev Cardiol. 2009;12:102–108. doi: 10.1111/j.1751-7141.2008.00012.x. [DOI] [PubMed] [Google Scholar]
- 7.Doran B, Zhu W, Muennig P. Gender differences in cardiovascular mortality by C-reactive protein level in the United States: Evidence from the National Health and Nutrition Examination Survey III. Am Heart J. 2013;166:45–51. doi: 10.1016/j.ahj.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15:321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 9.Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, et al. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation. 2007;115:221–227. doi: 10.1161/CIRCULATIONAHA.106.668921. [DOI] [PubMed] [Google Scholar]
- 10.Ferrario CM, Basile J, Bestermann W, Frohlich E, Houston M, Lackland DT, et al. The role of noninvasive hemodynamic monitoring in the evaluation and treatment of hypertension. Ther Adv Cardiovasc Dis. 2007;1:113–118. doi: 10.1177/1753944707086095. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM, Joyner J, Colby C, Exuzides A, Moore M, Simmons D, et al. The COSEHC Global Vascular Risk Management quality improvement program: first follow-up report. Vasc Health Risk Manag. 2013;9:391–400. doi: 10.2147/VHRM.S44950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, et al. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario CM, Smith RD. Cost-effectiveness of impedance cardiography testing in uncontrolled hypertension. Am Heart Hosp J. 2006;4:279–289. doi: 10.1111/j.1541-9215.2006.05728.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario CM, Smith RD, Brosnihan B, Chappell MC, Campese VM, Vesterqvist O, et al. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertens. 2002;15:557–564. doi: 10.1016/s0895-7061(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 15.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 16.Higashi H, Okayama H, Saito M, Morioka H, Aono J, Yoshii T, et al. Relationship Between Augmentation Index and Left Ventricular Diastolic Function in Healthy Women and Men. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt115. [DOI] [PubMed] [Google Scholar]
- 17.Hitsumoto T. Clinical significance of the augmentation index in patients with preserved kidney function. J Nippon Med Sch. 2012;79:422–429. doi: 10.1272/jnms.79.422. [DOI] [PubMed] [Google Scholar]
- 18.Jeppesen J, Asferg C. Positive relationship between plasma leptin levels and hypertension from an epidemiological perspective. Hypertension. 2010;56:573–574. doi: 10.1161/HYPERTENSIONAHA.110.158519. [DOI] [PubMed] [Google Scholar]
- 19.Joyner J, Moore AR, Mount DL, Simmons DR, Ferrario CM, Cline DM. Emergency department patients self-report higher patient inertia, hopelessness, and harmful lifestyle choices than community counterparts. J Clin Hypertens (Greenwich) 2012;14:828–835. doi: 10.1111/jch.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan NM. The treatment of hypertension in women. Arch Intern Med. 1995;155:563–567. [PubMed] [Google Scholar]
- 21.Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12:1135–1141. doi: 10.1016/0196-9781(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005a;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Sutton-Tyrrell K, Patel AS, Matthews KA, Pasternak RC, Everson-Rose SA, et al. Ethnic variation in hypertension among premenopausal and perimenopausal women: Study of Women’s Health Across the Nation. Hypertension. 2005b;46:689–695. doi: 10.1161/01.HYP.0000182659.03194.db. [DOI] [PubMed] [Google Scholar]
- 24.Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107:158–161. doi: 10.7326/0003-4819-107-2-158. [DOI] [PubMed] [Google Scholar]
- 25.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127:1254–29. doi: 10.1161/CIR.0b013e318287cf2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ. Twelve-year follow-up of American women’s awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okin PM, Gerdts E, Kjeldsen SE, Julius S, Edelman JM, Dahlof B, et al. Gender differences in regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy. Hypertension. 2008;52:100–106. doi: 10.1161/HYPERTENSIONAHA.108.110064. [DOI] [PubMed] [Google Scholar]
- 29.Rabkin SW, Chan SH, Sweeney C. Ankle-brachial index as an indicator of arterial stiffness in patients without peripheral artery disease. Angiology. 2012;63:150–154. doi: 10.1177/0003319711410307. [DOI] [PubMed] [Google Scholar]
- 30.Rossi R, Nuzzo A, Iaccarino D, Lattanzi A, Origliani G, Monopoli DE, et al. Effects of antihypertensive treatment on endothelial function in postmenopausal hypertensive women. A significant role for aldosterone inhibition. J Renin Angiotensin Aldosterone Syst. 2011;12:446–455. doi: 10.1177/1470320311415134. [DOI] [PubMed] [Google Scholar]
- 31.Safar ME, Smulyan H. Hypertension in women. Am J Hypertens. 2004;17:82–87. doi: 10.1016/s0895-7061(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 32.Saito M, Kasuya A. Relationship between the subendocardial viability ratio and risk factors for ischemic heart disease. Sangyo Eiseigaku Zasshi. 2003;45:114–119. doi: 10.1539/sangyoeisei.45.114. [DOI] [PubMed] [Google Scholar]
- 33.Salmi AA, Zaki NM, Zakaria R, Nor Aliza AG, Rasool AH. Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. Vasa. 2012;41:96–104. doi: 10.1024/0301-1526/a000171. [DOI] [PubMed] [Google Scholar]
- 34.Shankar A, Syamala S, Xiao J, Muntner P. Relationship between Plasma Leptin Level and Chronic Kidney Disease. Int J Nephrol. 2012;2012:269532. doi: 10.1155/2012/269532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma AC, Fogelson BG, Nawas SI, Vigneswaran WT, Sam AD, Alden KJ, et al. Elevated coronary endothelin-1 but not nitric oxide in diabetics during CABG. Ann Thorac Surg. 1999;67:1659–1663. doi: 10.1016/s0003-4975(99)00287-8. [DOI] [PubMed] [Google Scholar]
- 36.Siebenhofer A, Kemp C, Sutton A, Williams B. The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J Hum Hypertens. 1999;13:625–629. doi: 10.1038/sj.jhh.1000887. [DOI] [PubMed] [Google Scholar]
- 37.Simova I, Katova T, Kostova V, Hristova K, Dimitrov N. Reproducibility of arterial stiffness indices in different vascular territories and between different observers. Echocardiography. 2011;28:448–456. doi: 10.1111/j.1540-8175.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith RD, Levy P, Ferrario CM. Value of noninvasive hemodynamics to achieve blood pressure control in hypertensive subjects. Hypertension. 2006a;47:771–777. doi: 10.1161/01.HYP.0000209642.11448.e0. [DOI] [PubMed] [Google Scholar]
- 39.Smith RD, Yokoyama H, Averill DB, Cooke L, Brosnihan KB, Schiffrin EL, et al. The protective effects of angiotensin II blockade with olmesartan medoxomil on resistance vessel remodeling (The VIOS study): rationale and baseline characteristics. Am J Cardiovasc Drugs. 2006b;6:335–342. doi: 10.2165/00129784-200606050-00006. [DOI] [PubMed] [Google Scholar]
- 40.Smith RD, Yokoyama H, Averill DB, Schiffrin EL, Ferrario CM. Reversal of vascular hypertrophy in hypertensive patients through blockade of angiotensin II receptors. J Am Soc Hypertens. 2008;2:165–172. doi: 10.1016/j.jash.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Thoenes M, Neuberger HR, Volpe M, Khan BV, Kirch W, Bohm M. Antihypertensive drug therapy and blood pressure control in men and women: an international perspective. J Hum Hypertens. 2010;24:336–344. doi: 10.1038/jhh.2009.76. [DOI] [PubMed] [Google Scholar]
- 42.Tomiyama H, Yamashina A. Vascular function tests to assess risk for cardiovascular diseases. Rinsho Byori. 2011;59:787–792. [PubMed] [Google Scholar]
- 43.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47:C19–C31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008a;336:1121–1123. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–958. doi: 10.1097/HJH.0b013e3280bad9b4. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008b;29:2669–2680. doi: 10.1093/eurheartj/ehn427. [DOI] [PubMed] [Google Scholar]
- 47.Ugrinska A, Miladinova D, Trajkovska M, Zdravkovska M, Kuzmanovska S, Tripunoski T, et al. Correlation of serum leptin with anthropometric parameters and abdominal fat depots determined by ultrasonography in overweight and obese women. Prilozi. 2013;34:115–119. [PubMed] [Google Scholar]
- 48.Yang WI, Park S, Youn JC, Son NH, Lee SH, Kang SM, et al. Augmentation index association with reactive hyperemia as assessed by peripheral arterial tonometry in hypertension. Am J Hypertens. 2011;24:1234–1238. doi: 10.1038/ajh.2011.132. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Agnoletti D, Safar ME, Wang JG, Topouchian J, Xu Y, et al. Comparison study of central blood pressure and wave reflection obtained from tonometry-based devices. Am J Hypertens. 2013;26:34–41. doi: 10.1093/ajh/hps031. [DOI] [PubMed] [Google Scholar]