Abstract
We estimate the contribution of influenza to hospitalizations for acute exacerbations of chronic obstructive pulmonary disease (AECOPD) in Kashmir, India. Prospective surveillance for influenza among patients hospitalized with AECOPD was conducted at a tertiary care hospital. Patients had clinical data collected and nasal/throat swabs tested for influenza viruses. Outcomes among patients with and without influenza were compared with logistic regression adjusting for age and underlying conditions. During October 2010–September 2012, 498 patients hospitalized with AECOPD were enrolled, of whom 40 (8%) had received influenza vaccine. Forty (8%) had influenza; influenza virus detection peaked in winter (January–March). Patients with influenza were more likely to die during hospitalization (adjusted OR 3·4, CI 1·0–11·4) than those without.
Keywords: Chronic obstructive lung disease, chronic obstructive, hospitalization, influenza viruses, pulmonary disease
Introduction
Globally, an estimated 64 million people have chronic obstructive pulmonary disease (COPD), with 90% of COPD deaths occurring in low- and middle-income countries.1 In India, estimates of COPD prevalence in India have varied from 2 to 20% depending upon method of diagnosis. In Kashmir, India, the prevalence of spirometrically diagnosed COPD is 17% in males and 15% in females aged ≥40 years.2 Against a backdrop of high COPD prevalence, measures to prevent COPD exacerbations in India would have substantial public health impact. However, seasonal influenza vaccines are not routinely recommended for any target group in India due in part to a lack of national data on influenza.3 We used data from prospective surveillance for influenza among patients hospitalized with acute exacerbations of COPD (AECOPD) to estimate the contribution of influenza to the overall burden of AECOPD and describe the epidemiology of AECOPD at a tertiary care hospital in Kashmir, India.
Methods
Sher-i-Kashmir Institute of Medical Sciences (SKIMS) is the main tertiary care hospital for persons with COPD in Kashmir, India and has 650 beds and an intensive care unit. During October 2010–September 2012, surveillance was conducted at SKIMS for patients aged ≥40 years hospitalized with AECOPD. AECOPD was defined as ≥2 major symptoms (increased dyspnea, sputum purulence, or sputum amount) or ≥1 major and ≥1 minor symptom (nasal discharge/congestion, wheezing, sore throat, or cough) for ≥2 consecutive days in a patient with COPD.4,5 Data were collected using a standardized case report form through interview of consenting patients or their family members and review of patients' medical records. Surveillance staff also telephoned patients or their families at least 30 days after hospital discharge to collect data on readmission or death within 30 days after discharge.
Laboratory specimen collection and testing
Nasal and throat swabs were collected from participants at enrollment for testing by reverse-transcription polymerase chain reaction for influenza A and B viruses, and those positive for influenza A viruses were further subtyped for A/H1N1pdm09, A/H3N2, and seasonal H1N1 using US Centers for Disease Control and Prevention (CDC) protocols.
Human subjects review
The surveillance protocol was reviewed and approved by the institutional review board of SKIMS. The protocol was also reviewed at the US CDC and determined to be for public health surveillance purposes exempted from further CDC IRB review.
Data analysis
Frequencies were calculated to describe baseline characteristics, treatment, and outcomes among patients. Illness outcomes (i.e., readmission within 30 days of discharge and death during or within 30 days after hospitalization) were compared among those with and without influenza in multivariate analysis using logistic regression to adjust for age and presence of ≥1 underlying medical conditions. Statistical analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
During October 2010–September 2012, 498 patients hospitalized with AECOPD were enrolled. Of these, 308 (62%) patients were male and 400 (80%) had ≥1 additional underlying medical condition; hypertension (58%) and heart disease (19%) were the most common conditions (Table 1). Adverse environmental exposures were frequent with 66 (13%) patients reporting current tobacco use, 118 (24%) reporting secondhand tobacco smoke exposure, and 406 (82%) reporting use of indoor kitchens, 121 (30%) of whom used biomass fuels (i.e., wood, crop residues, or dung). Only 40 (8%) patients reported receiving seasonal trivalent influenza vaccine during the preceding year.
Table 1.
Baseline characteristics of patients hospitalized with acute exacerbations of COPD, Kashmir, October 2010–September 2012 (N = 498)
| n | % | |
|---|---|---|
| Male | 308 | 62 |
| Age group (years) | ||
| ≤39 | 1 | 0 |
| 40–59 | 97 | 19 |
| 60–69 | 183 | 37 |
| ≥70 | 217 | 44 |
| Rural | 299 | 60 |
| Urban | 199 | 40 |
| ≥1 additional underlying medical condition | 400 | 80 |
| Environmental exposures | ||
| Current smoker | 66 | 13 |
| Secondhand smoke exposure | 118 | 24 |
| Indoor kitchen | 406 | 82 |
| Biomass fuels for cooking* | 146 | 29 |
| Biomass fuels for personal (kangri) or space heaters (bukhari)** | 461 | 93 |
| Chronic supplemental oxygen | 167 | 34 |
| Chronic steroid therapy | 16 | 3 |
| Received seasonal influenza vaccine during past year | 40 | 8 |
| Median symptom onset to specimen collection (days) | 8 | IQR 5–12 |
| Influenza virus positive | 40 | 8 |
Biomass fuels for cooking include wood, cow dung, and crop residue.
Biomass fuels for heating include wood or charcoal.
The median time from symptom onset to respiratory specimen collection was 8 days (IQR 5–12 days). Overall, 40 (8%) patients had respiratory specimens positive for influenza viruses, of which 39 were subtyped (12 (31%) with A(H1N1)pdm09, 17 (44%) with A(H3N2), and 10 (26%) with B). Detection of influenza viruses and overall number of exacerbations peaked during January–March 2011 and January–February 2012; during peak periods, influenza was associated with 14 and 33% of AECOPD hospitalizations in 2011 and 2012, respectively (Figure 1). The proportion of patients hospitalized with influenza-associated AECOPD was similar during October 2010–September 2011 and October 2011–September 2012 (21/280 (8%) versus 19/218 (9%), P = 0·6).
Figure 1.
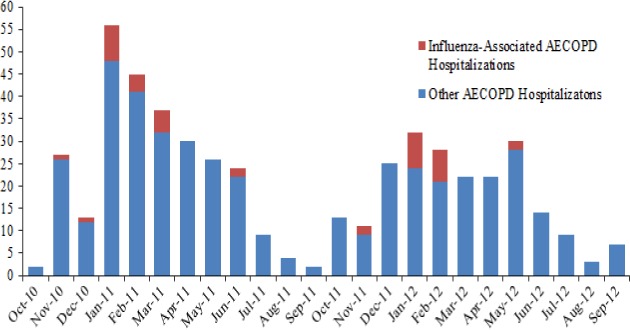
Acute exacerbation of chronic obstructive pulmonary disease hospitalizations by month, Kashmir, India, October 2010-September 2012.
Fifty-four (11%) patients with AECOPD required non-invasive mechanical ventilation with continuous positive airway pressure and 5 (1%) required invasive mechanical ventilation. While the majority of patients with AECOPD were treated with antibiotics (466/498, 94%) and steroids (352/498, 71%), only 1 (3%) of 40 patients with influenza-associated AECOPD was treated with an influenza antiviral medication.
Data on readmission within 30 days of hospital discharge and death during or within 30 days after hospital discharge were available for 317 patients (22 with influenza and 295 without influenza). The likelihood of readmission and death within 30 days of discharge was similar among patients with and without influenza after adjusting for age and underlying conditions [readmission 14% versus 4%, adjusted OR 3·3 (95% CI 0·9–12·8); death 17% versus 16%, adjusted OR 1·0 (0·3–3·8)]. However, patients with influenza were more likely to die during hospitalization [18% versus 6%, adjusted OR 3·4 (95% CI 1·0–11·4)].
Discussion
During a 2-year period, influenza was associated with 8% of all hospitalizations for AECOPD at the main tertiary care hospital in Kashmir, India. The majority of influenza detection occurred during a defined 2- to 3-month period in the winter, when influenza was associated with 14–33% of hospitalizations for AECOPD. Few patients reported receiving influenza vaccines, and influenza antiviral treatment was uncommon. A substantial proportion of patients hospitalized with AECOPD required invasive or non-invasive mechanical ventilation and died during hospitalization or within 30 days after hospital discharge. Death during hospitalization was more common among patients with influenza than those without.
Our estimate of influenza virus detection prevalence among patients hospitalized with COPD exacerbations is consistent with previously published reports documenting influenza detection in 5–22% of patients hospitalized with exacerbations.6–9 Of the viral infections implicated in AECOPD, influenza is currently the only vaccine-preventable infection. The World Health Organization recommends influenza vaccination for groups at increased risk for severe influenza, such as persons with chronic lung disease, and influenza vaccination has been shown to reduce the incidence of COPD exacerbations.10,11 However, only 8% of patients in our study reported receiving influenza vaccine, consistent with limited data on uptake in other high-risk groups in India.12 Our findings, along with emerging data on influenza virus circulation and influenza-associated hospitalization incidence in other parts of India,13,14 suggest that influenza is a substantial contributor to severe respiratory illness in India and that recommendations for influenza vaccination in certain high-risk groups may be warranted.
Several issues should be considered when interpreting the results of our analysis. First, respiratory specimens were collected a median of 8 days from symptom onset, so we may have missed some cases of influenza and underestimated the prevalence of influenza among patients with COPD exacerbations. Second, we tested only for influenza viruses as influenza is currently the only vaccine-preventable respiratory virus infection.
Funding
No external funding was received for this work.
Conflict of interest
The authors do not have any conflict of interests to declare.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty–a BOLD analysis. Thorax. 2014;69:465–473. doi: 10.1136/thoraxjnl-2013-204460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Group of the Association of Physicians of India on Adult Immunization in I. The Association of Physicians of India evidence-based clinical practice guidelines on adult immunization. J Assoc Physicians India. 2009;57:345–356. [PubMed] [Google Scholar]
- 4.Patel IS, Seemungal TA, Wilks M, et al. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 6.Beckham JD, Cadena A, Lin J, et al. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50:322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko FW, Ip M, Chan PK, et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132:900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102:1575–1580. doi: 10.1016/j.rmed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. The Cochrane database of systematic reviews. 2006:CD002733. doi: 10.1002/14651858.CD002733.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based review. Ont Health Technol Assess Ser. 2012;12:1–64. [PMC free article] [PubMed] [Google Scholar]
- 12.Bali NK, Ashraf M, Ahmad F, et al. Knowledge, attitude, and practices about the seasonal influenza vaccination among healthcare workers in Srinagar, India. Influenza Other Respir Viruses. 2013;7:540–545. doi: 10.1111/j.1750-2659.2012.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadha MS, Hirve S, Dawood FS, et al. Burden of Seasonal and Pandemic Influenza-Associated Hospitalization during and after 2009 A(H1N1)pdm09 Pandemic in a Rural Community in India. PLoS ONE. 2013;8:e55918. doi: 10.1371/journal.pone.0055918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V, Dawood FS, Rai SK, et al. Validity of clinical case definitions for influenza surveillance among hospitalized patients: results from a rural community in North India. Influenza Other Respir Viruses. 2013;7:321–329. doi: 10.1111/j.1750-2659.2012.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


