Abstract
Adult survivors of childhood brain tumors experience multiple, significant, life-long deficits as a consequence of their malignancy and therapy. Current survivorship literature documents the substantial impact such impairments have on survivors’ physical health and quality of life. Psychosocial reports detail educational, cognitive, and emotional limitations characterizing survivors as especially fragile, often incompetent, and unreliable in evaluating their circumstances. Anecdotal data suggests some survivors report life experiences similar to those of healthy controls. The aim of our investigation was to determine whether life satisfaction in adult survivors of childhood brain tumors differs from that of healthy controls and to identify potential predictors of life satisfaction in survivors. This cross-sectional study compared 78 brain tumor survivors with population–based matched controls. Chi-square tests, t-tests, and linear regression models were used to investigate patterns of life satisfaction and identify potential correlates. Results indicated life satisfaction of adult survivors of childhood brain tumors was similar to that of healthy controls. Survivors’ general health expectations emerged as the primary correlate of life satisfaction. Understanding life satisfaction as an important variable will optimize the design of strategies to enhance participation in follow-up care, reduce suffering, and optimize quality of life in this vulnerable population.
Keywords: pediatric brain tumors, survivorship, life satisfaction, quality of life
Introduction
As a result of improvements in diagnosis and aggressive, multimodality treatment, there is a growing population of long-term survivors of childhood brain tumors. Because curative therapy for central nervous system tumors typically involves intensive multimodality therapy, survivors are at risk for numerous complications related to the injury of normal central nervous system tissues that have a limited capacity for self-repair. Extended follow-up of large cohorts has confirmed that this growing group of survivors is at high risk for profound, life-altering late effects. A recent analysis from the St. Jude Lifetime Cohort study indicated a high prevalence of clinically ascertained chronic health conditions among cohort members (median age, 32 years); 98.2% of survivors had one or more chronic medical conditions, and a severe/disabling or life-threatening condition occurred in 67.6% (Hudson et al., 2013).
Epidemiologic research has detailed the permanent, sometimes progressive metabolic deficits and psychopathology that negatively affect the health and functioning of brain tumor survivors and may diminish life satisfaction (Armstrong et al., 2009). Endocrine and neurologic dysfunctions are most common. Endocrine late effects include growth hormone deficiency, diabetes insipidus, abnormal thyroid function, obesity, and adrenocorticotropic hormone deficiency (Crom et al., 2010; Shaw, 2009) . Brain tumor survivors also struggle with a spectrum of neurologic sequelae such as sensory impairment, seizures, sleep dysregulation, cognitive deficits, apathy, executive dysfunction, inattention or processing speed impairment, and emotional deficits (Armstrong et al., 2011; Conklin et al., 2012; Di Pinto, Conklin, Li, & Merchant, 2012; Nolan et al., 2013). These substantial adverse consequences lead to disabilities that affect the daily lives of survivors who grapple with poor educational attainment, unemployment/underemployment (Kirchhoff et al., 2011), lower rates of marriage (Kirchhoff, Yi, Wright, Warner, & Smith, 2012), depression or anxiety (Gurney et al., 2009), and physical performance limitations resulting in restricted access to the environment (Brinkman, Li, et al., 2013; Ness et al., 2010). Cumulatively, these deficits impede successful integration into society and may adversely affect life satisfaction.
Despite considerable disability among brain tumor survivors, emergent data suggest many of them report a quality of life that is similar to that of sibling or normative controls. In a study examining psychological outcomes in long-term survivors of childhood brain cancer, Childhood Cancer Survivor Study investigators, observed that the prevalence of distress in survivors was similar to that in siblings and consistent with that in the general population (Zebrack et al., 2004). Musial-Bright and colleagues reported that despite tumor and treatment late morbidity, low-grade glioma survivors rated their quality of life higher than did their peers. (Musial-Bright, Panteli, & Hernaiz Driever, 2011). Similarly, an assessment of health-related quality of life after brachytherapy indicated patients and caregivers rated survivors’ lives as not different from that of the general population (Korinthenberg et al., 2011). Data suggest that subjective perceptions of physical conditions are better predictors of well-being than objective health status measures (Zebrack, Yi, Petersen, & Ganz, 2008) and that well-being in adult survivors of childhood cancer is a function of the individuals’ perception of how cancer has affected or continues to affect them in both positive and negative ways. (Zebrack et al., 2010). Surprisingly, Zebrack and Landier found that endorsement of a perceived positive effect of cancer was significantly greater among survivors exposed to at least one intense therapy such as radiation therapy (Zebrack & Landier, 2011). Collectively, these studies show that consideration of survivors’ subjective perspective is crucial to a balanced understanding of the status of adult survivors of pediatric brain tumors.
Life satisfaction measures the subjective perspective of how people evaluate their life as a whole. No external standard or rubric is applied. It is an assessment of which life circumstances and conditions are important for personal well-being. This evaluation of subjective well-being is built on the premise that how people experience a set of circumstances is as important as the circumstances themselves, and that individuals are the best judges of how their own lives are going. (Ryff & Singer, 1996). Ed Diener (Diener, Emmons, Larsen, & Griffin, 1985; Diener, Lucas, & Scollon, 2006) in his development of the Satisfaction with Life Scale, stressed that a single overall evaluation of life is best, rather than considering satisfaction with different domains of living such as coping, happiness, and adaptation.
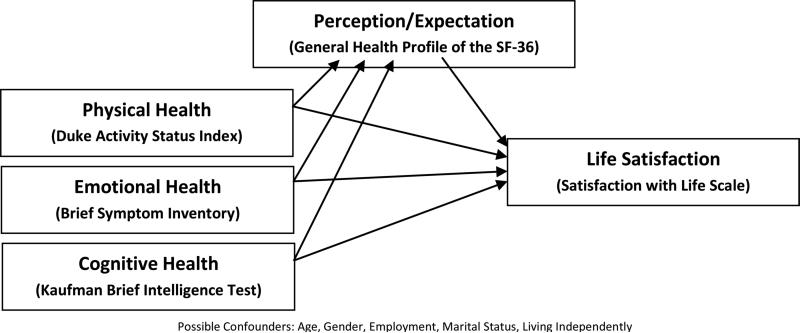
As a part of a larger study that explored physical performance limitations among adult survivors of childhood brain tumors, this investigation focused on findings specific to understanding the unique experience of life satisfaction. Based on a review of published studies and our clinical experience, we anticipated that childhood brain tumor survivors would report life satisfaction similar to that of the control group. We examined possible correlates of life satisfaction including physical health, emotional health, cognitive health, and perception/expectation. The influence of patient-related variables (e.g., sex, marital status, socioeconomic status, and co-morbid conditions) and cancer-related variables (e.g., age at diagnosis, and time since diagnosis) was also considered. Figure 1 illustrates concepts and conceptual linkages in our proposed theoretical framework. We hypothesized that life satisfaction would be the result of physical, emotional, and cognitive health mediated by a survivor's expectations and perceptions.
Figure 1.
Theoretical Framework: Concepts and Conceptual Linkages
Methods
Subjects and Procedures
Details of the current study design, participant recruitment, and cohort characteristics have been previously reported (Ness et al., 2010) Briefly, childhood brain tumor survivors who were receiving medical follow-up in the oncology outpatient clinic at St. Jude Children's Research Hospital or the University of Minnesota Children's Hospital were invited to participate. St. Jude Children's Research Hospital in Memphis, Tennessee, is an international referral center specializing in the treatment of catastrophic diseases of childhood. It is the national coordinating center for the National Cancer Institute-funded Pediatric Brain Tumor Consortium. About 180 new brain tumor patients are seen each year. The Children's Hospital in Minneapolis is a comprehensive regional referral center and is associated with the University Of Minnesota Department Of Pediatrics. More than 100 brain tumor survivors are seen each year. Study inclusion criteria included: treatment for a primary brain tumor at age 21 or younger, 18 years of age or older at the time of evaluation, and survival of at least 5 years. We excluded patients with recurrent disease, patients who were currently pregnant, and those who did not speak English. A population-based comparison group matched by age, sex, and ZIP Code was also recruited using Melissa Data services (http://melissadata.com). Evaluations were completed during home visits to eliminate the potential bias of inability to travel to the hospital. Study subjects were seen by a licensed physical therapist or study nurse at their respective study sites. Study personnel were trained to administer the tests by the principal investigator and practiced examination procedures until 95% agreement was obtained. The study nurse or physical therapist reviewed with each subject the study purpose (investigating physical performance, disability and quality of life in childhood brain tumor survivors), procedures, and plans for the evaluation. Study subjects received compensation for participation. The study was approved by the institutional review boards of both participating institutions and participants orlegal guardians provided informed consent.
Predictors and Covariates
Demographic and treatment information including age at diagnosis, time since diagnosis, sex, tumor type, and details of the extent of surgery, chemotherapy, and radiation, were abstracted from medical records. Information regarding employment, marital status and living arrangements was obtained from study participants at the time of evaluation.
Physical health and performance were evaluated by the Duke Activity Status Index. The self-administered 12-item questionnaire assesses function based on activities of daily living (Can you take care of yourself? Can you do light work around the house?) and cardiovascular fitness (Can you climb a flight of stairs or walk up a hill? Can you run a short distance?). Scores on the measure correlate with exercise capacity. Test scores are correlated with peak oxygen intake during graded exercise testing (r=.81). Test scores are converted to VO2 max values and compared with population norms provided by the American College of Sports Medicine (Hlatky et al., 1989; Medicine, 2000). This index has been compared to and validated with exercise testing in both healthy and sick populations (Alonso et al., 1997; Carter et al., 2002) . Study participants were classified as impaired if their peak oxygen intake was ≥ 2.5 standard deviations below age- and sex-predicted values.
Cognitive functioning was assessed by the Kaufman Brief Intelligence Test, version. Verbal and nonverbal intelligence are measured quickly. Internal consistency of the Kaufman is reported at 0.94 and test-retest reliability from 0.92 to 0.95. Each test item has been checked for validity by comparing it to the Wechsler Adult Intelligence Scale-Revised. The composite intelligence quotient score has a general population mean of 100 and standard deviation of 15, with a range of 40 to 160 (Kaufman & Kaufman, 2004). Scores ≤ the 10th percentile were classified as impaired.
Emotional health was assessed by the Brief Symptom Inventory (BSI-18) which evaluates symptoms over the previous week. This 18-item instrument yields a summary score of global distress. Test items are rated on a 5-point Likert scale that ranges from “no distress” to “extreme distress.” Scores are dichotomized using a cut point of 63. T Scores ≥ 63 reflected poor emotional health (Derogotis, 2000). Reliability and validity of the BSI-18 has been established in persons with traumatic brain injury, adult survivors of childhood cancer and others (Meachen, Hanks, Millis, & Rapport, 2008; Recklitis & Rodriguez, 2007).
Perception of physical health was measured by the General Health subscale of the SF-36. The SF-36 is a widely used generic health profile that provides age-and sex-specific norms. Reulen and colleagues studied the psychometrics of the SF-36 in more than 10,000 adult survivors of childhood cancer, which confirmed its validity and reliability for this population (Reulen et al., 2006) . The General Health sub-scale consists of 4 statements: I get sick a little easier; I am as healthy as any; I expect my health to get worse; and my health is excellent. T scores ≤ 40 were classified as poor health (Ware, Snow, & Kosinski, 2000).
Life satisfaction was assessed by having participants complete the Satisfaction with Life Scale (Diener et al., 1985), a 5-item, 7-point Likert scale measure of subjective well-being. It compares one's circumstances to what is thought to be appropriate without an externally imposed standard. The scale does not assess specific life domains such as health or finances, but allows patients to integrate and weigh different dimensions of life in any way they choose. Items include: my life is close to ideal; conditions of my life are excellent; I am satisfied with my life; so far I have gotten important things I want; and if I could live my life over I would change nothing. Scores range from 5 (extremely dissatisfied) to 35 (highly satisfied). Scores ≤ 14 indicated poor life satisfaction (Diener, 1984). The test –retest reliability of the Scale has been reported to range from 0.83 to 0.50 for intervals ranging from 2 weeks to 4 years; in general higher reliabilities were associated with shorter test intervals (W Pavot & Diener, 1993). The validity of the Scale has been documented by convergence with an array of both self-reported and external criteria ((Diener et al., 1985; W. Pavot, Diener, Colvin, & Sandvik, 1991).
Statistical Analysis
Descriptive statistics were calculated (frequency, percent, mean, standard deviation, median, and range) to characterize the study population. Categorical outcomes were compared among survivors and controls with chi-square statistics; continuous outcomes were compared with two sample t-tests, or with the non-parametric Kruskal-Wallis test in instances of non-normality. General linear regression was used to evaluate associations between physical health, emotional health, and life satisfaction among brain tumor survivors. SAS version 9.2 (Cary, N.C.) was used for all analyses.
Results
Participants
Participants included 78 randomly selected brain tumor survivors. The population-based comparison group included 78 individuals who responded to a mailed invitation. Age, sex, and race distributions for brain tumor survivors and individuals in the comparison group were statistically indistinguishable. Descriptive characteristics of the brain tumor survivors who participated in the study are summarized in Table 1. Fifty-four percent were male, and a majority of the cohort was white. Survivors age at the time of the study ranged from 18.4 to 58.3 years (mean, 22 years). More than half of the survivors were younger than 10 years of age at diagnosis, and 84.6% had survived for more than 10 years. The most common tumor type was astrocytic, and the most common tumor location was the cerebellum. Most survivors were treated with combination multimodality therapy. Seventy-seven percent had surgical resection, 66.7% received cranial radiation, and 30.8% received chemotherapy. Survivors were more likely than controls to be unemployed or have sheltered employment, were less likely to live independently, and were more likely to be single (Table 2, all p-values <0.01).
Table 1.
Characteristics of brain tumor survivors (N=78)
| N | % | |
|---|---|---|
| Age at Diagnosis, years | ||
| <5 | 22 | 28.2 |
| 5-9 | 26 | 33.3 |
| 10-14 | 21 | 26.9 |
| 15-20 | 9 | 11.5 |
| Time since Diagnosis, years | ||
| 5-9 | 12 | 15.4 |
| 10-14 | 30 | 38.5 |
| 15-19 | 22 | 28.2 |
| ≥20 | 14 | 17.9 |
| Sex | ||
| Male | 42 | 54 |
| Female | 36 | 46 |
| Tumor Type | ||
| Astrocytic | 40 | 51.3 |
| Medulloblastoma | 13 | 16.7 |
| Ependymoma | 9 | 11.5 |
| Other | 16 | 20.5 |
| Primary Tumor Location | ||
| Cerebrum | 11 | 14.1 |
| Thalamus | 5 | 6.4 |
| Sellar/parasellar/hypothalamic | 18 | 23.1 |
| Pineal | 4 | 5.1 |
| Brainstem | 8 | 10.3 |
| Brainstem and spine | 2 | 2.6 |
| Cerebellum | 25 | 32.1 |
| Optic Nerve | 5 | 6.4 |
| Extent of Surgery | ||
| None/ Biopsy | 18 | 23.1 |
| Partial/Near Total Resection | 24 | 30.8 |
| Gross Total Resection | 36 | 46.1 |
| Chemotherapy | ||
| Yes | 24 | 30.8 |
| No | 54 | 69.2 |
| Radiation | ||
| None | 26 | 33.3 |
| Cranial | 52 | 66.7 |
| Craniospinal | 23 | 29.5 |
Table 2.
Comparison of employment, living situation, and marital status
| Survivors (N=78) | Comparison Group (N=78) | ||||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Employment | <0.001 | ||||
| Employed/Student | 51 | 65.4 | 73 | 93.6 | |
| Sheltered Employment | 4 | 5.1 | 0 | 0 | |
| Unemployed | 23 | 29.5 | 5 | 6.4 | |
| Living Situation | <0.001 | ||||
| Independent | 58 | 74.4 | 78 | 100 | |
| Family Support | 18 | 23.1 | 0 | 0 | |
| Custodial Care | 2 | 2.6 | 0 | 0 | |
| Marital Status | <0.01 | ||||
| Married | 13 | 16.7 | 35 | 44.9 | |
| Single | 65 | 83.3 | 43 | 55.1 | |
Physical, Cognitive, and Emotional Functioning
Comparisons of survivors and controls on the primary outcomes of interest are detailed in Table 3. Differences between survivors and controls were observed in physical health and performance, cognitive functioning and perception/expectation of physical health. Survivors had significantly poorer physical health (p<0.001), including sensory deficits, reduced muscle strength, and poor exercise tolerance. Specifically, 51.3% of survivors had peak oxygen uptake estimates more than 2.5 standard deviations below age and sex-predicted values vs. only 11.5% of controls (p<0.001). Survivors, on average, had more impaired cognition than controls (survivor mean = 82.7 vs. control mean = 103; p<0.001). Perception/expectation of physical health indicated that survivors rated their health as significantly worse than controls did, with 23.4% of survivors reporting impaired physical health vs. only 1.3% of controls (p<0.001). Symptoms of emotional distress were similar for survivors and controls (p=0.32).
Table 3.
Comparison of survivors and controls in terms of life-satisfaction, perception/expectations, physical health, emotional health, and cognitive health.
| Case | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% Confidence Interval | Percent Impaired | Mean | SD | 95% Confidence Interval | Percent Impaired | Continuous variable comparison P-value | Binary variable comparison P-value | |
| Physical Health/Functiona | 35.5 | 21.2 | 30.7-40.3 | 51.3 | 52.7 | 10.2 | 50.4-55.0 | 11.5 | <0.001 | <0.001 |
| Cognitive Statusb | 82.7 | 21.8 | 77.6-87.9 | 31.9 | 102.7 | 14.6 | 99.4-106.0 | 1.3 | <0.001 | <0.001 |
| Perception/Expectationc | 49.5 | 11.1 | 47.0-52.0 | 23.4 | 55.8 | 6.5 | 54.4-57.3 | 1.3 | 0.001 | <0.001 |
| Emotional Health Global Scored | 49.1 | 11.0 | 46.6-51.6 | 7.8 | 46.8 | 8.3 | 45.0-48.7 | 3.9 | 0.32 | 0.49 |
| Life Satisfactione | 26.8 | 7.3 | 25.2-28.5 | 7.9 | 27.2 | 6.4 | 25.7-28.6 | 6.4 | 0.74 | 0.76 |
Since scores distributions were non-normal, Wilcoxon tests were used to compare case and control for continuous variables. Chi-square or exact Fisher tests were used to calculate p-values for binary variables.
DASI scores ≥ 2.5 standard deviations below age and sex-predicted values indicates poor physical performance
KBIT-18 scores ≤ the 10th percentile classified as impaired cognition
SF-36 scores ≤ 40 indicates perceived poor health
BSI-18 scores ≥ 63 indicates poor emotional health functioning
SWLS scores ≤ 14 indicates poor life satisfaction
Life Satisfaction
No significant differences were seen in life satisfaction between survivors and controls. In a multivariate linear regression model, restricted to survivors and adjusted for age, sex, and socioeconomic status, only survivor perception/expectation of physical health contributed significantly to life satisfaction. For each standard deviation (10 point) increase in the SF-36 physical function subscale, survivors, on average, reported a 4.06 point increase in life satisfaction. While the overall model accounted for 38% of the variance in life satisfaction, cognitive status and physical functioning were not significantly associated with life satisfaction among survivors.
Discussion and Implications for Practice
Three primary findings emerge from our analysis: (1) adult survivors of childhood brain tumors reported an increased burden of multiple physical functional and cognitive deficits; (2) despite this long-term morbidity, survivors’ life satisfaction was similar to that of sex- and age-matched controls; and (3) survivors’ perceptions/expectations of health status are important predictors of life satisfaction.
Our finding that adult survivors of childhood brain tumors reported diminished health, more physical deficits, and cognitive limitations than individuals in the control group is not surprising. Reports from the Childhood Cancer Survivor Study have documented that adult survivors of childhood brain tumors are an especially vulnerable group who experience more limitations than their siblings and more medical complications than any other group of childhood cancer survivors (Armstrong et al., 2009; Crom et al., 2010). In our cohort, cognitive performance was, on average, below that observed for controls, but still within the low average range of functioning. This is important because as Zebrack and colleagues note, survivors must have the cognitive capacity to acknowledge the severity of the life disruption caused by the disease to perceive a positive life influence after experiencing cancer (Zebrack et al., 2012). Socially, our survivors reported lower rates of independent living, marriage, and employment, similar to rates noted in past studies (Crom et al., 2007; Kirchhoff et al., 2011; Kirchhoff et al., 2012; Ness et al., 2010).
Emotional health did not differ between survivors and controls, as reflected by the BSI-18 global score. This finding is somewhat inconsistent with historical reports. Although most childhood cancer survivors are noted to be psychologically well and do not report symptoms of distress or depression that exceed sibling controls or population norms, brain tumor survivors appear to be an exception. Zebrack and Zeltzer hypothesized that factors contributing to increased anxiety and depression in this sub-group of survivors include disproportionate rates of learning disabilities, unemployment, behavioral concerns, and social limitations resulting in daily frustration (Zebrack et al., 2004; Zeltzer et al., 2009). Brinkman, et al. noted in their study of longitudinal patterns of psychological distress that patients treated with cranial radiation reported increased symptoms of distress over time and were more likely to use pharmacologic therapy (Brinkman, Ullrich, et al., 2013; Brinkman, Zhu, et al., 2013). Furthermore, nearly 11% of brain tumor survivors reported suicidal ideations (Recklitis et al., 2010). Depression, pain, and poor physical health have been associated with an increased likelihood of suicidal thoughts in adult survivors of childhood brain tumors (Brinkman, Liptak, et al., 2013). Taken together, these data appear to suggest that adult survivors of childhood brain tumors are vulnerable to symptoms of psychological distress. An important difference between our study and previous reports that may account for observed differences is the method of data collection. We collected data through in-person interviews whereas most previous studies have relied on self-report data from questionnaires completed by mail. Face-to-face reporting may minimize the frequency and severity with which psychological symptoms are reported.
Despite considerable morbidity, survivors in our study reported life satisfaction similar to that of healthy controls. Although this finding seems somewhat counterintuitive, it aligns with the findings of Dietz and Mulrooney, who emphasized that survivors of childhood cancers may be more resilient than expected and may be able to overcome many of the barriers forced upon them (Dietz & Mulrooney, 2011). However, other researchers contend that brain tumor survivors have less positive life satisfaction outcomes and experience loneliness, loss, and isolation. For example, Zebrack and colleagues argued that debilitating cognitive and behavioral limitations experienced by survivors may preclude them from perceiving a positive effect of their cancer (Zebrack et al., 2012). Qualitative researchers debate the statistical versus clinical significance of such reports and assert that quantitative analyses in large survivor cohorts obscure the full range of possible psychosocial outcomes and trivialize the complexity of cancer survivorship. After interviewing 50 adult survivors of childhood cancers, including brain tumor survivors, Parry and Chesler concluded that many outcomes are possible, including resilience and even thriving (Parry & Chesler, 2005). Boydell and colleagues (Boydell, Stasiulis, Greenberg, Greenberg, & Spiegler, 2008) found “I'll show them” was a frequent theme as brain tumor survivors struggled to reintegrate into society and establish realistic goals. Survivors expressed new determination and strength, but also needed to give additional attention to obtaining help when necessary.
Elements of post-traumatic growth have also been considered as an explanation for why childhood cancer survivors report being satisfied with their lives (Zeltzer et al., 2008). As one element of such growth, many survivors communicate a deeper empathy for the suffering of others (Crom, 2009). However, they fail to integrate other components of potential post-traumatic growth, such as developing new directions or consciously changing life priorities. Half of study participants were less than 10 years of age at diagnosis making post-traumatic growth developmentally improbable. Also, post-traumatic growth may require formal operational conceptualization that would be challenging for many brain tumor survivors with cognitive impairments.
It is also possible that a phenomenon of “response shift” explains the lack of variance in life satisfaction between survivors and controls. Response shift occurs when individuals alter their internal standards, values, and determinants of what makes life satisfying based on changes in their physical, cognitive, and emotional health. It is an unconscious process of recalibration, reconceptualization, and reprioritization of internal standards and references utilized for self-appraisal (Barclay-Goddard, Epstein, & Mayo, 2009). Such shifts have been examined in breast cancer survivors (Dabakuyo et al., 2013), in patients who have had a stroke (Barclay-Goddard, Lix, Tate, Weinberg, & Mayo, 2011), and in the assessment of outcomes after spinal surgery (Schwartz, Sajobi, Lix, Quaranto, & Finkelstein, 2013). Researchers stress adaptation and adjustment are gradual, ongoing processes--a change of life trajectory. This may occur over the course of several decades of cancer survivorship. Response shift enables patients to reframe a negative event. Such reframing may require that they compartmentalize losses and reevaluate formerly important domains of life (Massey, Cameron, Ouellette, & Fine, 1998).
Survivors’ perceptions and expectations emerged in multivariate regression analysis as the key determinant of life satisfaction. Responses suggest survivors realistically do not deny their poor health but integrate their limitations, gradually and subconsciously redefining what makes life satisfying. Pemberger, et al. attributed childhood cancer survivors’ report of subjective well-being to a better appreciation of being alive and to considering possible impairments of present health status less important (Pemberger et al., 2005). Recent research, specific to childhood cancer survivors, corroborates that subjective perceptions of health status are better predictors of distress or well-being than are objective functional measures (Zebrack et al., 2008).
These findings must be considered in the context of several study limitations. First, the generalizability of these findings is limited by the small sample size. In addition, we note that this is a cross-sectional study and understanding the changing lives of adult survivors of childhood brain tumors would be improved by longitudinal data. We acknowledge that the relationship between perception/expectation of health and life satisfaction is associative and not necessarily causal. Generic measures were utilized to capture the experience of cancer survivors, which may not be the best way to understand their life satisfaction. No qualitative data were available to add additional insight. Finally, the use of self-reported data and field testing are subject to measurement error and may have reduced the precision of our estimates.
Despite these limitations, this study offers important information to nursing practice as clinicians help organize follow-up care for adult survivors of childhood brain tumors. If survivors perceive that their health status is what they would expect it to be, they may be reluctant to adhere to recommended health behaviors such as eliminating tobacco use or participating in physical activities. This perception would explain the poor attendance at multidisciplinary clinics (Krull et al., 2011) where aging adult survivors could benefit from screening evaluations such as those defined by the Children's Oncology Group (available at http://www.survivorship-guidelines.org).
The finding that perception/expectation of health is a sentinel mediator of life satisfaction is a call to action for oncology nurses to become advocates for adult survivors of childhood brain tumors. Oncology nurses are in an ideal position to talk deeply with survivors. We have previously shown that oncology nurses can help to create a positive listening environment in which survivors relate complete and honest information about their life experiences, motivations, and expectations (Crom, Hinds, Gattuso, Tyc, & Hudson, 2005). Armed with a better understanding of the complex reality of this most vulnerable group of aging cancer survivors, we are better able to address barriers to care and design interventions to promote quality of life.
These results must be interpreted with caution. Pediatric oncology nurses remain concerned regarding the limitations, poor societal integration, and isolation reported by many adult survivors. Our findings provide the opportunity to ask more questions and better questions that seek to identify critical correlates of life satisfaction in survivors. The appropriateness of evaluating survivors with general measures must be addressed if we are to identify future directions for this population. New, disease-specific/population-specific, measures are emerging based on qualitative studies identifying important dimensions of life satisfaction in survivors. Our report underscores the importance of evaluating life satisfaction based on the perceptions of the survivor and not as measured by an externally imposed standard.
Acknowledgements
We thank David Galloway, ELS, for editorial assistance.
Contributor Information
Deborah B. Crom, Family Nurse Practitioner, After Completion of Therapy Clinic St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105.
Zhenghong Li, Department of Epidemiology and Cancer Control St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105 Zhenghong.Li@stjude.org.
Tara M. Brinkman, Department of Epidemiology and Cancer Control St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105 Tara.Brinkman@stjude.org.
Melissa M. Hudson, Department of Oncology St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105 Melissa.Hudson@stjude.org.
Gregory T. Armstrong, Departments of Oncology, Epidemiology and Cancer Control St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105 Gregory.Armstrong@stjude.org.
Joseph Neglia, Department of Pediatrics University of Minnesota, 2450 Riverside Avenue, 8952A, Minneapolis, MN 55454 jneglia@umn.edu.
Kirsten K. Ness, Department of Epidemiology and Cancer Control St. Jude Children's Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105 Kiri.Ness@stjude.org.
References
- Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI). Eur Heart J. 1997;18(3):414–419. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, Morris EB. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–234. doi: 10.1093/neuonc/noq178. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Packer RJ. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. doi: 10.1093/jnci/djp148. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay-Goddard R, Epstein JD, Mayo NE. Response shift: a brief overview and proposed research priorities. Qual Life Res. 2009;18(3):335–346. doi: 10.1007/s11136-009-9450-x. doi: 10.1007/s11136-009-9450-x. [DOI] [PubMed] [Google Scholar]
- Barclay-Goddard R, Lix LM, Tate R, Weinberg L, Mayo NE. Health-related quality of life after stroke: does response shift occur in self-perceived physical function? Arch Phys Med Rehabil. 2011;92(11):1762–1769. doi: 10.1016/j.apmr.2011.06.013. doi: 10.1016/j.apmr.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Boydell KM, Stasiulis E, Greenberg M, Greenberg C, Spiegler B. I'll show them: the social construction of (in)competence in survivors of childhood brain tumors. J Pediatr Oncol Nurs. 2008;25(3):164–174. doi: 10.1177/1043454208315547. doi: 10.1177/1043454208315547. [DOI] [PubMed] [Google Scholar]
- Brinkman TM, Li Z, Neglia JP, Gajjar A, Klosky JL, Allgood R, Ness KK. Restricted access to the environment and quality of life in adult survivors of childhood brain tumors. J Neurooncol. 2013;111(2):195–203. doi: 10.1007/s11060-012-1001-6. doi: 10.1007/s11060-012-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman TM, Liptak CC, Delaney BL, Chordas CA, Muriel AC, Manley PE. Suicide ideation in pediatric and adult survivors of childhood brain tumors. J Neurooncol. 2013;113(3):425–432. doi: 10.1007/s11060-013-1130-6. doi: 10.1007/s11060-013-1130-6. [DOI] [PubMed] [Google Scholar]
- Brinkman TM, Ullrich NJ, Zhang N, Green DM, Zeltzer LK, Lommel KM, Krull KR. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7(1):104–114. doi: 10.1007/s11764-012-0250-x. doi: 10.1007/s11764-012-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman TM, Zhu L, Zeltzer LK, Recklitis CJ, Kimberg C, Zhang N, Krull KR. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br J Cancer. 2013;109(5):1373–1381. doi: 10.1038/bjc.2013.428. doi: 10.1038/bjc.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Holiday DB, Grothues C, Nwasuruba C, Stocks J, Tiep B. Criterion validity of the Duke Activity Status Index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2002;22(4):298–308. doi: 10.1097/00008483-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Ashford JM, Howarth RA, Merchant TE, Ogg RJ, Santana VM, Xiong X. Working memory performance among childhood brain tumor survivors. J Int Neuropsychol Soc. 2012;18(6):996–1005. doi: 10.1017/S1355617712000793. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crom DB. “I think you are pretty; i don't know why everyone can't see that”: reflections from a young adult brain tumor survivor camp. J Clin Oncol. 2009;27(19):3259–3261. doi: 10.1200/JCO.2009.22.1978. doi: 10.1200/JCO.2009.22.1978. [DOI] [PubMed] [Google Scholar]
- Crom DB, Hinds PS, Gattuso JS, Tyc V, Hudson MM. Creating the basis for a breast health program for female survivors of Hodgkin disease using a participatory research approach. Oncol Nurs Forum. 2005;32(6):1131–1141. doi: 10.1188/05.ONF.1131-1141. doi: 10.1188/05.ONF.1131-1141. [DOI] [PubMed] [Google Scholar]
- Crom DB, Lensing SY, Rai SN, Snider MA, Cash DK, Hudson MM. Marriage, employment, and health insurance in adult survivors of childhood cancer. J Cancer Surviv. 2007;1(3):237–245. doi: 10.1007/s11764-007-0026-x. doi: 10.1007/s11764-007-0026-x. [DOI] [PubMed] [Google Scholar]
- Crom DB, Smith D, Xiong Z, Onar A, Hudson MM, Merchant TE, Morris EB. Health status in long-term survivors of pediatric craniopharyngiomas. J Neurosci Nurs. 2010;42(6):323–328. doi: 10.1097/jnn.0b013e3181f8a59d. quiz 329-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabakuyo TS, Guillemin F, Conroy T, Velten M, Jolly D, Mercier M, Bonnetain F. Response shift effects on measuring post-operative quality of life among breast cancer patients: a multicenter cohort study. Qual Life Res. 2013;22(1):1–11. doi: 10.1007/s11136-012-0135-5. doi: 10.1007/s11136-012-0135-5. [DOI] [PubMed] [Google Scholar]
- Derogotis L. Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual. 3rd edition ed. NCS Pearson, Inc.; Minneapolis: 2000. [Google Scholar]
- Di Pinto M, Conklin HM, Li C, Merchant TE. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2012;84(3):e363–369. doi: 10.1016/j.ijrobp.2012.03.066. doi: 10.1016/j.ijrobp.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E. Subjective well-being. Psychol Bull. 1984;95(3):542–575. [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Diener E, Lucas RE, Scollon CN. Beyond the hedonic treadmill: revising the adaptation theory of well-being. Am Psychol. 2006;61(4):305–314. doi: 10.1037/0003-066X.61.4.305. doi: 10.1037/0003-066X.61.4.305. [DOI] [PubMed] [Google Scholar]
- Dietz AC, Mulrooney DA. Life beyond the disease: relationships, parenting, and quality of life among survivors of childhood cancer. Haematologica. 2011;96(5):643–645. doi: 10.3324/haematol.2011.042606. doi: 10.3324/haematol.2011.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, Ness KK. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2390–2395. doi: 10.1200/JCO.2008.21.1458. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. doi: 10.1001/jama.2013.6296. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. 2nd edition ed. AGS Publishing; Circle Pines, Minnesota: 2004. [Google Scholar]
- Kirchhoff AC, Krull KR, Ness KK, Park ER, Oeffinger KC, Hudson MM, Leisenring W. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer. 2011;117(13):3033–3044. doi: 10.1002/cncr.25867. doi: 10.1002/cncr.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff AC, Yi J, Wright J, Warner EL, Smith KR. Marriage and divorce among young adult cancer survivors. J Cancer Surviv. 2012;6(4):441–450. doi: 10.1007/s11764-012-0238-6. doi: 10.1007/s11764-012-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinthenberg R, Neuburger D, Nikkhah G, Teske C, Schnabel K, Calaminus G. Assessing quality of life in long-term survivors after (1)(2)(5)I brachytherapy for low-grade glioma in childhood. Neuropediatrics. 2011;42(3):110–115. doi: 10.1055/s-0031-1283111. doi: http://dx.doi.org/10.1055/s-0031-1283111 10.1055/s-0031-1283111. [DOI] [PubMed] [Google Scholar]
- Krull KR, Annett RD, Pan Z, Ness KK, Nathan PC, Srivastava DK, Hudson MM. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47(9):1380–1388. doi: 10.1016/j.ejca.2011.03.001. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey S, Cameron A, Ouellette S, Fine M. Qualitative approaches to the study of thriving: What can be learned? Journal of Social Issues. 1998;54(2) [Google Scholar]
- Meachen SJ, Hanks RA, Millis SR, Rapport LJ. The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):958–965. doi: 10.1016/j.apmr.2007.12.028. doi: 10.1016/j.apmr.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Medicine A. C. o. S. ACSM's Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- Musial-Bright L, Panteli L, Hernaiz Driever P. Pediatric low-grade glioma survivors experience high quality of life. Childs Nerv Syst. 2011;27(11):1895–1902. doi: 10.1007/s00381-011-1467-0. doi: 10.1007/s00381-011-1467-0. [DOI] [PubMed] [Google Scholar]
- Ness KK, Morris EB, Nolan VG, Howell CR, Gilchrist LS, Stovall M, Neglia JP. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116(12):3034–3044. doi: 10.1002/cncr.25051. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan VG, Gapstur R, Gross CR, Desain LA, Neglia JP, Gajjar A, Ness KK. Sleep disturbances in adult survivors of childhood brain tumors. Qual Life Res. 2013;22(4):781–789. doi: 10.1007/s11136-012-0208-5. doi: 10.1007/s11136-012-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C, Chesler MA. Thematic evidence of psychosocial thriving in childhood cancer survivors. Qual Health Res. 2005;15(8):1055–1073. doi: 10.1177/1049732305277860. doi: 10.1177/1049732305277860. [DOI] [PubMed] [Google Scholar]
- Pavot W, Diener E. Review of the Satisfaction with Life Scale. Psychological Assessments. 1993;5(2):164–172. [Google Scholar]
- Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the Satisfaction with Life Scale: evidence for the cross-method convergence of well-being measures. J Pers Assess. 1991;57(1):149–161. doi: 10.1207/s15327752jpa5701_17. doi: 10.1207/s15327752jpa5701_17. [DOI] [PubMed] [Google Scholar]
- Pemberger S, Jagsch R, Frey E, Felder-Puig R, Gadner H, Kryspin-Exner I, Topf R. Quality of life in long-term childhood cancer survivors and the relation of late effects and subjective well-being. Support Care Cancer. 2005;13(1):49–56. doi: 10.1007/s00520-004-0724-0. doi: 10.1007/s00520-004-0724-0. [DOI] [PubMed] [Google Scholar]
- Recklitis CJ, Diller LR, Li X, Najita J, Robison LL, Zeltzer L. Suicide ideation in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(4):655–661. doi: 10.1200/JCO.2009.22.8635. doi: 10.1200/JCO.2009.22.8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recklitis CJ, Rodriguez P. Screening childhood cancer survivors with the brief symptom inventory-18: classification agreement with the symptom checklist-90-revised. Psychooncology. 2007;16(5):429–436. doi: 10.1002/pon.1069. doi: 10.1002/pon.1069. [DOI] [PubMed] [Google Scholar]
- Reulen RC, Zeegers MP, Jenkinson C, Lancashire ER, Winter DL, Jenney ME, Hawkins MM. The use of the SF-36 questionnaire in adult survivors of childhood cancer: evaluation of data quality, score reliability, and scaling assumptions. Health Qual Life Outcomes. 2006;4:77. doi: 10.1186/1477-7525-4-77. doi: 10.1186/1477-7525-4-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Singer B. Psychological well-being: meaning, measurement, and implications for psychotherapy research. Psychother Psychosom. 1996;65(1):14–23. doi: 10.1159/000289026. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Sajobi TT, Lix LM, Quaranto BR, Finkelstein JA. Changing values, changing outcomes: the influence of reprioritization response shift on outcome assessment after spine surgery. Qual Life Res. 2013 doi: 10.1007/s11136-013-0377-x. [DOI] [PubMed] [Google Scholar]
- Shaw S. Endocrine late effects in survivors of pediatric brain tumors. J Pediatr Oncol Nurs. 2009;26(5):295–302. doi: 10.1177/1043454209343180. doi: 10.1177/1043454209343180. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Quality Metric Incorporated; Lincoln RI: 2000. [Google Scholar]
- Zebrack BJ, Donohue JE, Gurney JG, Chesler MA, Bhatia S, Landier W. Psychometric evaluation of the Impact of Cancer (IOC-CS) scale for young adult survivors of childhood cancer. Qual Life Res. 2010;19(2):207–218. doi: 10.1007/s11136-009-9576-x. doi: 10.1007/s11136-009-9576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrack BJ, Gurney JG, Oeffinger K, Whitton J, Packer RJ, Mertens A, Zeltzer LK. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22(6):999–1006. doi: 10.1200/JCO.2004.06.148. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Landier W. The perceived impact of cancer on quality of life for post-treatment survivors of childhood cancer. Qual Life Res. 2011;20(10):1595–1608. doi: 10.1007/s11136-011-9893-8. doi: 10.1007/s11136-011-9893-8. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Stuber ML, Meeske KA, Phipps S, Krull KR, Liu Q, Zeltzer LK. Perceived positive impact of cancer among long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Psychooncology. 2012;21(6):630–639. doi: 10.1002/pon.1959. doi: 10.1002/pon.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008;17(9):891–900. doi: 10.1002/pon.1300. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Lu Q, Leisenring W, Tsao JC, Recklitis C, Armstrong G, Ness KK. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):435–446. doi: 10.1158/1055-9965.EPI-07-2541. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, Krull K. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396–2404. doi: 10.1200/JCO.2008.21.1433. doi: 10.1200/jco.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]