Abstract
Background
Diet may substantially alter prostate cancer initiation and progression. However, large-scale clinical trials of diet modification have yet to be performed for prostate cancer. The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) is investigating the effect of increased vegetable consumption on clinical progression in men with localized prostate cancer.
Study Design
MEAL is a randomized, Phase III clinical trial designed to test whether an intervention that increases vegetable intake will decrease the incidence of clinical progression in men with clinically localized prostate cancer on active surveillance. We are randomizing 464 patients to either a validated telephone-based diet counseling intervention or a control condition in which patients receive a published diet guideline. The intervention will continue for two years. The primary outcome variable is clinical progression defined by serum prostate-specific antigen (PSA) and pathological findings on follow-up prostate biopsy. Secondary outcome variables include incidence of surgical and non-surgical treatments for prostate cancer, prostate-cancer related patient anxiety and health-related quality of life.
Conclusion
The MEAL Study is assessing the effectiveness of a high-vegetable diet intervention for preventing clinical progression in men with localized prostate cancer on active surveillance.
Keywords: Diet, Prostate Cancer, Outcomes, Active Surveillance, Carotenoids, Nutrition
Introduction
Due to widespread prostate-specific antigen (PSA) screening, approximately 50% of men diagnosed with prostate cancer present with relatively indolent disease.1,2 Many of these patients nevertheless undergo surgery, radiation, or other aggressive treatments associated with chronic—and substantial—side effects.3-5 Active surveillance, which entails careful monitoring of selected patients with early stage prostate cancer and treatment of those who demonstrate evidence of disease progression, provides a viable and safe alternative to immediate treatment.6-8 However, approximately 30% to 35% of patients pursuing active surveillance will clinically progress and undergo aggressive treatment with surgery or radiation within 5 years, while others will opt for treatment even though they do not meet the objective criteria for progression.7-9
A novel strategy of potentially decreasing the number of active surveillance patients who require aggressive treatment is diet modification. Diet may substantially influence prostate cancer initiation and progression,10-12 and altering dietary intake— specifically, switching to a diet that emphasizes vegetable intake and de-emphasizes meat and fat intake—might decrease the risk of clinical progression.10,13 Prostate cell line and animal studies demonstrate that components of cruciferous vegetables (isothiocyanates) and tomatoes (lycopene) induce apoptosis of prostate cancer cells, inhibit carcinogenesis, and promote the expression of cytoprotective enzymes in prostate tissue.14-16
Early clinical evidence supporting these epidemiological and laboratory data are limited, but promising. Three small trials have evaluated diet change as a therapy for prostate cancer, two of which observed favorable results.17-19 In one of these studies, a small (n = 93) group of active surveillance patients who implemented extreme lifestyle changes—including a low-fat, plant-based diet—experienced decreased serum PSA concentrations and rates of progression to standard treatment for up to 2 years following the intervention.18,20 Gene expression profiling in a sample (n=30) of these men comparing pre- and post-intervention prostate biopsy tissue identified significant post-intervention changes in biological processes related to carcinogenesis, suggesting the possibility that nutritional and other lifestyle changes may alter tumorigenesis.21
Additional follow-up studies of these patients have also hinted at intriguing links between lifestyle change in prostate cancer patients and telomeres, protective DNA-protein complexes at the end of chromosomes that promote chromosomal stability. Shorter telomere length is a prognostic marker of disease, aging, and premature morbidity; telomere shortening is counteracted by the cellular enzyme telomerase. Analyses in 24 and 10 of these patients demonstrated significantly increased telomerase activity22 and longer telomeres,23 respectively, in peripheral blood mononuclear cells in response to the lifestyle intervention, intimating that nutritional changes may beneficially influence chromosome stability.
To further test the potential clinical benefits of diet change in men with localized prostate cancer, we designed and successfully pilot tested a telephone-based diet intervention for prostate cancer patients based on well-established principles of social cognitive theory. This intervention produced robust diet changes and led to increased plasma carotenoids—a biomarker for vegetable intake—in prostate cancer patients, including those on active surveillance.24,25
The Men’s Eating and Living (MEAL) Study is a Phase III clinical trial designed to assess the efficacy of our dietary intervention to prevent clinical progression in men with localized prostate cancer on active surveillance
Research Design and Methods
Eligibility and exclusion criteria
Eligible patients are 50 to 80 years of age with biopsy-proven adenocarcinoma of the prostate who were diagnosed within 24 months prior to presentation, had ≥ 10 biopsy tissue cores procured at the time of the diagnostic biopsy, had < 25% of biopsy tissue cores positive for cancer, had ≤ 50% of any single biopsy tissue core positive for cancer and chose to pursue active surveillance. Biopsy eligibility criteria also include biopsy Gleason sum ≤ 6 for men ≤ 70 years and biopsy Gleason sum ≤ (3 + 4) = 7 for men > 70 years. These inclusion criteria are typical for allowing patients with localized prostate cancer to safely pursue active surveillance and represent the current standard of care in the community.6-10 Centralized pathology review is conducted on the prostate biopsy tissue cores by a single pathologist (PH) to confirm eligibility.
Other eligibility criteria are clinical stage ≤ T2a, serum PSA < 10 ng/mL, life expectancy at least 3 years, and the ability to read and comprehend English language text and to understand spoken English over the telephone.
Exclusion criteria include prostate cancer with distant metastases; prior treatment for prostate cancer by surgery, radiation, minimally-invasive local ablation (i.e., cryosurgery or high-intensity focused ultrasound), or androgen deprivation therapy; current oral anticoagulation therapy with warfarin; a history of non-cutaneous malignancy (other than non-melanoma skin cancer) in the previous 5 years; current consumption of ≥ 6 servings per day of fruits and vegetables (not including juices); unwillingness to adopt a vegetable-rich diet; intolerance of cruciferous vegetables; psychiatric illness precluding compliance with the intervention and/or obtainment of informed consent; and medical conditions that in the opinion of the treating physician would make the protocol unreasonably hazardous.
Patients taking dietary supplements, including lycopene and beta-carotene, are eligible. Intake of supplements by study participants is also permitted while on study. Patients receiving treatment with the 5-alpha reductase inhibitors finasteride or dutasteride within 90 days are not eligible. Should they wish to enroll, patients will become eligible after discontinuation and then a 90-day washout period. Patients who begin taking these medications while on study will be censored at time of medication initiation.
Study design
The MEAL Study is a randomized, phase III clinical trial. The intervention is a validated, telephone-based counseling program.24,25 We are randomizing 464 patients on active surveillance to either the telephone-based counseling intervention or the control condition in which patients receive printed materials from the Prostate Cancer Foundation that recommend consumption of a healthy diet (Figure 1).
Figure 1.
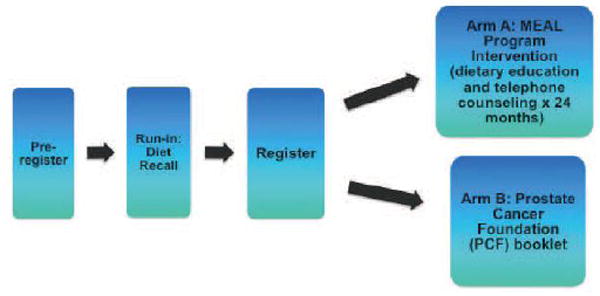
The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) schema
This study utilizes the centralized administrative infrastructure of the Alliance for Clinical Trials in Oncology (formerly known as CALGB), a National Cancer Institute (NCI)-funded cancer trials cooperative group. Each member site must obtain regulatory approval from its local IRB to be eligible to participate. Study personnel at participating institutions obtain written informed consent from interested and potentially eligible patients. During pre-registration, study personnel at participating institutions utilize a secure, Web-based patient registration system that captures relevant demographic and medical data. When the patient is preregistered, the system generates a patient identification number for record keeping that is shared with the Alliance statistical center.
During the subsequent run-in period, patients will complete an initial evaluation for eligibility and provide 6-hour fasting blood samples. Once eligibility is confirmed, eligible patients will be contacted by staff of the UC San Diego Moores Cancer Center within one week to schedule a series of three 24-hour dietary recalls, all of which will be completed within 3 weeks. Eligible patients who complete the run-in and submit a blood sample are then registered and randomized by site. Randomization is accepted only through Alliance member institutions, at-large members, selected affiliate institutions, and Community Clinical Oncology Programs using the Web-based Patient registration system. Registration must occur prior to the initiation of therapy.
Telephone counseling intervention
All counseling is performed by telephone from UC San Diego Moores Cancer Center. The telephone counseling protocol follows a step-wise, phased approach that employs strategies adopted from social cognitive theory.26 Motivational interviewing techniques27 are utilized to help participants assume and maintain responsibility for their own behavior change.
After randomization, each intervention participant is assigned to a personal counselor. Counselors work morning, afternoon, or evening shifts, and every effort is made to assign the participant to a counselor working when he prefers to receive calls.
The dietary targets for the intervention arm are designed to be challenging yet achievable. Men randomized to the intervention diet are encouraged to consume daily at least 7 servings of vegetables (including at least 2 servings of cruciferous vegetables and 2 servings of tomatoes), 2 servings of fruit, 2 servings of whole grains, and 1 serving of legumes. To maximize the intake of potentially beneficial bioactive food components, intervention participants are encouraged to consume “bold” (“big color” and “strong flavor”) vegetables and fruit. In addition to cruciferous vegetables and tomatoes, these foods include dark green leafy vegetables, deep orange vegetables and fruits, allium vegetables (onions, garlic), berries, and citrus fruit. Servings are defined as a half-cup cut up raw or cooked vegetables, fruit, or 100% vegetable juice; one cup raw leafy green vegetable; a half cup cooked whole grain, or legume; 1 slice whole-grain bread. Because of lower content of potentially beneficial bioactive food components, iceberg lettuce, white potatoes, and fruit juices are not counted toward the daily goals.
The telephone counseling intervention is divided into 4 phases over a 24-month period. Telephone calls are scheduled more frequently at the beginning of the intervention when participants need the most support. During the first phase, counseling sessions are focused on setting and achieving short-term goals to build self-efficacy. Each call during this phase starts with a 24-hour recall to monitor dietary intake. Performance is discussed with the participant and positive aspects of achievements highlighted prior to negotiating a new set of sub-goals. To maximize the probability of success, counselors focus on helping participants identify barriers to achieving goals and ensuring that participants take these barriers into consideration when setting their iterative set of short-term goals.
The second phase focuses on consolidation of the new dietary pattern into the daily lifestyle of the participant. Emphasis is placed on goals for establishing an environment that is conducive to achieving the new dietary pattern, such as altering the type of food available in the house, modifying recipes and food preparation, and focusing on appropriate portion sizes, eating patterns, and eating behaviors. During this phase, participants are encouraged to self-monitor their adherence to the study dietary goals by each day recording the number of servings they ingested of vegetables (cruciferous, tomato, other), fruit, whole grains, and beans/legumes on the studyspecific Weekly Food Checklist. Counselors measure self-efficacy at the end of each counseling call by asking the participant to rate his confidence that he will meet the incremental behavioral goals set during the call for the time period ending with the next call. If the counselor rates self-efficacy as low, the counselor helps the participant adjust the goal to maximize the potential for behavior change.
The third phase focuses on relapse prevention. As vulnerability to relapse is known to be associated with a declining self-efficacy,27 the counselor checks selfefficacy at each call and revisits earlier goal setting strategies if needed.
The fourth phase focuses on providing positive feedback on achievements and maintaining participant accountability, and their commitment to their lifestyle changes and to the study. The counselor continues to monitor self-efficacy and help problem solve barriers that arise. To ensure intervention fidelity, counselors completed an intensive 80-hour training program that reviews the rationale for the study, protocols for conducting 24-hour recalls, and extensive role-playing using a computerized structured protocol. The intervention focuses on a series of questions, and responses are entered into the study database in real time. A registered dietitian supervises the intervention team and conducts regular performance reviews. Counselors attend a monthly case management meeting, sharing issues and solutions, as well as reviewing recent relevant literature.
To further standardize the intervention and minimize the potential for bias, we have developed a detailed, relational database that provides counselors with a computer-assisted coaching protocol for their participant contacts. All contacts will be recorded in the database, and the database will generate the call schedule for each counselor each day. Calls will then follow a script that includes suggested question phrasing and responses to key questions inserted into the database in real time; these standardize intervention delivery. Automatic range checks will ensure quality in the dataset. At the completion of each call, the database prompts the counselor for detailed comments for use in the next contact. The supervisor reviews these comments as a component of performance review. The supervisor will compare each counselor’s performance to that of his or her peers, in terms of achieving dietary change toward study goals and in keeping the database complete.
Primary outcome
The primary outcome of MEAL is clinical progression, a composite outcome defined as PSA > 10 ng/nL, or PSA doubling time (PSADT) < 3 years, or any of the following findings on repeat prostate biopsy: > 25% of biopsy tissue cores positive for cancer, > 50% of any biopsy tissue core positive for cancer, Gleason sum ≥ 7 for men ≤ 70 years, or Gleason sum ≥ (4 + 3) = 7 for men > 70 years. We based these definitions of progression on prior studies of patients with localized prostate cancer on active surveillance. 6-10
Secondary outcomes
Secondary outcomes include incidence of surgical and non-surgical treatment in patients whose disease does not meet the definition of clinical progression, anxiety as measured by the Memorial Anxiety Scale for Prostate Cancer (Max-PC), urinary symptoms as measured by the International Prostate Symptom Score (I-PSS), and quality of life (QoL) as measured by the Functional Assessment of Cancer Therapy Scale-Prostate (FACT-P) and Expanded Prostate Cancer Index Composite 26 (EPIC- 26).
Outcome evaluation
Each patient will be followed for 24 months, and PSA is evaluated every 3 months starting from baseline. Pursuant to established Alliance practice for prostate cancer clinical trials, PSA measures are performed in the clinical laboratories of each individual study site using standard platforms. Site personnel enter the PSA results into the Alliance Web-based patient registration system, which compiles the data. PSADT will be calculated as log2 divided by the slope (the least squares estimator) of log (PSA) observations over time using the latest 3 PSA measurements at months 0, 3 and 6. To ensure comparability, the PSADT will be calculated at the Alliance Statistics and Data Center.
Consistent with current standards of care for the treatment of patients with localized prostate cancer on active surveillance, all participants who do not undergo definitive treatment (i.e., surgery or radiation) during the study period will receive an end-of-study biopsy 24 months after baseline. In addition, at the treating physician’s discretion, additional for-cause biopsies may be performed prior to 24 months as clinically indicated. Centralized pathology review is conducted on the prostate biopsy tissue cores by a single pathologist (PH) to evaluate progression. A separate team of telephone assessors evaluate the diets of all study participants at baseline, 12 months, and 24 months by a series of 3 separate 24-hour dietary recalls collected via telephone interview, which uses the Nutrition Data Systems for Research (NDS-R, current version 2010, University of Minnesota Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) software and nutrient database. Fasting blood samples are collected at baseline, 12 months, and 24 months and analyzed for plasma carotenoid concentrations—a biomarker of vegetable and fruit intake and thus an effective method for measuring compliance—using high-performance liquid chromatography methodology.25 We are measuring the following carotenoids as biomarkers: total carotenoids, α-carotene, β-carotene, lutein, lycopene, and cryptoxanthin.
Statistics
We are randomizing patients 1:1 to receive the dietary intervention (experimental arm) or dietary information (control arm). The log-rank test with a two-sided α = 5% and a sample size of 418 has 80% power to detect a difference in progression rate of 20% in the control versus 10% in the experimental arm during the 24-month follow-up period. The expected progression rate is based on prior studies of patients with localized prostate cancer on active surveillance. In these studies, progression rates ranged from 20% to 35%, depending on the population under study.6-10 We chose 20% for the sample size calculation because it was the most conservative definition of the expected event rate.
Under the exponential distribution assumption for the time to progression, the 2- year progression rate of 20% versus 10% corresponds to a hazard ratio (HR) of 2.1. Assuming a 10% dropout rate (including patients who are treated before progression), we are enrolling a total of 464 patients. We based the expected 10% dropout rate on our pilot study, in which the observed dropout rate was 2%; for this trial, we conservatively assigned an expected 5-fold increase in the dropout rate compared to the pilot. 21 We are stratifying randomization by age (< 70 years versus ≥ 70 years), race (African American versus Other) and—to account for any additional prostate biopsies performed after the diagnostic baseline prostate biopsy but prior to study entry—time since baseline prostate biopsy (≤12 months prior to registration versus >12 months prior to registration).
We are comparing time to clinical progression between the two arms using the log-rank test for univariate analysis and Cox’s proportional hazards regression for multivariate analysis adjusting for stratification and other prognostic factors. We are censoring the patients who proceed to treatment with surgery, radiation, local ablative therapy or androgen deprivation therapy before progression within the 2-year follow-up period at the time of treatment.
We are also comparing the probability to proceed to treatment within 2 years using the chi-squared test. We are estimating the time trajectory of QoL using the generalized estimating equation method based on working independent correlation structure and comparing the slopes for the two arms of the time trajectory,28 adjusting for multiple testing among different QoL subscales using the methodology of Bang, Jung and George.29
We will compare the changes from baseline in mean daily intakes of total vegetables, crucifers, tomato products, beans/legumes and fat between the two study arms with two-sample t-tests at 12 and 24 months. We will compare the changes in plasma carotenoid concentrations from baseline between the two arms using a twosample t-test. We will correlate carotenoid concentrations with PSADT, using descriptive analysis with scatter plots and regression analysis, and adjusting intervention and known predictors including the stratification factors in multivariate analysis.
We will consider logarithmic transformations of the data to improve normality of the distributions and variance stabilization as necessary.
Discussion
The MEAL Study is the first large-scale clinical trial of a dietary intervention for prostate cancer. Assessing the therapeutic efficacy of diet requires the accumulation of data from rationally designed trials focused on feasible interventions that do not place undue burdens on patients. The MEAL Study promotes robust diet changes through a telephone counseling system that has previously been shown to effectively promote dietary change in breast cancer survivors.30 The use of a centralized service to conduct the intervention by telephone has a number of strengths that include intervention fidelity and significant economies of scale. The intervention could likely be applied to relatively large patient populations cheaply and efficiently.
Our innovative telephone-based intervention focuses on beneficial dietary components that have been associated with decreased prostate cancer incidence and progression in observational studies. The intervention is inexpensive enough to be considered a population intervention. The counseling protocol is stepped and utilizes principles of social cognitive theory31,32 and motivational interviewing.27 We cannot definitively predict whether the changes in dietary intake and plasma carotenoid concentrations over a 2-year period will be maintained over a longer period. However, in the Women’s Healthy Eating and Living (WHEL) study for breast cancer, which utilized a similar dietary intervention over a similar period of time, participants maintained diet changes for at least 4 years.33
Reducing the number of active surveillance patients who receive treatment represents an important opportunity to minimize treatment-associated morbidity, improve quality of life, and contain health care costs. Diet change represents an innovative approach to treating prostate cancer with the potential to promulgate a novel therapeutic paradigm: dietary management, without curative intent, of early stage prostate cancer.
Moreover, because prostate cancer diagnosis is a source of considerable anxiety and diminished quality of life,34 dietary intervention also may encourage patients without clinical progression to remain on active surveillance rather than choosing treatment. Many patients with no objective PSA or pathologic criteria for progression will nonetheless opt for treatment, presumably due to anxiety associated with their diagnosis.35 For these patients, diet change potentially provides an intervention or therapy on which to focus, possibly dissuading otherwise lower risk men from pursuing unnecessarily aggressive, morbidity-generating treatments.
Conclusion
In summary, the MEAL Study uses a centralized, telephone-based counseling intervention to assess the effectiveness of a high-vegetable diet for preventing clinical progression, improving health-related QoL, and decreasing anxiety in men with localized prostate cancer on active surveillance. This study holds the potential to substantively inform the treatment of early stage prostate cancer.
Acknowledgments
The authors acknowledge the contributions of the MEAL UC San Diego Counseling Team.
Funding: National Cancer Institute 1R01 CA132951-01A1, Department of Defense PC073412, and The Prostate Cancer Foundation. The study is also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 4.Kopp RP, Marshall LM, Wang PY, Bauer DC, Barrett-Connor E, Parsons JK. The Burden of Urinary Incontinence and Urinary Bother Among Elderly Prostate Cancer Survivors. Eur Urol. 2013;64:672–679. doi: 10.1016/j.eururo.2013.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 6.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of longterm follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 8.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 9.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 10.Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis. 2005;8(4):304–310. doi: 10.1038/sj.pcan.4500825. [DOI] [PubMed] [Google Scholar]
- 11.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23(32):8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 12.Nelson WG, Demarzo AM, Yegnasubramanian S. The diet as a cause of human prostate cancer. Cancer Treat Res. 2014;159:51–68. doi: 10.1007/978-3-642-38007-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jian L, Du CJ, Lee AH, Binns CW. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. 2005;113(6):1010–1014. doi: 10.1002/ijc.20667. [DOI] [PubMed] [Google Scholar]
- 14.Barber NJ, Zhang X, Zhu G, et al. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006;9(4):407–413. doi: 10.1038/sj.pcan.4500895. [DOI] [PubMed] [Google Scholar]
- 15.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10(9):949–954. [PubMed] [Google Scholar]
- 16.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspasemediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25(1):83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 17.Spentzos D, Mantzoros C, Regan MM, et al. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res. 2003;9(9):3282–3287. [PubMed] [Google Scholar]
- 18.Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–1069. doi: 10.1097/01.ju.0000169487.49018.73. discussion 1069-1070. [DOI] [PubMed] [Google Scholar]
- 19.Saxe GA, Major JM, Nguyen JY, Freeman KM, Downs TM, Salem CE. Potential attenuation of disease progression in recurrent prostate cancer with plant-based diet and stress reduction. Integr Cancer Ther. 2006;5(3):206–213. doi: 10.1177/1534735406292042. [DOI] [PubMed] [Google Scholar]
- 20.Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319–1323. doi: 10.1016/j.urology.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. PNAS, USA. 2008;105(24):8369–8374. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. The Lancet Oncology. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 23.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The Lancet Oncology. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JK, Newman V, Mohler JL, Pierce JP, Paskett E, Marshall J. The Men’s Eating and Living (MEAL) study: a Cancer and Leukemia Group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology. 2008;72(3):633–637. doi: 10.1016/j.urology.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101:1227–1231. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 26.Pierce JP, Newman VA, Flatt SW, et al. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr. 2004;134(2):452–458. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- 27.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York: Guilford Press; 2002. [Google Scholar]
- 28.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 29.Bang H, Jung SH, George SL. Sample size calculation for simulation-based multiple-testing procedures. J Biopharm Statistics. 2005;15(6):957–967. doi: 10.1080/10543400500265710. [DOI] [PubMed] [Google Scholar]
- 30.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahneman D, Tversky A, editors. Choices, Values, and Frames. New York: Cambridge University Press and Russell Sage Foundation; 2000. [Google Scholar]
- 32.Bandura A. Self-Efficacy: The Exercise of Self-Control. New York, New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 33.Pierce JP, Newman VA, Natarajan L, et al. Telephone counseling helps maintain long-term aderence to a high-vegetable dietary pattern. J Nutr. 2007;137:2291–2296. doi: 10.1093/jn/137.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korfage IJ, Essink-Bot ML, Janssens AC, Schroder FH, de Koning HJ. Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow-up. Br J Cancer. 2006;94(8):1093–1098. doi: 10.1038/sj.bjc.6603057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23(32):8165–8169. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]


