Abstract
We report the design of polyelectrolyte multilayers (PEMs) that can be prefabricated on an elastomeric stamp and mechanically transferred onto biomedically-relevant soft materials, including medical-grade silicone elastomers (E′~450–1500 kPa; E′-elastic modulus) and the dermis of cadaver-skin (E′~200–600 kPa). Whereas initial attempts to stamp PEMs formed from poly(allylamine hydrochloride) and poly(acrylic acid) resulted in minimal transfer onto soft materials, we report that integration of micrometer-sized beads into the PEMs (thicknesses of 6–160 nm) led to their quantitative transfer within 30 seconds of contact at a pressure of ~196 kPa. To demonstrate the utility of this approach, PEMs were impregnated with a range of loadings of silver-nanoparticles and stamped onto the dermis of human cadaver-skin (a wound-simulant) that was subsequently incubated with bacterial cultures. Skin-dermis stamped with PEMs that released 0.25±0.01 μg cm−2 of silver ions caused a 6 log10 reduction in colony forming units of Staphylococcus epidermidis and Pseudomonas aeruginosa within 12 h. Significantly, this level of silver release is below that which is cytotoxic to NIH 3T3 mouse fibroblast cells. Overall, this study describes a general and facile approach for the functionalization of biomaterial surfaces without subjecting them to potentially deleterious processing conditions.
Keywords: Polymer-multilayers, silver-nanoparticles, microparticles, stamping, wounds
1. Introduction
Silver-based compounds and formulations have been widely used to functionalize a range of materials and surfaces.[1–8] Silver exerts antimicrobial activity against a broad spectrum of bacterial, yeast, fungal, and viral species, including several antibiotic resistant bacterial strains.[9] Silver works by damaging bacterial cell membranes and interfering with basic metabolic functions of microbes.[10] Unlike antibiotics, silver does not provoke microbial resistance and is therefore used broadly in wound management.[10, 11] More recently, silver salts, nanocrystalline silver, and silver-compounds have been incorporated into wound care materials and devices to provide long-term antibacterial activity in the wounds without the need for frequent dressing changes.[12–14] However, existing silver ointments and dressings contain high loadings of silver and typically lead to the delivery of a large excess of silver (~100 μg cm−2)[13] to wounds and surrounding tissue, causing tissue toxicity and impaired wound healing.[15–18] Management of the microbial burden of wounds using materials and treatments that contain loadings of silver that do not cause significant tissue-toxicity would represent an important advance in the design of functional materials for wound care.
To address the challenge of delivering loadings of silver that are non-toxic but antibacterial to wound-beds, we recently reported that nanoscopic localization of carefully controlled loadings of silver-nanoparticles within molecularly-thin multilayer films of polymers on a surface can lead to antibacterial activity without any measurable cytotoxicity to mammalian cells.[19] This approach built on prior reports[20, 21] that polyelectrolyte multilayer (PEM) films of oppositely charged polyelectrolytes poly(allylamine hydrochloride) (PAH) and poly(acrylic acid) (PAA) can be prepared by ‘layer-by-layer’ assembly on glass substrates and impregnated with tailored loadings of silver nanoparticles. In our prior study,[19] we demonstrated that the loadings of silver nanoparticles in nanometer-thick PEMs supported on glass coverslips could be systematically tailored to levels as low as 0.4 μg cm−2 and still achieve a 6 log10 decrease in the colony forming units (CFU) of Staphylococcus epidermidis with no measurable cytotoxicity to mouse fibroblast (NIH 3T3) cells that were cultured on the PEMs. Achieving antibacterial activity without mammalian cell cytotoxicity via nanoscopic localization of precise loadings of silver nanoparticles on a surface contrasts with past approaches to management of the microbial burden of wounds in which wound dressing materials (including textiles and fabrics) have been used as macroscopic reservoirs of silver (~100 μg cm−2).[13] In this latter case, silver ions are delivered to the wound bed by diffusion across a fluid film in the wound. The highly reactive nature of silver ions makes them susceptible to inactivation by proteins and chloride ions within the wound fluid environment, thus contributing to the need for high loadings of silver in macroscopic dressings. As noted above, accumulation of excess silver (from silver dressings) in wounds impairs wound healing.[15–17] In this paper, we report the fabrication of PEMs containing precisely defined and minimal loadings of silver nanoparticles that can be deposited onto soft biomedically-relevant material interfaces, including biological tissues, to achieve antibacterial activity.
Fabrication of PEMs and their impregnation with silver nanoparticles is a laborious process that involves numerous (hundreds) adsorption and rinsing steps and the use of non-physiological solutions such as reducing agents.[19] For this reason, it is generally not practical to fabricate PEMs directly on biological tissues, including wound-beds. To address this limitation, herein we report methods that allow the pre-fabrication of PEMs loaded with silver-nanoparticles on elastomeric stamps and the mechanical transfer of the silver-loaded PEMs onto the surfaces of biological tissues. This approach avoids exposure of the biological materials to extensive chemical processing, and likely can be extended to the integration of a range of bioactive molecules (hosted within PEMs) onto the surfaces of biological tissues so as to confer useful functionality. In this report, we test the hypothesis that integration of PEMs preloaded with silver-nanoparticles onto the surfaces of model wounds can lead to antimicrobial activity using significantly less silver than found in conventional textile/fabric-based silver dressings.
The approach to polymer multilayer transfer reported here was inspired by past demonstrations that PEMs fabricated on elastomeric stamps can be contact printed onto glass or metal surfaces for the fabrication of electro/optical devices.[22] In our initial studies, we attempted to use contact printing as described by Park et al[22] to mechanically transfer pre-fabricated PEMs of PAA and PAH onto the dermis of human-cadaver skin-graft. The dermis of cadaver-skin was used as a model of a partial-thickness skin wound that is susceptible to microbial colonization. We found that the procedures reported by Park et al did not allow the transfer of PEMs onto soft biological tissue (elastic modulus E′~200–600 kPa [23]) or other soft-substrates such as medical grade soft silicone sheets (E′~450–1500 kPa). We established that the mechanical properties (softness) of these materials prevented the successful contact printing of the PEMs, and in the course of that investigation, we made the unexpected observation that incorporation of polymeric microspheres (1–2 μm in diameter) into the PEMs greatly facilitated the mechanical transfer of the PEMs onto a range of soft materials (E′~450–1500 kPa; as illustrated in Fig. 1). This initial observation led us to subsequently demonstrate the utility of a microspheres-based approach for contact transfer of PEMs onto soft materials through measurement of antibacterial activity conferred on the dermis of cadaver-skin functionalized with PEMs impregnated with silver-nanoparticles. Specifically, we measured an up to 6 log10 decrease within 12 h in the CFU of gram-positive bacteria Staphylococcus epidermidis and gram-negative bacteria Pseudomonas aeruginosa on stamped skin-grafts. Moreover, the concentration of silver-ions extracted from the functionalized skin samples (~0.25 μg cm−2) was below the level we had previously reported to be cytotoxic (~0.4 μg cm−2) to NIH 3T3 mouse fibroblast cells.[19]
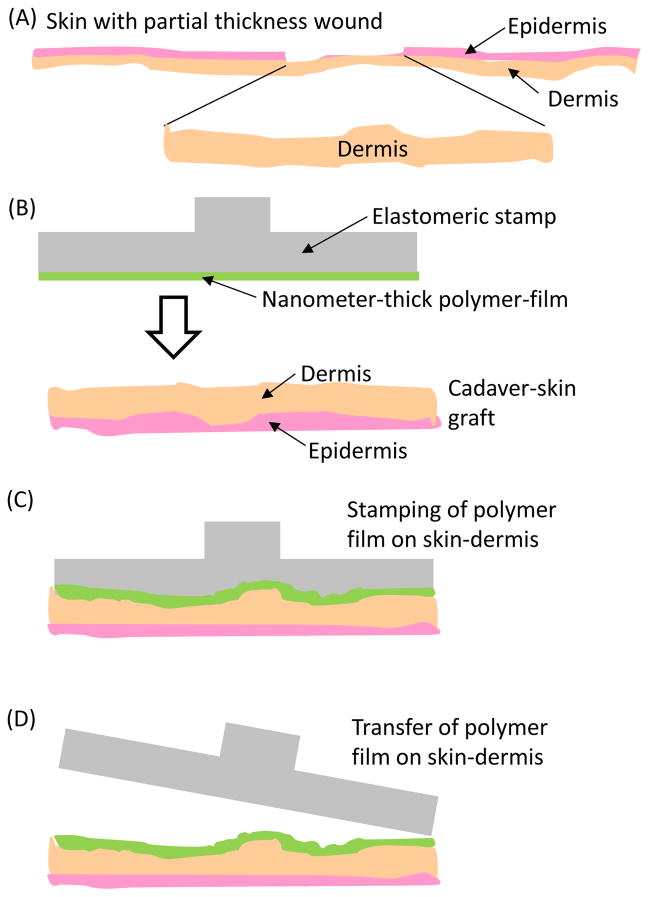
Figure 1.
(A) Schematic illustration of a partial thickness skin wound that exposes the dermis. (B–D) Schematic illustration of the mechanical transfer of nanometer-thick polymer-films, prefabricated on PDMS stamps, onto the dermis of human-cadaver skin-graft. As described in the text, integration of microspheres into the PEMs makes possible the transfer of the PEMs from the PDMS stamps onto soft materials such as skin-dermis.
Overall, this report describes a general and facile methodology that permits functionalization of the surfaces of biological tissues found in wound-beds and soft synthetic materials used in medical devices. In particular, the ability to directly stamp pre-fabricated PEMs onto biological tissue circumvents exposure of the tissues to processing conditions that may cause tissue damage. It also provides the opportunity to achieve precise control over the loading and spatial placement of bioactive factors on these materials.
2. Results
2.1. Stamping of PEMs onto skin dermis
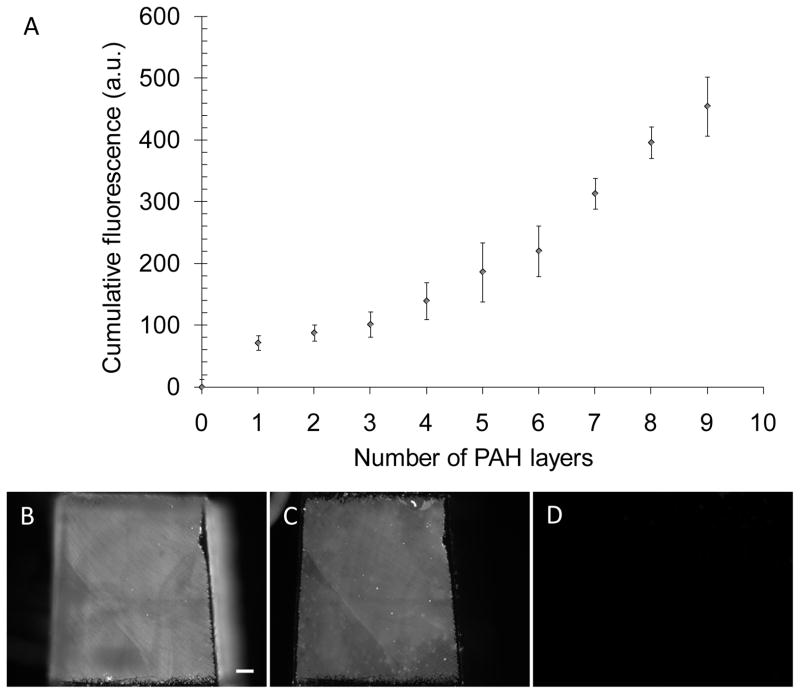
PEMs of PAA and PAH (prepared using solutions at pH 7.5) were assembled on poly(dimethylsiloxane) (PDMS) stamps by layer-by-layer deposition, and impregnated with tailored loadings of silver nanoparticles, as described in the Methods section. Assembly of (PAH/PAA)10 multilayers on PDMS stamps was confirmed by fluorescence measurements using fluorescein-5-isothiocyanate (FITC) (Ext/Em-488/510 nm)-labeled PAH. The total fluorescence intensity from the PEMs on the PDMS surface increased with the deposition of each additional fluorescently labeled PAH layer, confirming the cumulative growth of PEMs (Fig. 2A). The ellipsometric thickness of a (PAH/PAA)10 multilayer deposited on a plasma-cleaned silicon wafer (using PlasmaTherm 1441 RIE Instrument; 8 sccm O2, 20 s, 100 W) was measured to be 30±2 Å, consistent with past reports.[24]
Figure 2.
Characterization of the fabrication of PEMs on PDMS stamps, and demonstration of the absence of contact transfer of the PEMs from the PDMS stamps onto skin-dermis. (A) Growth in the cumulative fluorescence of a (PAH/PAA)10 multilayers, containing FITC-labeled PAH, during fabrication on a PDMS substrate. (B, C, D) Micrographs of the fluorescent PEMs comprised of (PAH/PAA)10 on PDMS before the stamping, PDMS after stamping skin-dermis, and skin-dermis after the stamping, respectively. Scale bar=200 μm.
Mechanical transfer of (PAH/PAA)10 multilayers (assembled on PDMS stamps) was investigated on gamma-irradiated human-cadaver skin-allograft comprised of intact dermal and epidermal layers (see schematic in Fig. 1). The dermis of the skin was used in our study as a wound simulant where the epidermis of the skin is lost and underlying dermis is exposed to microbial colonization. As described in the Introduction, our initial attempts to achieve mechanical transfer of PEMs onto the dermis of the cadaver skin were based on previous reports describing transfer of PEMs from PDMS stamps onto glass-substrates for the fabrication of electro-optical devices.[22] In those prior reports, the PEMs were formed on PDMS stamps by first priming the hydrophobic PDMS surface with PAH and then assembling PEMs of (SPS/PDAC)7 (SPS is sulfonated polystyrene; PDAC is polydiallyldimethylammonium chloride) over the PAH layer. The PEMs on the PDMS and glass surfaces were exposed to a stream of water vapor and immediately brought in contact with each other. The PDMS stamps were removed from the glass surfaces after 30 min. The authors interpreted their results to suggest that hydrophobic interactions between the PDMS stamp and the base layer of the PEMs were weak compared to the strong electrostatic forces acting between the charged glass surface and the PEMs, thus leading to detachment of the PEMs from the stamp following contact with glass.
To determine if this previously-described method would also lead to the transfer of PEMs from PDMS stamps to skin-dermis, PAH(PAA/PAH)10 multilayers were assembled as described above on PDMS stamps using Alexa Fluor 633 carboxylic acid succinimidyl ester (Ext/Em-633/650 nm)-labeled PAH. PAH labeled with the far-red dye Alexa Fluor 633 was used (instead of FITC) to reduce the intensity of auto-fluorescence from the cadaver-skin during imaging. PEMs supported on PDMS stamps and the dermis of the cadaver-skin were exposed to water vapor and brought in contact with each other. The stamps were left in contact with the skin-graft for 30 min (with no external pressure applied to the stamps) and then removed by peeling one edge of the stamp from the skin. PEMs that were fabricated with a terminal layer of either PAH or PAA were tested for transfer to the skin-dermis. The area of each stamp was ~2.56 mm2 (i.e. ~1.6 mm × 1.6 mm).
Fluorescent micrographs of PEMs on PDMS stamps before and after stamping onto the skin-dermis indicated that less than 1% of the fluorescent area on the surface of the stamp was lost following contact with the skin-demis (Fig. 2B, C, D). There was also no significant increase in fluorescence from the skin-dermis after stamping. The experiment was repeated on several days, each time with more than ten samples. We note that quantification of the intensity of fluorescence of the PEMs on the PDMS stamps before and after stamping was determined to be an unreliable method to characterize the transfer of the PEMs because some level of bleaching of the fluorophores was observed during stamping and imaging. Instead, as described above, we quantified the transfer of the PEMs by calculating the area of the surface of the PDMS stamp that exhibited loss of fluorescence due to detachment of the PEMs.
Extensive experimentation revealed that PAH(PAA/PAH)10 multilayers ending with either PAH or PAA could not be transferred from PDMS stamps onto the skin-dermis using procedures described in previous reports.[22] We note that skin-dermis possesses surface chemical functionality, mechanical properties, and topography that substantially differs from inorganic substrates (e.g., glass) onto which PEMs have been transferred previously by stamping.[22, 25] Specifically, the skin dermis is comprised of an extra-cellular matrix of proteins and lipids with a bulk elastic modulus (E′) in the range of 200–600 kPa [23, 26] while the elastic modulus of glass is ~65 GPa. The E′ of the PDMS stamps used in our study was ~2.5 MPa. To provide insight into factors underlying the absence of PEMs transfer onto the skin-dermis, we investigated the transfer of (PAA/PAH)10 multilayers onto a range of synthetic substrates with varying surface chemistries and elastic moduli.
2.2. Stamping of PEMs onto rigid glass substrates and soft silicone sheets
To determine if the surface chemistry of a substrate influences the efficiency of transfer of PEMs from PDMS stamps onto the substrate, glass slides with hydrophilic (high surface energy) or hydrophobic (low surface energy) surfaces were tested. Hydrophilic glass surfaces were prepared by piranha cleaning, and hydrophobic glass surfaces were prepared by coating piranha-cleaned glass slides with octadecyltrichlorosilane (OTS), as described in the Methods section.
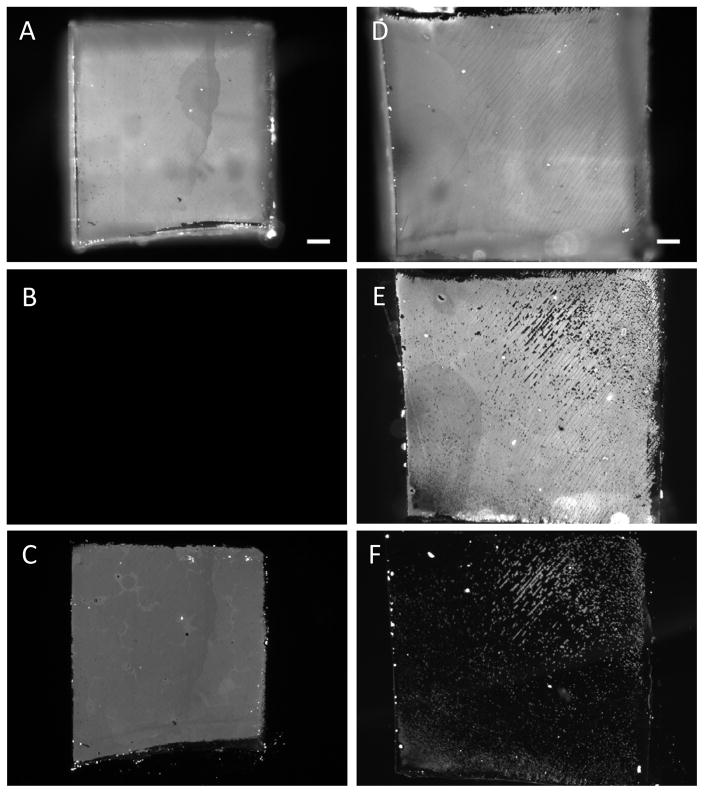
PAH(PAA/PAH)10 multilayers assembled on PDMS stamps using FITC-PAH were stamped onto glass slides following the procedures outlined above. Fluorescent micrographs of a stamp before and after stamping onto a glass-slide with high surface energy are shown in Fig. 3A, B. These micrographs show that following stamping there was complete loss of fluorescence from almost the entire area of the stamp that contacted the glass. A fluorescent image of the glass slide after stamping (Fig. 3C) confirms the transfer of FITC-PAH fluorescence from the stamps onto the glass surface. These experiments were repeated on several days and at least six samples were tested each time. Analysis of the images using ImageJ software indicates that the fraction of fluorescent area transferred from PDMS stamps to glass slides was ~97±2 % (averaged over 10 samples). Similar efficiencies of PAH(PAA/PAH)10 multilayer transfer were obtained on low-energy glass surfaces (OTS-coated glass) (supplementary Fig. 1). Also, no transfer of fluorescent multilayers was observed when stamps were brought in contact with glass slides without pre-exposure to water vapors, as was also shown in previous reports.[22] Because the PEMs were observed to transfer onto the glass surfaces in a manner that was independent of the surface energy, these results suggested that factors related to surface energetics were unlikely to be the reason for the absence of transfer of PAH(PAA/PAH)10 multilayers from the PDMS stamps to the skin-dermis (as reported above).
Figure 3.
Influence of mechanical properties of substrates on the transfer of PEMs upon contact with PDMS stamps on which PEMs were fabricated. Fluorescent micrographs that characterized the mechanical transfer of PEMs comprised of PAH(PAA/PAH)10 onto either glass slides or soft silicone sheets (E′~1500 kPa). Micrographs of fluorescent PEMs on PDMS (A) before stamping glass slide, (B) PDMS after stamping, and (C) glass slide after stamping. Micrographs of fluorescent PEMs on (D) PDMS before stamping the silicone sheet, (E) PDMS after stamping, and (F) silicone sheet after stamping. Scale bar=200 μm.
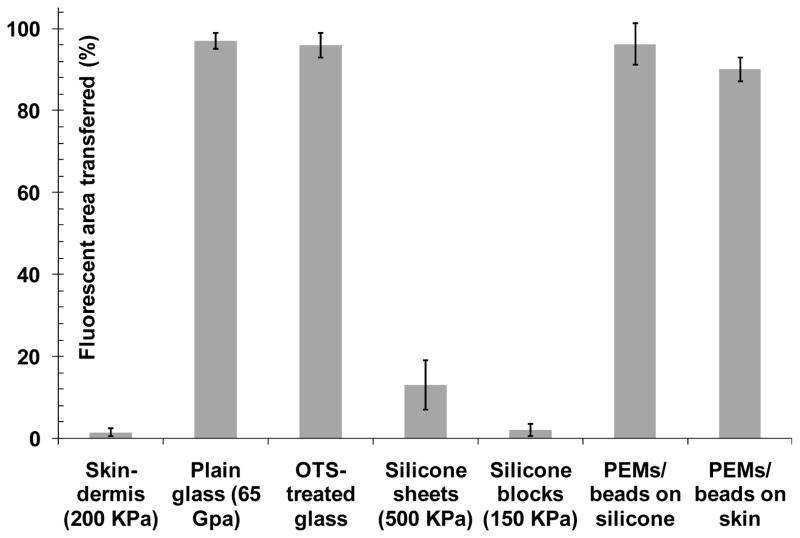
Next, to determine if the mechanical properties of the substrates influenced PEMs transfer efficiencies, we investigated the transfer of PEMs from PDMS stamps onto silicone sheets with elastic moduli comparable to skin-tissues. Medical-grade silicone-sheets and blocks with hardness of 10, 30 or 40 Shore A Durometer (E′~450 kPa, 1050 kPa and 1500 kPa, respectively) were used. For comparison, the hardness of skin has been reported[26] to be ~25 Shore O Durometer (E′~600 kPa). PAH(PAA/PAH)10 multilayers, assembled on PDMS stamps using FITC-PAH, were stamped onto the silicone sheets following the same procedures outlined above. After stamping, fluorescence from the stamps and silicone sheets was imaged (Fig. 3D, E, F). Analysis of the images using ImageJ software showed that the fraction of fluorescent area transferred from PDMS to the silicone sheets (E′~1500 kPa) was 13±6% (averaged over 10 samples): Even lower levels of transfer were observed when using silicone blocks with E′~450 kPa (only 2±1.5%). The percentage of fluorescent area transferred from the PDMS stamps onto the different substrates is summarized in Fig. 4. Plasma oxidization of silicone sheets prior to stamping (PlasmaTherm 1441 RIE; 8 sccm O2, 20 s, 100 W) did not lead to an improvement in the transfer of fluorescent PEMs onto the silicone sheets. Application of external pressure of up to 2 kgf cm−2 (~196 kPa) during stamping also did not affect the transfer of PEMs. These results suggest that the mechanical properties of a substrate play a dominant role in determining the transfer of (PAH/PAA)10 multilayers from the PDMS stamps, and that the absence of transfer of PEMs onto the skin-dermis, as reported above, likely results from the soft nature of the skin-dermis.
Figure 4.
Incorporation of beads dramatically improves transfer of PEMs onto compliant (soft) substrates. Average (n=6) extent of transfer of PEMs (quantified as fluorescent area) from PDMS stamps onto various substrates by contact printing, as described in the text. Fluorescence was from FITC-PAH incorporated into PEMs formed on the PDMS stamps, except in case of PEMs with beads, where fluorescence from the fluorescently labeled beads was measured.
2.3. Microspheres facilitate the stamping of PEMs onto silicone sheets and skin-dermis
In the course of performing experiments that were aimed at quantifying the transfer of PEMs onto the dermis of cadaver-skin, we incorporated crimson-fluorescent polystyrene (PS) microspheres within the PEMs as a fluorescent label that could be readily imaged against the tissue auto-fluorescence. These experiments led to the unexpected observation of the transfer of the microspheres embedded within the PEMs onto the skin-dermis. Subsequently, we performed an investigation to determine if the PEMs were being transferred along with the microspheres and, more generally, if the incorporation of microspheres within PEMs may provide the basis of a general methodology that facilitates the transfer of PEMs onto skin-dermis and soft-substrates with E′ in the range of 200–1500 kPa.
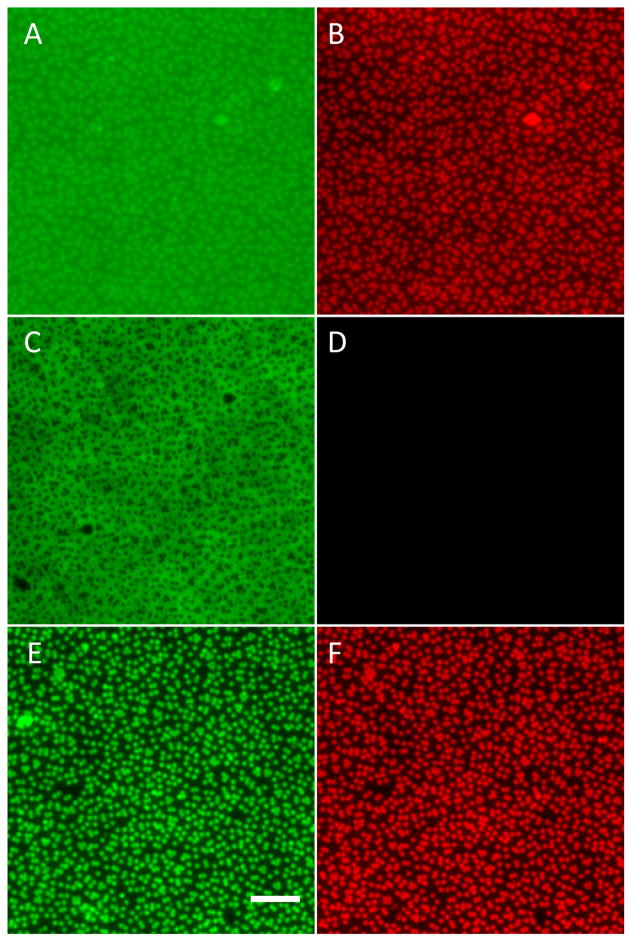
In subsequent experiments, negatively charged, crimson fluorescent (Ext/Em-625/645 nm) carboxylate-modified polystyrene microspheres, 1 μm in diameter, were incorporated into the PEMs as follows (elastic modulus of PS is E′~3 GPa). PAH(PAA/PAH)5 multilayers terminating in a positively charged PAH layer were deposited on PDMS stamps, and then a monolayer of negatively charged PS microspheres was adsorbed onto the PEMs. Subsequently, another set of PAH(PAA/PAH)5 multilayers was deposited over the microspheres. In the text below, we denote these PEMs as PAH(PAA/PAH)5(PS-microspheres)(PAH/PAA)5PAH. The incorporation of microspheres into the PEMs was quantified by fluorescent imaging (supplementary Fig 2). PDMS stamps supporting PEMs containing microspheres were brought into contact with skin-dermis after exposing each surface to water vapor. An external pressure of ~196 kPa was applied to the stamps in contact with the skin-graft for 30 s and the stamps were then left in contact for 30 min (without any external pressure) until they were peeled from the skin-dermis (following the procedures described above). Imaging of the fluorescently labeled microspheres on the PDMS stamps and skin-dermis after stamping confirmed a high level of transfer of fluorescent-microspheres onto the skin-dermis (Fig. 5). Quantitatively, the fraction of the fluorescent area transferred from the PDMS stamps onto the skin-dermis was 90±3% (averaged over 10 samples). Experiments were repeated on several days and at least ten samples were tested each time.
Figure 5.

Fluorescent micrographs of PEMs comprised of (PAH/PAA)5(PS-microspheres)(PAH/PAA)5 transferred by contract printing onto the dermis of cadaver-skin grafts. (A) PDMS before stamping; (B) PDMS after stamping; (C) skin-dermis after stamping. The fluorescent signal was generated by 1 μm diameter crimson-fluorescent PS microspheres incorporated into PEMs. Scale bar=400 μm.
Additional experiments revealed that high levels of transfer of the fluorescent-microspheres embedded in PEMs were achieved onto the skin-dermis without pre-exposing each surface to a stream of water vapor. Furthermore, removal of the stamps immediately following the application of an external pressure of ~196 kPa for 30 s also resulted in efficient transfer of the microspheres. A similar efficiency of transfer was observed for fluorescently labeled microspheres stamped onto soft-silicone sheets (E′ ~450 kPa, 1050 kPa and 1500 kPa) (supplementary Fig. 3). In aggregate, these results indicate that microspheres incorporated in (PAH/PAA)n multilayers can be mechanically transferred onto skin-dermis and soft-substrates with E′ as low as 200 kPa. The only parameter that was measured to influence significantly the transfer was the magnitude of the external pressure applied onto the stamps, which was required to be greater than 196 kPa to achieve a high level of transfer. Supplementary Fig. 4 shows representative fluorescent images illustrating the partial transfer of PEMs containing microspheres onto skin-dermis when an external pressure of ~50 kPa was applied to the PDMS during the process of stamping.
Next, to determine if the PEMs were also transferred along with the microspheres onto the skin-dermis and the silicone sheets during stamping, we fabricated PEMs using FITC-labeled PAH and crimson-fluorescent microspheres. In one set of experiments, FITC-labeled PAH was incorporated into the PEMs formed beneath, but not above, the crimson-fluorescent microspheres, resulting in PEMs with the structure (PAA/FITC-PAH)5(PS-microspheres)(PAH/PAA)5. Micrographs of stamps obtained with 40× magnification (Fig. 6) show that after mechanical contact with silicone sheets with an external applied pressure of ~196 kPa for 30 s, both crimson-fluorescent PS microspheres (Fig. 6b, d) and FITC-PAH were transferred from the stamps to the silicone sheets (Fig. 6a, c). Furthermore, micrographs of the stamped silicone sheets confirmed that the fluorescent PEMs beneath the fluorescent microspheres were transferred onto the silicone sheets (Fig. 6e) and co-localized with fluorescent microspheres (Fig. 6f). Inspection of Fig. 6c also reveals that although the fluorescent PEMs under the microspheres were transferred onto the silicone sheets (generating dark patches on the PDMS stamp after transfer of the PEMs), PEMs between the microspheres were retained on the stamps. The average area projected by PS microspheres on the PDMS stamps was estimated from Fig. 6b, c and f using ImageJ software to be ~40%.
Figure 6.
Fluorescent micrographs showing that microspheres integrated into PEMs facilitate the transfer of the PEMs above and below the microspheres onto silicone sheets (E′~450 kPa). Micrographs show fluorescence from PAH (green) and PS microspheres (red) on- (A,B) PDMS stamp before printing; (C,D) PDMS stamp after stamping; and (E,F) silicone sheet after printing. Scale bar=10 μm.
In an additional set of experiments, we fabricated PEMs containing crimson-fluorescent microspheres using AF633-labeled PAH in the PEMs formed above the microspheres. Following contact of the PEMs with the skin-dermis, we again measured the loss of fluorescent PAH from the stamps (supplementary Fig. 5). Results verify the transfer of PEMs above the microspheres onto the skin-dermis. Taken together, these results demonstrate that incorporation of microspheres makes possible the transfer of PEMs (present above and below the microspheres) onto soft-substrates with E′ ranging from 450 to 1500 kPa.
We note that in the experiments described above, the diameters of the microspheres (1 μm) were large compared to the thickness of the PEMs (6 nm). Thus the microspheres protruded above the PEMs in direct contact with the PDMS stamp, making mechanical contact with the soft substrate. We propose that the soft substrate deforms around the microspheres and thus generates adhesive interactions that lead to transfer of the microspheres to the soft substrate. As described below, we found that larger microspheres are required to achieve the transfer of thicker PEMs, a result that provides further support for our conclusion that the topographical characteristics of PEMs containing the microspheres play a central role in the transfer of the PEMs onto soft substrates. Ongoing studies seek to provide a fuller understanding of the mechanical interactions involved in the transfer of PEMs containing microspheres onto soft substrates.
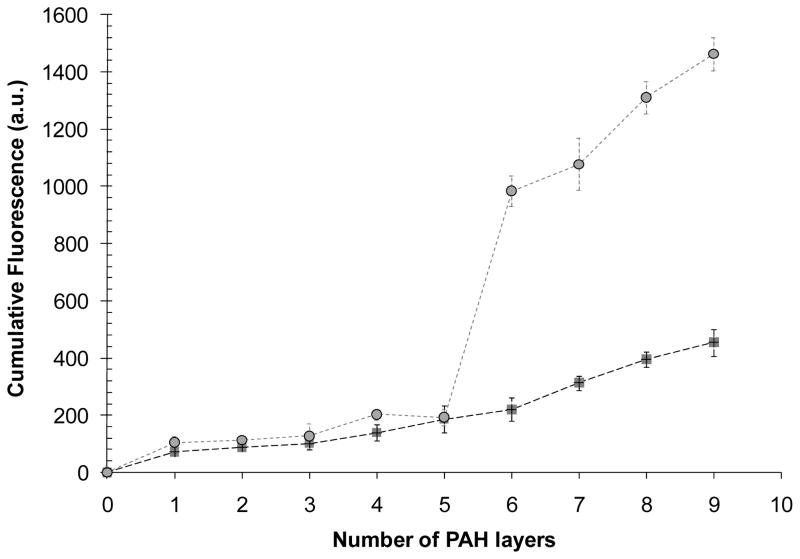
Because the incorporation of the microspheres into the PEMs changes the topography of the PEM-decorated surface, we suspected that incorporating microspheres into PEMs may also impact the amount of polymer deposited on each surface during the formation of the PEMs. To characterize the growth of PEMs that incorporated a monolayer of PS microspheres, PAH(PAA/PAH)5(PS-microspheres)(PAH)(PAA/PAH)5 multilayers were assembled on PDMS stamps using non-fluorescent microspheres and FITC-PAH. Total fluorescence from the multilayers was measured after deposition of each FITC-labeled PAH layer. As shown in Fig. 7, the total fluorescence of PEMs on PDMS stamps increased with the deposition of each layer of labeled PAH. However, after deposition of the microspheres onto the surface, there was a pronounced rise in the rate of increase of fluorescence upon deposition of each subsequent FITC-PAH layers. This result supports our conclusion that the deposition of the monolayer of microspheres results in an increase in the surface area onto which FITC-PAH and PAA can adsorb.
Figure 7.
Influence of microspheres integrated into PEMs on the growth of the PEMs during fabrication on PDMS stamps. Growth of (PAH/PAA)10 multilayer (squares) and PAH(PAA/PAH)4(PS microspheres)(PAH/PAA)5 multilayer (circles), containing FITC-labeled PAH, on PDMS substrates.
As noted above, analysis of Fig. 6b, c leads to the conclusion that ~40% of the projected area of the PDMS stamp was occupied by PS microspheres. Since the total surface area of a microsphere is 4× its cross sectional area, we calculated the increase in total surface area upon deposition of the microspheres to be = (40%) × (4) = 160%. Thus, we estimate that the total surface area available for polyelectrolytes deposition on a monolayer of microspheres is 260% of the surface area of the flat PDMS surface (i.e. 2.6 times). This ratio (2.6) is close to the ratio of the slopes of the fluorescence plots in Fig. 7 (2.46). This result (along with Fig. 5 and Fig. 6) suggests that the microspheres have PEMs adsorbed over their surfaces.
2.4. Integration and transfer of silver nanoparticles with PEMs containing microspheres
A central goal of this study was to develop methods to transfer PEMs impregnated with silver-nanoparticles onto soft biological tissue and demonstrate functionalization of the tissue surface with antibacterial activity. Towards that goal, (PAH/PAA)n(PS-microspheres)(PAH/PAA)n multilayers were assembled on PDMS stamps and impregnated with silver-nanoparticles as described in the Methods section. Briefly, PEMs were incubated in silver nitrate solutions to incorporate silver-ions via exchange with the acidic protons of the PAA; this was followed by reduction of silver-ions to silver-nanoparticles by incubating the PEMs in a solution of reducing agent sodium borohydride. Reduction of silver ions regenerates the carboxylic acid groups within the PEMs.[20] As described elsewhere,[20] cycles of silver ion exchange and reduction were repeated to achieve the desired loadings of silver in the PEMs. The loadings of silver in the PEMs were determined by extracting the silver from the PEMs into dilute nitric acid and performing elemental analysis using inductively-coupled plasma-emission spectroscopy, as described in the Methods section.
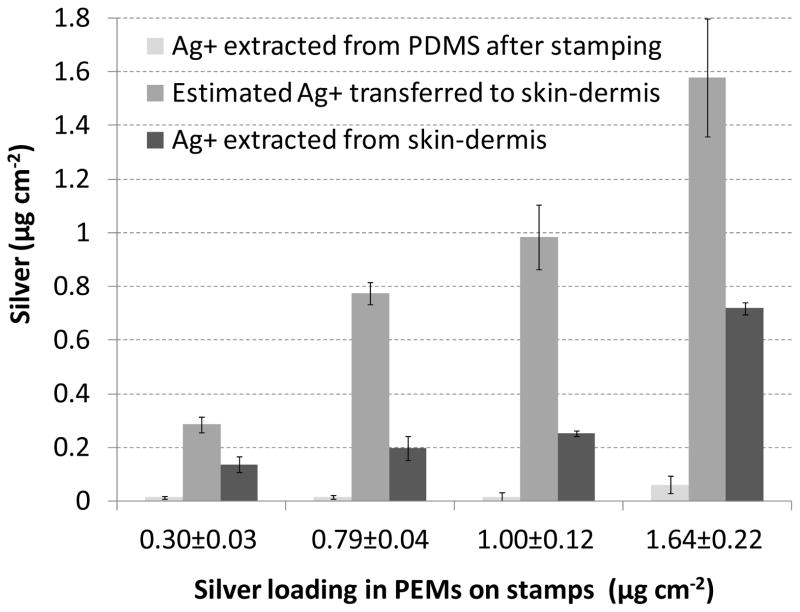
In our previous report,[19] we demonstrated that by tuning the pH of the PAA assembly solution between pH 2.5 to pH 7.5, the loading of silver in PEMs of (PAA/PAH)10 on glass substrates could be tailored between 0.4 to 22 μg cm−2. In initial experiments reported here, we fabricated (PAA/PAH)10(PS-microspheres)(PAA/PAH)10 on PDMS stamps with PAA solutions at pH 7.5 and subsequently impregnated the PEMs with silver nanoparticles using six cycles of silver ion exchange and reduction. The amount of silver ions extracted from the PEMs was determined to be 0.26±0.01 μg cm−2. The silver-loaded PEMs were stamped onto the skin-dermis and the amount of silver transferred was quantified by extracting the silver remaining on the PDMS stamps. This was determined to be 0.02±0.01 μg cm−2. Thus, the amount of silver transferred from the PDMS stamps onto the skin-dermis was inferred to be 0.24±0.01 μg cm−2. We note that the high level of transfer of the silver to the skin-dermis indicates that PEMs between the microspheres on the stamp are likely also transferred to the skin-dermis along with the PEMs on the microspheres. To investigate the dose-response to the silver, (PAA/PAH)10(PS-microspheres)(PAA/PAH)10 multilayers were assembled on PDMS using PAA in solution at pH 5.5 (instead of pH 7.5 used in all the PEMs mentioned above) to increase the amount of silver transferred onto skin-dermis.[19] As shown in Table 1, PEMs assembled with PAA at pH 5.5 could be loaded with 0.62±0.06 μg cm−2 after 4 cycles of silver-ion exchange and reduction. However, we found that PEMs prepared under these conditions did not transfer onto the silicone sheets or skin-dermis when 1 μm diameter microspheres were incorporated into the PEMs. Ellipsometric measurements revealed the thickness of a (PAH/PAA)10 multilayer assembled using a PAA solution of pH 5.5 (stamped on plasma-oxidized silicon-wafers using methods described above for PEMs transfer onto glass-slides) to be ~160 nm. As mentioned above, we postulated that the thickness of the PEMs prepared at pH 5.5 partially masked the presence of 1 μm microspheres incorporated into the PEMs, thus preventing the transfer of these PEMs to the skin dermis. To test this proposition, 2 μm diameter carboxylate-functionalized PS microspheres were incorporated in (PAA/PAH)10(PS-microspheres)(PAA/PAH)10 multilayers assembled using PAA solution at pH 5.5. Stamping of these PEMs resulted in excellent transfer of microspheres onto both the silicone sheets and skin-dermis, as determined by imaging of fluorescent microspheres (supplementary Fig. 6). The amount of silver impregnated in these PEMs was 1.64±0.22 μg cm−2 after four cycles of silver ion exchange and reduction (see Table 1). We note that the 2 μm diameter beads lead to higher loadings of silver in the PEMs than the 1 μm diameter beads, suggesting that the bead-size may also influence the structure and composition of the PEM/microparticle/silver nanoparticle construct. Finally, to study the dose-response of silver on skin-dermis, multilayers containing microspheres and impregnated with silver-nanoparticles using 1 to 4 cycles of silver ion exchange and reduction were fabricated on PDMS stamps (Table 1) and stamped onto skin-dermis. Fig. 8 shows the amount of silver incorporated in these PEMs (on the x-axis of the plot), the amount of silver extracted from PEMs remaining on the PDMS stamps after stamping onto the skin-dermis, and the amount of silver transferred onto skin-dermis (calculated from the difference between silver on the PDMS before and after stamping).
Table 1.
Loadings of silver nanoparticles incorporated into PEMs of (PAH/PAA)10(PS-microspheres)(PAH/PAA)10 fabricated on PDMS.
| pH of PAA | Diameter of microspheres | Number of silver loading cycles | [Ag+] μg cm−2 |
|---|---|---|---|
| 7.5 | 1 μm | 4 | 0.12±0.01 |
| 7.5 | 1 μm | 6 | 0.26±0.01 |
| 5.5 | 1 μm | 4 | 0.62±0.06 |
| 5.5 | 2 μm | 1 | 0.30±0.03 |
| 5.5 | 2 μm | 2 | 0.79±0.04 |
| 5.5 | 2 μm | 3 | 1.00±0.12 |
| 5.5 | 2 μm | 4 | 1.64±0.22 |
Figure 8.
Characterization of the transfer of silver onto skin-dermis by stamping with PEMs containing microspheres and a range of silver-loadings. The loading indicated under each set of bars is the silver in each PEM prior to stamping. The bars (left to right) show the amount of silver remaining on the PDMS after stamping skin-dermis; the amount of silver transferred onto skin-dermis (calculated as difference between the loading of silver extracted from PDMS before and after stamping); and the amount of silver extracted from stamped skin graft.
2.5. Antibacterial activity of skin-dermis functionalized by stamping with silver-impregnated PEMs
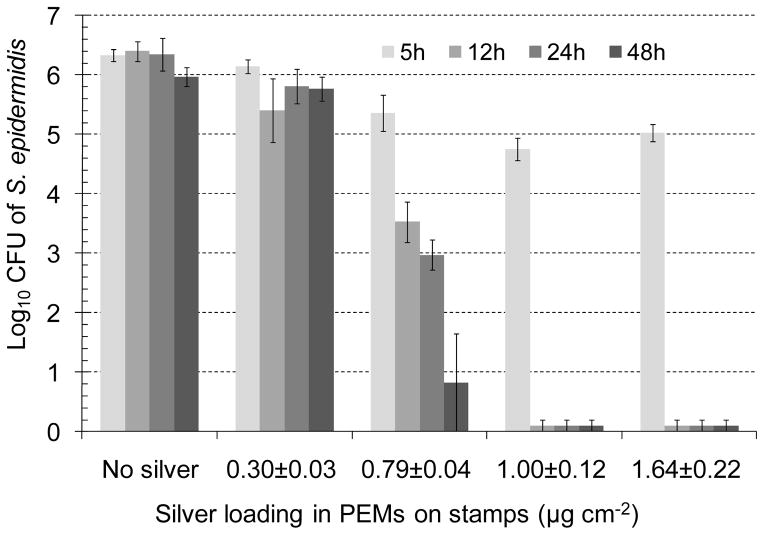
To demonstrate that PEMs containing microspheres can be used to functionalize the surface of biological tissues, the dermis of cadaver-skin was stamped with silver-loaded PEMs and tested for antibacterial activity against gram-positive bacteria Staphylococcus epidermidis or gram-negative bacteria Pseudomonas aeruginosa, the two pathogens commonly isolated from infected wounds.[19] To this end, test substrates (skin-dermis stamped with PEMs) were cut using biopsy punches to fit into the wells of 96-well polystyrene plates (~6 mm diameter wells), placed at the bottom of the wells, and incubated with 100 μL of PBS buffer containing 107 colony forming units (CFU) of bacteria. The plates were incubated on a shaker at 100 rpm within an incubator at 37°C. After pre-determined periods of incubation, bacteria were rinsed from the wells of the plate using ice-cold PBS and collected in vials to determine the bacterial cell counts using a surface spread-plate method, as described in the Methods section. As controls in each experiment, bacterial suspensions were incubated in wells containing untreated skin-dermis or wells to which skin-dermis was not added. Fig. 9 shows the counts of S. epidermidis in buffer solutions after incubation for times that ranged for 5 h to 48 h, as a function of the silver loading in the PEMs (characterized in Fig. 8). The results clearly show that higher loadings of silver lead to higher levels of the antibacterial activity on the dermis of cadaver-skin. In particular, we note that the skin-dermis stamped with PEMs containing 1.0±0.12 μg cm−2 silver caused a 1 log10 CFU decrease in bacterial counts within 5 h and a 6 log10 CFU decrease within 12h; i.e. more than 99.999% of bacteria were killed in 12h. In addition, we note that the bactericidal activity of the PEMs on the surface of the cadaver-skin was maintained over 48 h (longest duration investigated in the experiments).
Figure 9.
Influence of silver loading in PEMs stamped onto skin-dermis on the antibacterial activity measured as a function of time against S. epidermidis. The transfer of the PEMs onto the skin-dermis was achieved by integration of microparticles into the PEMs. Skin-dermis was incubated with bacterial suspensions in PBS, and the decrease in the viable bacterial counts in the suspensions was determined at the indicated time points between 5 to 48 h (n≥4).
Inspection of Fig. 9 also reveals that no significant reduction in the viable counts of S. epidermidis was observed when the bacteria were incubated with skin-dermis not stamped with silver-impregnated PEMs (i.e. no silver). These results confirm that the observed antibacterial activity on skin-dermis stamped with PEMs containing higher loadings of silver was due to the presence of silver, and it was silver dose-dependent. We also note in Fig. 9 that no significant reduction in bacterial counts was observed within 5 h on skin-dermis stamped with PEMs containing 0.79±0.04 μg cm−2 silver. However, up to 3 log10 CFU decrease in bacterial counts was observed after 12 h incubation and up to 6 log10 CFU decrease after 48 h. These results indicate sustained release of silver from the PEMs stamped onto the skin-dermis. Similar measurements of antibacterial activity were obtained using P. aeruginosa (supplementary Fig. 7).
In our previous work[19] with PEMs fabricated on glass substrates, we demonstrated that PEMs impregnated with silver nanoparticles at concentrations below 0.4 μg cm−2 provided a 6 log10 CFU decrease in S. epidermidis within 24 h. However, the results in Fig. 9 suggest that only skin-dermis stamped with PEMs containing 0.79±0.04 μg cm−2 or more of silver exhibited significant antibacterial activity. To provide insight into the apparent shift in the loading of silver needed to achieve antibacterial activity, skin-dermis stamped with silver-impregnated PEMs were incubated in 2% nitric acid for 24 h to determine the silver available for release from the PEMs on the skin-dermis. Inspection of Fig. 8 reveals that the amount of silver extracted from the stamped skin-dermis was significantly lower than that loaded into the PEMs (also shown in Fig. 8). In particular, the concentration of silver ions extracted from skin-dermis stamped with PEMs containing 1.0±0.12 μg cm−2 silver was only 0.25±0.01 μg cm−2. This level of silver release, while sufficient to cause 6 log10 CFU decrease in S. epidermidis within 12 hr, is lower than the concentration of silver ions extracted from PEMs that were previously found to be cytotoxic to NIH 3T3 mouse fibroblasts cells (>0.4 μg cm−2).[19] This observation suggests that the dermis of cadaver-skin functionalized with PEMs containing 1.0±0.12 μg cm−2 of silver released silver at levels that were antibacterial but not-cytotoxic. Supplemental experiments (see supplementary Fig. 8) suggest that proteins and molecular constituents of skin-dermis (such as collagen fibers, cell debris) can strongly bind silver ions, effectively reducing the silver ions available in solutions to exert antibacterial activity.
3. Discussion
A key finding of the study reported in this paper is that mechanical transfer of PEMs of (PAH/PAA)n from elastomeric stamps onto the surfaces of the soft substrates is dependent on the mechanical properties of the substrates. Specifically, previously published methods for stamping (PAH/PAA)n multilayers onto glass surfaces[22] do not lead to the transfer of PEMs onto the surfaces of soft substrates such as skin-dermis and medical-grade silicone sheets with E′ values ranging from 450 to 1500 kPa. We have discovered, however, that incorporation of a monolayer of rigid microspheres (1–2 μm in diameter) into PEMs of (PAH/PAA)n leads to reproducible transfer of PEMs above and below the microspheres onto the dermis of cadaver skin. When using the microspheres, mechanical transfer is achieved by contact of the PDMS stamps with the soft-substrate of interest for approximately 30 s with an external applied pressure of ~196 kPa. Our results illustrate the utility of this mechanical transfer process by describing compositions and methods that allow integration of PEMs impregnated with silver-nanoparticles onto the dermis of cadaver-skin and demonstrating antibacterial activity of the modified skin-dermis. Overall, the approach presented here provides general and facile methods to engineer surface properties of soft, biologically relevant materials including tissues.
Although details of the mechanics of the transfer process that take place when microspheres are incorporated into PEMs remains to be elucidated, our results suggest that a necessary condition for transfer of the PEMs is that the microspheres must protrude substantially from the PEMs. We postulate that protrusion of the microspheres above the PEMs in contact with the PDMS stamps allows the soft, elastic substrate to conformally contact a threshold surface area of each PEM-coated microsphere, thus leading to capture of the microsphere by the substrate upon removal of the stamp. Our observation that incorporation of larger microspheres was necessary to transfer thick PEMs provides additional support for this proposed physical mechanism of transfer. We also comment that the microspheres used in these studies were composed of polystyrene but that the mechanism of transfer is not strongly dependent on the material composition (or surface properties) of the microspheres. For example, we have also demonstrated that biodegradable, 2 μm diameter poly(lactic-co-glycolic acid) microspheres (from Phosphorex Inc, Fall River, MA) integrated into 160 nm-thick PEMs also lead to the transfer of PEMs onto the dermis of cadaver-skin (data not shown). This class of microspheres is particularly promising as a range of bioactive molecules have been demonstrated to be released over time from the microspheres via hydrolytic degradation.[27]
The ability to mechanically transfer PEMs onto soft biological materials is significant because a range of bioactive molecules can be incorporated into PEMs. In this paper, we report that PEMs loaded with silver-nanoparticles (and containing microspheres) can be mechanically transferred onto the skin-dermis to functionalize the dermis surface with antibacterial activity. Significantly, the antibacterial activity is observed with immobilized loadings of silver that are 100-fold less than those used in fabric-based, silver dressings. Commercially available textile/fabric-based silver dressings such as Acticoat® release up to 100 μg cm−2 of silver ions in aqueous solutions in 24 h.[13] In contrast, the total amount of silver released from PEMs stamped on the dermis of cadaver-skin that killed 6 log10 CFU of S. epidermidis and P. aeruginosa (Fig. 9) was only 0.25±0.01 μg cm−2. This study thus goes beyond previous reports[19] by demonstrating that nanoscopic localization of silver-nanoparticles on a biological tissue can exert antibacterial activity below silver concentrations at which cytotoxicity has been reported for mammalian cells (>0.4 μg cm−2). Whereas the ability to use PEMs to engineer antibacterial activity into the surface of model wounds is the focus of the current study, we comment that the approach is a general one that we believe can be used to achieve nanoscopic localization of a range of bioactive agents on a variety of tissues and biomedical devices. In future studies, we propose to investigate the mechanical transfer of PEMs containing bioactive factors including growth factors and antibacterial peptides onto model wound surfaces to exploit the therapeutic efficiency of bioactive agents that otherwise cannot be used for wound-healing because of their toxicology profile at higher concentrations.
4. Conclusion
In summary, we report a general and facile method to fabricate and integrate molecularly-thin polymer films loaded with silver-nanoparticles onto the surface of the dermis of cadaver-skin such that the skin-dermis exhibits bactericidal activity for at least 48 h. A key innovation underlying the approach presented in this paper is a procedure that permits the mechanical transfer of pre-fabricated polymer thin films onto soft-biological tissue such as skin-dermis (serving as a skin wound simulant). We have found that incorporation of microspheres within the PEMs facilitates the transfer of otherwise non-transferrable PEMs onto soft substrates such as silicone sheets and soft biological tissues. The ability to directly stamp pre-fabricated PEMs onto soft-substrates, such as dermal tissue of wounds, circumvents the need to expose tissue to non-physiological conditions and provides precise control over the loading and spatial placement of bioactive factors. The ability to integrate PEMs onto the surfaces of tissue further allows nanoscopic localization of bioactive factors impregnated in PEMs. Our results suggest that nanoscopic localization can be used to achieve bioactivity at loadings of bioactive compounds that are significantly lower than those typically employed in topical applications. The approach described in this paper provides a general methodology for chemical and physical modification of the surfaces of wound-beds and soft medical devices, and can be used to generate surfaces with tailored functional properties (e.g., antibacterial activity) with a high level of control.
5. Experimental
Materials
PAH (Mw= 70 kDa), silver nitrate, and sodium borohydride were obtained from Sigma Aldrich (St. Louis, MO), and PAA (Mw = 60 kDa) from Polysciences (Warrington, PA). Polished silicon wafers were purchased from Silicon Sense (Nashua, NH). Terminally gamma-irradiated human cadaver skin allograft, GammaGraft®, was obtained from Promethean LifeSciences Inc, Pittsburgh, PA. All work with GammaGraft® was performed under sterile conditions in an air-flow hood.
Cleaning of glass slides
Glass microscope slides were cleaned sequentially in piranha solution [70:30 (% v/v) H2SO4:H2O2] and alkaline solution (70:30 (% v/v) KOH:H2O2) for 1 h at ~80 °C, according to published procedures [28]. If required, cleaned glass slides were coated with octadecyltrichlorosilane (OTS) as described earlier [29] to generate a low-energy hydrophobic surface.
Preparation of PEMs
Poly(dimethylsiloxane) (PDMS) stamps were fabricated by curing Sylgard 184 (10:1 base to catalyst; Dow Chemical, Midland, MI) on top of OTS-coated glass slides at 60°C for 24 h. After curing, PDMS stamps were released from the glass slides and PEMs were assembled on the face of the stamps that previously were in contact with the OTS-coated glass. OTS-coated glass was used to ensure formation of a smooth surface on the PDMS stamps.
PEMs with desired number of multilayers were assembled on PDMS stamps in petri-dishes (Polystyrene, non-tissue culture treated, BD, Franklin Lakes, NJ) by sequential incubation in solutions of PAA and PAH (0.01 M by repeat unit) for 10 min each, as described elsewhere [19]. Polyelectrolytes solutions were adjusted to the desired pH using either HCL (1 M) or NaOH (1 M). The formation of the PEMs was initiated by the adsorption of PAH onto the PDMS. Stamps were rinsed with DI water 3 times for 1 min each after immersion in each polyelectrolyte solution. After assembly, the PEMs were dried in vacuum at 60°C for 1 h.
PAH was labeled with either fluorescein-5-isothiocyanate (FITC) (Ext/Em-488/510) or Alexa Fluor® 633 carboxylic acid succinimidyl ester (Ext/Em-633/650) (cat# F1906 and A20005, respectively, Invitrogen) using procedures described elsewhere [30]. Crimson fluorescent (Ext/Em-625/645) FluoSpheres® carboxylate-modified polystyrene (PS) microspheres, 1 μm in diameter (cat# F8816, Invitrogen), or non-fluorescent carboxyl latex microspheres, 1 μm in diameter (cat# C37274, Invitrogen) were used to prepare PEMs containing microspheres. The microspheres were washed three times with DI water in Eppendorf tubes by centrifuging for 5 min at 9000g, and re-suspended in fresh DI water by sonication and vortexing before use. To deposit a monolayer of microspheres on the PEMs, PAH/PAA multilayers with PAH as the top layer were incubated with a suspension of microspheres for 30 min, and then washed 3× with water. Additional layers of polyelectrolytes were assembled subsequently over the microspheres, starting with deposition of a PAH layer.
Characterization of PEMs
Fluorescence from FITC or Alexa-Fluor 633-labeled PAH incorporated into PEMs was quantified on a Synergy™ multi-mode microplate reader (BioTek Instruments, Winooski, VT). An Olympus IX70 inverted microscope equipped with Chroma Technology Corp. (Rockingham, VT) fluorescence filter cubes was used to image the fluorescence from fluorescent PAH or PS microspheres. Images were captured and analyzed using the Metavue version 7.1.2.0 software package (Molecular Devices, Toronto, Canada). A Gaertner LSE ellipsometer (λ=632.8 nm, ψ=70°) was used to measure the thickness of PEMs prepared on the silicon wafers. Silicon wafers were piranha cleaned and RCA cleaned as described above [28]. The effective substrate parameters (ns= 3.85, ks= −0.02) were measured by averaging six measurements on six different silicon-wafer pieces. The refractive index of the polymers was assumed to be 1.55 [31].
Loading of silver nanoparticles in PEMs
Synthesis of the silver nanoparticles (Ag NPs) within the PEMs was initiated by incubation of the pre-assembled PEMs on PDMS in an aqueous solution of silver nitrate (5 mM) for 1 h. As described in past reports [20, 32], Ag+ ions diffuse into the PEMs and exchange with the acidic protons of the PAA. The carboxylate-bound Ag+ within the PEMs were subsequently reduced to zero-valent Ag NPs by incubating PEMs in NaBH4 (5 mM) aqueous solution (pH 7.0) for 15 min [33]. In addition to forming the Ag NPs, this procedure regenerates the carboxylic acid groups within the PEMs. This permits additional Ag+ to be loaded into the PEMs. Thus, repeated cycles of incubation in Ag+ solutions and reducing agent solutions were used to increase the loading of Ag NPs in the PEMs. The loading of silver incorporated into the PEMs was determined by extracting the silver into 3 mL diluted nitric acid by incubation for 3 h. The concentration of Ag+ extracted from the PEMs was measured by elemental analysis using an inductively-coupled plasma (ICP) emission spectrometer (Perkin Elmer Optima 3000DV) at the wavelength of 328.068 nm [34]. The detection limit of the instrument was specified to be ~0.1 ppb. Details regarding the analytical method can be found elsewhere [19].
Antibacterial activity
The strain of S. epidermidis was a clinical isolate from the University of Wisconsin-Madison Veterinary Hospital (provided by Professor R. D. Schultz), and P. aeruginosa (ATCC 27853) was obtained from ATCC (Manassas, VA). Bacteria were grown in Tryptic Soy Broth Yeast Extract (TSBYE) (BD, Franklin Lakes, NJ) overnight at 37°C with shaking at 200 rpm until a cell density of approximately 4×109 CFU/mL was reached. The latter was calculated from a standard curve for optical density (600 nm) of bacterial suspensions prepared using a UV–vis spectrometer (Beckman Coulter, Fullerton, CA). The bacterial suspensions were centrifuged at 2700 rpm for 10 min and the pellet washed and resuspended in PBS. In antibacterial assays, test substrates in the wells of 96-well plates were incubated with 100 μL PBS buffer (pH 7.4) containing 107 CFU of bacteria. Plates were incubated with shaking (100 rpm) at 37°C for 5 to 48 h. After incubation, the buffer in each well was collected, and the wells were washed 3× in 200 μL ice cold PBS to rinse out remaining bacteria. For each well, washings were pooled and made to 1 mL using PBS. The viable bacterial cells in washings were determined by a surface spread-plate method [25]. Serial dilutions of the samples were prepared in PBS and 0.1 mL of each diluted sample was spread onto Trypticase Soy Blood Agar plates (#221261, BD, Franklin Lakes, NJ,). After incubation in a 37°C incubator for 24 h, bacterial colonies were counted and used to calculate the mean colony forming units (CFU) per mL. All assays were carried out on at least three different days, with at least three replicates of each test sample performed on each day.
Statistical Analyses
Where appropriate, the data is presented as means with standard deviations (SD) as error bars, calculated over three or more data points. Significant differences between two groups were established by student’s t-test and between more than two groups by one-way ANOVA analysis of variance, followed by Tukey’s test. The level of significance was set at p<0.05.
Supplementary Material
Acknowledgments
The authors thank Tyler Nelson and Adeyinka A Lesi for assistance in the fabrication of PEMs, Nancy Faith and Harpreet Singh for assistance with antibacterial experiments, and Chris Worley for assistance with the ICP spectrophotometer. The funding for this study was provided by NIH grant# 1RC2AR058971-01 from NIAMS and the Wisconsin Institute for Discovery. A.A. acknowledges support via a fellowship from the Ewing Marion Kauffman Foundation. Supporting Information is available online from Wiley InterScience or from the author.
Contributor Information
Dr. Ankit Agarwal, Department of Chemical and Biological Engineering, University of Wisconsin, 1415 Engineering Drive, Madison, WI 53706 (USA)
Kathleen M Guthrie, Department of Surgery, School of Veterinary Medicine, University of Wisconsin-Madison, 2015 Linden Dr, Madison, WI 53706 (USA).
Prof. Charles J. Czuprynski, Department of Pathobiology, School of Veterinary Medicine, University of Wisconsin-Madison, 2015 Linden Dr, Madison, WI 53706 (USA)
Prof. Michael J. Schurr, Department of Surgery, School of Medicine, University of Wisconsin-Madison, 600 Highland Ave, Madison, WI 53706 (USA)
Prof. Jonathan F. McAnulty, Department of Surgery, School of Veterinary Medicine, University of Wisconsin-Madison, 2015 Linden Dr, Madison, WI 53706 (USA)
Prof. Christopher J. Murphy, Email: cjmurphy@ucdavis.edu, Department of Ophthalmology and Vision Sciences, School of Medicine, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California-Davis, 1423 Tupper Hall, Davis, CA 95616 (USA)
Prof. Nicholas L. Abbott, Email: abbott@engr.wisc.edu, Department of Chemical and Biological Engineering, University of Wisconsin, 1415 Engineering Drive, Madison, WI 53706 (USA)
References
- 1.Vasilev K, Sah V, Anselme K, Ndi C, Mateescu M, Dollmann Br, Martinek P, Ys H, Ploux L, Griesser HJ. Nano Lett. 2009;10:202. doi: 10.1021/nl903274q. [DOI] [PubMed] [Google Scholar]
- 2.Babu R, Zhang J, Beckman EJ, Virji M, Pasculle WA, Wells A. Biomaterials. 2006;27:4304. doi: 10.1016/j.biomaterials.2006.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho CH, Tobis J, Sprich C, Thomann R, Tiller JC. Adv Mater. 2004;16:957. [Google Scholar]
- 4.Sambhy V, MacBride MM, Peterson BR, Sen A. J Am Chem Soc. 2006;128:9798. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]
- 5.Yu DG, Lin WC, Yang MC. Bioconjug Chem. 2007;18:1521. doi: 10.1021/bc060098s. [DOI] [PubMed] [Google Scholar]
- 6.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. Biomaterials. 2004;25:4383. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 7.Mao JY, Belcher AM, Van Vliet KJ. Adv Funct Mater. 2010;20:209. [Google Scholar]
- 8.Podsiadlo P, Paternel S, Rouillard JM, Zhang Z, Lee J, Lee JW, Gulari E, Kotov NA. Langmuir. 2005;21:11915. doi: 10.1021/la051284+. [DOI] [PubMed] [Google Scholar]
- 9.Wright JB, Lam K, Burrell RE. Am J Infect Control. 1998;26:572. doi: 10.1053/ic.1998.v26.a93527. [DOI] [PubMed] [Google Scholar]
- 10.Warriner R, Burrell R. Adv Skin Wound Care. 2005;18:2. doi: 10.1097/00129334-200510001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Burns. 2007;33:139. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Lu S, Gao W, Gu HY. Burns. 2008;34:623. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Taylor PL, Ussher AL, Burrell RE. Biomaterials. 2005;26:7221. doi: 10.1016/j.biomaterials.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Blaker JJ, Nazhat SN, Boccaccini AR. Biomaterials. 2004;25:1319. doi: 10.1016/j.biomaterials.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Kristiansen S, Ifversen P, Danscher G. Histochem Cell Biol. 2008;130:177. doi: 10.1007/s00418-008-0415-x. [DOI] [PubMed] [Google Scholar]
- 16.Van Den Plas D, De Smet K, Lens D, Sollie P. Eur J Dermat. 2008;18:416. doi: 10.1684/ejd.2008.0437. [DOI] [PubMed] [Google Scholar]
- 17.Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. J Trauma. 2006;60:648. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- 18.Asharani PV, Sethu S, Vadukumpully S, Zhong S, Lim CT, Hande MP, Valiyaveettil S. Adv Funct Mater. 2010;20:1233. [Google Scholar]
- 19.Agarwal A, Weis TL, Schurr MJ, Faith NG, Czuprynski CJ, McAnulty JF, Murphy CJ, Abbott NL. Biomaterials. 2010;31:680. doi: 10.1016/j.biomaterials.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TC, Rubner MF, Cohen RE. Langmuir. 2002;18:3370. [Google Scholar]
- 21.Li Z, Lee D, Sheng X, Cohen RE, Rubner MF. Langmuir. 2006;22:9820. doi: 10.1021/la0622166. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Hammond PT. Adv Mater. 2004;16:520. [Google Scholar]
- 23.Diridollou S, Patat F, Gens F, Vaillant L, Black D, Lagarde JM, Gall Y, Berson M. Skin Res Technol. 2000;6:214. doi: 10.1034/j.1600-0846.2000.006004214.x. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI, Rubner MF. Biomacromolecules. 2003;4:96. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
- 25.Kohli N, Worden RM, Lee I. Chem Commun. 2005;289:316. doi: 10.1039/b406430e. [DOI] [PubMed] [Google Scholar]
- 26.Romanelli M, Falanga V. J Am Acad Dermatol. 1995;32:188. doi: 10.1016/0190-9622(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed F, van der Walle CF. J Pharm Sci. 2008;97:71. doi: 10.1002/jps.21082. [DOI] [PubMed] [Google Scholar]
- 28.Skaife JJ, Abbott NL. Chem Mater. 1999;11:612. [Google Scholar]
- 29.Brake JM, Abbott NL. Langmuir. 2002;16:6101. [Google Scholar]
- 30.Gupta JK, Tjipto E, Zelikin AN, Caruso F, Abbott NL. Langmuir. 2008;24:5534. doi: 10.1021/la800013f. [DOI] [PubMed] [Google Scholar]
- 31.Lockwood NA, Cadwell KD, Caruso F, Abbott NL. Adv Mater. 2006;18:850. [Google Scholar]
- 32.Joly S, Kane R, Radzilowski L, Wang T, Wu A, Cohen RE, Thomas EL, Rubner MF. Langmuir. 2000;16:1354. [Google Scholar]
- 33.Logar M, Jancar B, Suvorov D, Kostanjsek R. Nanotechnology. 2007;18:325601. [Google Scholar]
- 34.Shi Z, Neoh KG, Zhong SP, Yung LYL, Kang ET, Wang W. J Biomed Mater Res A. 2006;76A:826. doi: 10.1002/jbm.a.30597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.