Abstract
Background
CpG methylation in the O6-methylguanine-DNA methyltransferase (MGMT) promoter is associated with better outcome following alkylating agent chemotherapy in glioblastoma (GBM) and anaplastic glioma (AG). To what extent improved response reflects low or absent MGMT activity in glioma tissue has not been unequivocally assessed. This information is central to developing anti-resistance therapies.
Methods
We examined the relationship of MGMT activity in 91 GBMs and 84 AGs with progression-free survival (PFS) following alkylator therapy and with promoter methylation status determined by methylation-specific PCR (MSP).
Results
Cox regression analysis revealed that GBMs with high activity had a significantly greater risk for progression in dichotomous (P ≤ 0.001) and continuous (P ≤ 0.003) models, an association observed for different alkylator regimens, including concurrent chemo-radiation with temozolomide. Analysis of MGMT promoter methylation status in 47 of the GBMs revealed that methylated tumors had significantly lower activity (P ≤ 0.005) and longer PFS (P ≤ 0.036) compared to unmethylated tumors, despite overlapping activities. PFS was also significantly greater in methylated vs. unmethylated GBMs with comparable activity (P ≤ 0.005), and among unmethylated tumors with less than median activity (P ≤ 0.026), suggesting that mechanisms in addition to MGMT promote alkylator resistance. Similar associations of MGMT activity with PFS and promoter methylation status were observed for AGs.
Conclusions
Our results provide strong support for the hypotheses that MGMT activity promotes alkylator resistance and reflects promoter methylation status in malignant gliomas.
General significance
MGMT activity is an attractive target for anti-resistance therapy regardless of methylation status.
Abbreviations: AG, anaplastic glioma; BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea; CCNU, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea; GBM, glioblastoma; HR, hazard ratio; O6-meG, O6-methylguanine; MGMT, O6-methylguanine-DNA methyltransferase; MSP, methylation-specific PCR; PFS, progression-free survival; RT, radiation therapy; TMZ, temozolomide
Keywords: Brain tumor, DNA repair, Drug resistance, Clinical outcome
Highlights
-
•
Largest study to date of association of MGMT activity with treatment response.
-
•
MGMT activity is inversely associated with alkylator response in malignant gliomas.
-
•
Mean activity is significantly lower in MGMT promoter-methylated tumors.
-
•
Better response in methylated tumors is unlikely due to lower MGMT activity alone.
-
•
Supports the use of MGMT inhibitors to improve responsiveness to alkylator therapy.
1. Introduction
Glioblastoma (GBM; WHO grade 4) is among the most lethal human cancers with approximately 13,000 individuals newly diagnosed in the U.S. annually [1]. Therapy has long included surgery to the safest extent possible followed by involved-field radiotherapy (RT). Chemotherapy with nitrosourea-based methylating and/or chloroethylating agents given after RT has done little to improve clinical outcome [2]. In contrast, inclusion of the triazene methylator temozolomide (TMZ) concurrently with RT, and continued as a single agent after completing RT (chemo-RT) adds an average of 12 weeks overall survival in newly diagnosed GBMs, and as a result is now the contemporary standard of care [2], [3]. Nevertheless, the majority of GBMs are not responsive to chemo-RT, and tumors that initially respond inevitably become refractory. Consequently, the prognosis for GBM remains dismal with most patients dying within 9 to 15 months [3]. Chemo-RT is also used in the treatment of anaplastic (i.e., WHO grade 3) gliomas (AGs), a heterogeneous group of gliomas that are histologically, genetically and clinically distinct from GBM [4]. AGs generally show greater responsiveness to adjuvant RT and/or alkylating agent therapy, an attribute that is believed to be due, at least in part, to genetic alterations not found in GBMs. As a consequence most AGs have a better prognosis and longer overall survival than GBMs [4], [5], [6], [7], [8]. While chemo-RT is increasingly used in the adjuvant therapy of AGs, its efficacy compared to the contemporary standard of care of first line RT or alkylators awaits demonstration in ongoing clinical trials [4].
Extensive preclinical data demonstrate that the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) limits the efficacy of TMZ and other clinical alkylators [9], [10]. MGMT is the sole human activity that excises O6-methylguanine (O6-meG) [11], a minority (~ 5–7%), cytotoxic base adduct produced by TMZ and other methylators [12]. MGMT covalently transfers the methyl group to an internal cysteine residue, yielding an inactive S-alkylcysteine-modified protein and guanine [11]. This “suicide” mechanism constrains the number of O6-meG adducts that can be removed from DNA in vivo to the number of MGMT molecules in cells and the rate of re-synthesis of the protein. MGMT also excises O6-chloroethylguanine, the progenitor of cytotoxic inter-strand crosslinks [9], [11].
MGMT activity varies widely (~ 300-fold) in GBMs and other gliomas, with a minority lacking biochemically detectable activity [9], [13]. Absence of MGMT activity in GBM-derived cell lines and xenografts is associated with hypermethylation of CpG dinucleotides in the MGMT promoter region [3], [9], indicating that expression can be epigenetically silenced. Hypermethylation of the MGMT promoter has also been observed in an appreciable fraction of GBMs and other gliomas [3]. Importantly, numerous studies have shown that MGMT promoter methylation is associated with longer survival in newly diagnosed GBMs following chemo-RT [3]. Prolonged survival has also been observed in GBMs, and in lower-grade gliomas treated with adjuvant alkylating agents following completion of RT [3], [14], [15], [16]. These observations provide evidence that promoter methylation accompanies a treatment-sensitive phenotype and has utility as a prognostic and, in some instances, as a predictive marker [15], [17].
The association of promoter methylation status with clinical response strongly suggests that absence or low expression of MGMT promotes alkylator sensitivity in vivo. This hypothesis has been examined primarily by immunohistochemistry (IHC) to determine the fraction of MGMT-expressing GBM cells in sections of formalin-fixed paraffin embedded tissue [reviewed in [17], [18]]. However, low MGMT expression has not consistently accompanied better treatment response in GBMs with MGMT promoter methylation [13], [19]. The disparity among these studies has been attributed to a variety of limitations, including lack of standardized scoring criteria and small sample size [discussed in 18]. MGMT biochemical activity provides a direct and objective means of quantifying MGMT expression in human tumors. However, MGMT activity has also not consistently shown an association with either clinical response to alkylating agents [13], [20], [21] or promoter methylation status [22], [23]. The discordance between these studies likely reflects, in part, the limited number of samples analyzed in some cases, differences in reporting MGMT activity (e.g., normalization to cell protein vs. cell number) and, in some instances, the inclusion of both GBMs and AGs in analyses.
Here we analyzed the association of MGMT biochemical activity [24] with progression-free survival (PFS) following alkylator-based chemotherapy in the largest cohort of GBMs and anaplastic gliomas (AGs) reported to date. Univariate Cox regression models, with activity entered either as a dichotomous variable using median activity as the cut point or as a continuous variable, revealed an inverse association between MGMT activity and PFS in 91 GBMs and in 84 AGs. The association was observed in tumor sub-groups segregated by demographic, clinical and genetic markers associated with treatment response. In all groups examined, less than median MGMT activity was associated with longer PFS following alkylators therapy. Significantly, lower MGMT activity and longer PFS following alkylator therapy were each associated with promoter methylation [determined by methylation-specific PCR (MSP)] in the 47 GBMs and 34 AGs that were examined. Additional findings not previously reported are that PFS for methylated tumors was significantly greater than that for unmethylated tumors with comparable activity, and that among unmethylated GBMs, lower than median MGMT activity was associated with significantly lower risk for progression following alkylator therapy. Overall, our data strongly indicate that MGMT activity mediates resistance to alkylator therapy and that low MGMT activity is responsible, at least in part, for the better response that accompanies promoter methylation. Our data also suggest that suppression of MGMT activity may promote better response to alkylators regardless of promoter methylation status.
2. Materials and methods
2.1. Tissue and treatment outcome
One hundred forty-two GBMs and 133 AGs were obtained with informed consent from adult patients operated at the University of Washington Medical Center between 1991 and 2012. Diagnoses were in accord with WHO criteria [5] and reflected the consensus of a panel of neuropathologists. GBMs harboring mutant IDH1/2 or with histological evidence of progression from a lower grade glioma (so-called secondary GBM) were excluded from this study. Course of treatment and radiologic documentation of progression was reviewed by the multidisciplinary UW Neuro-Oncology Tumor Board comprised of physicians providing patient care. Tumor, treatment and demographic information were obtained in accordance with protocols approved by the Institutional Review Board at the University of Washington. Care was taken to ensure that tumor tissue samples obtained for MGMT activity were adjacent to tumor submitted for pathology and were distant from the margins of normal brain. Multiple single-use aliquots of tumors were snap frozen immediately after excision and subsequently stored under liquid nitrogen. In our experience, tumors stored in this fashion retain enzymatic activity and DNA integrity for more than 20 years.
2.2. Radiation and alkylating agent-based chemotherapy
All 275 tumors received standard, conformal RT (~ 60 Gy in 30 fractions over 6 weeks). Ninety-one GBMs (64% of all GBMs) and 84 AGs (63% of all AGs) also received alkylating agent-based chemotherapy. The total number of GBM and AG patients receiving each alkylator regimen is described below: the number of individual GBMs and AGs treated is entered in Table 1, Table 4, respectively. Fifty-six patients were given daily TMZ (75 mg/m2) during RT followed by adjuvant TMZ (200 mg/m2) daily for the first 5 days of a 28 day cycle for as many as 6 cycles for GBM (EORTC 26981; [25]) and as many as 19 cycles for AG. Six tumors received a single dose of BCNU (200 mg/m2) immediately prior to the first fraction of RT followed by up to 6 cycles of adjuvant BCNU (200 mg/m2) every 6 to 8 weeks after completing RT. The remaining gliomas completed RT prior to receiving one of the following alkylating agent regimens: TMZ (200 mg/m2) daily for the first 5 days of a 28 day cycle, the cycle repeated until tumor progression; procarbazine, CCNU and vincristine (PCV), given as described [26]; PCV plus 6-thioguanine, dibromodulcitol, 5-fluorouracil and hydroxyurea using the doses and schedules previously described [27]; single agent BCNU (200 mg/m2) every 6 to 8 weeks for up to 6 cycles; BCNU (80 mg/m2) with cisplatin (33 mg/m2) daily for 3 days every 6 weeks for 3 cycles followed by BCNU (80 mg/m2) daily for 3 days every 6 weeks for 2 cycles. Chemotherapy was terminated upon radiologic evidence of tumor progression (see below). All patients had a Karnofsky performance score ≥ 70 at time of operation. Approximately 60% of GBMs and 80% of AGs that recurred after alkylator-based chemotherapy received additional surgery, RT and/or chemotherapy.
Table 1.
GBM MGMT activity and PFS following alkylator therapy.
| Tumor sample |
PFS post alkylator therapy |
MGMT (fmol/10a cells) |
||
|---|---|---|---|---|
| N | Failures | Median (mo) | Mean ± SD; [median]; (range) | |
| All alkylator-treated GBM | 91 | 82 (90%) | 6 | 9.4 ± 11; [5.8]; (< 0.25–57) |
| Exclude outlier MGMT activitiesb | 81 | 73 (90%) | 6.5 | 6.4 ± 4.9; [5]; (< 0.25–22) |
| Exclude upper and lower 10% of PFS | 73 | 67 (92%) | 6 | 9.9 ± 11; [5.6]; (0.25–57) |
| Prior treatment | ||||
| None and biopsy onlyc | 69 | 63 (91%) | 6.5 | 9.4 ± 11; [5.4]; (< 0.25–57) |
| Surgery or biopsy followed by RTd | 22 | 19 (86%) | 5 | 9.5 ± 9.8; [5.9]; (1.2–39) |
| Age | ||||
| Younger (< 50) | 41 | 37 (90%) | 6 | 9.4 ± 11; [5.7]; (0.8–57) |
| Older (≥ 50) | 50 | 45 (90%) | 6 | 9.6 ± 10; [6.3]; (< 0.25–43) |
| Progression after RTe | ||||
| No | 50 | 44 (88%) | 8 | 8.6 ± 11; [5]; (0.7–57) |
| Yesf | 41 | 38 (93%) | 4.5 | 11 ± 11; [6.3]; (< 0.25–40) |
| Alkylator therapy | ||||
| Chemo-RT | 33 | 28 (85%) | 7.5 | 9.9 ± 12; [5.4]; (0.7–57) |
| RT then alkylatorsa | 58 | 54 (93%) | 5 | 9.2 ± 9.8; [5.8]; (< 0.25–40) |
| None (i.e., RT only) | 51 | 50 (98%) | 3 | 8.6 ± 8.9; [6.1]; (< 0.25–40) |
Includes 22 tumors treated with TMZ, 20 treated with PCV-based chemotherapy and 16 treated with BCNU.
Limiting activities to those within the 95% confidence interval for the data eliminated the 10 highest MGMT activities.
Includes 60 newly operated tumors and 9 that were previously biopsied; MGMT activity is that obtained at debulking operation.
MGMT activity determined in recurrent tumor re-operated after RT.
Prior to initiating alkylator therapy.
Includes 22 tumors re-operated for recurrence after surgery or biopsy and RT.
Table 4.
AG MGMT activity and PFS following alkylator therapy.
| Tumor sample |
PFS post alkylator therapy |
MGMT (fmol/106 cells) |
||
|---|---|---|---|---|
| N | Failures | Median (mo) | Mean ± SD; [median]; (range) | |
| All alkylator-treated AG | 84 | 60 (71%) | 16 | 5.9 ± 6.7; [4.1]; (< 0.25–32) |
| Exclude outlier MGMT activitiesa | 78 | 54 (69%) | 21 | 4.4 ± 3.6; [3.6]; (< 0.25–14) |
| Exclude upper and lower 10% of PFS | 68 | 51 (75%) | 16 | 5.6 ± 6.6; [3.8]; (< 0.25–32) |
| Without 1p and/or19q deleted AO/AOA | 71 | 54 (76%) | 16 | 6.3 ± 7.1; [4.1]; (< 0.25–32) |
| Diagnosis | ||||
| Anaplastic astrocytoma | 35 | 28 (80%) | 15 | 6.2 ± 7.1; [3.2]; (< 0.25–32) |
| Anaplastic oligodendroglioma | 24 | 17 (71%) | 13 | 5.6 ± 6.4; [4.2]; (< 0.25–32) |
| Anaplastic oligo-astrocytoma | 25 | 15 (60%) | 24 | 5.8 ± 6.7; [3.8]; (< 0.25–29) |
| Prior treatment | ||||
| None and surgery or biopsy onlyb | 57 | 37 (65%) | 16 | 5.8 ± 6.5; [4.1]; (< 0.25–32) |
| Surgery or biopsy followed by RTc | 27 | 23 (85%) | 19 | 6.2 ± 7.1; [4.1]; (< 0.25–32) |
| Progression after RT | ||||
| No | 45 | 27 (60%) | 26 | 4.7 ± 4.2; [3.8]; (< 0.25–23) |
| Yesd | 39 | 33 (85%) | 16 | 7.3 ± 8.6; [4.3]; (< 0.25–32) |
| Prior low-grade glioma | ||||
| No | 54 | 38 (70%) | 14 | 6.7 ± 7.1; [4.8]; (< 0.25–32) |
| Yes | 30 | 22 (73%) | 22 | 4.6 ± 5.8; [3.1]; (< 0.25–29) |
| Alkylator therapy | ||||
| PCV | 42 | 42 (100%) | 16 | 4.8 ± 5.9; [3.1]; (< 0.25–32) |
| All other alkylatorse | 42 | 28 (67%) | 14 | 7.1 ± 7.3; [4.9]; (< 0.25–32) |
| None (i.e., RT only) | 49 | 30 (61%) | 18 | 7.3 ± 8.9; [4.0]; (< 0.25–49) |
Limiting activities to those within the 95% confidence interval for the data eliminated the 6 highest MGMT activities.
Includes 11 tumors previously operated or biopsied.
MGMT activity assayed in recurrent tumor re-operated after RT.
Includes the 27 tumors re-operated for recurrence after surgery or biopsy and RT.
Includes 23 tumors treated with chemo-RT, 15 with TMZ and 4 with BCNU.
2.3. MGMT activity
MGMT activity in extracts of whole tissue was measured in a standard biochemical assay that quantifies the transfer of radioactivity from a DNA substrate containing [methyl-3H]O6-methylguanine (specific activity, 17–80 Ci/mmol) to protein, as we have previously described [21], [24]. Supernatants were prepared either by homogenization followed by sonication of tissue in isotonic buffer [21], or by extraction of powdered tissue with non-ionic detergents in the presence of 600 mM NaCl [28]. In both instances, crude homogenates were cleared by centrifugation at 10,000 ×g for 30 min. The insoluble pellet was saved for DNA extraction for MSP analysis described below. MGMT activity in supernatants prepared from the same tumor using both extraction techniques differed by less than 3-fold in all cases, a difference which did not affect the relationship between activity and PFS (see below). DNA was quantified in crude homogenates by the diphenylamine method that measures deoxyribose following degradation of DNA with heat and acid [29]. Activity was normalized to cell number using a conversion factor of 6 pg DNA per human cell. All activities are the mean of at least 4 determinations that generally differed by no more than 20%, and MGMT-expressing specimens displayed linearity of activity with added extract. Control experiments validating assay specificity, the range of activities, and absence of detectable activity as well as parameters defining the limit of detection of the assay (< 0.25 fmol/106 cells or 151 molecules/cell) have been described in detail [21], [24]. Whenever possible, tumors were re-extracted to confirm absence of detectable activity.
2.4. MGMT promoter methylation
The CpG methylation status of the MGMT promoter was determined by MSP of bisulfite-treated DNA [3], [30]. Tumor DNA was isolated from the insoluble material pelleted during non-ionic detergent tissue extractions (above) by solubilization in SDS followed by serial salt and alcohol precipitation. Bisulfite treatment, PCR primers and reaction conditions were essentially those described elsewhere [31]. Five μg of genomic DNA was used for each MSP assay. For each sample, methylation status is the concordant results of at least two separate bisulfite reactions and a minimum of two amplification reactions for each aliquot of bisulfite-treated DNA. DNA from MGMT-expressing (SF767) and MGMT-deficient (SNB19) human glioma cell lines [32] served as controls. MGMT activity and CpG methylation were determined using the same piece of tumor tissue.
2.5. Statistical analysis
The outcome variable PFS, i.e., the interval between the initiation of alkylator treatment and tumor progression, was assessed by radiologic imaging. Overall survival (OS) was the interval between initial surgery/biopsy to date of death. Tumor progression was defined as the appearance of new tumor growth in the case of gross total resection (i.e., excision of all contrast enhancing tissue), or increase in the product of the orthogonal diameters of residual tumor by at least 25% and/or tumor growth at a different site in the case of sub-total resection. Observations were censored at the last documented follow-up time if progression had not occurred. Median PFS was determined by the method of Kaplan and Meier and compared using log-rank test. The hazard ratio (HR) for tumor progression as a function of MGMT activity was determined by Cox proportional hazards regression analysis with activity entered as either a dichotomous or a continuous variable. In the case of dichotomous analyses, the median MGMT activity of each group examined was used as the cut point. Adjusting for extraction method had no effect on the association between activity and PFS in either dichotomous or continuous models (data not shown). For purposes of calculation, MGMT-non-expressing tumors were assigned a value of 0.125 fmol/106 cells, one-half the limit of detection; using 0 or 0.25 fmol/106 cells did not alter the results of the analyses. All analyses were performed using Stata software (Stata Corporation, College Station, TX).
3. Results
3.1. GBM: tumor characteristics, treatment and MGMT activity
We assayed MGMT activity in 91 GBMs that were treated with upfront RT and alkylating agents. Patients ranged in age from 21 to 79 years (mean ± SD = 52 ± 12); 59 tumors (65%) were from males and 32 (35%) from females. Distribution of tumors by prior treatment, age and response to RT prior to beginning alkylator treatment is summarized in Table 1. Approximately equal numbers of GBMs were from patients younger than 50 and those 50 and older. Sixty-nine GBMs were either newly operated (N = 60) or had been biopsied (N = 9) prior to definitive surgical resection; MGMT activity was determined in the specimen obtained from surgical resection. Another 22 were re-operated following recurrence after previous surgery and RT; MGMT activity was determined in the specimen obtained at re-operation. Adjuvant alkylator treatment began immediately after completing RT in 50 tumors, and commenced only after tumor progression in the remaining 41 GBMs. Thirty-three GBMs received chemo-RT while the remaining 58 tumors completed RT prior to treatment with TMZ (N = 22), PCV (N = 20) or BCNU (N = 16). Eighty-two tumors (90%) had documented progression after alkylator therapy and 9 were lost to follow up. Median progression-free survival (PFS) was 6 months.
As shown in Table 1, mean MGMT activity for the 91 alkylator-treated GBMs was 9.4 ± 11 fmol/106 cells (i.e., ~ 5700 ± 6650 molecules/cell). Activity ranged more than 225-fold from < 0.25 to 57 fmol/106 cells. Table 1 also shows that activity was not affected by recurrence after previous surgery and RT, age, or withholding alkylator therapy until recurrence after RT, results consistent with our previously reported findings [21], [24]. MGMT activity was also assayed in 51 newly operated GBMs that were subsequently treated with RT alone: Patient age (52 ± 12 years) and fraction of treatment failures in the RT only population were comparable to that of the alkylator-treated GBMs.
3.2. MGMT activity is inversely associated with alkylating agent response in GBM
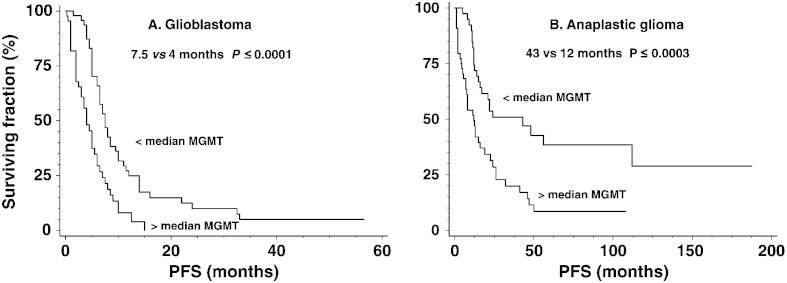
The inverse association between MGMT activity and response to alkylator treatment in 91 GBMs is illustrated in Fig. 1 that compares survival, estimated by the Kaplan–Meier method, for tumors with less than or greater than median activity (5.8 fmol/106 cells, i.e., ~ 3500 MGMT molecules/cell). As shown in Table 2, GBMs with greater than median MGMT activity had an approximately 2.4-fold higher hazard ratio (HR) for progression which was accompanied by significantly shorter median PFS (4 vs. 7.5 months; P ≤ 0.0001; Fig. 1; Table 3) following alkylating agent therapy. Entering MGMT activity as a continuous variable also revealed a significant inverse relationship and a ≥ 5.0-fold (i.e., 1.029(57–0.25)) difference in risk of progression between tumors with the lowest and highest MGMT.
Fig. 1.
Progression-free survival (PFS) for 91 glioblastomas (GBM) and 84 anaplastic gliomas (AG) according to MGMT activity. Tumors were dichotomized by median MGMT activity (5.8 fmol/106 cells for GBMs; 4.1 fmol/106 cells for AGs), and survival curves were calculated by the method of Kaplan–Meier. Greater than median MGMT activity was accompanied by significantly shorter median PFS for GBM (4 vs. 7.5 months; P ≤ 0.0004) and for AGs (12 vs. 43 months; P ≤ 0.0003).
Table 2.
Hazard ratio estimates for association between MGMT activity and PFS in GBMa.
| Tumor sample |
Dichotomousb |
Continuous |
|||||
|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P≤ | HR | 95% CI | P≤ | |
| All alkylator-treated GBM | 91 | 2.43 | [1.535; 3.85] | 0.001 | 1.029 | [1.010; 1.048] | 0.003 |
| Exclude outlier MGMT activitiesc | 81 | 2.02 | [1.257; 3.26] | 0.004 | 1.094 | [1.036; 1.156] | 0.001 |
| Exclude upper and lower 10% of PFS | 73 | 1.76 | [1.072; 2.88] | 0.025 | 1.026 | [1.005; 1.046] | 0.013 |
| Prior treatment | |||||||
| None and biopsy onlyd | 69 | 2.56 | [1.501; 4.36] | 0.001 | 1.033 | [1.012; 1.054] | 0.002 |
| Surgery or biopsy followed by RTe | 22 | 8.70 | [2.31; 32.7] | 0.001 | 1.024 | [0.980; 1.071] | 0.29 |
| By age | |||||||
| Younger (< 50 years) | 41 | 2.07 | [1.048; 4.10] | 0.036 | 1.037 | [1.007; 1.067] | 0.015 |
| Older (≥ 50 years) | 50 | 2.77 | [1.455; 5.30] | 0.002 | 1.029 | [1.002; 1.058] | 0.037 |
| Progression after RT | |||||||
| No | 50 | 2.41 | [1.258; 4.62] | 0.008 | 1.035 | [1.008; 1.063] | 0.012 |
| Yesf | 41 | 4.60 | [2.09; 9.97] | 0.001 | 1.032 | [1.003; 1.062] | 0.030 |
| Alkylator therapy | |||||||
| Chemo-RT | 33 | 2.39 | [1.048; 5.44] | 0.038 | 1.032 | [1.002; 1.062] | 0.034 |
| RT then alkylatorsg | 58 | 2.41 | [1.379; 4.21] | 0.002 | 1.038 | [1.010; 1.068] | 0.009 |
| None (i.e., RT only) | 51 | 1.059 | [0.603; 1.86] | 0.84 | 1.022 | [0.986; 1.060] | 0.23 |
| Overall survivalh | 83 | 1.270 | [0.805; 2.00] | 0.30 | 1.002 | [0.981; 1.023] | 0.88 |
The relationship between MGMT activity in GBMs and PFS following alkylating agent therapy was examined by using Cox proportional hazards regression analysis.
Tumor activity was dichotomized at the median of each group examined.
Limiting activities to those within the 95% confidence interval for the data eliminated the 10 highest MGMT activities.
Includes 60 newly operated tumors and 9 that were previously biopsied; MGMT activity is that obtained at debulking operation.
MGMT activity assayed in recurrent tumor re-operated after RT.
Includes the 22 tumors re-operated for recurrence after biopsy and RT.
Includes 22 tumors treated with TMZ, 20 treated with PCV-based chemotherapy and 16 treated with BCNU.
Date of first operation was unavailable for 8 tumors that had recurred after prior surgery followed by RT.
Table 3.
GBM PFS dichotomized by median MGMT activity.
| Tumor sample |
N |
PFS (mo) |
P≤a |
|
|---|---|---|---|---|
| < median MGMT | > median MGMT | |||
| All alkylator-treated GBM | 91 | 7.5 | 4 | 0.0001 |
| Exclude outlier MGMT activitiesb | 81 | 7.5 | 5 | 0.003 |
| Exclude upper and lower 10% of PFS | 73 | 7 | 5 | 0.020 |
| Prior treatment | ||||
| None and biopsy onlyc | 69 | 8.5 | 5 | 0.0003 |
| Surgery or biopsy followed by RTd | 22 | 6.5 | 4 | 0.0002 |
| Age | ||||
| Younger (< 50) | 41 | 8 | 4 | 0.030 |
| Older (≥ 50) | 50 | 7 | 4.5 | 0.0008 |
| Progression after RT | ||||
| No | 50 | 9.5 | 6 | 0.005 |
| Yese | 41 | 5 | 2 | 0.0001 |
| Alkylator therapy | ||||
| Chemo-RT | 33 | 8.5 | 6 | 0.030 |
| RT then alkylatorsf | 58 | 7 | 3 | 0.008 |
| None (i.e., RT only) | 51 | 3 | 3 | 0.83 |
| Overall survivalg | 73 | 17 | 14 | 0.30 |
Determined by log rank test.
Limiting activities to those within the 95% confidence interval for the data eliminated the 10 highest MGMT activities.
Includes 60 newly operated tumors and 9 that were previously biopsied; MGMT activity is that obtained at debulking operation.
MGMT activity assayed in recurrent tumor re-operated after RT.
Includes the 22 tumors re-operated for recurrence after biopsy and RT.
Includes 22 tumors treated with TMZ, 20 treated with PCV-based chemotherapy and 16 treated with BCNU.
Date of first operation was unavailable for 8 tumors that had recurred after prior surgery followed by radiotherapy.
Further analysis indicated that the inverse association did not reflect the presence of a small number of tumors with outlier MGMT activity or PFS. Limiting the analysis to the 81 tumors within the 95% confidence interval for MGMT activities (Table 1) revealed a statistically significant relationship in both dichotomous and continuous models (Table 2). Significant relationships also persisted when tumors with the highest and lowest 10% of PFS were excluded (Table 2). Prior treatment with RT also had no effect on the association. Restricting the analysis to the 69 newly operated or previously biopsied GBMs (median activity 5.4 fmol/106 cells, i.e., ~ 3250 MGMT molecules/cell) produced HR comparable to that observed for all 91 tumors in both dichotomous and continuous regression models (Table 2), and revealed a ≥ 6.3-fold (i.e., 1.033(57–0.25)) increase in risk of progression between the lowest and highest MGMT activity. These observations indicate that the association between MGMT and PFS is not biased by the shorter PFS of 22 GBMs in our sample that were re-operated at recurrence following RT (5 vs. 6.5 months; P ≤ 0.035; Table 1). Altogether, these results indicate that the correlation between MGMT and PFS is not determined by a limited number of outliers and that MGMT activity in re-operated tumors retains the association with PFS. These conclusions are supported by the significantly longer PFS that accompanied less than median activity in each population (Table 3).
3.3. Association of MGMT with PFS is independent of age, clinical history or alkylator therapy
Increasing age is a prognostic factor associated with worse clinical outcome in GBM [1]. However, as shown in Table 2, the association between MGMT and PFS was very similar in tumors from younger (< 50) and older (≥ 50) patients, suggesting that age did not significantly affect the correlation. That age does not influence the association was further evidenced by the significantly longer PFS observed for tumors with less than median MGMT activity for both younger and older patients (Table 3).
GBMs in our study did not all receive the same course of treatment (Table 1). An appreciable fraction of tumors (41/91, 45%) recurred after RT prior to starting alkylating agent-based chemotherapy, a factor associated with significantly reduced PFS (4.5 vs. 8 months; P ≤ 0.001; Table 1). However, as shown in Table 2, intervening recurrence had no effect on the association of MGMT with PFS in both dichotomous and continuous regression models. Moreover, less than median MGMT activity was associated with longer PFS (Table 3) further strengthening the conclusion that recurrence after RT had no effect on the association of MGMT activity with response. Similar results were observed for GBMs treated with chemo-RT or with alkylating agents after completing RT (Table 2, Table 3). Importantly, MGMT activity showed no relationship with PFS in GBMs treated solely with RT, and with overall survival in GBMs treated with both RT and alkylators (Table 2, Table 3). Together, the foregoing findings strongly indicate that MGMT is not merely a marker for a broader resistance phenotype in GBM, but that the association with outcome reflects a specific function of MGMT, namely excision of cytotoxic O6-alkylguanine adducts.
3.4. AG: tumor characteristics, treatment and MGMT activity
As alkylators are frequently a component of the adjuvant therapy for AGs [4], [5], [6], [7], [8], we also analyzed the association between MGMT activity and PFS in 84 AGs treated with upfront RT and alkylating agents. Patient age was 42 ± 11 years (range 20 to 74) with 40 tumors from males and 44 from females. The distribution of tumors by diagnosis, prior treatment, response to RT, and prior diagnosis of low-grade (i.e., grade 2) glioma is summarized in Table 4. The AGs included 35 anaplastic astrocytomas (AA), 24 anaplastic oligodendrogliomas (AO) and 25 anaplastic mixed oligo-astrocytomas (AOA). Fifty-seven AGs were either newly operated (N = 46) or had been previously operated (N = 7) or biopsied (N = 4); MGMT activity was determined in the specimen obtained at re-operation. Twenty-seven tumors were re-operated at recurrence after previous surgery and RT; MGMT activity was determined in the specimen obtained at re-operation. Of the 38 AGs re-operated at recurrence after surgery/biopsy alone or surgery followed by RT, 30 (79%) had a diagnosis of low-grade (i.e., grade 2) glioma at first surgery. Alkylator treatment began immediately after completing RT in 45 tumors, and commenced only after tumor progression in the remaining 39 AGs. Twenty-three tumors received TMZ concurrently during RT followed by adjuvant TMZ, and the remaining 61 AGs completed RT before beginning treatment with PCV (N = 42), TMZ (N = 15), or BCNU (N = 4). Sixty tumors (71%) had documented progression after alkylator therapy, 12 have stable disease and 12 were lost to follow up. Median progression-free survival for all AGs treated with alkylators was 16 months, significantly longer than the 6 months observed for GBM (P ≤ 0.001) and in accord with the better prognosis that accompanies AGs [4], [5].
As summarized in Table 4, mean MGMT activity for all alkylator-treated AGs was 5.9 ± 6.7 fmol/106 cells (i.e., ~ 3550 ± 4000 molecules/cell) and ranged ~ 130-fold from < 0.25 to mol/fmol/106 cells. Mean activity was significantly lower in AGs compared to GBMs (9.4 ± 11 vs. 5.9 ± 6.7 fmol/106 cells; P ≤ 0.01), in accord with our previously reported findings [21], [24]. Table 4 also shows that activity was not affected by recurrence after previous surgery and RT, withholding alkylator therapy until recurrence after RT, or prior diagnosis of low-grade glioma. MGMT activity was also assayed in 49 AGs that were treated with RT alone; patient age (42 ± 13 years), fraction of treatment failures and MGMT activity were comparable to that in the alkylator-treated AGs.
3.5. MGMT activity is inversely associated with alkylating agent response in AG
The inverse relationship between MGMT activity and PFS following alkylator therapy in 84 AGs is shown in Fig. 1 that compares survival, estimated by the Kaplan–Meier method, for tumors with MGMT activity less than or greater than the median (4.1 fmol/106 cells, i.e., ~ 2500 molecules/cell). AGs with greater than median activity had a 2.6-fold greater risk of progression (Table 5) and an ~ 3.5-fold shorter median PFS after alkylating agent therapy (Fig. 1; Table 6). A significant inverse relationship was also observed when MGMT activity was entered as a continuous variable (Table 5); the risk for progression differed by ~ 9.1-fold (1.072(32–0.25)) between the tumors with the lowest and highest MGMT activity. Restricting analysis to the 78 AGs within the 95% confidence interval for MGMT activities and the 68 tumors excluding the upper and lower 10% of PFS also yielded statistically significant associations (Table 5), indicating that the relationship was not determined by a limited number of outliers. Limiting analysis to the 57 AGs that had received no RT prior to surgery (median activity = 4.1 fmol/106 cells) in a dichotomous model, revealed that tumors with greater than median MGMT activity had an elevated risk for progression (Table 5). A continuous model also yielded a significant inverse relationship (Table 5) that showed a 41-fold (1.124(32–0.25)) difference in risk for progression between the tumors with the lowest and highest MGMT activity. Near significant associations were also observed for AGs that were re-operated at recurrence after surgery/biopsy followed by RT (Table 5). These findings indicate that the correlation between MGMT and PFS is not determined by a limited number of outliers and that MGMT activity in re-operated tumors retains the association with PFS. These conclusions are supported by the significantly longer PFS that accompanied less than median activity in each population (Table 6).
Table 5.
Hazard ratio estimates for association between MGMT activity and PFS in AGsa.
| Tumor sample |
Dichotomousb |
Continuous |
|||||
|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P ≤ | HR | 95% CI | P≤ | |
| All alkylator-treated AG | 84 | 2.57 | [1.505; 4.39] | 0.001 | 1.072 | [1.039; 1.107] | 0.001 |
| Exclude outlier MGMTc | 78 | 2.34 | [1.343; 4.07] | 0.003 | 1.109 | [1.032; 1.192] | 0.005 |
| Exclude upper and lower 10% of PFS | 68 | 1.88 | [1.074; 3.28] | 0.03 | 1.064 | [1.025; 1.104] | 0.01 |
| Exclude 1p 19q deleted | 71 | 2.94 | [1.664; 5.19] | 0.001 | 1.072 | [1.038; 1.109] | 0.001 |
| Prior treatment | |||||||
| None and surgery or biopsy aloned | 57 | 2.57 | [1.309; 5.03] | 0.006 | 1.124 | [1.071; 1.180] | 0.001 |
| Surgery or biopsy followed by RT | 27 | 2.26 | [0.911; 5.63] | 0.08 | 1.045 | [0.933; 1.10] | 0.09 |
| By diagnosis | |||||||
| Anaplastic Astrocytoma | 35 | 2.40 | [1.102; 5.21] | 0.027 | 1.050 | [1.005; 1.097] | 0.029 |
| Anaplastic oligodendroglioma | 24 | 3.58 | [1.283; 9.92] | 0.015 | 1.110 | [1.027; 1.199] | 0.008 |
| Anaplastic oligo-astrocytoma | 25 | 2.90 | [1.010; 8.31] | 0.048 | 1.130 | [1.036; 1.234] | 0.006 |
| Progression after RT | |||||||
| No | 45 | 2.50 | [1.113; 5.62] | 0.027 | 1.112 | [1.027; 1.204] | 0.009 |
| Yese | 39 | 3.51 | [1.620; 7.61] | 0.001 | 1.065 | [1.027; 1.106] | 0.001 |
| Prior low-grade glioma | |||||||
| No | 54 | 2.43 | [1.252; 4.71] | 0.009 | 1.061 | [1.023; 1.100] | 0.002 |
| Yes | 30 | 2.39 | [1.000; 5.77] | 0.054 | 1.127 | [1.036; 1.225] | 0.005 |
| By alkylator treatment | |||||||
| PCV | 42 | 3.72 | [1.825; 7.58] | 0.001 | 1.112 | [1.051; 1.176] | 0.001 |
| All other alkylatorsf | 42 | 2.07 | [0.959; 4.85] | 0.064 | 1.061 | [1.019; 1.106] | 0.004 |
| None (i.e., RT only) | 49 | 0.746 | [0.357; 1.556] | 0.43 | 0.985 | [0.943; 1.029] | 0.50 |
| Overall survivalg | 83 | 1.02 | [0.614; 1.69] | 0.94 | 1.028 | [0.988; 1.070] | 0.17 |
The relationship between MGMT activity in AGs and PFS following alkylating agent therapy was examined by using Cox proportional hazards regression analysis.
Tumor activity was dichotomized at the median of each diagnosis (Table 4).
Limiting activities within the 95% confidence interval for the data eliminated the 6 highest MGMT activities.
Includes seven previously operated and 4 previously biopsied tumors.
Includes the 27 tumors re-operated for recurrence after surgery or biopsy and RT.
Includes 23 tumors treated with chemo-RT, 15 with TMZ and 4 with BCNU.
Date of initial surgery not available for one tumor.
Table 6.
AG PFS dichotomized by median MGMT activity.
| Tumor sample |
N |
PFS (mo) |
P≤a |
|
|---|---|---|---|---|
| < median MGMT | > median MGMT | |||
| All alkylator-treated AG | 84 | 43 | 12 | 0.0003 |
| Exclude outlier MGMT activitiesb | 78 | 43 | 14 | 0.002 |
| Exclude upper and lower 10% of PFS | 68 | 22 | 13 | 0.025 |
| Without 1p and/or 19q deleted AO/AOA | 71 | 26 | 8 | 0.0001 |
| Prior treatment | ||||
| None and surgery or biopsy onlyc | 57 | 48 | 12 | 0.004 |
| Surgery or biopsy followed by RT | 27 | 21 | 13 | 0.068 |
| By diagnosis | ||||
| Anaplastic astrocytoma | 35 | 21 | 8 | 0.022 |
| Anaplastic oligodendroglioma | 24 | 47 | 12 | 0.009 |
| Anaplastic oligo-astrocytoma | 25 | 43 | 13 | 0.038 |
| Prior treatment | ||||
| None and surgery or biopsy onlyc | 57 | 48 | 12 | 0.004 |
| Surgery or biopsy followed by RT | 27 | 21 | 13 | 0.068 |
| Progression after RT | ||||
| No | 45 | 112 | 15 | 0.020 |
| Yesd | 39 | 22 | 7 | 0.0006 |
| Prior low-grade glioma | ||||
| No | 54 | 47 | 8 | 0.006 |
| Yes | 30 | 24 | 19 | 0.044 |
| Alkylator therapy | ||||
| PCV | 42 | 24 | 8 | 0.0001 |
| Other than PCVe | 42 | 26 | 13 | 0.055 |
| None (i.e., RT only) | 49 | 18 | 19 | 0.43 |
| Overall survivalf | 84 | 68 | 53 | 0.58 |
Determined by log rank test.
Limiting activities to those within the 95% confidence interval for the data eliminated the 6 highest MGMT activities.
Includes seven previously operated and 4 previously biopsied tumors.
Includes the 27 tumors re-operated for recurrence after surgery or biopsy and RT.
Includes 23 tumors treated with chemo-RT, 15 with TMZ and 4 with BCNU.
Date of initial surgery not available for one tumor.
3.6. Association of MGMT with PFS is independent of diagnosis, clinical history or alkylator therapy
As shown in Table 5, MGMT activity was inversely correlated with PFS in both dichotomous and continuous regression models in each of the three AG diagnoses. This observation suggests that the association of outcome with MGMT in all 84 AGs does not merely reflect the contribution of treatment-responsive oligodendroglial tumors harboring deletions on chromosomes 1p and 19q [17]. In accord, eliminating the 13 tumors with deletions had little effect on the association (Table 5). The contribution of MGMT to alkylator resistance is also evidenced by the significantly longer PFS accompanying less than median MGMT activity for all diagnoses (Table 6).
As indicated in Table 4, withholding alkylator treatment until progression following RT was associated with a greater risk of progression and significantly shorter PFS (16 vs. 26 months; P ≤ 0.014). However, MGMT was significantly inversely associated with PFS in both dichotomous and continuous regression models regardless of intervening progression after RT (Table 5). The relationship of MGMT activity with PFS was also comparable between AGs that had progressed from low-grade glioma and those that had not, suggesting that malignant progression did not affect the contribution of MGMT to resistance. As gliomas are graded to the most malignant histology observed, our findings also suggest that the presence of low-grade tumor cells in AGs does not affect the role of MGMT in determining response to alkylators. Finally, analysis of groups by alkylator regimen revealed significant inverse associations in dichotomous and continuous models between MGMT activity and PFS for the 42 tumors receiving PCV, the most frequent treatment regimen, as well as significant associations for the 42 tumors treated with other alkylating agents (Table 5). In all cases, these associations were reflected in the longer PFS for tumors with less than median MGMT activity (Table 6). Importantly, MGMT activity showed no relationship with PFS in AGs treated solely with RT, and with overall survival in AGs treated with both RT and alkylators (Table 5, Table 6). These findings strongly suggest that that the association between MGMT and PFS reflects the repair of O6-alkylguanine adducts, and that the contribution of MGMT to alkylator resistance is independent of prognostic markers associated with therapeutic response.
3.7. MGMT activity and CpG methylation status
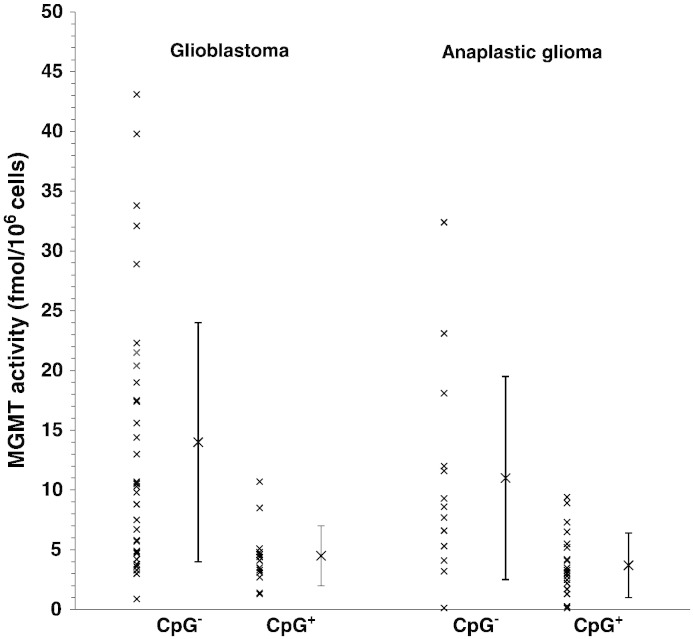
As noted earlier, CpG methylation of the MGMT promoter is associated with better outcome following alkylator therapy in GBMs, and methylation has been associated in some, but not all, studies, with low or absent MGMT activity [22], [23]. Here, we analyzed the relationship of MGMT activity with MGMT promoter methylation status, determined by MSP [30], [31], in 47 of the GBMs described above for which sufficient DNA was available. This subgroup is similar to the larger sample from which it was drawn in MGMT activity (11 ± 10 vs. 9.4 ± 11 fmol/106 cells) and comparable in PFS (5.5 vs. 6 months). In the 47 GBMs, greater than median MGMT activity was accompanied by greater risk of progression (HR = 1.98; P ≤ 0.04) and shorter PFS (5 vs. 6.5 months; P ≤ 0.03). Twenty-seven percent (12/45) of GBMs displayed methylated promoters; these tumors had a lower risk of progression (HR = 0.297; P ≤ 0.009) and longer PFS than the unmethylated tumors (7.5 vs. 5 months; P ≤ 0.002), in accord with previous observations [3]. Importantly, Fig. 2 shows that promoter methylation was accompanied by significantly lower mean MGMT activity (4.7 ± 2.6 vs. 14 ± 11 fmol/106 cells; P ≤ 0.001), suggesting that the longer PFS following alkylator therapy of methylated GBMs is associated with lower capacity to remove cytotoxic O6-alkylguanine adducts.
Fig. 2.
MGMT activity and promoter methylation status in GBM and AG. MGMT activities for 45 GBMs and 34 AGs are shown, together with the mean ± SD, for tumors displaying either unmethylated or methylated MGMT promoters, determined by MSP. Mean activity was significantly lower in methylated GBMs (4.4 ± 2.7 vs. 14 ± 11 fmol/106 cells; P ≤ 0.001) and AGs (3.7 ± 2.7 vs. 11 ± 8.6 fmol/106 cells; P ≤ 0.0001).
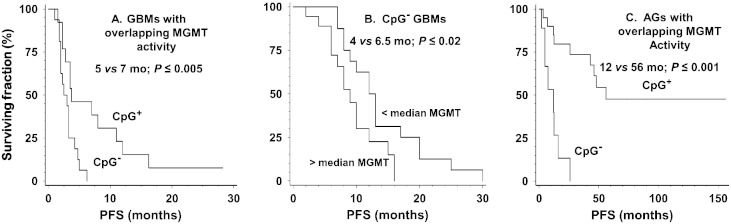
Fig. 2 also shows that the activities of 56% (19/34) of the unmethylated GBMs fell within the range observed for methylated tumors. As illustrated in Fig. 3A, PFS for these unmethylated tumors with low MGMT activity remained significantly shorter than that for methylated tumors (5 vs. 7.5 months; P ≤ 0.005). This finding suggests that low MGMT activity alone is not sufficient to account for the difference in survival between unmethylated and methylated tumors. As shown in Fig. 3B, further analysis restricted to the 34 unmethylated GBMs revealed that tumors with greater than median MGMT activity (10 fmol/106 cells for this sample) had shorter PFS (4 vs. 6.5 months; P ≤ 0.015) and a significantly higher risk for progression than tumors with less than median activity in a univariate model (HR = 2.45; P ≤ 0.030). This finding suggests that among unmethylated GBMs lower MGMT activity is accompanied by greater sensitivity to alkylators. The small number of methylated GBMs precluded comparable analysis.
Fig. 3.
Comparison of survival of MGMT promoter methylated and unmethylated GBM (A) and AG (C) with overlapping MGMT activities and of (B) promoter unmethylated GBM dichotomized by MGMT activity. Survival was calculated by the method of Kaplan–Meier. As illustrated in A and C, unmethylated GBMs and AGs had significantly shorter PFS despite having MGMT activity comparable to that of methylated tumors (6.3 ± 3.6 vs. 4.4 ± 3.76 fmol/106 cells for GBMs and 5.9 ± 3.4 vs. 3.8 ± 2.7 fmol/106 cells for AGs). Panel B illustrates the significantly shorter PFS of unmethylated GBM expressing greater than median MGMT activity (10 fmol/106 cells).
We also determined promoter methylation status in 34 of the AGs for which sufficient DNA was available. These tumors were comparable to the larger group from which they were drawn in MGMT activity (6.6 ± 6.7 vs. 5.9 ± 7.0 fmol/106 cells), and PFS (26 vs. 17 months) and proportion of diagnoses. This subgroup was also comparable in that greater than median MGMT activity was accompanied by significantly higher risk of progression (HR = 2.81; P ≤ 0.02) and shorter PFS (8 vs. 48 months; P ≤ 0.015). Promoter methylation was displayed by 59% (20/34) of AGs and was accompanied by significantly lower risk for progression (HR = 0.113; P ≤ 0.001) and longer PFS (56 vs. 8 months; P ≤ 0.001). As illustrated in Fig. 2, mean MGMT activity was 2.3-fold lower in methylated tumors (3.7 ± 2.7 vs. 11 ± 8.6 fmol/106 cells; P ≤ 0.0001). Fig. 2 also shows that 64% (9/14) of unmethylated AGs had MGMT activity that fell within the range observed for methylated tumors. However, low activity in these unmethylated tumors was not accompanied by the longer PFS observed for methylated AGs (12 vs. 56 months; P ≤ 0.001; Fig. 3C), suggesting that mechanisms in addition to MGMT are responsible for the better response to alkylators in methylated AGs.
4. Discussion
The role of MGMT in the response of GBM and other gliomas to treatment has been the focus of intensive investigation by the international neuro-oncology community during the last decade [2], [3], [4]. The association of improved response to chemo-RT with MGMT promoter methylation [3], a marker of epigenetic silencing of expression, suggests that impaired ability to remove cytotoxic O6-meG and other O6-alkylguanine adducts imparts better outcome following alkylator-based therapies. However, whether the better clinical outcome primarily reflects removal of cytotoxic O6-alkylguanine adducts remains to be confirmed, as previous studies have failed to consistently show that tumor MGMT expression or activity is inversely correlated with glioma response to alkylating agent-based therapies [9], [18], [19]. Elucidation of the underlying mechanism is critical if MGMT is to be used to direct treatment decisions and to serve as a target for anti-resistance therapies. Here we present evidence that low MGMT activity is associated with better response to alkylating agent therapy and with promoter methylation in high-grade gliomas.
A primary goal of our study was to examine the relationship of MGMT activity with PFS following alkylator-based therapy in 91 GBMs, the most frequently diagnosed glioma. We found a strong inverse association between PFS and activity following alkylating agent treatment in Cox regression models (Table 2). In the dichotomous model, GBMs with greater than median MGMT activity were more than twice as likely to progress relative to tumors with lower activity, resulting in a significantly shortened PFS (Fig. 1; Table 3). These observations are in accord with an earlier report that high MGMT activity is accompanied by significantly shorter PFS in 24 GBMs treated concomitantly with radiation and either TMZ or chloroethylating agents [22]. Of note, an analysis of MGMT expression by IHC in 418 GBMs found results comparable to ours for tumors harboring greater than the median fraction (30%) of immunopositive cells [19]. We also observed a significant inverse association between MGMT activity and PFS in continuous Cox regression models (Table 2), which revealed that GBMs with the highest and lowest activities differed in risk of progression by more than 5- to 7-fold. To our knowledge, this is the first examination of the association of MGMT activity with alkylator response in a continuous model that allows the evaluation of the relative risk of progression among individual GBMs rather than between two tumor cohorts.
We used PFS after initiating alkylator therapy as an endpoint in our analyses to provide evidence that the association between activity and clinical outcome is a reflection of MGMT-mediated removal of potentially lethal O6-alkylguanine lesions. This premise is supported by the lack of correlation of MGMT activity with PFS in GBM treated with RT alone or with overall survival (Table 2). Further evidence is provided by the independence of the relationship on prior clinical course and on the alkylator chemotherapy employed (Table 2). These observations suggest a causal relationship that is specific to alkylation and that low MGMT activity is not simply a non-specific marker for GBMs that are inherently more susceptible to any therapeutic intervention. The persistence of the relationship in smaller sub-groups of GBMs indicates that the association of activity with clinical response does not reflect the influence of a small number of unrepresentative samples, a conclusion validated by analysis of groups that either excluded specimens with outlier MGMT or extreme values for PFS (Table 2). These considerations, together with the reduced risk of progression that accompanies low activity, support the development of clinically tractable methods to inhibit MGMT expression in order to suppress resistance to alkylating agents in GBM and improve therapeutic outcome.
In accord with our results for GBM, we found that MGMT activity is inversely associated with response to alkylating agent therapy in AGs, regardless of diagnosis and clinical course, in both dichotomous and continuous regression models (Table 5). Notably, anaplastic tumors have appreciably lower mean MGMT activity than GBM (5.9 ± 7.0 vs. 10 ± 11 fmol/106 cells; P ≤ 0.004), suggesting that the longer post-alkylator PFS observed for AGs (17 vs. 6 months; P ≤ 0.001), reflects, at least in part, a greater dependence on MGMT for resistance. This conclusion is supported by the significant difference in HR between newly operated AGs and GBMs (HR = 1.124; CI [1.071; 1.180] vs. HR = 1.033 CI [1.012; 1.054]; note non-overlapping CIs). This difference in risk persists when analysis excludes AGs known to harbor 1p and/or 19q deletions (HR = 1.117; CI [1.061; 1.175]), suggesting the greater risk does not reflect the presence of treatment hypersensitive deleted tumors. While the difference in alkylator sensitivity likely involves a multiplicity of intrinsic factors, AGs may not be as proficient in dealing with the cytotoxic consequences of unrepaired O6 = alkylguanine (e.g., replication fork collapse; double-strand break formation [11], [33]), thus increasing the vulnerability of tumors with low MGMT activity. While it is difficult to definitively assign significance to these findings because of the small cohort size and the divergent clinical behavior for each AG diagnosis, they do suggest that suppression of MGMT activity in AGs may be as or more effective than in GBMs.
We also found that the reduced risk of progression in GBMs and AGs displaying MGMT promoter methylation was accompanied by lower MGMT activity (Fig. 2). Our findings are in accord with those of others [13] as well as the recent report that the fraction of cells immunopositive for MGMT is significantly lower in methylated GBMs [19]. These results support the hypothesis that the better treatment outcome associated with promoter methylation is due, at least in part, to decreased removal of O6-alkylguanine adducts. However, we found that the range of MGMT activities overlapped appreciably between methylated and unmethylated GBMs and AGs. Such overlap has been reported previously for both MGMT activity [22] and MGMT protein expression [19] in GBMs. Detectable activity in methylated GBMs may in part reflect the presence of contaminating normal cells that express MGMT. However, emerging data strongly indicate that MGMT expression in GBMs is directly correlated with the number of methylated CpG dinucleotides in the MGMT promoter [13], [19], a feature not evaluated by the MSP assay. Thus, tumor cells harboring incompletely methylated promoters may contribute to the activity in GBMs judged to be methylated by MSP. The overlap of activities may also reflect induction of MGMT expression in methylated tumors in response to exposure to corticosteroids, as suggested by work of Weiler et al. [4]. In accord with this hypothesis, Weiler et al. found that PFS was reduced for elderly patients with promoter methylated GBMs following concomitant treatment with corticosteroids and alkylators compared to treatment with alkylators alone. Nevertheless, we found that the prolonged PFS that accompanies low MGMT activity in methylated gliomas was not observed in unmethylated tumors with comparable activity (Fig. 3 A & C). This novel finding suggests that MGMT promoter methylation is associated with an alkylation sensitive phenotype that is absent in unmethylated gliomas with low MGMT activity. While the identity of the genes critical for clinical response remains to be completely elucidated, promoter CpG island methylation appears to silence the expression of a number of DNA repair activities in addition to MGMT, suggesting possible candidate genes that influence sensitivity to alkylating agent-based treatment [34]. These include components of base excision repair that excise cytotoxic N-alkylpurines and abasic sites [10], [19], and of post-replication repair pathways that either prevent or repair the double-strand breaks that can arise when DNA replication forks encounter unrepaired O6-alkylguanine adducts [33], [35]. Suppression of these repair activities has been shown to enhance alkylator sensitivity in human glioma cell lines [10], [28], [36], [37]. Demonstration of concomitant suppression of MGMT and one or more of these DNA repair activities accompanying better response to therapy would further elucidate the mechanism(s) responsible for alkylation hypersensitivity accompanying MGMT promoter methylation in malignant gliomas and identify new targets for anti-resistance therapies.
A second novel finding of our study is that the inverse association between MGMT activity and PFS persists in unmethylated GBMs (Fig. 3B). This result demonstrates the relationship between MGMT activity and PFS in the presence of resistance mechanisms that counter the cytotoxicity of unrepaired O6-meG in unmethylated GBMs that are absent or reduced in methylated tumors. This finding also suggests that MGMT activity may be used to stratify risk of progression within groups of unmethylated and methylated tumors as has been proposed for MGMT protein expression evaluated by IHC [19]. While the high sensitivity of biochemical assay and the wide range of activities displayed by GBMs and AGs (~ 300-fold) support the use of MGMT activity to further stratify risk of progression, the nature of the biochemical assay, requiring extract preparation from intact tissue and the use of a radioactive substrate that is not commercially available, make it unsuitable for routine clinical use. Nonetheless, our results suggest that biochemical assay has an important role in characterizing the contribution of MGMT to resistance to alkylating agent treatment and in corroborating promoter methylation assays.
5. Conclusions
The biochemical activity of MGMT is inversely associated with PFS following alkylating-agent based chemotherapy in GBM and AGs. The relationship is specific to alkylating agents and is independent of clinical course and alkylator regimen indicating that the association reflects the removal of O6-alkylguanine adducts. Significantly lower mean activity is positively correlated with MGMT promoter CpG methylation despite considerable overlap of activity between methylated and unmethylated tumors. The shorter PFS displayed by unmethylated tumors with overlapping activity suggests that resistance mechanisms in addition to MGMT are suppressed in methylated tumors. Our findings emphasize the need to develop strategies to inhibit MGMT activity in order to improve clinical outcome in malignant gliomas.
Acknowledgments
We thank S. Fazal, A. Goesling, C. Rostomily and E. King for theirtechnical assistance. This work was supported by NIH grants CA82622 and CA104593 (JR Silber) and from donations to the Brain Tumor Research Fund in memory of Ro Jean Mount. All authors declare no conflict of interest,
References
- 1.Schwartzbaum J.A., Fisher J.L., Aldape K.D., Wrensch M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain M.C. Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert. Rev. Neurother. 2010;10:1537–1544. doi: 10.1586/ern.10.32. [DOI] [PubMed] [Google Scholar]
- 3.Wick W., Weller M., van den Bent M., Sanson M., Weiler M., von Deimling A., Plass C., Hegi M., Platten M., Reifenberger G. MGMT testing—the challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 4.Weller M., van den Bent M., Hopkins K., Tonn J.C., Stupp R., Falini A., Cohen-Jonathan-Moyal E., Frappaz D., Henriksson R., Balana C., Chinot O., Ram Z., Reifenberger G., Soffietti R., Wick W. European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 5.Louis D.N., Ohgaki H., Wiestler O.D. International Agency for Research on Cancer (IARC) Press; Lyon: 2007. WHO Classification of Tumours of the Central Nervous System. [Google Scholar]
- 6.Walker C., Baborie A., Crooks D., Wilkins S., Jenkinson M.D. Biology, genetics and imaging of glial cell tumours. Br. J. Radiol. 2011;84(Spec No 2):S90–S106. doi: 10.1259/bjr/23430927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wick W., Hartmann C., Engel C., Stoffels M., Felsberg J., Stockhammer F., Sabel M.C., Koeppen S., Ketter R., Meyermann R., Rapp M., Meisner C. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross G., Wang M., Shaw E., Jenkins R., Brachman D., Buckner J., Fink K., Souhami L., Laperriere N., Curran W., Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silber J.R., Bobola M.S., Blank A., Chamberlain M.C. O6-Methylguanine-DNA methyltransferase in glioma therapy: promise and problems. Biochim. Biophys. Acta. 2012;1826:71–82. doi: 10.1016/j.bbcan.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobola M.S., Kolstoe D.D., Blank A., Chamberlain M.C., Silber J.R. Repair of 3-methyladenine and abasic sites by base excision repair mediates glioblastoma resistance to temozolomide. Front Oncol. 2012;2 doi: 10.3389/fonc.2012.00176. (Article176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaina B., Christmann M., Naumann S., Roos W.P. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair. 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Marchesi F., Turriziani M., Tortorelli G., Avvisati G., Torino F., De Vecchis L. Triazene compounds: mechanism of action and related DNA repair systems. Pharmacol. Res. 2007;56:275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Wiewrodt D., Nagel G., Dreimüller N., Hundsberger T., Perneczky A., Kaina B. MGMT in primary and recurrent human glioblastomas after radiation and chemotherapy and comparison with p53 status and clinical outcome. Int. J. Cancer. 2008;22:1391–1399. doi: 10.1002/ijc.23219. [DOI] [PubMed] [Google Scholar]
- 14.van den Bent M.J., Gravendeel L.A., Gorlia T., Kros J.M., Lapre L., Wesseling P., Teepen J.L., Idbaih A., Sanson M., Smitt P.A., French P.J. A hypermethylated phenotype is a better predictor of survival than MGMT methylation in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. Clin. Cancer Res. 2011;17:7148–7155. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 15.Wick W., Platten M., Meisner C., Felsberg J., Tabatabai G., Simon M., Nikkhah G., Papsdorf K., Steinbach J.P., Sabel M., Combs S.E., Vesper J., Braun C., Meixensberger J., Ketter R., Mayer-Steinacker R., Reifenberger G., Weller M., NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 16.Shaw E.G., Wang M., Coons S.W., Brachman D.G., Buckner J.C., Stelzer K.J., Barger G.R., Brown P.D., Gilbert M.R., Mehta M.P. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J. Clin. Oncol. 2012;30:3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weller M., Stupp R., Hegi M.E., van den Bent M., Tonn J.C., Sanson M., Wick W., Reifenberger G. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro. Oncol. 2012;14(S4):iv100–iv108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason S., McDonald K. MGMT testing for glioma in clinical laboratories: discordance with methylation analyses prevents the implementation of routine immunohistochemistry. J. Cancer Res. Clin. Oncol. 2012;138:1789–1797. doi: 10.1007/s00432-012-1312-1. [DOI] [PubMed] [Google Scholar]
- 19.Lalezari S., Chou A.P., Tran A., Solis O.E., Khanlou N., Chen W., Li S., Carrillo J.A., Chowdhury R., Selfridge J., Sanchez D.E., Wilson R.W., Zurayk M., Lalezari J., Lou J.J., Ormiston L., Ancheta K., Hanna R., Miller P., Piccioni D., Ellingson B.M., Buchanan C., Mischel P.S., Nghiemphu P.L., Green R., Wang H.J., Pope W.B., Liau L.M., Elashoff R.M., Cloughesy T.F., Yong W.H., Lai A. Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro. Oncol. 2013;15:370–381. doi: 10.1093/neuonc/nos308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineura K., Izumi I., Watanabe K., Kowada M. Influence of O6-methylguanine-DNA methyltransferase activity on chloroethylnitrosourea chemotherapy in brain tumors. Int. J. Cancer. 1993;55:76–81. doi: 10.1002/ijc.2910550115. [DOI] [PubMed] [Google Scholar]
- 21.Silber J.R., Blank A., Bobola M.S., Ghatan S., Kolstoe D.D., Berger M.S. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin. Cancer Res. 1999;5:807–814. [PubMed] [Google Scholar]
- 22.Christmann M., Nagel G., Horn S., Krahn U., Wiewrodt D., Sommer C., Kaina B. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int. J. Cancer. 2010;127:2106–2118. doi: 10.1002/ijc.25229. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell J.A., Johnson S.P., Quinn J.A., McLendon R.E., Ali-Osman F., Friedman A.H., Herndon J.E., II, Bierau K., Bigley J., Bigner D.D., Friedman H.S. Quantitative analysis of O6-alkylguanine-DNA alkyltransferase in malignant glioma. Mol. Cancer Ther. 2006;5:2531–2539. doi: 10.1158/1535-7163.MCT-06-0106. [DOI] [PubMed] [Google Scholar]
- 24.Silber J.R., Bobola M.S., Ghatan S., Blank A., Kolstoe D.D., Berger M.S. O6-methylguanine-DNA methyltransferase activity in adult gliomas: relation to patient and tumor characteristics. Cancer Res. 1998;58:1068–1073. [PubMed] [Google Scholar]
- 25.Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A.A. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 26.Levin V.A., Edwards M.S., Wright D.C., Seager M.L., Schimberg T.P., Townsend J.J., Wilson C.B. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat. Rep. 1980;64:237–244. [PubMed] [Google Scholar]
- 27.Levin V.A., Prados M.D. Treatment of recurrent gliomas and metastatic brain tumors with a polydrug protocol designed to combat nitrosourea resistance. J. Clin. Oncol. 1992;10:766–771. doi: 10.1200/JCO.1992.10.5.766. [DOI] [PubMed] [Google Scholar]
- 28.Blank A., Bobola M.S., Gold B., Varadarajan S., Kolstoe D., Meade E.H., Rabinovitch P.S., Loeb L.A., Silber J.R. The Werner syndrome protein confers resistance to the DNA lesions N3-methyladenine and O6-methylguanine: implications for WRN function. DNA Repair. 2004;3:629–638. doi: 10.1016/j.dnarep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Gendimenico G.J., Bouquin P.L., Tramposch K.M. Diphenylamine-colorimetric method for DNA assay: a shortened procedure by incubating samples at 50 degrees C. Anal. Biochem. 1988;173:45–48. doi: 10.1016/0003-2697(88)90156-x. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M., Garcia-Foncillas J., Andion E., Goodman S.N., Hidalgo O.F., Vanaclocha V., Baylin S.B., Herman J.G. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 31.Hegi M.E., Diserens A.C., Gorlia T., Hamou M.F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., Bromberg J.E., Hau P. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 32.Bobola M.S., Tseng S.H., Blank A., Berger M.S., Silber J.R. Role of O6-methylguanine-DNA methyltransferase in resistance of human brain tumor cell lines to the clinically relevant methylating agents temozolomide and streptozotocin. Clin. Cancer Res. 1996;2:735–741. [PubMed] [Google Scholar]
- 33.Roos W.P., Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Toyota M., Suzuki H. Epigenetic drivers of genetic alterations. Adv. Genet. 2010;70:309–323. doi: 10.1016/B978-0-12-380866-0.60011-3. [DOI] [PubMed] [Google Scholar]
- 35.Alexander B.M., Pinnell N., Wen P.Y., D'Andrea A. Targeting DNA repair and the cell cycle in glioblastoma. J. Neurooncol. 2012;107:463–477. doi: 10.1007/s11060-011-0765-4. [DOI] [PubMed] [Google Scholar]
- 36.Agnihotri S., Gajadhar A.S., Ternamian C., Gorlia T., Diefes K.L., Mischel P.S., Kelly J., McGown G., Thorncroft M., Carlson B.L., Sarkaria J.N., Margison G.P. Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Invest. 2012;122:253–266. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C.C., Taniguchi T., D'Andrea A. The Fanconi anemia (FA) pathway confers glioma resistance to DNA alkylating agents. J. Mol. Med. 2007;85:497–509. doi: 10.1007/s00109-006-0153-2. [DOI] [PubMed] [Google Scholar]