Abstract
Aim
To describe the rationale and design of a pilot program to implement and evaluate pharmacogenetic (PGx) testing in a primary care setting.
Study rationale
Several factors have impeded the uptake of PGx testing, including lack of provider knowledge and challenges with operationalizing PGx testing in a clinical practice setting.
Study design
We plan to compare two strategies for the implementation of PGx testing: a pharmacist-initiated testing arm compared with a physician-initiated PGx testing arm. Providers in both groups will be required to attend an introduction to PGx seminar.
Anticipated results
We anticipate that providers in the pharmacist-initiated group will be more likely to order PGx testing than providers in the physician-initiated group.
Conclusion
Overall, we aim to generate data that will inform an effective delivery model for PGx testing and to facilitate a seamless integration of PGx testing in primary care practices.
Keywords: clinical utility, pharmacist support, pharmacogenetics, pharmacogenetic testing, primary care
Background
Although genetics has long been recognized to play a role in drug response [1], the tremendous amount of research recently has catapulted understanding of the impact of genetic variation on drug targets and risk of adverse drug response (ADR), leading to the development of several clinical pharmacogenetic (PGx) tests. Genetic variation is estimated to account for 20–95% of the differences in individual responses to medications [2] and 137 drug labels currently include information about the impact of PGx variants [3]. More than half (59%) of the drugs with frequently reported ADRs are metabolized by at least one CYP450 enzyme that is highly polymorphic, resulting in a range of metabolic activity [4]. Thus, analysis of particular genetic variants through PGx testing may help optimize pharmaceutical therapy by identifying patients with these polymorphisms and better informing the selection of the proper drug and dosage and reducing risk of ADRs. The substantial healthcare costs associated with ADRs [5–8], the increasing use of prescription drugs in the USA [9] and other potential benefits of PGx testing such as increased medication adherence [10,11] provide further support for the use of PGx testing to personalize care.
Uptake and use of new clinical applications are determined by numerous factors [12] and many groups are developing and testing tailored implementation strategies to maximize uptake and appropriate use. Health providers have expressed interest in applying new genetic technologies to improve therapeutic decision-making [13–15], including PGx testing. Primary care providers (PCPs) prescribe a substantial proportion of drugs [16,17], many known to be impacted by PGx variants [17–20]. For example, one study estimated that 29% of patients in primary care practices were taking at least one of 16 drugs that are metabolized by the polymorphic CYP450 enzymes [20]. Thus, primary care patients may particularly benefit from PGx testing [21]. However, utilization of PGx tests has been limited, reportedly owing to lack of provider awareness about available tests, lack of knowledge about genetics and drug response [22–27], lack of conclusive evidence of clinical utility [28], and concerns regarding coverage and reimbursement [29]. In addition, it is uncertain how PGx testing may be most effectively integrated into the current healthcare system. Testing may be ordered upon initiation of a particular drug or prospectively, as part of routine examination, each with their own advantages and disadvantages including potential delay of treatment and reimbursement [30]. Some pharmacy benefit managers have promoted PGx testing [31]; although healthcare providers still appear uncertain about the use of the test results [32]. Overall, the integration of PGx testing has been slower than anticipated.
To address some of the barriers to the use of PGx testing in the primary care setting, particularly provider knowledge and operationalization, we developed a study to implement and evaluate two educational and clinical support interventions to facilitate integration of PGx testing into primary care: provider-initiated and pharmacist-initiated PGx testing. Both interventional strategies include provider education, but one arm is a provider-initiated PGx testing program with on-call pharmacist support and the other is a pharmacist-initiated PGx testing program with a clinic-based pharmacist making suggestions for PGx testing at the point of care. In this paper, we describe the study rationale, study design and outcome assessments.
Study rationale
While the translational framework of genomic medicine has been described optimally as four sequential phases (gene discovery to health application to evidence-based guidelines to health practice to health impact) [33], the practical translation (in a nonresearch setting) is more difficult to define owing to multiple potential paths and other challenges. The integration of PGx testing into clinical practice will not only require careful consideration of effects on physician practices (particularly with respect to any additional time needed to consent and/or communicate results to patients, which may ultimately serve as a barrier to uptake of PGx testing), but also on the impact of test results on patient behaviors, such as medication adherence. Using two potential delivery systems, this study aims to address the barrier of limited physician knowledge about PGx, as well as operational issues (e.g., when to order testing, from what laboratory and for which drug). We have chosen to focus on primary care practices given the extensive number of drugs impacted by PGx variants prescribed in these settings, the potential to substantially improve treatment for a large number of patients and the paucity of studies in this important community.
Study design
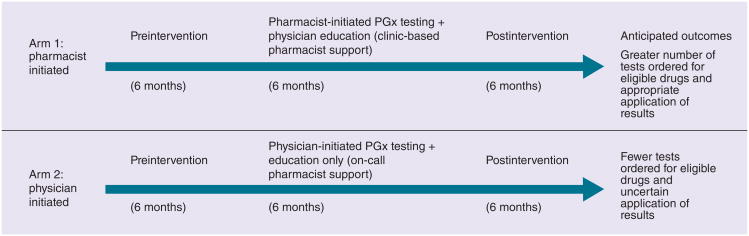
The study aims to assess two delivery models of PGx testing for commonly prescribed drugs in primary care practices and to evaluate the delivery of testing from three perspectives: physician, patient and practice setting. As shown in Figure 1, the study aims to compare a 6-month intervention in which physicians order PGx testing according to their delivery system (pharmacist initiated or physician initiated), and surveying participating physicians and patients for whom testing was ordered at two time points in the study. We chose to have a pharmacist serve as the main clinical decision support for providers in the primary care practices during the intervention period given their experience with providing many different clinical services within different practice settings [34,35] and their knowledge and training in pharmacokinetics and pharmacodynamics, including PGx. In addition, pharmacists have played an instrumental role in the delivery of PGx testing in other clinic settings [36,37]. Prior to the start of the intervention period, all providers are required to attend an educational session about PGx. The study has been approved by the Duke University Health System Institutional Review Board and registered at clinicaltrials.gov (NCT01600846).
Figure 1. Overview of pharmacogenetic delivery model study design and timeline.
PGx: Pharmacogenetic.
Provider education
Two types of provider education/support were developed for the study: printed/online materials and a continuing medical education (CME) presentation. As CME courses on genetics and PGx have been demonstrated to be an effective way to introduce new applications and even increase personal interest [38–42], we developed a 1-h CME-accredited presentation on PGx testing presented by one of the investigators (G Ginsburg and A Cho). The CME presentation is required for all physician participants. The learning objectives of the CME presentation include genetic processes and terminology, history of pharmacogenetics, two PGx case studies (warfarin and codeine), overview of recommendations and clinical guidelines from the US FDA, and relevant ethical, legal and social issues. Printed resources are also provided, including a pocket guide of each of the drugs for which PGx testing is provided in the study, information about the major genetic variants in the relevant genes, and how the test result could impact drug selection or dosing. For more convenient access, the information in the pocket guide as well as additional resources was also available online. Additionally, in order to assist providers in discussing PGx testing with their patient, an educational brochure was developed to be given to patients describing personalized medicine, and specifically PGx testing. The patient materials were evaluated and revised through a series of cognitive interviews conducted with 12 members of the public, recruited from Durham, NC, USA, prior to being used by providers.
PGx testing
A total of six genetic tests associated with the metabolism of 12 drugs are available in this study: CYP2D6, CYP2C19, HLA-B*1502, CYP2C9, SLC01B1 and VKORC1/CYP2C9 (Table 1). We selected these 12 drugs based on the list of 16 ADR-associated medications identified by Grice et al. [18] and commonly prescribed drugs used in primary care. All but one of the eligible medications for the study included PGx information in the drug label [3]. The one drug that did not have PGx information in the drug label was simvastatin; however, multiple papers have validated the association of the SLCOB1 variant and risk of myopathy [43–46]. With the prevalent use of statins in the primary care setting, it was thus deemed an important drug to include in the study. In addition, 58% of these medications listed had Clinical Pharmacogenetic Implementation Consortium (CPIC) guidelines available to further aid in optimizing drug therapy for the patient. Furthermore, the medications listed in Table 1 were selected based off the Table of Pharmacogenomic Biomarkers in Drug Labeling [3]. A saliva sample for DNA extraction is collected using the Oragene-DNA® kit from patients who consented to testing based on their physician's recommendation. All testing is performed by the Mayo Medical Laboratory (MN, USA).
Table 1.
List of medications and genes eligible for the study.
| Drug | Gene | CPIC or other guideline available |
| Codeine | CYP2D6 | Yes |
| Fluoxetine | CYP2D6 | No |
| Nortriptyline | CYP2D6 | Yes |
| Imipramine | CYP2D6 | Yes |
| Metoprolol | CYP2D6 | No |
| Warfarin | VKORC1/CYP2C9 | Yes |
| Clopidogrel | CYP2C19 | Yes |
| Carbamazepine | HLA-B*1502 | Yes |
| Esomeprazole | CYP2C19 | No |
| Atomoxetine | CYP2D6 | No |
| Celecoxib | CYP2C9 | No |
| Simvastatin | SLCO1B1 | Yes |
Testing is provided at no cost to the patient for select drugs with PGx evidence to support adjustment to drug or dosing decisions. We recognize that covering the costs of testing may artificially increase use of testing. However, given the uneven coverage of testing in the US by public and private insurers [47], the study population would be potentially biased to those patients with insurance coverage or able to afford testing expenses out-of-pocket without enabling all patients access to testing if indicated.
Pharmacist-initiated intervention
In the pharmacist-initiated intervention, a pharmacist is based at the practice location to screen patients prescribed a new targeted medication during their clinic visit and provide on-site consultation to providers about testing. Specifically, the pharmacist alerts the provider about the availability of PGx testing for patients prescribed one of the targeted drugs via electronic messaging through the electronic medical record. As a result, the provider receives the pharmacist recommendation after the prescription is written, similar to the current pharmacy benefit manager model where notification about testing is carried out after prescribing [48]. Decisions about testing, interpretation of test results, communication of results with patients, and any decision to continue or change therapy are at the sole discretion of the PCP, although physicians can consult the pharmacist with questions. All pharmacist interactions with physicians are noted for data analysis, including the nature of the interaction and time spent per interaction.
Provider-initiated intervention
In the provider-initiated practice, the decision to offer PGx testing is determined by the PCP unassisted by the pharmacist. If contacted by the PCP, the on-call pharmacist provides support and responds to questions or issues related to testing processes/procedures, interpretation of test results and/or treatment recommendation. Decisions about testing, interpretation of test results, communication of results with patients and any decision to continue or change therapy are at the sole discretion of the PCP. All pharmacist interactions with physicians are noted for data analysis, including the nature of the interaction and time spent per interaction.
For both the pharmacist-initiated and provider-initiated arm, the ordering of PGx testing at the point of care (when medication is needed) may impact decisions about whether to order testing or when/how to incorporate changes to therapeutic decisions. In particular, the turnaround time for test results ranges from 3–7 days and therefore, providers will need to decide if delaying treatment is feasible or medically necessary until the results are available or if they should prescribe a standard or lower dose while awaiting the results.
Study population
Owing to the nature of this study, two groups are considered research participants: PCPs and patients.
Providers
This pilot study focuses on patients and providers from two internal medicine clinics affiliated with Duke University and located in Durham, NC, USA. Both clinics are part of Duke Primary Care, a network of community-based practices. The two clinics have eight and nine full-time physician faculty, respectively. Only providers that attend the CME course are eligible to participate.
Patients
All patients who are offered PGx testing at one of the two primary care clinics are eligible to participate in the study. Patients in both clinics represent a diverse patient population with respect to age, sex, race and payer mix. The clinic for the pharmacist-initiated arm sees approximately 24,000 patients annually; the patient population is predominately white (72%), black (26%) and Asian (2%), and most are covered by a managed care plan (39%), Blue Cross/Blue Shield (25%) or Medicare (25%). Most patients are 50–64 years of age (28%); 65% are 65+ and 21% are 35–49 years old. The clinic for the provider-initiated arm serves approximately 21,000 patients annually; most are covered by Medicare (32%), a managed care plan (31%) or Blue Cross/Blue Shield (29%). Eligible patients must be 18 years or older, prescribed a drug listed in Tab le 1, able to consent on their own, and able to read and write in English.
Assessments
Physician survey
To assess provider attitudes, knowledge and experience of genetic and PGx testing, an online survey is administered to providers prior to the CME session (Supplementary Material; see online at: www.futuremedicine.com/doi/suppl/10.2217/pgs.14.109). Specifically, the preintervention survey includes two questions about provider and practice history (year of graduation from medical school and number of years in practice), and six questions about experience with general genetic testing including how often they ordered genetic diagnostic or susceptibility tests, how often they referred patients to genetic specialists and how comfortable they feel ordering and discussing genetic testing results. A similar set of questions is asked regarding PGx testing specifically. Physicians are also asked about their awareness of PGx testing, where they learned about PGx testing and questions about their perceptions of the usefulness of PGx testing. To assess provider knowledge of genomics and PGx, we developed a nine-question knowledge component. All knowledge questions are multiple choice, two of the questions are based on hypothetical case reports and one is about genetic discrimination protections. The survey also includes eight questions about factors impacting use of PGx testing and clinical support for and education about PGx testing, many adapted from our previous study [15]; in particular, providers are asked about the perceived roles of various providers (physicians, pharmacists and genetic counselors) in the delivery of PGx.
To assess their experience with PGx testing and changes in attitudes and knowledge, a second survey is administered after completion of the intervention phase. All providers who complete the preintervention survey and attend the CME presentation are emailed a link to the online follow-up survey. In the post intervention survey, providers are asked to indicate the perceived value of the CME presentation, the educational materials, and their interactions (if any) with the pharmacist. They are also asked to provide feedback regarding the PGx laboratory test report. Questions regarding providers' perceptions of and comfort with using PGx testing are slightly modified from the preintervention and repeated to assess change over time. Changes in providers' knowledge about PGx are assessed using the same nine-question survey from preintervention. Providers are asked what barriers they experienced when using PGx testing, factors that could improve/reduce those barriers and their likelihood of continued use of PGx testing after the study. Finally, providers are asked to report their perceptions of patient preferences and value about testing.
Patient survey
To gather data on patient attitudes and experiences with PGx testing, patients are also invited to complete online surveys (Supplementary Material). Providers who discuss PGx testing with eligible patients ask patients to participate in an online survey to gather patient feedback on attitudes and interest in PGx testing, regardless of whether they underwent testing or not. The study brochure developed for patients provides a link to the first online survey. A consent statement at the beginning of the survey provides information about the study, eligibility criteria, a description of participant requirements, associated risks (i.e., loss of confidentiality), benefits, reimbursement and contact information for study staff. If the patient wishes to complete the survey, they are asked to click-through: “To consent to participate and complete the survey, please click below to continue to the next page.” Continuing to the survey is an assumption of consent.
The patient survey collects demographic information including gender, age and race. Patients are asked about their personal and family history of side effects from prescribed medication as well as their personal perceived knowledge and familial experience with genetics and genetic testing. In addition, the survey includes questions about patients' beliefs about the need for and related concerns about medications using the validated Beliefs about Medicines Questionnaire (BMQ) [49]. Patients are also asked to report what factors influenced their decision to have or not to have PGx testing. Upon completion of the baseline survey, patients who consent to PGx testing are invited to participate in a follow-up survey 3 months later. Those willing to participate provide contact information and are sent a link via email to complete the online follow-up survey. The goal of the follow-up survey is to understand the patients' experiences with PGx testing including whether or not they received results, how they received them, what changes if any they recalled being made to their care and their general understanding of the results. Their perceptions of PGx are also assessed regarding their satisfaction, trust in the results and perceived helpfulness of the PGx results. Patients' information-seeking behavior, medication adherence and overall awareness of side effects are also assessed. To measure drug adherence, we use the validated Morisky Medication Adherence Scale [50]. The BMQ instrument is also included in the post-testing survey to assess the impact of PGx testing on patient beliefs or concerns about medication use as well.
Chart review
To assess provider behaviors before, during and after the study with respect to the number of prescriptions written for drugs known to be impacted by PGx variants and the use of PGx testing, patient charts are reviewed at three time points in the study: 6-month preintervention period; 6-month intervention period; and 6-month postintervention period. The preintervention review assesses new and recent (within 1 month of the reviewed date) prescriptions for drugs listed in Table 1, number of PGx tests ordered, by which physician (using a unique identifier code) and for which drugs. The intervention review assesses the effectiveness of the intervention during a 6-month period based on number of PGx tests ordered. Following the intervention period, several data points are to be abstracted from a chart review of patients seen during the intervention period to determine the number of targeted drugs prescribed, the number of patients prescribed targeted drugs, the number of targeted drugs per patient, the number of PGx tests ordered for each drug and for each patient, which physician ordered the test, whether the pharmacist was consulted pre- or post-testing, physician counseling/follow-up visit to discuss test results, and how the results were applied to treatment decisions including whether treatments were changed owing to nonresponse or side effects, and occurrence of drug-related side effects. For many of the drugs on the study medication list, we anticipate that patients newly prescribed these drugs will have a follow-up visit with the provider within several months, and therefore, we expect to be able to capture most of these data. The postintervention review assesses the durability of the intervention through the following data abstractions: the number of new prescriptions for drugs listed in Table 1, the frequency with which PGx testing is ordered, by which provider and for which drugs.
Study measures
Four groups of study measures will be evaluated (Table 2). First, we will evaluate the effectiveness of two delivery models on provider behaviors by assessing change and durability of any changes based on the number of tests ordered, dosage adjustments, adverse responses and drug discontinuations of target drugs with available PGx testing during the 6-month intervention period compared with the 6 months prior and the 6 months following the intervention. The data to inform these study measures will be obtained through chart review. Given the lack of in-depth knowledge and expertise of most providers regarding PGx testing and in light of their heavy workload, we hypothesize that the pharmacist-initiated approach will result in greater and appropriate use of PGx testing given the hands-on educational approach with the pharmacist providing expert guidance. Thus, through chart reviews, we will gather data on the number of tests ordered during each study phase, for which drugs (to determine if testing was indicated) and how the results were applied to therapeutic decisions.
Table 2.
Groups of study measures to be evaluated.
| Study categories | Measures |
| Effectiveness of delivery model | Number of tests ordered, changes in drug selection, dosage adjustments, adverse drug reactions and drug discontinuations |
| Provider knowledge and attitudes | Provider knowledge, perceived value of testing |
| Patient attitudes and beliefs | Beliefs about medications, information-sharing and seeking behavior, medication adherence and overall awareness of side effects |
| Economic outcomes | Pharmacist consultation time, costs associated with alternative medications, monitoring costs and adverse drug events |
Second, we will assess and compare provider knowledge, attitudes, perceived value and self-reported use of PGx testing in each practice before completion of the educational CME and after the intervention period. The data obtained from provider surveys will be used assess this group of study measures. We hypothesize that greater utilization will be influenced by increased provider knowledge and awareness of drugs with PGx testing, comfort in discussing PGx testing with patients and applying results to therapeutic decision-making and positive attitudes towards the testing process. We hypothesize that having a pharmacist with expertise in PGx testing as part of the medical team will result in greater physician comfort with test ordering and application of results as compared with the physician-initiated model.
As patient perceptions are increasingly used to assess quality of care [51–53], we will assess the impact of PGx testing on patients with respect to information sharing and seeking, medication adherence and overall awareness of side effects. We will assess their perceived value of PGx testing, potential concerns about testing, and experience with the testing process and impact on care and medication adherence. The patient surveys will be used for this group of measures. While perceived safety or efficacy may increase as a result of PGx testing, we hypothesize that perceptions of drug safety or efficacy may be negatively affected in those with very high or low metabolizing status and therefore, medication adherence may worsen.
Fourth, we will perform an economic analysis to evaluate the costs associated with the physician-initiated and pharmacist-initiated delivery models as well as costs associated with targeted medications, associated monitoring costs, and adverse drug events. We will combine primary data collection and sound costing methods to compare associated costs between the PGx delivery models and between the study's time periods to evaluate changes in costs associated with the PGx delivery models. These comparisons will provide preliminary information about whether potential savings owing to avoided adverse drug events, reduced follow-up care and/or reduced medication costs could offset the costs associated with PGx testing.
Analysis
Data collected through chart review and surveys will be analyzed using a software package such as STATA. We will calculate frequency data for all survey questions, and perform χ2 to assess associations between respondent characteristics and knowledge and attitudes. Analysis of the association of categorical variables will be performed using Pearson χ2 tests and Fisher's exact test. Binary variables will be created from the Likert response scales by clustering responses (e.g., very or somewhat likely). Odds ratios and 95% CIs will be generated to assess strength of associations adjusting for covariates. For most drugs, we do not anticipate having adequately large sample sizes, and thus, will combine data between the two clinic sites to analyze for trends regarding physician and patient knowledge and attitudes.
Discussion
In order to determine the effectiveness of delivering and/or incorporating PGx testing into primary care settings, we seek to evaluate two delivery models of pharmacist-assisted delivery of PGx testing. Pharmacists can serve as valuable members of the clinic or medical home, collaborating with PCPs to perform medication-related assessments, including test ordering, monitoring and adjusting therapies to improve overall patient care, and now PGx consultations [54–56]. Obviously, it will not be practical or cost effective to place a pharmacist in every primary care setting and therefore, other clinical delivery approaches are needed both to assist healthcare providers and perform patient care. To broaden outreach and increase efficiency, one option may be phone-based support by a central pharmacist group to provide both clinical decision support and patient services. As we are assessing in this pilot study, this option may be feasible once PGx testing awareness increases among providers, but in-person consultation may be necessary during the early stages of clinical use. One hospital has implemented a pharmacy on-call assistance program for test interpretation and application, working closely with the molecular testing laboratory [57]. Thus, it is possible that different settings will warrant different clinical decision support and electronic decision support may become a primary tool in initiating PGx testing and dosing medications based on PGx results.
It will be important to consider the costs and outcomes associated with any delivery model and thus, we designed the study to enable prospective analysis of economic factors. The published literature reporting economic outcomes associated with PGx testing is beginning to mature [58,59]. Most of the literature pertains to use of PGx testing in oncology, wherein several studies demonstrate good value with PGx testing in early breast cancer [60–62] and variable findings for other cancers [63,64]. Several recent papers have focused on the cost–effectiveness of PGx testing in primary care with variable findings about the potential value of specific PGx tests [65–69]. Although the quality of economic evaluations of PGx testing appears to be increasing with more studies based on data from randomized trials [70,71], the lack of robust data on the clinical utility of many PGx tests limits the development of high-quality evidence on their cost–effectiveness [59].
Conclusion
In conclusion, we aim to evaluate delivery models for PGx testing implemented in a primary care clinic. We anticipate that our study will help provide insight about provider barriers to the use of PGx testing and effective approaches to overcome these barriers. Given the number of factors affecting the use of PGx testing, including both provider and health system factors, it is unlikely that a single approach will be sufficient and feasible in all clinical settings. Therefore, different or a combination of approaches may be necessary depending on the clinical setting and resources available. Using data gathered from this study, we hope to inform decisions regarding delivery models for PGx testing and increase the appropriate utilization of PGx.
Future perspective
We expect that the use of PGx testing will increase in the future as testing has more evidence of utility generated. Thus, it is important to explore the potential delivery options of PGx testing to identify effective approaches to facilitate the appropriate use and applications of testing. Pharmacists may serve as effective clinical decision support to PCPs to promote the appropriate use and application of PGx testing. With this study, we will better understand the challenges, benefits and feasibility of integrating PGx into clinical care and using a pharmacist as a first-hand resource for providers.
Supplementary Material
Executive summary.
Background study rationale
Several factors have been identified as contributing to the slow integration of pharmacogenomic (PGx) testing in clinical care, including provider knowledge and operational issues.
Integration of PGx testing into clinical practice will not only require careful consideration of effects on physician practices (particularly with respect to any additional time needed to consent and/or communicate results to patients), but also on the impact of test results on patient behaviors, such as medication adherence.
Study design
The study aims to assess two delivery models of PGx testing (physician initiated and pharmacist initiated) for commonly prescribed drugs in primary care practices and to evaluate the relative impact of the delivery models from three perspectives: physician, patient and practice setting.
To address provider knowledge barriers, two types of provider education/support will be developed and evaluated in the study: continuing medical education seminar and pharmacist support.
Four groups of study measures will be evaluated for the provider-initiated and pharmacist-initiated testing delivery models: PGx test ordering and durability of any changes; provider attitudes and knowledge about PGx testing before and after the intervention; impact on patients with respect to information sharing and seeking, medication adherence and overall awareness of side effects; and associated costs with the delivery models, targeted medications, associated monitoring costs and adverse drug events.
Acknowledgments
This work was supported by the NIH (R01-GM081416). SB Haga is a paid consultant to the nonproft Inova Transla-tional Medicine Institute.
Footnotes
Financial competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1••.Scott SA. Personalizing medicine with clinical pharmacogenetics. Genet Med. 2011;13(12):987–995. doi: 10.1097/GIM.0b013e318238b38c. Excellent review of the history of pharamacogenetics and current applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WE, McLeod HL. Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 3.US FDA. Table of Pharmacogenomic Biomarkers in Drug Labels. www.pharmgkb.org/view/drug-labels.do.
- 4.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286(18):2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 5.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Monguio R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21(9):623–650. doi: 10.2165/00019053-200321090-00002. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Hooft CS, Sturkenboom MC, Van Grootheest K, Kingma HJ, Stricker BH. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Safety. 2006;29(2):161–168. doi: 10.2165/00002018-200629020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41(2):192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 9.Gu QP, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 10.Haga SB, Lapointe NM. The potential impact of pharmacogenetic testing on medication adherence. Pharmacogenomics J. 2013;13(6):481–483. doi: 10.1038/tpj.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charland SL, Agatep BC, Herrera V, et al. Providing patients with pharmacogenetic test results affects adherence to statin therapy: results of the Additional KIF6 Risk Offers Better Adherence to Statins (AKROBATS) trial. Pharmacogenomics J. 2013;14(3):272–280. doi: 10.1038/tpj.2013.27. [DOI] [PubMed] [Google Scholar]
- 12.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. doi: 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in Type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299–2305. doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields AE, Blumenthal D, Weiss KB, Comstock CB, Currivan D, Lerman C. Barriers to translating emerging genetic research on smoking into clinical practice. Perspectives of primary care physicians. J Gen Int Med. 2005;20(2):131–138. doi: 10.1111/j.1525-1497.2005.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians' knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82(4):388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl Health Stat Rep. 2008;3:1–39. [PubMed] [Google Scholar]
- 17.Lockhart P, Guthrie B. Trends in primary care antidepressant prescribing 1995–2007: a longitudinal population database analysis. Br J Gen Pract. 2011;61(590):e565–e572. doi: 10.3399/bjgp11X593848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grice GR, Seaton TL, Woodland AM, McLeod HL. Defining the opportunity for pharmacogenetic intervention in primary care. Pharmacogenomics. 2006;7(1):61–65. doi: 10.2217/14622416.7.1.61. [DOI] [PubMed] [Google Scholar]
- 19.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 20.Visser LE, Trienekens PH, De Smet PA, et al. Patients with an ApoE epsilon4 allele require lower doses of coumarin anticoagulants. Pharmacogenet Genom. 2005;15(2):69–74. doi: 10.1097/01213011-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett G, Antoun J, Zgheib NK. Theranostics in primary care: pharmacogenomics tests and beyond. Expert Rev Mol Diagn. 2012;12(8):841–855. doi: 10.1586/erm.12.115. [DOI] [PubMed] [Google Scholar]
- 22.Hunter A, Wright P, Cappelli M, Kasaboski A, Surh L. Physician knowledge and attitudes towards molecular genetic (DNA) testing of their patients. Clin Genet. 1998;53(6):447–455. doi: 10.1111/j.1399-0004.1998.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 23.Wideroff L, Vadaparampil ST, Greene MH, Taplin S, Olson L, Freedman AN. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Gen. 2005;42(10):749–755. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA. Physicians' knowledge of genetics and genetic tests. Acad Med. 1993;68(8):625–632. doi: 10.1097/00001888-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians' preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomark. 2013;17(3):219–225. doi: 10.1089/gtmb.2012.0165. [DOI] [PubMed] [Google Scholar]
- 26.Houwink EJF, Henneman L, Westerneng M, et al. Prioritization of future genetics education for general practitioners: a Delphi study. Genet Med. 2012;14(3):323–329. doi: 10.1038/gim.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houwink EJF, Van Luijk SJ, Henneman L, Van Der Vleuten C, Dinant GJ, Cornel MC. Genetic educational needs and the role of genetics in primary care: a focus group study with multiple perspectives. BMC Fam Pract. 2011;12:5. doi: 10.1186/1471-2296-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikediobi ON, Shin J, Nussbaum RL, et al. Addressing the challenges of the clinical application of pharmacogenetic testing. Clin Pharmacol Ther. 2009;86(1):28–31. doi: 10.1038/clpt.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swen JJ, Guchelaar HJ. Just how feasible is pharmacogenetic testing in the primary healthcare setting? Pharmacogenomics. 2012;13(5):507–509. doi: 10.2217/pgs.12.19. [DOI] [PubMed] [Google Scholar]
- 30.Haga SB, Moaddeb J. Comparison of delivery strategies for pharmacogenetic testing services. Pharmacogenet Genom. 2013;24(3):139–145. doi: 10.1097/FPC.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topol EJ. Pharmacy benefit managers, pharmacies, and pharmacogenomic testing: prescription for progress? Sci Transl Med. 2010;2(44):44cm22. doi: 10.1126/scitranslmed.3001067. [DOI] [PubMed] [Google Scholar]
- 32.Desai NR, Canestaro WJ, Kyrychenko P, et al. Impact of CYP2C19 genetic testing on provider prescribing patterns for antiplatelet therapy after acute coronary syndromes and percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2013;6(6):694–699. doi: 10.1161/CIRCOUTCOMES.113.000321. [DOI] [PubMed] [Google Scholar]
- 33.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9(10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 34.Bond CA, Raehl CL. Adverse drug reactions in United States hospitals. Pharmacotherapy. 2006;26(5):601–608. doi: 10.1592/phco.26.5.601. [DOI] [PubMed] [Google Scholar]
- 35.Rupp MT, Deyoung M, Schondelmeyer SW. Prescribing problems and pharmacist interventions in community practice. Med Care. 1992;30(10):926–940. doi: 10.1097/00005650-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 36••.Shuldiner AR, Palmer K, Pakyz RE, et al. Implementation of pharmacogenetics: The University of Maryland personalized anti-platelet pharmacogenetics program. Am J Med Genet Part C Semin Med Genet. 2014;166(1):76–84. doi: 10.1002/ajmg.c.31396. Example of one approach to integrate pharmacogenetic testing into the clinical setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet Part C Semin Med Genet. 2014;1661:56–67. doi: 10.1002/ajmg.c.31390. Example of one approach to integrate pharmacogenetic testing into the clinical setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll JC, Rideout AL, Wilson BJ, et al. Genetic education for primary care providers: improving attitudes, knowledge, and confidence. Can Fam Physician. 2009;55(12):e92–e99. [PMC free article] [PubMed] [Google Scholar]
- 39••.Mrazek DA, Lerman C. Facilitating clinical implementation of pharmacogenomics. JAMA. 2011;306(3):304–305. doi: 10.1001/jama.2011.1010. Nice overview of key factors critical to clinical use of pharmacogenetic testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clyman JC, Nazir F, Tarolli S, Black E, Lombardi RQ, Higgins JJ. The impact of a genetics education program on physicians' knowledge and genetic counseling referral patterns. Med Teach. 2007;29(6):e143–e150. doi: 10.1080/01421590701477373. [DOI] [PubMed] [Google Scholar]
- 41.Houwink EJ, Muijtjens AM, Van Teeffelen SR, et al. Effectiveness of oncogenetics training on general practitioners' consultation skills: a randomized controlled trial. Genet Med. 2014;16(1):45–52. doi: 10.1038/gim.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houwink EJ, Van Teeffelen SR, Muijtjens AM, et al. Sustained educational effect after online training in oncogenetics: a randomized controlled trial. Eur J Hum Genet. 2014;22(3):310–316. doi: 10.1038/ejhg.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy – a genomewide study. N Engl J Med. 2008;359(8):789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 44.Scarpini F, Cappellone R, Auteri A, Puccetti L. Role of genetic factors in statins side-effects. Cardiovasc Hematol Disord Drug Targets. 2012;12(1):35–43. doi: 10.2174/187152912801823138. [DOI] [PubMed] [Google Scholar]
- 45.Voora D, Shah SH, Spasojevic I, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54(17):1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilke RA, Ramsey LB, Johnson SG, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92(1):112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hresko A, Haga S. Insurance coverage policies for personalized medicine. J Pers Med. 2012;2(4):201–216. doi: 10.3390/jpm2040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell Teagarden J, Stanek EJ. On pharmacogenomics in pharmacy benefit management. Pharmacotherapy. 2012;32(2):103–111. doi: 10.1002/PHAR.1039. [DOI] [PubMed] [Google Scholar]
- 49.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 50.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Berkman ND, Lohr KN, Ansari M, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2013. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the agency for healthcare research and quality: an update. Prepared by the RTI-UNC Evidence-based Practice Center under Contract No. 290-2007-10056-I). AHRQ Publication No. 13(14)-EHC130-EF. [PubMed] [Google Scholar]
- 52.Carnevale A, Scaringi C, Scalabrino G, et al. Radiation therapy after breast reconstruction: outcomes, complications, and patient satisfaction. Radiol Med. 2013;118(7):1240–1250. doi: 10.1007/s11547-013-0947-6. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. doi: 10.1186/1472-6963-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerrald KR, Dixon DL, Barnette DJ, Williams VG. Evaluation of a pharmacist-managed lipid clinic that uses point-of-care lipid testing. J Clin Lipidol. 2010;4(2):120–125. doi: 10.1016/j.jacl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with Type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012;18(7):516–526. doi: 10.18553/jmcp.2012.18.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziman ME, Bui HT, Smith CS, et al. The pharmacists' role in improving guideline compliance for thyroid function testing in patients with heart failure. J Pharm Pract. 2012;25(2):195–200. doi: 10.1177/0897190011416008. [DOI] [PubMed] [Google Scholar]
- 57.Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health-Syst Pharm. 2011;68(2):143–150. doi: 10.2146/ajhp100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne K, Shabaruddin FH. Cost–effectiveness analysis in pharmacogenomics. Pharmacogenomics. 2010;11(5):643–646. doi: 10.2217/pgs.10.45. [DOI] [PubMed] [Google Scholar]
- 59.Wong WB, Carlson JJ, Thariani R, Veenstra DL. Cost effectiveness of pharmacogenomics: a critical and systematic review. Pharmacoeconomics. 2010;28(11):1001–1013. doi: 10.2165/11537410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Lamond NW, Skedgel C, Rayson D, Lethbridge L, Younis T. Cost-utility of the 21-gene recurrence score assay in node-negative and node-positive breast cancer. Breast Cancer Res Treat. 2012;133(3):1115–1123. doi: 10.1007/s10549-012-1989-5. [DOI] [PubMed] [Google Scholar]
- 61.Paulden M, Franek J, Pham B, Bedard PL, Trudeau M, Krahn M. Cost–effectiveness of the 21-gene assay for guiding adjuvant chemotherapy decisions in early breast cancer. Value Health. 2013;16(5):729–739. doi: 10.1016/j.jval.2013.03.1625. [DOI] [PubMed] [Google Scholar]
- 62.Reed SD, Dinan MA, Schulman KA, Lyman GH. Cost–effectiveness of the 21-gene recurrence score assay in the context of multifactorial decision making to guide chemotherapy for early-stage breast cancer. Genet Med. 2013;15(3):203–211. doi: 10.1038/gim.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Lima Lopes G, Jr, Segel JE, Tan DS, Do YK, Mok T, Finkelstein EA. Cost–effectiveness of epidermal growth factor receptor mutation testing and first-line treatment with geftinib for patients with advanced adenocarcinoma of the lung. Cancer. 2012;118(4):1032–1039. doi: 10.1002/cncr.26372. [DOI] [PubMed] [Google Scholar]
- 64.Obradovic M, Mrhar A, Kos M. Cost–effectiveness of UGT1A1 genotyping in second-line, high-dose, once every 3 weeks irinotecan monotherapy treatment of colorectal cancer. Pharmacogenomics. 2008;9(5):539–549. doi: 10.2217/14622416.9.5.539. [DOI] [PubMed] [Google Scholar]
- 65.Assimes TL, Holm H, Kathiresan S, et al. Lack of association between the Trp719Arg polymorphism in kinesin-like protein-6 and coronary artery disease in 19 case–control studies. J Am Coll Cardiol. 2010;56(19):1552–1563. doi: 10.1016/j.jacc.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iakoubova OA, Tong CH, Rowland CM, et al. Association of the Trp719Arg polymorphism in kinesin-like protein 6 with myocardial infarction and coronary heart disease in 2 prospective trials: the CARE and WOSCOPS trials. J Am Coll Cardiol. 2008;51(4):435–443. doi: 10.1016/j.jacc.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 67.Sorich MJ, Wiese MD, O'Shea RL, Pekarsky B. Review of the cost effectiveness of pharmacogenetic-guided treatment of hypercholesterolaemia. Pharmacoeconomics. 2013;31(5):377–391. doi: 10.1007/s40273-013-0045-6. [DOI] [PubMed] [Google Scholar]
- 68.Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost–effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150(2):73–83. doi: 10.7326/0003-4819-150-2-200901200-00005. [DOI] [PubMed] [Google Scholar]
- 69.Perlis RH, Patrick A, Smoller JW, Wang PS. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost–effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology. 2009;34(10):2227–2236. doi: 10.1038/npp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. Pharmacoeconomics. 2010;28(1):61–74. doi: 10.2165/11318240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Leey JA, McCabe S, Koch JA, Miles TP. Cost–effectiveness of genotype-guided warfarin therapy for anticoagulation in elderly patients with atrial fibrillation. Am J Geriatr Pharmacother. 2009;7(4):197–203. doi: 10.1016/j.amjopharm.2009.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.