Abstract
Context
New biomarkers are needed in acetaminophen (APAP) hepatotoxicity. Plasma argininosuccinate synthetase (ASS) is a promising candidate.
Objective
Characterize ASS in APAP hepatotoxicity.
Methods
ASS was measured in plasma from rodents and humans with APAP hepatotoxicity.
Results
In mice, ASS increased before injury, peaked before ALT, and decreased rapidly. Fischer rats had a greater increase in ASS relative to ALT. Patients with abnormal liver test results had very high ASS compared to controls. ASS appeared to increase early in some patients, and declined rapidly in all.
Conclusions
: ASS may be a useful biomarker of acute cell death in APAP hepatotoxicity.
Keywords: Acetaminophen, Hepatotoxicity, Diagnosis, Prognosis, Mitochondria
INTRODUCTION
Acetaminophen (APAP; paracetamol) is a popular and widely used analgesic. Unfortunately, although the drug is safe and effective at therapeutic doses, accidental or intentional overdose can cause severe liver injury and this is a major problem in the U.S., the U.K., and many other Western countries. In the U.S. alone, more than 78,000 emergency department visits per year may be attributed to APAP overdose (Budnitz et al., 2011). Although most of these patients survive, the sheer volume of cases makes APAP overdose not only the chief cause of acute liver injury in the U.S., but also the number one etiology of acute liver failure-related deaths (Ostapowicz et al., 2002).
The mechanisms of APAP-induced liver injury have been thoroughly studied using rodent models. Toxicity is initiated by reaction of N-acetyl-p-benzoquinone imine (NAPQI), an electrophilic metabolite of APAP, with protein (Jollow et al., 1973; McGill et al., 2013a). This protein binding causes mitochondrial damage and oxidative stress (Meyers et al., 1988; Tirmenstein and Nelson, 1989; Jaeschke et al., 2012), which leads to activation of the c-Jun N-terminal kinase (JNK) (Gunawan et al., 2006; Saito et al., 2010; Jaeschke et al., 2012). One recent study has shown that the receptor interacting protein kinase 3 (RIP3) is increased during APAP hepatotoxicity in mice and may also act upstream of JNK (Ramachandran et al., 2013). In any case, JNK exacerbates the mitochondrial dysfunction (Jaeschke et al., 2012). The end result of all this is opening of the mitochondrial membrane permeability transition pore and oncotic cell necrosis (Kon et al., 2004; Jaeschke et al., 2012). Importantly, recent data suggested that the mechanisms of APAP-induced liver injury in humans are similar (Antoine et al., 2012; McGill et al., 2012a; Woolbright et al., 2012).
Currently, the diagnosis of APAP overdose in humans is based largely on patient history and serum APAP levels. Liver biopsy is not usually performed in these cases, in part because of the risk of bleeding due to coagulopathy. Hence, serum alanine aminotransferase (ALT) and aspartate aminostransferase (AST) activity have become the standard clinical markers of liver injury during APAP hepatotoxicity. Unfortunately, serum aminotransferase activities do not increase until hours after overdose, and generally do not peak until 24-48 h, after significant liver injury is already present (Singer et al., 1995; McGill et al., 2012a). Morevoer, serum levels of these enzymes are not predictive of patient outcome (Antoine et al., 2012). There is a clear need for earlier and more prognostic biomarkers in APAP hepatotoxicity. Although N-acetylcysteine (NAC) is a very effective treatment, many APAP overdose patients develop acute liver failure (ALF). Liver transplantation for ALF caused by APAP overdose is much less common than for ALF from other causes (Ostapowicz et al., 2002; Simpson et al., 2009). There are a number of reasons for this, including psychiatric contraindications and the overall higher rate of spontaneous survival after APAP overdose compared with other causes (Simpson et al., 2009). Medical contraindications, principally hepatic encephalopathy, also contribute significantly to the low transplantation rates (Ostapowicz et al., 2002; Simpson et al., 2009). Unfortunately, the rapid progression of acute liver injury after APAP overdose means that many patients develop encephalopathy and may become comatose within days or even just hours of the first evidence of liver damage. The window for decision-making with regard to life-saving liver transplantation is short. Earlier biomarkers, which become detectable before ALT, and which hopefully help to predict outcome, would be helpful in such cases. Many attempts have been made to discover and develop novel biomarkers of liver damage; however, while some of these biomarkers have greatly improved our understanding of disease pathogenesis in humans, most have demonstrated only limited potential for clinical use. Using a proteomics/degradomics approach, a more promising candidate was recently identified (Svetlov et al., 2006). Argininosuccinate synthetase (ASS) is an important enzyme for the synthesis of arginine, as it catalyzes the reaction of citrulline and aspartate to form argininosuccinate which can then be broken down to arginine and fumurate. As a result, ASS is a critical enzyme in several metabolic processes requiring this arginine (Haines et al., 2011). In a particularly interesting study, ASS was shown to be an earlier and more sensitive biomarker than ALT in certain rodent models of acute liver injury (Svetlov et al., 2006; Prima et al., 2013). However, ASS has not been measured in an APAP model, and has not yet been well validated in humans with liver injury. The goal of the present work was to determine whether or not ASS could be a clinically useful plasma biomarker of liver injury during APAP hepatotoxicity in rodents and humans. Hepatotoxicity was also induced by furosemide, as a negative control for mitochondrial damage in acute liver injury (Wong et al., 2000),
METHODS
Patient enrollment
Patients were enrolled at the University of Kansas Hospital in Kansas City, KS and the Banner Good Samaritan Medical Center in Phoenix, AZ and studied prospectively. The study protocol was approved by the Institutional Review Boards (IRB) of both campuses. Diagnosis and determination of APAP overdose were made by a physician on-site. All patients were required to sign a consent form in order to participate in the study. Blood samples were drawn upon enrollment and approximately every 24 h thereafter. All patients received standard- of-care treatment with N-acetylcysteine (NAC). NAC was administered before the first study samples were obtained. Patients were included in the study if they met at least two of the three following criteria: a history of APAP overdose, high serum APAP levels based on the Rumack-Matthews nomogram, and elevated ALT. Subjects were placed in the abnormal liver test results (“Abnormal LT”) group if they had peak ALT > 1,000 U/L and peak prothrombin time (PT) > 18 s (all but 2 patients in this group recovered normally), or into the “Normal LT” group if they had peak ALT < 100 U/L and peak PT < 18s. Additional blood samples were obtained from healthy volunteers.
Animals
Eight-to-twelve week old male C57Bl/6 mice (20-25 g) were purchased from Jackson Laboratories (Bar Harbor, ME). In addition, 8-12 week old F344 and SD rats (200-250 g) were purchased from Charles River (Wilmington, MA) and Harlan Laboratories (Indianapolis, IN), respectively. All animals were housed in groups of four in an environmentally-controlled facility with a 12 h light/dark cycle, filtered air, and ad libitum access to standard laboratory chow and water until the commencement of each experiment. For APAP experiments, food was withdrawn 12-16 h before treatment. Mice were treated i.p. with 300 mg/kg APAP, which consistently causes acute liver injury in mice (McGill et al., 2013c), dissolved in warm phosphate-buffered saline, or with 500 mg/kg furosemide, similar to doses that have been previously used to cause liver injury in mice (Wong et al., 2000), dissolved in 1x PBS at pH 8.0. In both cases, the animals received a total volume of 20 mL/kg. Untreated mice were used for the 0 h time point. PBS controls in the dose-response experiment received the same volume of PBS per g bodyweight. Rats were treated i.p. with 1 g/kg (F344) or 2 g/kg (SD) APAP dissolved in 20% Tween-80 for a total volume of 12.5 mL/kg. Control rats received vehicle alone. All animals were sacrificed under anesthesia at the indicated time points (0, 0.5, 2, 6, 12, and 24 h) following treatment. Heparinized blood and livers were collected. Plasma was separated from the blood and collected after centrifugation at 1,000 g for 8 min. Some liver pieces were flash frozen in liquid nitrogen for later analysis, while others were fixed in 10% phosphate-buffered formalin for histology. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Biochemistry
For animal experiments, ALT was measured in plasma using a kit (Pointe Scientific, Canton, MI). For human samples, ALT, PT, and other clinical parameters were measured on-site in a hospital laboratory using standard methods and instrumentation. ASS was measured in plasma using a sandwich ELISA (Banyan Biomarkers, Alachua, FL), according to previously described methods (Prima et al., 2013). The ASS antibody was generated using an immunogenic peptide with 100% sequence homology with both human and rat ASS, and has similar binding affinity for human, rat, and mouse ASS. For all species, the lower limit of detection is 0.34 ng/mL. Validation data for the ASS ELISA can be found in Prima et al., 2011.
Histology
Fixed sections of liver were parafinized and stained with hematoxylin and eosin according to standard methods.
Statistics
The Shapiro-Wilk test was used to assess normality. For normally distributed data, one-way analysis of variance (ANOVA) was used to determine whether or not statistically significant differences existed between groups, followed by the Student-Newman-Keul's test. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn's multiple comparisons test. Planned comparisons between 0 and 2 h after APAP treatment and between 0 and 6 h after furosemide treatment were performed using two-tailed Student's t-test. For all tests, p < 0.05 was considered significant.
RESULTS
Injury and plasma ASS in APAP-treated mice
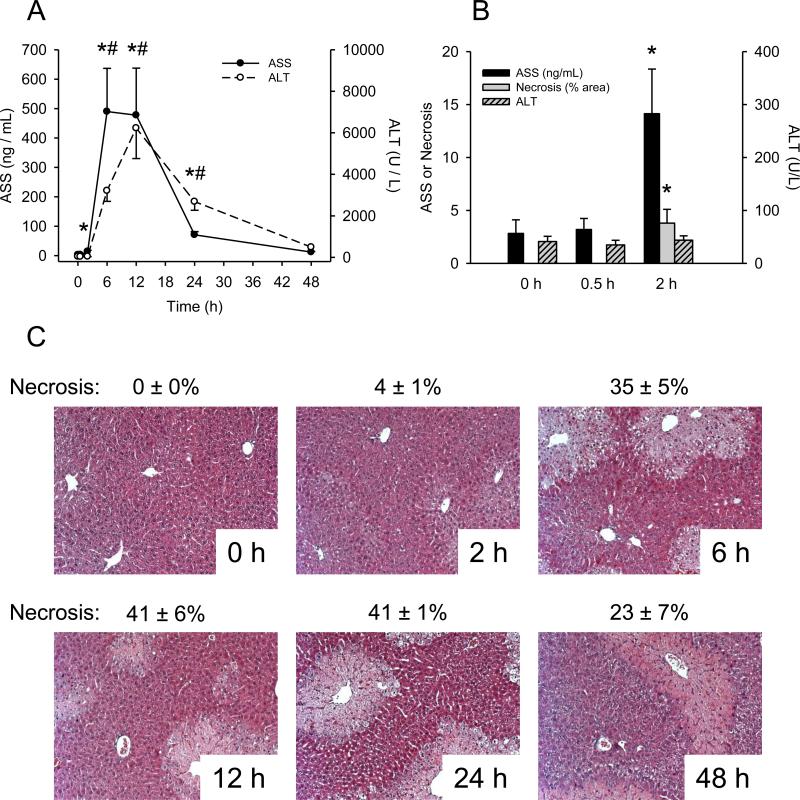
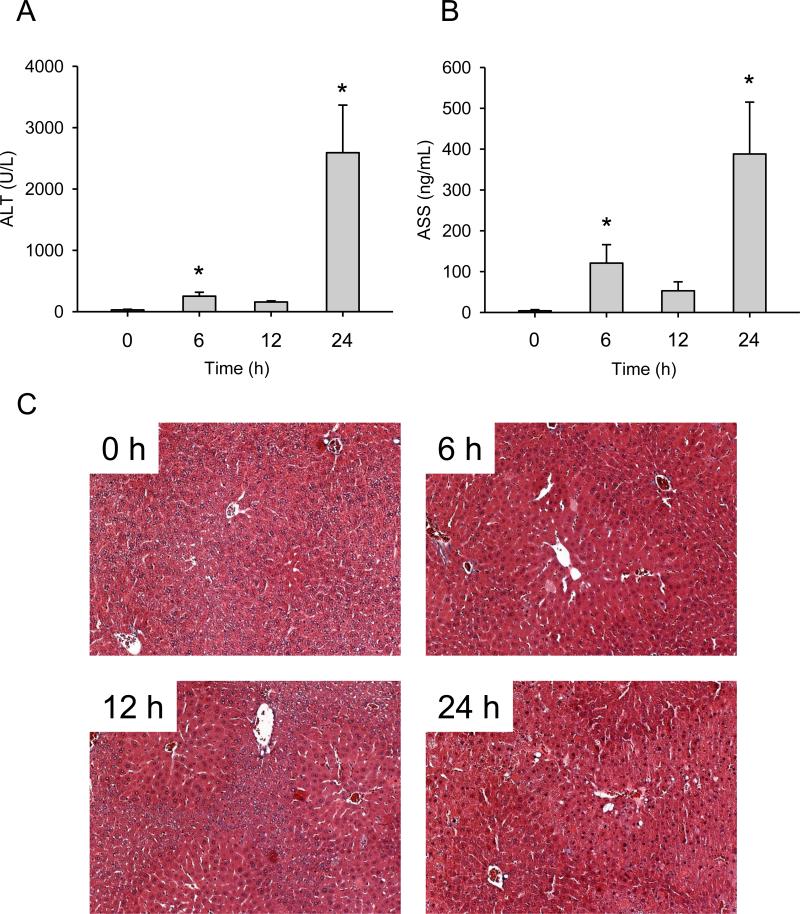
To determine whether or not ASS is elevated in mice with APAP-induced liver injury, animals were treated with 300 mg/kg APAP and blood and liver tissue were harvested at various time points. ALT increased by 6 h post-APAP and peaked at 12 h (Fig. 1A). Interestingly, a 5-fold increase in plasma ASS (from 2.8 ± 1.3 ng/mL at 0 h to 14.1 ± 4.2 ng/mL) was observed at 2 h, before any increase in ALT (Fig. 1B). The plasma ASS concentration peaked at 6 h, before the peak of ALT. Necrosis scores revealed that there was some histological evidence of tissue injury at the 2 h time point when ASS began to increase, but when ALT was still at baseline (Fig. 1B,C). These data suggest that ASS is a better biomarker of early liver damage, and possibly more sensitive, than ALT. Plasma ASS also decreased more rapidly than ALT. By 24 h, ASS concentration was just 15% of peak levels, while ALT was still 48% (Fig. 1A). This rapid decrease of plasma ASS is consistent with previous reports (Prima et al., 2013). The faster decline of ASS than ALT shows that the response of ASS to injury is more acute than that of ALT and is therefore a better indicator of active liver injury. Overall, these data show that increased ASS levels in plasma are detectable before increased ALT and that ASS better reflects active liver injury in mice.
Figure 1.
Liver injury and plasma ASS in APAP-treated mice. Mice were treated with 300 mg/kg acetaminophen (APAP) and sacrificed at various time points. (A) Time course of argininosuccinate synthetase (ASS) and alanine aminotransferase (ALT) in plasma from APAP-treated mice. (B) ASS, ALT, and % necrosis in H&E stained liver sections at early time points after APAP treatment in mouse plasma. (C) H&E stained liver sections from APAP-treated mice. Data are expressed as mean ± SEM for n = 3-6. *p < 0.05 for plasma ASS levels compared to 0 h. #p < 0.05 for plasma ALT compared to 0 h.
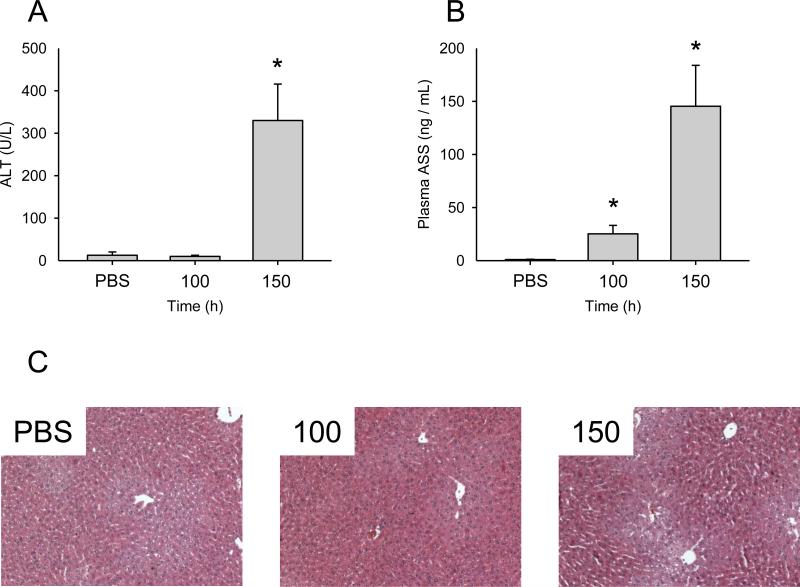
To further test the hypothesis that ASS is a more sensitive indicator of liver injury or stress than ALT, we performed a dose-response experiment with APAP. Mice were treated with PBS vehicle alone or with 100, 150, or 300 mg/kg APAP, and sacrificed 6 h later.
Importantly, plasma concentrations of ASS were significantly increased in mice treated with 100 and 150 mg/kg APAP (25 ± 16 and 145 ± 77 ng/mL, respectively, vs. 1 ± 0.3 ng/mL in PBS control samples), while ALT levels remained the same (Fig. 2).
Figure 2.
Dose-response of plasma ASS and ALT in APAP-treated mice. Mice were treated with PBS vehicle, 100, or 150 mg/kg acetaminophen (APAP) and sacrificed 6 h later. (A) Plasma alanine aminotransferase (ALT). (B) Plasma argininosuccinate synthetase (ASS). (C) H&E stained liver sections from the 100 and 150 mg/kg APAP-treated mice. Data are expressed as mean ± SEM for n = 3-4. *p < 0.05 compared with PBS vehicle.
Injury and plasma ASS in APAP-treated rats
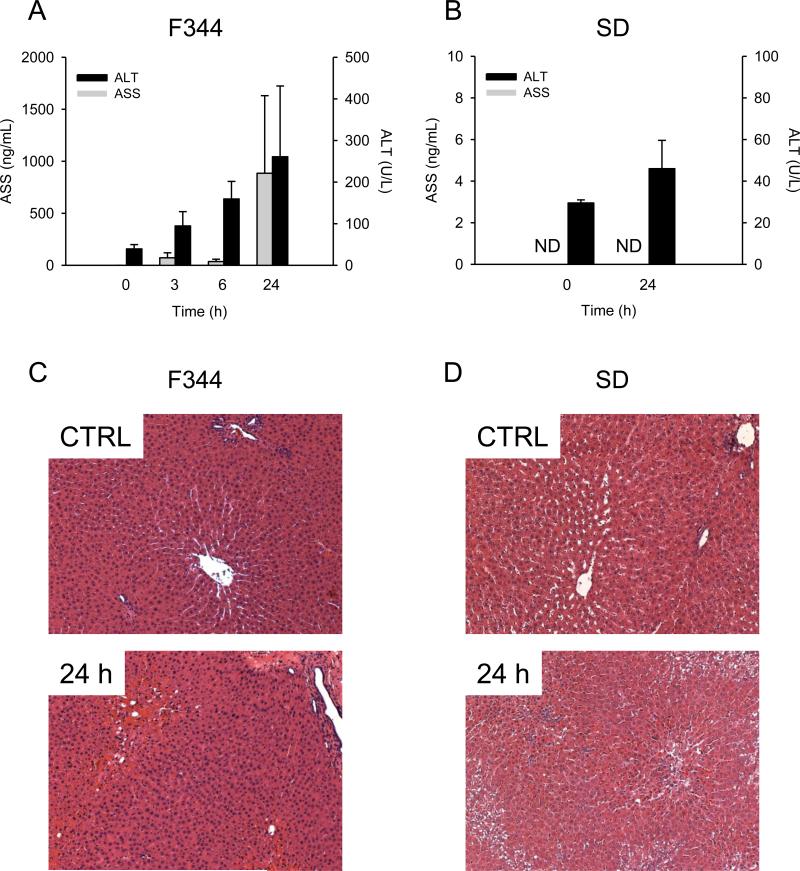
Rats are known to be less susceptible to APAP hepatotoxicity than mice and this is thought to be the result of lower mitochondrial protein binding and oxidative stress (McGill et al., 2012b). However, total liver protein binding is similar between mice and rats (McGill et al., 2012b) and other indicators of cell dysfunction, such as impaired energy metabolism and oxidative stress, have been reported by some in the latter species (Katayre and Satav, 1989; Yoshikawa et al., 2009). To determine whether or not ASS can detect injury or stress in a rat model of APAP overdose, rats were injected with 1 or 2 g/kg APAP and sacrificed at various times. Control ASS levels in rat plasma were similar to those in mouse plasma, but the maximum ASS levels were greater relative to ALT in F344 rats given 1 g/kg APAP than in mice, with the peak of both markers at 24 h, despite low overall hepatic injury (Fig.3A,C). These data suggest that increased ASS may be easier to detect as a biomarker of liver stress or damage than ALT. Unfortunately, in SD rats, we were unable to detect reproducible and significant increases in either ASS or ALT at 24 h, despite giving a higher 2 g/kg dose (Fig. 3B,D). The latter data, and the overall lower injury in rats compared to mice, are consistent with our earlier results showing that rats are resistant to APAP hepatotoxicity (McGill et al., 2012b).
Figure 3.
Hepatic injury and plasma ASS in APAP-treated rats. Rats were treated with large doses of APAP and sacrificed at various time points. (A) Plasma argininosuccinate synthetase (ASS) and alanine aminotransferase (ALT) in F344 rats treated with 1 g/kg of acetaminophen (APAP). (B) Plasma ASS and ALT in SD rats treated with 2 g/kg APAP. Data are expressed as mean ± SEM for n = 3. ND = Not Detectable.
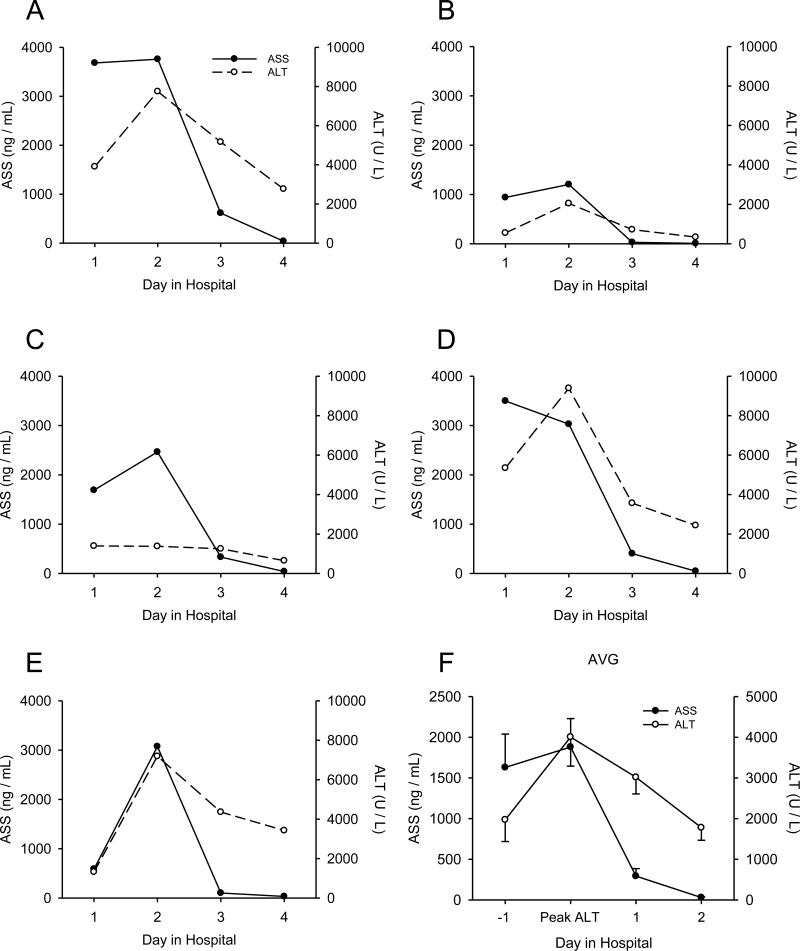
Injury and plasma ASS in APAP overdose patients
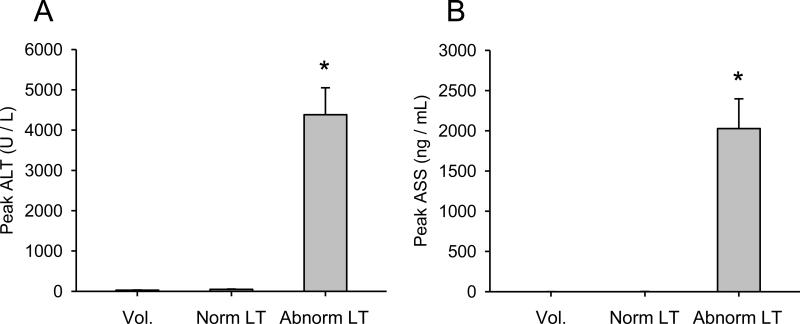
We next wanted to determine whether or not ASS could serve as a useful clinical biomarker of liver injury. APAP overdose patients studied prospectively were organized into two groups based on the results of liver function tests. Patients with abnormal liver test results (peak ALT > 1,000 U/L and peak PT > 18 s) were placed into the ”Abnormal LT” group, while patients with normal liver test results (peak ALT < 100 U/L and peak PT ≤ 18 s) were placed in the “Normal LT” group. Additional control samples were obtained from several healthy volunteers (“Vol.”). Patient information is shown in Table 1. When we measured ASS in plasma from these individuals we found a dramatic increase in the Abnormal LT group compared with both the Normal LT group and volunteer subjects (0.4 ± 0.3, 1.4 ± 0.9, and 2,027 ± 369 ng/mL in the volunteer, Normal LT, and Abnormal LT groups, respectively) (Fig. 4). Interestingly, similar to rats, the increase in ASS relative to ALT was much larger in humans compared with mice (>5,000-fold ASS increase and 156-fold ALT increase over control vs. 159-fold increase in ASS and 151-fold increase in ALT in humans and mice, respectively), suggesting that ASS may be an even more responsive biomarker in humans than mice. Importantly, when we looked at time courses from individual patients who presented before the peak of injury, we found that ASS appeared to have increased and reached a plateau faster than ALT in at least two cases (Fig. 5). Similar to mice, plasma ASS levels decreased before ALT in all of our patients (Fig. 5), demonstrating that ASS is a more acutely elevated biomarker and thus a biomarker of active liver injury in humans as well. Unfortunately, when we tested for correlation between plasma ASS in the first available sample drawn after study admission and later peak ALT or peak PT in these patients, we were unable to find a significant relationship (data not shown). This may be due to the small sample size. Altogether, these data suggest that ASS may be a promising biomarker of active injury in humans. Although the cohort of patients used in this study was too small to correlate ASS levels with final outcome (death, survival, or transplant), these results provide a strong basis for future studies to validate ASS as a useful biomarker in the clinical setting.
Table 1.
Patient Information.
| Parameter | Healthy Vol | Norm LT | Abnorm LT |
|---|---|---|---|
| Number per group | 6 | 9 | 13 |
| Mean age, range | 36, 23 – 51 | 41, 18 – 80 | 36, 21 – 62 |
| Sex (M/F) | 2 / 4 | 2 / 7 | 4 / 9 |
| Peak ALT (U/L) | 28 ± 6 | 49 ± 7 | 4,382 ± 668 |
| Peak PT (Seconds) | NA | 15.6 ± 0.8 | 60.4 ± 12.7 |
| Outcome (% Survival) | 100% | 85% |
PT = Prothrombin time. NA = Not available. Alanine aminotransferase (ALT) and prothrombin time (PT) are expressed as mean ± SEM.
Figure 4.
Injury and plasma ASS in APAP overdose patients. Plasma samples were collected from acetaminophen (APAP) overdose patients (A) Alanine aminotransferase (ALT) and (B) argininosuccinate synthetase (ASS) were measured in plasma from healthy volunteers (Vol.) (n = 6), APAP overdose patients with normal liver test results (Norm LT) (n = 9), and APAP overdose patients with abnormal liver test results indicating liver injury (Abnorm LT) (n = 13). Data are expressed as mean ± SEM. *p < 0.05 compared to healthy volunteers.
Figure 5.
Time courses of injury and plasma ASS in APAP overdose patients. Plasma samples were collected daily from acetaminophen (APAP) overdose patients with abnormal liver test results. (A-E) Time courses of plasma alanine aminotransferase (ALT) and argininosuccinate synthetase (ASS) from individual APAP overdose patients. (F) Average ASS and ALT levels over time, oriented around the day of peak ALT. Data in (F) are expressed as mean ± SEM for n = 13.
Injury and plasma ASS in furosemide-treated mice
Normally, ASS is present in mitochondria. Based on this, we hypothesized that plasma ASS could be a mechanistic biomarker of mitochondrial damage, not just cell injury or death. To test this idea, we measured ASS in plasma from mice treated with a hepatotoxic dose of the drug furosemide (FS). FS has been shown to induce liver injury similar to APAP without causing mitochondrial dysfunction (Wong et al., 2000), and we have used this approach in the past to identify possible markers of mitochondrial damage (McGill et al., 2012a; 2013b). We found that ASS was also elevated in plasma from FS-treated mice and the pattern of ASS accumulation closely followed the ALT profile (Fig. 6). These data suggest that ASS is not a specific mechanistic biomarker of liver injury involving mitochondria. This is likely due to the fact that ASS is anchored to the outside of mitochondria. Thus, ASS activity in an extra-mitochondrial compartment does not necessarily reflect damage or rupture of mitochondria.
Figure 6.
Injury and plasma ASS in furosemide-treated mice. Mice were treated with 500 mg/kg furosemide (FS) and sacrificed at various time points. (A) Alanine aminotransferase (ALT) and (B) argininosuccinate synthetase (ASS) were measured in plasma. Data are expressed as mean ± SEM for n = 3-6. *p < 0.05 compared to 0 h.
DISCUSSION
APAP overdose is a major clinical problem in the U.S. and U.K. Although most patients with APAP-induced liver injury survive, it still accounts for the largest number of acute liver failure related deaths in the U.S. (Ostapowicz et al., 2002). There is a need for new biomarkers which are capable of differentiating between patients who are likely to survive without a liver transplant and those who are not. Recent efforts by our group and others have identified a number of new biomarkers for use in APAP hepatotoxicity (Davern et al., 2006; Antoine et al., 2012; McGill et al., 2012a; Craig et al., 2013). Some of these markers have provided new information about the mechanisms of APAP-induced liver injury. For example, the presence of APAP-protein adducts in human serum showed that APAP-protein binding occurs in humans in a manner similar to rodents (Davern et al., 2006; McGill et al., 2013c); mitochondrial DNA, the mitochondrial enzyme glutamate dehydrogenase (GDH), and nuclear DNA fragments in plasma provided evidence for mitochondrial damage in humans (McGill et al., 2012a); and the ratio of caspase-cleaved to full-length keratin 18 demonstrated that the primary mode of cell death during APAP hepatotoxicity is oncotic necrosis (Antoine et al., 2012). Of greater interest to clinicians is the fact that peak levels of some of these markers have been shown to correlate with measures of injury and outcome. Recent work has shown that serum GDH, malate dehydrogenase, purine nucleoside phosphorylase, and paraxonase 1 levels correlate strongly with liver injury and could be used as powerful diagnostic tools (Schomaker et al., 2013). Moreover, HMGB1, full-length keratin 18, and microRNA 122 are associated with liver injury and with poor outcome in APAP overdose patients (Antoine et al., 2012; 2013). Unfortunately, where time course data from individual patients are available, it appears that many (though not all) of these biomarkers increase in plasma at later time points and/or simply mirror ALT (McGill et al., 2012a; Antoine et al., 2012).
ASS has been shown to increase earlier than ALT in rodent models of liver injury caused by ischemia-reperfusion and by galactosamine/endotoxin (Prima et al., 2013). Unlike aminotransferase levels, ASS also increased in serum after treatment with endotoxin alone, suggesting that ASS levels are more sensitive to liver damage (Prima et al., 2013). Based on these data, we hypothesized that ASS is an earlier and possibly more sensitive biomarker of liver damage than ALT during APAP hepatotoxicity, and therefore may have greater prognostic potential compared to ALT. We were able to show that ASS has a much greater diagnostic range, with fold-increases over control for ASS much greater than for ALT in humans. Although the size of our patient cohort was too small to assess the correlation between ASS and outcome, the data do show that increased ASS may be detected in plasma before increased ALT in mice. Unfortunately, the mechanism of ASS release is not yet known. In any case, the earlier increase may mean that ASS is an earlier biomarker of liver injury, and possibly outcome, in APAP hepatotoxicity. While ASS is also expressed in the kidney, western blot analysis revealed that ASS levels are about 5-fold higher per mg protein in the liver in rats and about 7-fold higher per mg protein in the liver in humans (Prima et al., 2011 and unpublished data). Given its much larger mass and the fact that the liver is the major target organ in APAP toxicity, it seems unlikely that the kidney contributed significantly to circulating levels of ASS in our model.
In addition to demonstrating that ASS could be an earlier biomarker of injury after APAP overdose, we found that it decreases much faster after the peak of injury and is therefore a more acutely elevated marker of liver damage. These data are consistent with earlier results from galactosamine/endotoxin-treated animals (Prima et al., 2013) and suggest that ASS is a better marker of active liver injury than ALT. This is likely due to a much shorter half-life in circulation. Prima et al. (2013) injected recombinant ASS and found that it was cleared from mouse circulation within 5-6 hours, much faster than ALT which has a very long serum half-life of ~ 47 hours in humans (Dufour et al., 2000). Because of the short circulating t1/2 of ASS, it is possible that it could yield false negatives in patients presenting late after APAP overdose. However, this is unlikely to be an issue, as ALT would likely be measured as well. In fact, the ratio of ALT to ASS may provide information about the time of patient presentation relative to the onset of injury. Moreover, this finding could make ASS a useful research tool for experiments in which the exact time of injury resolution needs to be defined.
Knowing whether or not active injury has ended could also help in the clinical setting. Surprisingly, we also found that plasma ASS levels were high relative to ALT in Fischer rats when compared with mice. Together with the early increase in mice before ALT, these data suggest that increases in ASS could be easier to detect than increases in ALT. Still, the levels of both ASS and ALT in rats were lower than those in mice after APAP treatment, and this is consistent with the considerable existing evidence that the rat is not a suitable species for studies of APAP hepatotoxicity (McGill et al., 2012b). One caveat of our work is that we have not yet investigated the effect of NAC treatment on circulating levels of ASS in APAP hepatotoxicity. We have previously shown that plasma acylcarnitines are decreased in APAP-treated mice after NAC (McGill et al., 2013b). It is possible that this could affect the levels of other biomarkers as well, including ASS. Future work should address this issue.
The need for more sensitive biomarkers of liver injury is debatable. There is some speculation that ALT is already too sensitive. For example, therapeutic use of APAP has been shown to elevate serum ALT levels in some people in the absence of other signs of liver injury and without progression to disease (Heard et al., 2011). On the other hand, it may be reasonable to expect that any marker that increases earlier than ALT and which could serve as an earlier biomarker of hepatotoxicity would have to be more responsive to the initial phases of hepatocellular injury, and therefore would be a more sensitive reporter of liver damage. Moreover, although the overall clinical utility of a biomarker of injury that is more sensitive than ALT is questionable, such a marker may be useful in pre-clinical and phase I testing of new drugs for the identification of potentially hepatotoxic compounds. Currently, liver injury is one of the most common reasons for post-approval withdrawal of drugs from the market and earlier identification of hepatotoxic drugs would be very useful. In addition, if it holds true that ASS increases earlier than ALT in humans, as it does in mice, then the early prognostic utility of ASS measurement could be greater than ALT. Characteristics of a useful biomarker for determining prognosis in APAP-induced hepatotoxicity would include an early rise in those destined to develop liver failure, and an early fall in patients who will recover (and have other evidence of lack of worsening illness). Thus, additional studies in humans to investigate this possibility with ASS are warranted.
From a methodological point of view, the measurement of ASS has some other advantages over existing biomarkers of liver injury. Our data show that the diagnostic window with ASS is very large, with a 5-fold increase in mouse plasma at 2 h and a more than 5,000-fold increase in humans at the time of peak ALT. Additionally, ASS measurements are not affected by some of the common sample collection and handling problems that afflict ALT, AST and certain other injury markers. For example, ASS is not found in erythrocytes, thus measurement of ASS in serum or plasma is not confounded by red blood cell hemolysis. Also, unlike some biomarkers, ASS can be measured in both serum and plasma.
CONCLUSIONS
Our rodent data show that ASS is an early plasma biomarker of liver injury. Moreover, the short half-life and rapid decline of ASS after peak injury in mice demonstrates that ASS is a more acutely elevated biomarker of active liver injury than ALT. Similar results were obtained with samples from APAP overdose patients. ASS increased in all patients, and declined faster than ALT. We conclude that ASS is an early biomarker of active liver injury during APAP hepatotoxicity. Future work with larger patient groups should confirm the earlier increase in plasma ASS levels and should test the ability of ASS levels to predict patient outcome.
ACKNOWLEDGEMENTS
Support was provided by grants from McNeil Consumer Health, Inc. (to H.J. and S.C.C.), the University of Kansas Medical Center Liver Center (to H.J.), the National Institutes of Health (R01 DK070195, and R01 AA12916 to H.J., and R44 DK074205 to S.I.S), from the National Center for Research Resources (5P20RR021940-07) and from the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health. Additional support was received from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20 GM12345) and the “Training Program in Environmental Toxicology” (T32 ES007079-26A2 to M.R.M.) from the National Institute of Environmental Health Sciences.
Footnotes
DECLARATION OF INTEREST
M.C., A.S., and S.I.S. are involved in the commercial development of the ASS assay. The remaining authors report no conflict of interest.
REFERENCES
- Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers of mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdose of acetaminophen-containing products. Am. J. Prev. Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Craig DG, Lee P, Pryde EA, Walker SW, Beckett GJ, Hayes PC, Simpson KJ. Elevated levels of the long pentraxin 3 in paracetamol-induced human acute liver injury. Eur. J. Gastroenterol. Hepatol. 2013;25:359–367. doi: 10.1097/MEG.0b013e32835ac77a. [DOI] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM, Acute Liver Failure Study Group Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin. Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthetase: at the center of arginine metabolism. Int. J. Biochem. Mol. Biol. 2011;2:8–23. [PMC free article] [PubMed] [Google Scholar]
- Heard K. Asymptomatic alanine aminotransferase elevations with therapeutic doses of acetaminophen. Clin. Toxicol. (Phila) 2011;49:90–93. doi: 10.3109/15563650.2011.553835. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Katyare SS, Satav JG. Impaired mitochondrial oxidative energy metabolism following paracetamol-induced hepatotoxicity in the rat. Br. J. Pharmacol. 1989;96:51–58. doi: 10.1111/j.1476-5381.1989.tb11783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013a;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 2013c;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Li F, Sharpe MR, Williams CD, Curry SC, Ma X, Jaeschke H. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2013b doi: 10.1007/s00204-013-1118-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012a;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012b;264:387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM, Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Prima V, Cao M, Svetlov SI. ASS and SULT2A1 are novel and sensitive biomarkers of acute hepatic injury – a comparative study in animal models. J. Liver. 2013;2:1000115. doi: 10.4172/2167-0889.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prima V, Wang A, Molina G, Wang KK, Svetlov SI. Inhibition of LPS toxicity by hepatic argininosuccinate sythase (ASS): novel roles for ASS in innate immune responses to bacterial infection. Int. Immunopharmacol. 2011;11:1180–1188. doi: 10.1016/j.intimp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. The receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomaker S, Warner R, Bock J, Johnson K, Potter D, Van Winke J, Aubrecht J. Assessment of emerging biomarkers of liver injury in human subjects. Toxicol. Sci. 2013;132:276–283. doi: 10.1093/toxsci/kft009. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, Pollok A, Masterton G, Hayes PC. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-acetaminophen etiologies. Liver Transpl. 2009;15:600–609. doi: 10.1002/lt.21681. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Carracio TR, Mofenson HC. The temporal profile of increased transaminase levels in patients with acetaminophen-induced liver dysfunction. Ann. Emerg. Med. 1995;26:49–53. doi: 10.1016/s0196-0644(95)70237-7. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Xiang Y, Oli MW, Foley DP, Huang G, Hayes RL, Ottens AK, Wang KK. Identification and preliminary validation of novel biomarkers of acute hepatic ischaemia/reperfusion injury using dual-platform proteomic/degradomic approaches. Biomarkers. 2006;11:355–369. doi: 10.1080/13547500600775110. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol. Lett. 2000;116:171–181. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
- Woolbright BL, Ramachandran A, McGill MR, Yan HM, Bajt ML, Sharpe MR, Lemasters JJ, Jaeschke H. Lysosomal instability and cathepsin B release during acetaminophen hepatotoxicity. Basic Clin. Pharmacol. Toxicol. 2012;111:417–425. doi: 10.1111/j.1742-7843.2012.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology. 2009;264:89–95. doi: 10.1016/j.tox.2009.07.017. [DOI] [PubMed] [Google Scholar]