Abstract
Consumption of sweet solutions has been associated with a reduction in withdrawal symptoms and alcohol craving in humans. The objective of the present study was to determine the effects of EtOH and saccharin (SACC) deprivations on operant oral self-administration. P rats were allowed to lever press concurrently self-administer EtOH (15% v/v) and SACC (0.0125% g/v) for 8 weeks. Rats were then maintained on daily operant access (non-deprived), deprived of both fluids (2 weeks), deprived of SACC and given 2 ml of EtOH daily, or deprived of EtOH and given 2 ml of SACC daily. All groups were then given two weeks of daily operant access to EtOH and SACC, followed by an identical second deprivation period. P rats responded more for EtOH than SACC. All deprived groups increased responding on the EtOH lever, but not on the SACC lever. Daily consumption of 2 ml EtOH decreased the duration of the ADE. Home cage access to 2 ml SACC also decreased the ADE but to a lesser extent than access to EtOH. A second deprivation period further increased and prolonged the expression of an ADE. These results show EtOH is a more salient reinforcer than SACC. With concurrent access to EtOH and SACC, P rats do not display a saccharin deprivation effect. Depriving P rats of both EtOH and SACC had the most pronounced effect on the magnitude and duration of the ADE, suggesting that there may be some interactions between EtOH and SACC in their CNS reinforcing effects.
Keywords: Alcohol deprivation effect, operant self-administration, alcohol-preferring P rats, repeated deprivations, Saccharin self-administration
INTRODUCTION
An association has been made between preference for sweet substances and high alcohol intake (for review, see Kampov-Polevoy et al., 1999). Sweetness-liking has been found to be correlated in humans with a paternal family history of alcoholism (Kampov-Polevoy et al., 2004). In rats, sweetness-liking and alcohol drinking have been linked by several studies. In Wistar rats, levels of saccharin drinking have been found to be positively related to later alcohol intake (Bell et al., 1994; Gosnell and Krahn, 1992). Saccharin consumption has been positively correlated with lines selectively bred for high alcohol consumption (alcohol-preferring, P; Alko Alcoholic, AA), and negatively correlated with lines selectively bred for low alcohol consumption (alcohol non-preferring, NP; Alko non-alcoholic, ANA) (Sinclair et al., 1992). Saccharin and alcohol intakes have been positively correlated in other rat lines known for their high alcohol drinking, e.g., Fawn-Hooded, Maudsley Reactive, as well as rats known for low alcohol drinking, e.g., Flinders Resistant, Flinders Sensitive, Maudsley Non-reactive (Overstreet et al., 1993).
In most rat lines studied, however, the preference for sweets tends to override the preference for EtOH. Availability of sweet substances has been shown to have an impact on the relative preference for ethanol in Fawn-hooded rats, which demonstrate an initial decrease in EtOH drinking behavior following the introduction of 0.1% saccharin (Kampov-Polevoy et al., 1995). Studies offering concurrent availability of sweetened water or a chocolate-flavored drink have shown a marked decrease in intake of EtOH, including the high-alcohol-drinking (HAD) rats (Lankford and Myers, 1994) and Sardinian alcohol-preferring (sP) rats (Colombo et al., 1997). The sP rats demonstrated suppressed acquisition and maintenance of EtOH intake with concurrent availability of 0.1 % and 1% saccharin (Colombo et al., 2005a, 2005b). In contrast, the AA rat line expresses stable levels of alcool consumption following the introduction of saccharin as an additional reinforcer (Sinclair, 1975).
When a chocolate or saccharin solution was presented as a third-choice to P rats, however, they continued to maintain a high level of EtOH self-administration, greater than 7 g/kg/day (Lankford et al., 1991). With 2-hr home cage access to 10% EtOH, the P rat has been found to consume pharmacologically relevant amounts of EtOH (Murphy et al., 1986). The P rat responds at an equal level for concurrently available 10% EtOH and 0.0125% SACC, in 2-hr alternate-day-access sessions at an FR-1 level of reinforcement (Nowak et al., 1999).
The finding that P rats drink EtOH in the presence of other palatable substances makes this selectively bred line suitable for research into experiments using concurrent access to EtOH and another palatable reinforcer. The P rat satisfies the criteria proposed as essential for an animal model of alcoholism (Cicero, 1979; Lester and Freed, 1973). This line of rats voluntarily consumes EtOH for its pharmacological effects, attains blood alcohol levels from 50 − 200 mg%, will work to obtain EtOH, and develops tolerance and dependence through free-choice alcohol drinking (reviewed in Murphy et al. 2002; Rodd et al., 2004). McBride and Li (1998) expanded on this model, further suggesting that an animal model of alcoholism should display characteristics associated with relapse, as research has shown that the drinking patterns of human alcoholics have multiple periods of abstinence and intake (Burish et al., 1981; Hilbrom, 1990; McMillen, 1997).
Operant techniques, examining alterations in the amount of work a subject will do to obtain a reinforcement, can be used to examine the effects of repeated deprivations on the reinforcing properties of EtOH, effectively modeling relapse (Ciccocioppo et al., 2001; Hodos, 1961; Rodd et al., 2004). Specifically, the deprivation effect, a temporary increase in a particular reward-seeking behavior seen after absence of the reward, can illuminate relapse-like behavior. A saccharin deprivation effect (SDE) is seen in rats, with increasing magnitude as length of deprivation increases, suggesting that deprivation effect is a general reward phenomenon, not involved with withdrawal, or simply relegated to drugs of abuse (Neznanova et al., 2002; Wayner et al., 1972; Sinclair and Senter, 1968).
An alcohol deprivation effect (ADE) is, therefore, a voluntary, temporary increase in the intake of EtOH, as evidenced by a change in ratio of EtOH to total fluid intake following a period of deprivation (Sinclair and Senter, 1967, 1968). The ADE has been used as model of alcohol craving to study the efficacy of drugs designed to prevent relapse drinking (Heyser et al., 1998; Kornet et al., 1991; Rodd et al., 2003, 2004, 2006; Sinclair and Li, 1989; Spanagel and Zieglgansberger, 1997; Vengeliene et al., 2005).
The P rat demonstrates an ADE after a single deprivation under 24-hr free-choice drinking and 4-hr operant access conditions (McKinzie et al., 1998). In these rats, repeated cycles of alcohol availability and deprivation prolonged the expression of an ADE (Rodd et al., 2003; Rodd-Henricks et al., 2000a, 2000b). Exposure to repeated cycles of EtOH access and deprivation increases the breakpoint obtained for EtOH during a progressive ratio test (Rodd et al., 2003) and reduced the concentration of EtOH required to support self-administration directly into the posterior ventral tegmental area (Rodd et al., 2005). These results suggest that alterations in the reinforcing properties of EtOH may be taking place with repeated deprivation cycles. However, the alternate solution in these studies was water. Thus for, studies have not been conducted to determine if the presence of an alternative reinforcing compound would influence expression of an ADE.
The objective of the present study was to determine the effects of concurrent access to EtOH and SACC on expression of an ADE. The hypothesis to be tested is that an ADE and SDE would be independently expressed following prolonged abstinence of either EtOH or SACC, and that a second deprivation would increase the magnitude and duration of the ADE and SDE.
METHODS
Animals
Adult male P rats (n = 42) from the 42nd – 43rd generations weighing 250−325g at the start of the experiment were used. Rats were maintained on a 12-hr reversed light-dark cycle (lights off at 0900 hr). Food and water were available in the home cage ad libitum throughout the experiment. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
Operant Apparatus
EtOH and SACC self-administration procedures were conducted in standard two-lever experimental chambers (Coulbourn Instruments) contained within ventilated, sound-attenuated enclosures. Two operant levers, located on the same wall, were 15 cm above a grid floor and 13 cm apart. A trough was directly beneath each lever, from which a dipper cup could raise to present fluid. Upon a reinforced response on the respective lever, a small light cue was illuminated in the drinking trough and 4 seconds of dipper cup (0.1 ml) access was presented. A personal computer controlled all operant chamber functions while recording lever responses and dipper presentations.
Operant Training
Naïve P rats were placed into the operant chamber, without prior training or previous substance experience. Operant sessions were 60 min in duration and occurred daily. The EtOH concentration used for operant administration was 15% (v/v), while the SACC concentration was 0.0125% (g/v). A previous study, examining the expression of an ADE under operant conditions, utilized 15% EtOH (Rodd et al., 2003); another study determined that 0.0125% SACC was a highly palatable solution for P rats (Nowak et al., 1999).
During the initial 4 weeks of daily operant access, both solutions (water and either EtOH or SACC) were reinforced on a fixed-ratio-1 (FR-1) schedule. Previous work with P rats indicated that the acquisition of operant oral self-administration of EtOH and SACC at an FR-1 schedule occurs within the 4th−6th session (60-min) when these fluids are paired with water (Rodd-Henricks et al., 2002a, 2002b). At the end of this time, rats were preferentially self-administering EtOH compared to SACC (6:1 ratio). The work requirement for EtOH was increased to an FR-3 schedule at session 29, and then to FR-5 schedule at session 43, as previously described (Rodd et al, 2003). SACC requirement remained at FR-1 throughout. This concurrent FR5-FR1 for EtOH-SACC was similar to the FR5-FR1 schedule previously used for 15% EtOH-water (Rodd et al, 2003).
Repeated Cycles of Deprivation, and EtOH and/or Saccharin Access
Following 8 weeks of operant access to EtOH and SACC, rats were randomly assigned to one of four groups. One group of rats continued daily operant sessions for the duration of the experiment (non-deprived). Three deprived groups consisted of rats maintained in their home cages for two weeks (1) without access to EtOH and SACC (deprived of EtOH and SACC); (2) given daily access to 2 ml SACC but deprived of EtOH; or (3) given daily access to 2 ml EtOH but deprived of SACC. Prior to the home-cage deprivation, P rats were, on the average, self-administering 6 ml of 15% EtOH and 1.5 ml of 0.0125 SACC. Thus, the amount received during the deprivation period was equivalent to approximately 30% of the “normal” EtOH intake and 130% of the “normal” SACC intake.
Fluid intakes were not measured in the present experiment, but it has previously been determined that under similar circumstances, non-deprived P rats consume 97% of the amount of 15% (v/v) EtOH presented by reinforcement, equivalent to 1.2 to 1.4 g/kg (Rodd et al., 2003). Body weights increased normally during the course of the experiment for all groups.
Following the initial deprivation period, P rats were given 14 consecutive daily operant sessions with both EtOH and SACC available. Subsequently, rats that were initially deprived were cycled through a second deprivation, receiving the same treatment as during the initial deprivation cycle. Non-deprived rats continued their daily operant sessions.
Statistical Analysis
Overall operant responding (60 min) data were analyzed with a mixed factorial ANOVA with a between subject factor of group and repeated measure of ‘session’, ‘lever’ and ‘cycle’ when applicable. The baseline measure for the factor of ‘day’ was the average number of responses on the EtOH lever for the 3 sessions immediately prior to deprivation. Post-hoc Tukey's b comparisons were performed to determine individual differences.
RESULTS
Acquisition and Maintenance
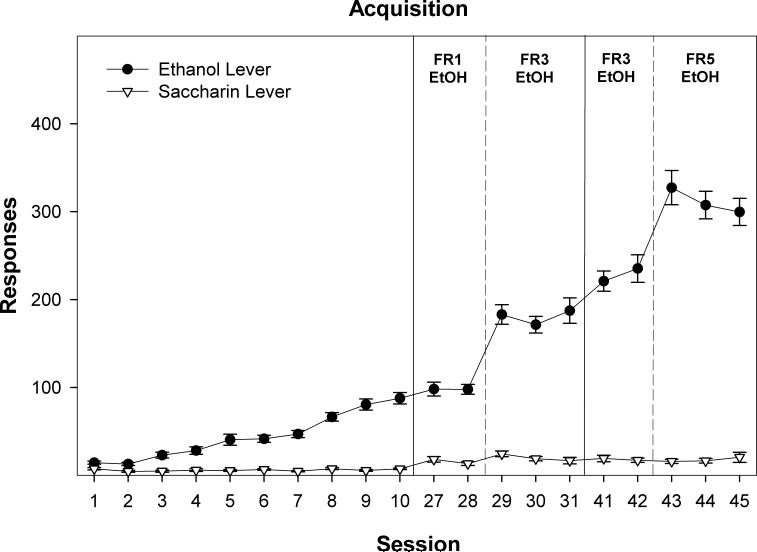
Within 3 sessions, P rats responded significantly more on the EtOH than SACC lever (Fig. 1). There was a significant effect of session, F(9,369) = 38.5; p < 0.0001, lever, F(1,41) = 322.4; p < 0.00001, and a session x lever interaction, F(9,369) = 39.8; p < 0.0001, during the initial 10 operant sessions. From the third session onward, P rats preferred to self-administer EtOH to the SACC solution (t-test conducted for each session, all p values < 0.002). Across the first 10 operant sessions, responding on the EtOH lever increased, F(9,369) = 40.9; p < 0.0001, with responding during sessions 4−10 being significantly greater than the average responding during sessions 1−3 (t-tests, all p values < 0.003). There was a significant effect of session on responding on the SACC lever, F(9,369) = 11.7; p < 0.0001. However, this effect was the result of responding on the SACC lever during sessions 3−5 being significantly lower than during sessions 1, 8, 9, and 10 (t-tests, p values < 0.05).
Figure 1.
Depicts the mean (± SEM) lever responses for P rats concurrently self-administering 15% EtOH and 0.0125 % saccharin (SACC). The graph also depicts the effects of increasing the work requirement for EtOH (FR1 increased to FR3, and then finally FR5) while the work requirement for SAC remained constant (FR1). As indicated in the graph, P rats preferred to self-administer EtOH from the 3rd session onward, regardless of the increase in the work requirement for EtOH (all p values < 0.002).
When the work requirement for EtOH increased from an FR-1 to an FR-3, there was a significant increase in the number of responses on the EtOH lever (contrasting responding from sessions 27−30; F(3,123) = 47.4; p < 0.0001. Responses on the SACC lever during this time was also altered, F(3, 123) = 4.6; p = 0.004. This effect was the result of a significant increase in responding for SACC during the initial day of FR-3 EtOH training (significantly greater responding for SACC during session 29 than during sessions 27 and 28; p values < 0.05), but there was no increase in responding during session 30 (p values > 0.12). The transient nature of this increase is also indicated by the fact that analyzing the SACC response data between session 27− 34 indicates no significant differences across sessions, F(7,287) = 0.4; p = 0.82. Increasing the work requirement for an EtOH reinforcer from an FR-3 to an FR-5 schedule of reinforcement significantly increased the number of responses on the EtOH lever, F(3,123) = 72.5; p < 0.0001, but during this time period there was no significant effect on responding on the SACC lever, F(3,123) = 0.6; p = 0.62.
The number of reinforcements obtained mirrored the number of responses on each lever. For example, during the 3rd session, P rats on average responded 23.0 times on the EtOH lever and received 21.3 reinforcers (an average of 2 non-reinforced responses – responses during the delivery of a reinforcer were recorded but not reinforced). Lever responses made during the 4 sec presentation of a reinforcer were recorded but did not result in additional presentation of a reinforcer (non-reinforced responses). During the 3rd operant session, P rats on average responded 4.8 times on the SACC lever and received 3.5 reinforcers. On the last day of FR1 responding (session 28) P rats responded 97.9 times on the EtOH lever and received 90.4 reinforcers. During the same session, P rats responded 13.4 times on the SACC lever, but only received on average 9.6 SACC reinforcers. On the last three FR5 sessions (baseline Fig. 1, non-deprived group), P rats responded on average 303 times on the EtOH lever, and received 58 reinforcers. In contrast, during the same 3 operant sessions, P rats responded on average 16 times on the SACC lever, but received only 10 reinforcers.
EtOH and SACC Responding Following a Single Deprivation
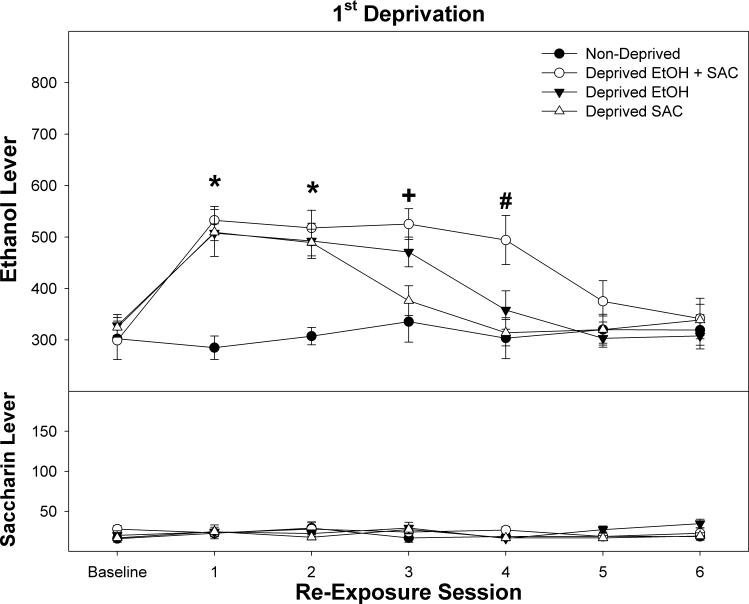
Responding on the EtOH lever was significantly increased following a 2 week deprivation period (Fig 2, top panel; session – F(6,228) = 15.5; p < 0.0001; group × session F(18,228) = 3.1; p < 0.0001. During the first 3 re-exposure sessions, there was a significant effect of group (F values (3,38) > 2.8; p values < 0.05). Post-hoc comparisons indicated that during the initial 2 re-exposure sessions all deprived groups responded significantly more than non-deprived controls; during the third re-exposure session, rats deprived of both EtOH and SACC, and rats deprived of EtOH alone responded more than the non-deprived rats and the group deprived of SACC. For the group deprived of both EtOH and SACC during the first re-exposure session, the rats responded on average 533 times on the EtOH lever, and received 105 reinforcers. In contrast, these rats responded 23 times on the SACC lever, but only received 13 reinforcers.
Figure 2.
Depicts the mean (± SEM) lever responses for P rats concurrently self-administering 15% EtOH (top) and 0.0125 % SACC (bottom) in rats maintained on self-administration (non-deprived) or following a 2 week deprivation period. Asterisks indicate that all groups are different from non-deprived and from baseline. The Plus symbol indicates that rats deprived of EtOH or EtOH and SACC responded significantly more than non-deprived rats and baseline. The Pound symbol indicates that rats deprived of EtOH and SACC responded more than all other groups and was significantly elevated compared to baseline.
In contrast to responses on the EtOH lever, responding on the SACC lever was not significantly altered in any of the groups following the 2 week deprivation period (Fig 2, bottom panel; session – F(6,228) = 1.5; p = 0.29; group × session F(18,228) = 1.1; p = 0.33.
EtOH and SACC Responding Following a Second Deprivation
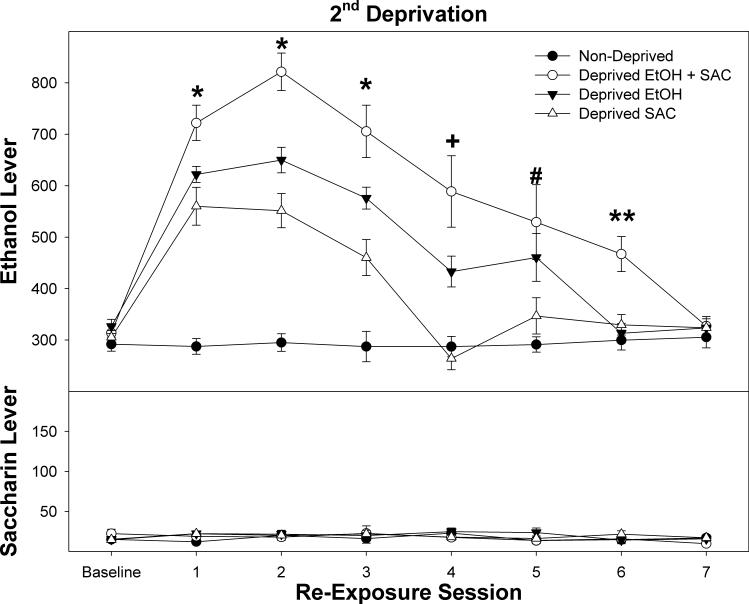
Responding on the EtOH lever for the 3 deprived groups returned to baseline levels by the end of the 2-week re-exposure period (Fig. 3, top panel; p values > 0.35). Responses on the EtOH lever were significantly increased following a 2nd deprivation period, and were significantly higher than responses following the first deprivation (cycle – F(1,38) = 17.4; p < 0.0001; cycle × session × group F(18,228) = 5.4; p < 0.001. Overall, the magnitude of responses on the EtOH lever during the second re-exposure period was greater in rats deprived of both EtOH and SACC, or deprived of EtOH alone, compared to the first re-exposure (increase in responding during the initial 4 and 3 re-exposure sessions, respectively; p values < 0.01). This increase in the magnitude of the responses on the EtOH lever was not observed in the SACC deprived group (p values > 0.12). Additionally, the higher responding on the EtOH lever was prolonged following the second deprivation period. In rats deprived of both EtOH and SACC, responding on the EtOH lever was increased for the initial 6 re-exposure sessions (p values < 0.008), compared to an increase in only 4 sessions following the first deprivation period (Fig. 2).
Figure 3.
Depicts the mean (± SEM) lever responses for P rats concurrently self-administering 15% EtOH (top) and 0.0125 % SACC (bottom) in rats maintained on self-administration (non-deprived) or following a 2 week deprivation period. Asterisks indicate that all groups are different from each other and all deprived groups are different from baseline. The Plus symbol indicates that rats deprived of EtOH or both EtOH and SACC were different from each other and responded significantly more than non-deprived rats and baseline. The Pound symbol indicates that rats deprived of EtOH and SACC responded more than all other groups and was significantly elevated compared to baseline. The Double Asterisks symbol indicates that rats deprived of EtOH and SACC responded significantly more than all other groups and was higher than baseline responding.
During the first 6 re-exposure sessions, there was a significant effect of group (F values (3,38) > 5.3; p values < 0.003). Post-hoc comparisons indicated that, during the initial 3 re-exposure sessions, all groups responded differently from each other, with the rats deprived of both EtOH and SACC exhibiting the highest responding of all groups. During the 4th re-exposure session, responses on the EtOH lever by rats deprived of both EtOH and SACC, or deprived of EtOH alone, were significantly different from each other, and were significantly different from the other two groups. During the 5th re-exposure session, rats deprived of both EtOH and SACC, or deprived of EtOH alone were different from the other two groups. During the 6th re-exposure session, rats deprived of both EtOH and SACC responded more than all other groups. For the group deprived of both EtOH and SACC during the first re-exposure session, the rats responded on average 722 times on the EtOH lever, and received 142 reinforcers. In contrast, these rats responded 19 times on the SACC lever, but only received 14 reinforcers.
In contrast to responses to the EtOH lever, responding on the SACC lever was not significantly altered following the 2nd deprivation period (cycle – F(1,38) = 2.0; p = 0.16; cycle × session × group F(18,228) = 1.1; p = 0.36, all other terms p values > 0.08). Responding on the SACC lever remained relatively constant (ranged between 16 ± 4 to 22 ± 6) following the second deprivation cycle.
DISCUSSION
The results of the current study indicate that in the presence of an alternate reinforcer, P rats express a robust ADE after a single deprivation, which increases in magnitude and duration with a second deprivation (Figs. 2 and 3). In addition, the present study supports previous work showing expression of an ADE by P rats (McKinzie et al., 1998; Rodd-Henricks et al., 2000a), and, furthermore, demonstrates that an ADE can be observed in the presence of SACC. Examination of data from the second deprivation cycle shows that results replicate a previous report from our lab where repeated deprivations using a single EtOH concentration in an operant paradigm results in an increase in both magnitude and duration of responding on the EtOH lever by the P rat (Rodd et al., 2003). This follows previous work that found an increase in both the magnitude and duration of expression of an ADE for P rats under 24-hr access to multiple EtOH concentrations conditions (Rodd-Henricks et al. 2001), and an increase in the duration of ADE after repeated deprivations, when a single EtOH concentration was available (Rodd-Henricks et al., 2000a).
This study shows that, when both SACC and EtOH are available concurrently in an operant setting, the P rat prefers 15% (v/v) EtOH over 0.0125% (g/v) SACC, as evidenced by the 6-to-1 ratio of EtOH self-administration over SACC at the FR-1 schedule, as well as by a continued willingness to increase responding for EtOH proportionately as the FR requirement increased. Additionally, the ratio of obtained reinforcers: predicted reinforcers was much higher for EtOH than for SACC in P rats (e.g., prior to deprivation - EtOH ratio 96%, SACC ratio 64%). This lower concentration of SACC was chosen because it had previously been shown that in an FR-5-alternate-day access paradigm, the P rat will work to self-administer the 0.0125% SACC at a comparable level to 15% EtOH, when the second reinforcer concurrently available is water (Melendez et al., 2004; Nowak et al., 1999). While self-administration paradigms qualitatively assess the reinforcing properties of a compound, quantitative assessment of the reinforcing properties of a compound can be determine through a progressive ratio test. Recent data indicated that P rats express identical breakpoints for the self-administration of 15% EtOH and 0.0125% SACC (Toalston et al., 2007). Therefore, when tested concurrently with water, 15% EtOH and 0.0125% SACC have the same reinforcing efficacy in P rats. Additional pilot studies were conducted to establish a concentration of SACC that would produce equal responding as concurrently available 15% EtOH. To date, all concentrations of SACC tested concurrently with 15% EtOH display a similar pattern (greatly suppressed) to that observed with the 0.0125% SACC concentration (Rodd et al., unpublished findings).
The specificity of preference of EtOH over another palatable solution is currently being assessed in our laboratory. An on-going research project is examining concurrent self-administration of sucrose and EtOH in P rats. The initial data set is very intriguing and markedly different from the current data set. During the acquisition of self-administration, P rats given concurrent access to EtOH (15%) and sucrose (1−8%) self-administer significantly more EtOH and sucrose (80 and 53% more, respectively) than P rats self-administering EtOH-water or sucrose-water (Rodd et al., unpublished data). Thus, P rats given concurrent operant access to EtOH and sucrose display a ‘positive contrast’ in self-administration behaviors. Positive-contrast is a well established learning phenomenon in which animals given two reinforcers increases the self-administration of both reinforcers compared to levels of self-administration when only a single reinforcer is available (Flaherty and Largen, 1975). In addition, P rats given concurrent access to sucrose and EtOH in operant situations will self-administer concentrations of EtOH that are not self-administered when EtOH is paired with water (e.g., P rats will self-administered 45 and 60% EtOH when 2% sucrose is concurrently available). Thus the preliminary data indicate that P rats will continual to self-administer EtOH, and at a higher rate, while another caloric reinforcer is available.
All deprived groups manifested an ADE following the initial deprivation period. Rats, which had daily access to 2 ml 15% EtOH in the home cage, returned to baseline responding earlier than those without EtOH, resulting in a decrease in the duration of the initial ADE. Following the second deprivation, an increase in the duration and magnitude of the observed ADE was observed in the EtOH deprived and the EtOH plus SACC deprived groups, but not in the group deprived of SACC (rats given 2 ml of 15% EtOH in their home cages during the deprivation period). This result supports the idea that repeated alcohol deprivations may produce long-term neuronal alterations different from any changes that may occur with continued access to EtOH. This study also observed that limited home cage access to SACC by the EtOH deprived group had a small depressive effect on EtOH responding during the re-exposure sessions, compared to responding on the EtOH lever by the EtOH plus SACC deprived group.
A potential influence of a sweetened solution (and other alternate, non-drug reinforcers) on craving behavior has previously been suggested. Taste of solutions presented, either separately or concurrently with EtOH, affects acquisition of EtOH drinking behavior (Wayner, 2002). In rhesus monkeys, both concurrent and between-session access to a 0.03% SACC solution markedly decreased responding for phencyclidine (Campbell and Carroll, 2000). Sucrose solutions given to rats prior to naloxone-precipitated withdrawal after a 6-day induction of morphine dependence decreased global withdrawal scores (Jain et al., 2004). Levels of sucrose used in this study were rather high (20−30%). A concentration of SACC higher than 0.0125% may therefore have stronger ADE-suppression effects.
Increasing the operant work requirement for access to EtOH during the study did not alter the amount of responding for SACC (approximately 20 responses/session throughout the experiment). Furthermore, following deprivations, responding for SACC remained the same. Although the data indicate that P rats will express an ADE in the presence of SACC, there was no observation of a SDE in the presence of EtOH. This suggests that EtOH, but not SACC, produced long-term CNS neuronal alterations. This idea is supported by microdialysis studies that reported increases in dopamine in the nucleus accumbens and ventral pallidum during anticipation and operant self-administration of EtOH, but not of SACC (Melendez et al., 2002; 2004). In addition, under operant conditions, P rats fail to display a SDE with higher concentration of SACC (0.025% and higher; Rodd et al., 2006 and unpublished findings).
Researchers have recently begun to address the neurobiological phenomena accompanying ADE in the P rat. In an intracranial self-administration study, a comparison between non-deprived and repeatedly deprived P rats with intra-cranial self-administration of EtOH into the posterior ventral tegmental area reported an increase in responding by the repeatedly deprived group (Rodd et al., 2005). Receptor binding assays have shown that following repeated deprivations of EtOH in inbred P (iP) rats, D1 and D2 receptor binding sites are differentially altered in the extended amygdala, accumbens, and dorsal striatum areas of the brain (Sari et al., 2006). Additional neurotransmitters systems (.i.e., glutamate and/or serotonin) probably mediate the neuroadaptions produced by exposure to a single or multiple periods of alcohol deprivation.
Overall, the results of this study indicate that an ADE effect can be observed for P rats in the presence of an alternate reinforcer, i.e., SACC, but a SDE was not observed in the presence of EtOH. In addition, because deprivation of EtOH plus SACC had a greater effect than deprivation of EtOH alone on expression of an ADE, these results suggest some overlap in the CNS circuitry mediating the reinforcing effects of EtOH and SACC.
Acknowledgments
This study was supported in part by NIAAA grants AA07462, AA07611, AA10721, AA16251 and AA11261.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bell SM, Gosnell BA, Krahn DD, Meisch RA. Ethanol reinforcement and its relationship to saccharin preference in Wistar rats. Alcohol. 1994;11:141–145. doi: 10.1016/0741-8329(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Burish TG, Maisto SA, Cooper AM, Sobell MB. Effects of voluntary short-term abstinence from alcohol on subsequent drinking patterns of college students. J. Stud. Alcohol. 1981;42:1013–1020. doi: 10.15288/jsa.1981.42.1013. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Reduction of drug self-administration by an alternative non-drug reinforcer in rhesus monkeys: magnitude and temporal effects. Psychopharmacology. 2000;147:418–425. doi: 10.1007/s002130050011. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol. Clin. Exp. Res. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cicero TJ. A critique of animal analogs of alcoholism. In: Majchrowich E, Noble EP, editors. Biochemistry and Pharmacology of Ethanol. Vol. 2. Plenum Press; New York: 1979. pp. 533–560. [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R, Gessa GL. Sardinian alcohol-preferring rats prefer chocolate and sucrose over ethanol. Alcohol. 1997;14:611–615. doi: 10.1016/s0741-8329(97)00075-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Maccioni P, Mascia MF, Orru A, Gessa GL, Carai MAM. Suppression of acquisition of alcohol-drinking behavior by the concurrent availability of saccharin in Sardinian alcohol-preferring (sP) rats. Alcohol. 2005a;35:27–33. doi: 10.1016/j.alcohol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Maccioni P, Mascia MF, Orru A, Gessa GL, Carai MAM. Suppression of maintenance of alcohol-drinking behavior by the concurrent availability of saccharin in Sardinian alcohol-preferring (sP) rats. Alcohol. 2005b;35:35–41. doi: 10.1016/j.alcohol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Largen J. Within-subjects positive and negative contrast effects in rats. J. Comp. Physiol. Psychol. 1975;88:653–664. doi: 10.1037/h0076416. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. The relationship between saccharin and alcohol intake in rats. Alcohol. 1992;9:203–206. doi: 10.1016/0741-8329(92)90054-e. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Hilbrom ME. Alcohol withdrawal seizures and binge versus chronic drinking. In: Port RJ, Mattson RH, Cramer JA, Diamond I, editors. Alcohol and Seizures: Basic Mechanisms and Clinical Concepts. F.A. Davis; Philadelphia: 1990. pp. 206–215. [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–4. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Jain R, Mukherjee K, Singh R. Influence of sweet tasting solutions on opioid withdrawal. Brain. Res. Bull. 2004;64:319–322. doi: 10.1016/j.brainresbull.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Overstreet DH, Rezvani AH, Janowsky DS. Suppression of ethanol intake in Alcohol-Preferring rats by prior voluntary saccharin consumption. Pharm. Biol. Behav. 1995;52:59–64. doi: 10.1016/0091-3057(94)00430-q. [DOI] [PubMed] [Google Scholar]
- Kamov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol. Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcohol status. Alcohol. Clin. Exp. Res. 2004;28:1290–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Kornet M, Goosen C, Van Ree JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology. 1991;104:367–376. doi: 10.1007/BF02246038. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- Lankford MF, Myers RD. Genetics of alcoholism: simultaneous presentation of a chocolate drink diminishes alcohol preference in high alcohol drinking HAD rats. Pharmacol. Biochem. Behav. 1994;49:417–425. doi: 10.1016/0091-3057(94)90443-x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit. Rev. Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li T-K. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol. Clin. Exp. Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- McMillen BA. Toward a definition of a valid animal model of alcoholism: multiple animal models for multiple diseases. Alcohol. 1997;14:409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol. Clin. Exp. Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Involvement of the mesopallidal dopamine system in ethanol reinforcement. Alcohol. 2004;32:137–144. doi: 10.1016/j.alcohol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li T-K. Effects of schedule access on ethanol intake by the alcohol-preferring P line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li T-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav. Genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Neznanova ON, Zvartau EE, Bespalov AY. Behavioral analysis of the saccharin deprivation effect in rats. Behav. Neurosci. 2002;116:747–756. doi: 10.1037/0735-7044.116.5.747. [DOI] [PubMed] [Google Scholar]
- Nowak KL, McKinzie DL, McBride WJ, Murphy JM. Patterns of ethanol and saccharin intake in P rats under limited-access conditions. Alcohol. 1999;19:85–96. doi: 10.1016/s0741-8329(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogenous rats as well as different rat strains. Alcohol. Clin. Exp. Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li T-K. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol. Clin. Exp. Res. 2000a;24:8–16. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li T-K. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol. Clin. Exp. Res. 2000b;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol. Clin. Exp. Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats. I. Periadolescent exposure. Alcohol. Clin. Exp. Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats. II. Adult exposure. Alcohol. Clin. Exp. Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol. Biochem. Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J. Pharmacol. Exp. Ther. 2005;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav. Brain. Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol. Clin. Exp. Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Li T-K. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following deprivation. Psychol. Sci. 1967;8:11–12. [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q. J. Stud. Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Sinclair JD. The use of animal models in the search for a cure for alcoholism: I. Developing animal models. Aust. J. Alcohol Drug Depend. 1975;2:47–49. [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li T-K. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol. Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- Toalston JE, Rodd ZA, Oster SM, Murphy JM, Bell RL, McBride WJ. Effects of saccharin or ethanol drinking by alcohol-preferring (P) rats during peri-adolescence on EtOH self-administration during adulthood. Alcohol. Clin. Exp. Res. 2007;31:73A. [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Wayner MJ. Craving for alcohol in the rat: adjunctive behavior and the lateral hypothalamus. Pharmacol. Biochem. Behav. 2002;73:27–43. doi: 10.1016/s0091-3057(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cotts A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol. Behav. 1972;8:345–362. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]