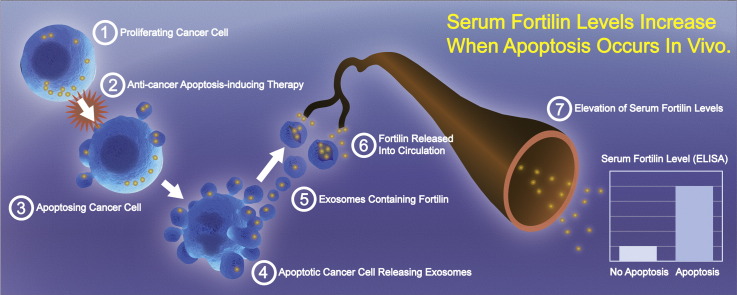
Abstract
Background
Billions of cells undergo apoptosis each day in the average normal adult. The ability to readily assess the degree of apoptosis in human diseases is hampered by the lack of sensitive and specific serum biomarkers of apoptosis. Fortilin is a novel prosurvival molecule that protects cells against various noxious stimuli. While fortilin is secreted into the extracellular space under certain conditions, the relationship between the serum concentration of fortilin and the presence and extent of apoptosis in vivo remains unknown.
Methods & results
Using a newly developed fortilin ELISA system, we show here that fortilin exists in the normal human and mouse circulation. We further demonstrate that fortilin serum levels are significantly elevated in patients with solid cancer, in response to anti-cancer chemo- or radiation therapy. The elevation of fortilin serum levels is more robust and sensitive than that of such previously-reported serum biomarkers of apoptosis as fragmented cytokeratin-18, cytochrome c, and nucleosomal DNA. In addition, targeted apoptotic liver damage induced by Jo2 anti-Fas (CD95) antibody consistently and significantly increased serum fortilin levels in C57BL/6J mice. Finally, when challenged by anti-human-Fas IgM antibody, Jurkat leukemic T cells apoptosed and released fortilin into the medium before plasma membrane integrity was compromised.
Conclusions
Taken together, these data suggest that serum fortilin levels reflect the degree and extent of apoptosis occurring in vivo.
General significance
Fortilin is a viable serum biomarker of in vivo apoptosis and can be utilized to noninvasively assess the status of in vivo apoptosis in humans.
Keywords: Apoptosis, Biomarker, Fortilin, Programmed cell death
Graphical abstract
Highlights
-
•
Ultra-sensitive fortilin ELISA has been developed. Fortilin circulates in blood.
-
•
Fortilin serum levels become highly elevated after apoptosis-inducing therapy.
-
•
Fortilin is more robust and sensitive than other serum apoptosis markers.
-
•
Fortilin is actively secreted before plasma membrane becomes disrupted.
-
•
Fortilin is an excellent serum biomarker of in vivo apoptosis.
1. Introduction
Approximately 50 to 70 billion cells undergo apoptosis each day in the average normal adult [1]. Serum biomarkers of apoptosis – molecules that can be readily and objectively measured as indicators of normally and pathologically occurring apoptosis at tissue and organ levels – would allow clinicians to easily monitor the status of apoptosis associated with the diseases and conditions they treat—such as apoptosis-induced skeletal muscle atrophy resulting from cancer (cachexia), aging (sarcopenia) [2], starvation, denervation, disuse, and inflammation [3]. Cancer cells undergo apoptosis at a higher rate than do normal cells and massively apoptose in response to radiation therapy and chemotherapy [4]. Serum biomarkers of apoptosis could thus allow clinicians to screen patients for certain cancers or to monitor the response of patients with cancer to anti-cancer chemo- or radiation therapy [5].
Thus far, three serum biomarkers of apoptosis have been reported in the literature including the fragmented cytokeratin-18 (fCK18, detectable by the M30 antibody), nucleosomally-cleaved genomic DNA (n-DNA), and cytochrome c (Cyt C) [5]—each with notable limitations to their utility. The utility of fCK18 is limited to apoptosis occurring in cells of epithelial origin [6]. The utility of circulating n-DNA is diminished because it can be rapidly degraded by serum DNases [7]. Cyt C is reportedly released from both apoptotic [8] and necrotic cells [9], depending on the extent of cellular damage, thus limiting its specificity. Further, these candidate serum apoptosis biomarkers have not been extensively characterized or validated at clinical, whole animal, and cellular levels.
Fortilin, also known as translationally controlled tumor protein (TCTP) and IgE-dependent histamine releasing factor (HRF), is a 172-amino acid nuclear-cytosolic shuttle protein that was originally cloned in 1989 by Gross and others as a molecule abundantly expressed in tumor cells [10]. A multifunctional protein implicated in various cellular functions [11], [12], [13], [14], [15], [16], fortilin possesses potent anti-apoptotic activity [12], [17], [18], [19], [20], [21], [22]. It binds the sequence-specific DNA binding domain of p53 and prevents p53 from transcriptionally activating the proapoptotic gene Bax [23]. Fortilin also binds to and stabilizes MCL1 [22], a macrophage survival factor [24], [25]. In addition to being intracellularly located, fortilin can be trafficked into exosomes – small secretory vesicles – and eventually be released into the extracellular space in an ER/Golgi-independent fashion [15]. However, it remains unknown if fortilin actually circulates in the blood of normal humans and animals. Further, it is not known if serum fortilin levels change in response to various pathophysiological conditions, including apoptosis occurring in body tissues.
Since it is a potent anti-apoptotic molecule that can be secreted into the extracellular space, we hypothesized that fortilin could be a viable serum apoptosis biomarker. Here, we report for the first time that fortilin is present in the blood of healthy humans and mice. We also show that anti-cancer chemo- or radiation therapy causes serum fortilin levels to increase, more robustly, sensitively and specifically than fCK-18, Cyt C, or n-DNA in humans. Strikingly, the release of fortilin from the cell precedes any signs of compromised plasma membrane integrity. We therefore propose that serum fortilin is a sensitive and robust biomarker of apoptosis occurring in vivo.
2. Materials and methods
2.1. Cell culture and cell lines
The Jurkat cell lines (Clone E6-1) were purchased from American Type Culture Collection (ATCC, Manassas, VA). All cell lines were maintained in high-glucose Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS) at 37 °C in an atmosphere containing 10% CO2.
2.2. Cell-based assay of biomarkers release and plasma membrane disruption
Jurkat cells were seeded in 18 wells of 6-well plates (5 × 105 cells/well) in RPMI medium with 5% FBS. The next day, the cells were washed once with phosphate buffered saline (PBS) and re-suspended in 1 mL of RPMI medium containing 12.5 ng/mL of anti-human Fas IgM (clone CH11). At each time point (0, 0.5, 1, 2, 4, and 8 h, N = 3 for each time point), we harvested 500 μL of cell suspension, centrifuged it at 100 g for 5 min, transferred the medium to fresh microfuge tubes, and froze both the media and cell pellets separately at − 80 °C until the assays for LDH, n-DNA, fortilin, Cyt C, and fCK-18, were performed. For 7-AAD staining, we added 20 μL of 7-AAD solution (BD Pharmingen) to 400 μL of cell suspension and incubated it for 10 min at room temperature, shielded from light. We counted total and 7-AAD-positive cells under the fluorescence microscope as described previously [26]. The integrity of the plasma membrane of the cells with positive 7-AAD signal is compromised. At least 200 cells were counted and the 7-ADD index was calculated as (the number of 7-AAD-positive cells) / (the number of total cells) ∗ 100.
2.3. Mouse model of targeted liver apoptosis
All animal procedures were performed according to a protocol approved by the UTMB Institutional Animal Care and Use Committee (IACUC), in accordance with the National Institutes of Health guidelines and the “Position of the American Heart Association on Research Animal Use.” We induced apoptosis in the liver of C57BL/6J male mice (12 weeks of age) by intraperitoneal administration of the Jo2 anti-Fas antibody (1.25 μg/body weight in gram, resulting in approximately 25 μg of antibody per mouse): PBS was used as a control. Once injected, the mice became ill within 3 h; half of them were dead within 6 h, as described previously [27]. At 5–9 h after anti-Fas injection, when they were clinically moribund, the mice were sacrificed, their blood collected, and the organs harvested for further analyses. Jo2 antibody binds the mouse Fas antigen and induces Fas-mediated apoptosis in the liver without affecting any other tissues as reported previously [27]. Transmission electron microscopic examination reportedly showed a lack of phagocytosis of apoptosed cells. Also, there was no gross leakage of cell contents into the extracellular space observed by the same examination [27].
2.4. Caspase-3 activity
Caspase-3 assays were performed as we described previously [28]. In brief, cells were suspended in cell lysis buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.01% Triton X-100), subjected to three freeze-thaw cycles, and centrifuged at 14,000 g. Aliquots of cleared cell lysates were incubated with 2.5 mM rhodamine 110 bis-(N-CBZ-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide)(Z-DEVD-R110) substrate (Invitrogen-Molecular Probes, Grand Island, NY). Caspase-3 activities were determined every 5 min for 90 min by measuring fluorescence (excitation/emission = 496/520 nm), using the SpectraMax Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA), and expressed as relative fluorescence units (RFU).
2.5. TUNEL staining
TUNEL staining was performed as we previously described [23], using the FragEL™ DNA Fragmentation Detection Kit (EMD Millipore, Calbiochem, Billerica, MA), according to the manufacturer's instructions. At least 600 cells were counted and TUNEL indices were calculated as the number of TUNEL-positive cells divided by the number of total cells counted and expressed as percentages.
2.6. Lactate dehydrogenase (LDH) activity assay
Serum LDH activity was measured by the LDH Activity Assay Kit (Sigma-Aldrich, St. Louis, MO; Catalog Number: MAK066) according to the manufacturer's instructions.
2.7. Serum Cyt C assay
Serum Cyt C was quantified by the Human Cytochrome C Quantikine ELISA kit (R&D Systems, Minneapolis, MN; Catalog Number: DCTC0) according to the manufacturer's instructions.
2.8. Serum alanine transaminase (ALT) assay
Serum ALT was quantified by the mammalian liver profile rotor and VETSCAN VS2 (Abaxis, Union City, CA) according to the manufacturer's instructions.
2.9. DNA fragmentation assay
The Cell Death Detection ELISA PLUS kit (Roche, Indianapolis, IN, Catalog Number: 11774425001) was used according to the manufacturer's instruction and modifications described previously [23]. We added 20 μL of serum from a patient into a well of a streptavidin-coated 96-well plate, in triplicate. We then added 80 μL of incubation buffer (PBS supplemented with 1% BSA, 0.5% Tween 20 and 1 mM EDTA), containing peroxidase-conjugated mouse anti-DNA antibody (MCA-33) and mouse biotinylated anti-histone antibody (H11-4) to the wells and incubated the plate for 2 h at room temperature on a shaker. The plate was then washed three (3) times with incubation buffer before we added 100 μL of 2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ATBS) solution to each well, incubated at room temperature on a shaker (300 rpm) until the color development was sufficient for reading (2–10 min), and added 100 μL of the ATBS Stop Solution.
2.10. Fragmented cytokeratin-18 (fCK-18) determination
Cytokeratin-18 (CK-18), a 48-kDa, 423 amino-acid polypeptide, is a major component of intermediate filaments of cells of epithelial origin. M30 is a monoclonal antibody produced by immunizing Balb/c mice with two purified CK18 fragments [29]. The epitope of M30 is the 387–396th amino acids of CK18 (EDFNLGDALD), representing the COOH-terminal amino acid residues of the intermediate 19-kDa fragment generated by caspases-3 and -7. M30 does not recognize the intact CK18 where it has not been cleaved between 396th asparatic acid (D) and 397th serine (S) by caspases [29], thus making M30 reactivity specific for apoptosis. The exact molecular mechanism by which CK-18 is released from epithelial cells undergoing apoptosis remains unknown [30]. Quantification of the caspase-generated neoepitope of CK-18 in serum samples was performed using the M30-Apoptosense ELISA kit (Peviva, Bromma, Sweden) as described previously and according to the manufacturer's instructions, in triplicates. The standard curves were generated by using synthetic immunogenic peptides provided with the kit.
2.11. ELISA to quantify the serum concentration of mouse and human fortilin
A polystyrene 96-well plate (BD Falcon, Bedford, MA) was coated with 50 μL of capture anti-fortilin antibody (Abnova, Taipei City, Taiwan; Clone 2C4; Catalog Number: H00007178-M03) diluted at 1 μg/mL in PBS and incubated at 4 °C overnight. After the wells were washed five (5) times with wash buffer (PBS with 0.1% Tween 20), they were blocked with 100 μL of blocking buffer (PBS with 1% BSA) for 1 h at room temperature. The wells were then washed five (5) times with wash buffer. 100 μL each of the samples, dissolved at an appropriate concentration in dilution buffer (PBS with 0.1% BSA), was then added to each well and incubated at 37 °C for 3 h. The wells were then washed five (5) times with wash buffer. 100 μL of biotinylated anti-fortilin detection antibody (Abnova; Clone 2A3; Catalog Number: H00007178-M06) diluted at 1 μg/mL in dilution buffer was added to each well, and the plate was incubated at 37 °C for 2 h. After wells were washed five (5) times with wash buffer, 100 μL of avidin-HRP (eBioscience, San Diego, CA, diluted at 1:500 in dilution buffer) was added to each well. The wells were then washed five (5) times with wash buffer. To detect bound antibody, 100 μL of Strep™ Ultra TMB-ELISA (Thermo Fisher Scientific, Waltham, MA) was added to each well and the plate was incubated at room temperature for 30 min before 50 μL of 2 M sulfuric acid was added to stop the reaction. The signal was read with a plate reader at 450 nm. The Coefficient of Variation (CV) was determined as %CV = SD/mean × 100 using the data obtained from normal human subjects. The limit of detection was defined as the fortilin concentration at which the current ELISA system gives a statistically significant value above that of the zero analyte (dilution buffer alone) at a 99% confidence level, i.e., the means of the quadruplicates of the zero analyte and those of fortilin at the detection-limit concentration must differ by three SDs.
2.12. Collection of human serum samples
Human sera were collected under protocols approved by the UTMB Internal Review Board. Detailed information on the samples from the patients undergoing anti-cancer therapy is shown in Table 1. Written informed consent was received from participants prior to sample collection. The samples were frozen at − 80 °C until the described assays were performed. All samples were free of any identifying information at the time of assay.
Table 1.
Summary of patients undergoing anti-cancer chemotherapy.
| Patient number | Age | Sex | Diagnosis |
Therapy |
Post-treatment sampling |
LDH | Fortilin | Established apoptosis biomarkers |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Location | Recurrent | Stage | Radiation therapy | Chemotherapy | (Days after the last therapy) | Anyone | Cyt C | n-DNA | CK-18 | |||||
| 1 | 49 | F | SCC | Cervical | Yes | IIIB | NO | Cisplatin | 26 | ↑ | ↑↑ | ↑ | ↑ | ||
| 2 | 35 | F | SCC | Cervical | Yes | IIIB | NO | Cisplatin | 11 | ↑ | |||||
| 3 | 40 | F | SCC | Cervical | Yes | IIIB | NO | Cisplatin | 50 | ↑ | ↑↑ | ↑ | ↑ | ||
| 4 | 48 | F | SCC | Cervical | No | IIIB | YES | Cisplatin | 16 | ↑ | ↑ | ||||
| 6 | 49 | F | SCC | Cervical | No | IIIB | YES | Cisplatin | 3 | ↑ | |||||
| 7 | 53 | F | SCC | Cervical | No | IIB | YES | Cisplatin | 7 | ↑ | ↑ | ↑ | |||
| 8 | 47 | F | SCC | Cervical | No | IV | YES | Cisplatin | 1 | ↑ | |||||
| 9 | 43 | F | SCC | Cervical | No | IIIB | YES | NO | 7 | ↑ | ↑ | ↑ | |||
| 10 | 48 | M | SCC | Retromolar trigone | No | T4bN2b | YES | Cisplatin | 20 | ↑ | |||||
| 11 | 62 | M | SCC | Hypopharynx | No | T3N2 | YES | Cisplatin | 44 | ↑ | |||||
| 12 | 71 | M | SCC | Larynx & NSCLC | No | T3N2 & T2N0 | YES | NO | 1 | ↑ | |||||
| 13 | 38 | M | ACC | Maxillary sinus | No | NA | YES | NO | 1 | ↑ | |||||
| 14 | 51 | F | SCC | Tonsil | No | T4aN1 | YES | TPF | 54 | ↑ | |||||
| 15 | 59 | M | SCC | Retromolar trigone | No | T4BbN2b | YES | Cisplatin | 7 | ↑ | |||||
| 16 | 53 | F | SCC | Base of the tongue | No | T4N2 | YES | NO | 1 | ↑ | |||||
| 17 | 35 | M | Mucoepidermoid carcinoma | Base of the tongue | No | IV | YES | NO | 1 | ↑ | ↑ | ↑ | |||
| 18 | 67 | M | SCC | Base of the tongue | No | T4bN2b | YES | Cisplatin | 1 | ↑ | ↑ | ↑ | |||
| 19 | 52 | M | SCC | Tonsil | No | IVa (T3N2) | YES | Cisplatin | 16 | ||||||
Abbreviation: F, female; M, male; SCC, squamous cell cardinoma; ACC, adenocystic carcinoma; NSCLC, non-small-cell lung carcinoma; TPF, docetaxel, cisplatin, 5-fluorouracil; NA, data not available. Patient #10: a sufficient amount of post-treatment sample available only for fortilin ELISA. Patient #5: excluded from the study as post-treatment sample volume was not sufficient for any assays.
2.13. Statistical analysis
The degree of spread of the data was expressed as the standard deviation (± SD). P < 0.05 was considered to be statistically significant. The Student's t-test was employed for comparing the means of two groups.
3. Results
3.1. Fortilin ELISA development
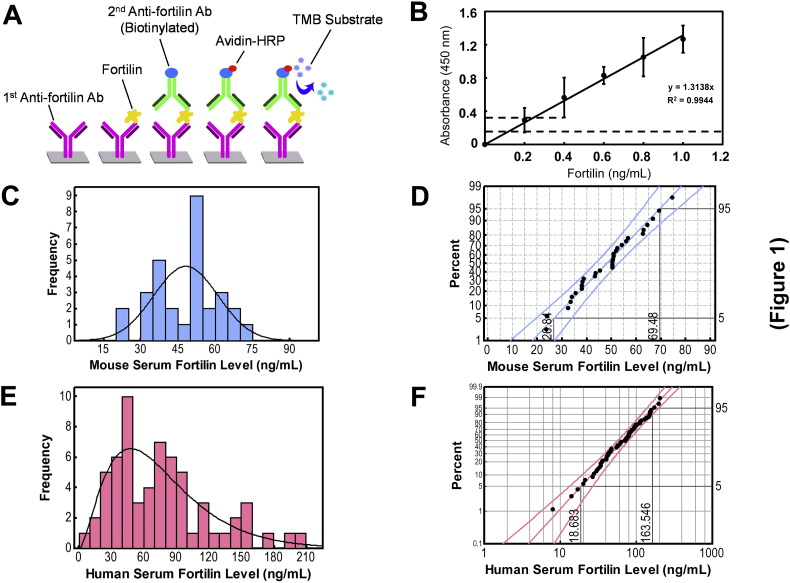
There have been no robust quantitative assays reported for fortilin. To evaluate fortilin as a serum apoptosis biomarker, we first developed a sandwich enzyme-linked immunosorbent assay (ELISA) of fortilin using two distinct anti-fortilin monoclonal antibodies and avidin-based signal amplification as described in detail in the Materials and methods section (Fig. 1A). The detection limit of the developed ELISA was 0.4 ng/mL (Fig. 1B) with an 8.6% coefficient of variation (CV).
Fig. 1.
Development and characterization of fortilin ELISA. Abbreviations: Ab, antibody; HRP, horseradish peroxidase; TMB,3,3′,5,5′-tetramethylbenzidine. A. The design of fortilin ELISA. B. Detection limits of the fortilin ELISA. Each span of the error bar represents 3 × standard deviation (SD). C. Histogram of mouse serum fortilin levels D. Probability plot of mouse serum fortilin levels using the normal distribution fit with the 5th and 95th percentile values of 26.84 and 69.48 ng/mL. E. Histogram of human serum fortilin levels. F. Probability plot of human serum fortilin levels using the gamma distribution fit with 5th and 95th percentile values of 18.68 and 163.55 ng/mL.
3.2. Fortilin circulates in the blood of normal humans and mice
To test the hypothesis that fortilin circulates in the blood, the sera from 12-week-old C57BL/6J male and female mice (n = 30) were subjected to the above fortilin ELISA assay, showing average mouse serum fortilin levels of 48.16 ± 12.96 ng/mL with no significant difference between male and female mice (males vs. females = 49.22 ± 14.48 vs. 47.10 ± 11.66 ng/mL, n = 15 each, P = 0.663; Fig. S1). The samples were distributed normally (Anderson–Darling [AD] normality test, AD value = 0.292, P = 0.581; Fig. 1C) with the 5 and 95 percentile values of 28.84 and 69.48 ng/mL (Fig. 1D). We then used the same ELISA assay to examine the sera from 63 patients presenting to clinic for routine examination. Their average fortilin serum concentration was 75.57 ± 45.79 ng/mL with no significant difference between male and female human subjects (males vs. females = 77.6 ± 49.9 vs. 74.1 ± 43.3 ng/mL, n = 26 and 37, respectively, P = 0.77; Fig. S2A). There was also no statistically significant correlation between age and fortilin levels (Fig. S2B). Unlike the mouse samples, the human samples did not distribute normally (Anderson–Darling [AD] normality test, P < 0.005; Fig. 1E). The data best fits the gamma distribution with the lowest AD value (0.182, P > 0.250), with the 5 and 95 percentile values of 18.68 and 163.546 ng/mL (Fig. 1 F). Taken together, these data suggest that fortilin circulates in the blood of both normal humans and mice.
3.3. Serum fortilin levels are significantly elevated after anti-cancer chemo- and radiation therapy
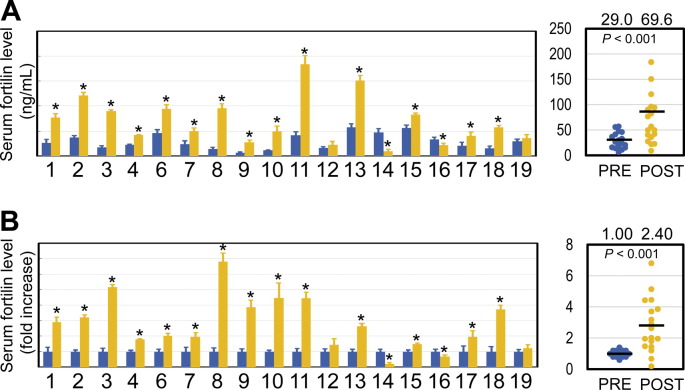
Fortilin has been shown to protect cells against apoptosis and to be released into the extracellular space via secretory exosomes [15]. To test the hypothesis that serum fortilin levels reflect apoptosis occurring at the tissue level, we quantified serum fortilin concentrations in pre- and post-treatment sera of patients with solid malignancies (cervical/neck cancers and squamous cell carcinoma [SCC]) who were undergoing anti-cancer chemo- or radiation therapy (“anti-cancer therapy” hereafter), as both anti-cancer drugs and ionizing radiation trigger apoptosis in cancer tissue (Table 1). The mean pre-treatment serum fortilin levels in these cancer patients were 29.0 ± 15.5 ng/mL. After chemotherapy, however, the mean serum fortilin levels increased 2.40 fold to 69.6 ± 47.2 ng/mL (P < 0.001, Paired Student's t-test; Fig. 2). Of 18 samples tested, 14 (77.8%) showed a statistically significant increase in fortilin levels after anti-cancer therapy while two (11.1%) were significantly decreased (Fig. 2). These data indicate that serum fortilin levels generally increase in response to anti-cancer therapy.
Fig. 2.
Serum fortilin levels are increased after anti-cancer therapy (radiation therapy, chemotherapy, or both). Abbreviations: *, statistically significant (P < 0.05); PRE, pre-anti-cancer therapy; POST, post-anti-cancer therapy. A. Change in serum fortilin levels [ng/mL] in 18 patients undergoing anti-cancer therapy. The details of the patients are in Table 1. B. Change in serum fortilin levels when pre-treatment levels were normalized to one. Patient #10: a sufficient amount of post-treatment sample was available only for fortilin ELISA, thus included for serum fortilin assay but excluded from the other assays. Patient #5: excluded from the study as post-treatment sample volume was not sufficient for any assays.
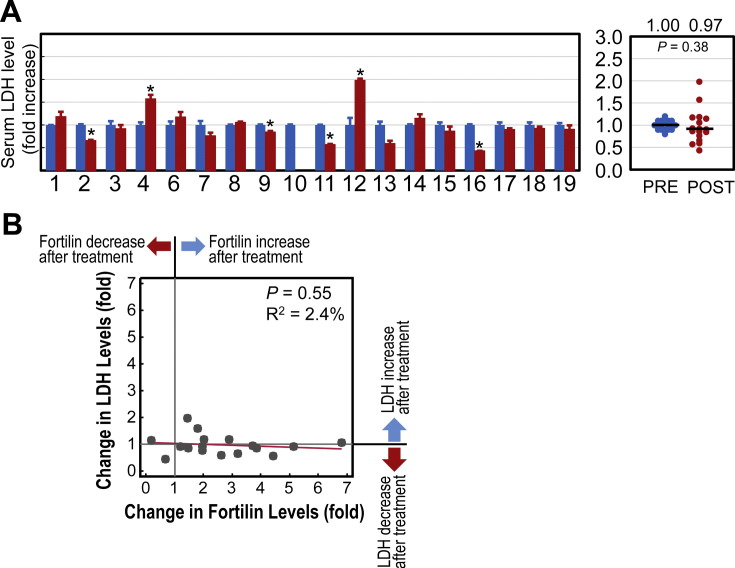
We also determined the lactate dehydrogenase (LDH) levels in these samples as these levels may reflect anti-cancer-therapy-induced tissue necrosis [8]. The mean pre-treatment serum LDH level was 33.36 ± 12.54 mU/mL, while the mean post-treatment serum LDH level was 30.46 ± 11.81 IU/mL (P = 0.38, NS). Out of 17 samples tested (the patient #10 was excluded as we did not have enough post-treatment sera remaining for assays), only two (11.8%) had a statistically significant increase in LDH levels after anti-cancer therapy while four (23.5%) were significantly decreased (Fig. 3A). Further, regression analysis showed no statistically significant association between the fold-change of serum fortilin levels and that of LDH levels after anti-cancer therapy (P = 0.552, R2 = 2.4%; Fig. 3B). These data suggest that anti-cancer treatment induced no significant degree of tissue necrosis and that fortilin elevation was not due to the necrotic plasma membrane damage in cancer cells (Fig. 3A & B).
Fig. 3.
Infrequent increases in serum lactate dehydrogenase (LDH) levels suggest a lack of extensive necrotic tissue damage in the study patients. Abbreviations: LDH, lactate dehydrogenase; *, P < 0.05; PRE, pre-anti-cancer therapy; POST, post-anti-cancer therapy. A. Change in serum LDH levels when pre-treatment levels were normalized to one. B. Serum LDH levels did not correlate significantly with serum fortilin levels.
3.4. Serum fortilin is superior to the other apoptosis biomarkers
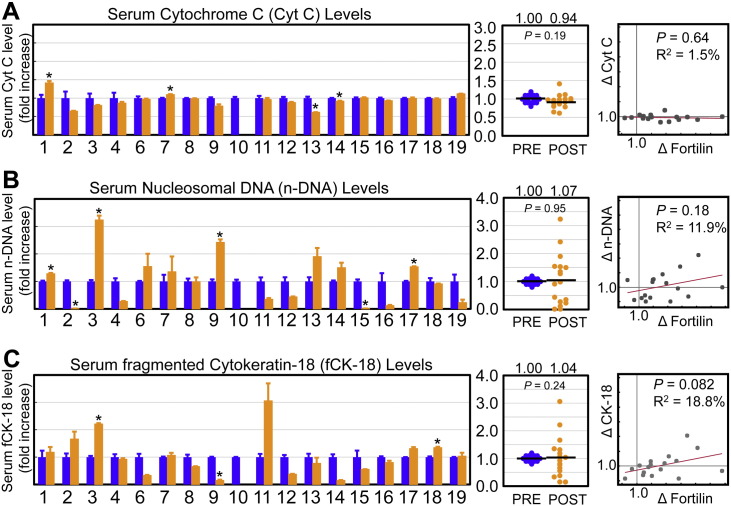
To compare serum fortilin levels with the other apoptosis biomarkers described in the literature, we assayed the same samples for Cyt C, n-DNA, and fCK-18 levels.
First, we found that the mean serum Cyt C levels did not statistically change (from 0.90 ± 0.26 to 0.83 ± 0.31 ng/μL, paired Student's t-test, P = 0.19) between before and after anti-cancer therapy. Out of 17 samples tested, only two (11.7%) showed a statistically significant increase in Cyt C levels after anticancer therapy while two (11.7%) had a statistically significant decrease (Fig. 4A).
Fig. 4.
Known serum markers of apoptosis do not increase as much as fortilin levels upon anti-cancer therapy. Abbreviations: Cyt C; cytochrome c; n-DNA, nucleosomal DNA; fCK-18, fragmented cytokeratin-18; *, P < 0.05; PRE, pre-anti-cancer therapy; POST, post-anti-cancer therapy; Δ, fold change. A. Changes in serum cytochrome c (Cyt C) levels when their pre-treatment levels were normalized to one. There was no statistically significant association between serum Cyt C and fortilin levels by regression analysis (P = 0.635, R2 = 1.5%). B. Changes in serum nucleosomal DNA (n-DNA) levels when their pre-treatment levels were normalized to one. There was no statistically significant association between serum n-DNA and fortilin levels by regression analysis (P = 0.18, R2 = 11.9%). C. Changes in serum fragmented cytokeratin-18 (fCK-18) levels when their pre-treatment levels were normalized to one. There was no statistically significant association between serum fCK-18 and fortilin levels by regression analysis (P = 0.082, R2 = 18.8%).
Next, we determined serum n-DNA levels before and after anti-cancer therapy and found that the mean serum n-DNA levels did not statistically change (from 0.23 ± 0.25 to 0.21 ± 0.38 ng/μL, paired Student's t-test, P = 0.820). Of 17 samples tested, only 4 (23.5%) showed a statistically significant increase in n-DNA levels after anti-cancer therapy, while 2 (11.7%) had a statistically significant decrease (Fig. 4B).
Upon assaying serum fCK-18 levels before and after anti-cancer therapy, we found that the mean serum fCK-18 levels did not statistically change (from 139.78 ± 98.1 to 148.4 ± 100.3 U/L, paired Student's t-test, P = 0.197). Of 17 samples tested, only 2 (11.7%) showed a statistically significant increase in fCK-18 levels after anti-cancer therapy while one (5.8%) had a statistically significant decrease (Fig. 4C).
Serum fortilin levels were significantly elevated in all patients who had at least one of three established biomarkers of apoptosis (Cyt C, n-DNA, or fCK-18) elevated (Patient #s 1, 3, 7, 9, 17, and 18) (Table 1). They were not elevated in patients whose established biomarkers were all negative (Patients # 12 and 19) (Table 1). These data presented above, when taken together, suggest that the elevation of serum fortilin levels accurately predicts apoptosis occurring in vivo and that serum fortilin levels more sensitively detect in vivo apoptosis than do Cyt C, n-DNA or fCK-18 levels.
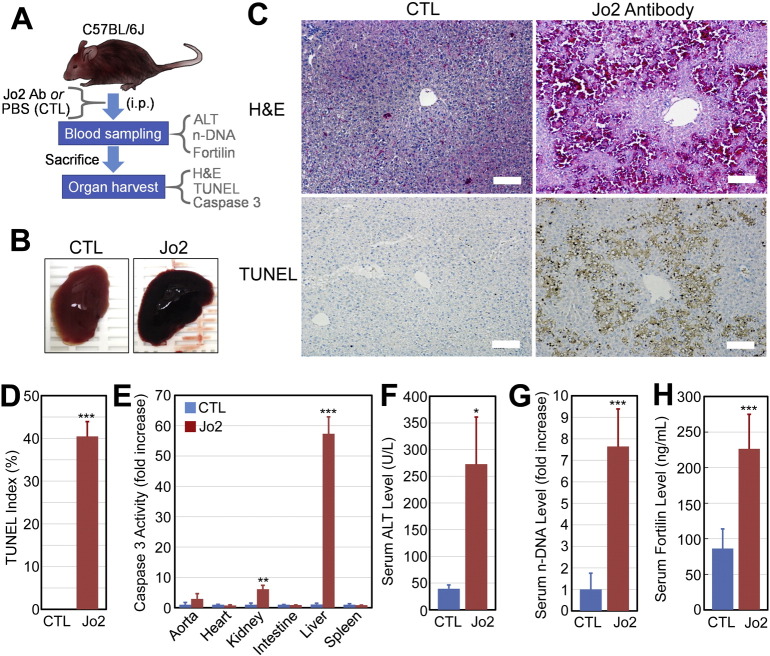
3.5. Serum fortilin levels are drastically elevated in mice with apoptosis-induced liver damage
To further validate the serum fortilin level as a biomarker of apoptosis in vivo, we turned to a mouse model of apoptosis-induced liver damage in which intraperitoneally administered Armenian hamster anti-Fas-antigen antibody (Jo2) rapidly and selectively induces Fas-mediated hepatocyte apoptosis, leading to severe liver damage without affecting other organs [27]. Since different strains of mice exhibit a vastly different response to Jo2 antibody [31], we first characterized the liver injury in C57BL/6J mice to which we intraperitoneally administered Jo2 antibody (Fig. 5A). Within 3 h of injection, mice became grossly ill and were dead within 9 h. The livers of mice treated with Jo2 antibody were hemorrhagic, both grossly and at a microscopic level, resembling human fulminant hepatitis (Fig. 5B and C–H & E). TUNEL staining (Fig. 5C-TUNEL, Fig. 5D) showed numerous TUNEL-positive, apoptotic hepatocytes suggesting that fas-mediated hepatocyte apoptosis predominantly resulted in liver damage in the current system (TUNEL indices: control (CTL) vs. Jo2 = 0.00 ± 0.00 vs. 40.46 ± 3.46%, n = 5, P < 0.005). The vast majority of Jo2 antibody's effects were on the liver, as the caspase 3 activity of the liver increased 55.33-fold while the kidney, the second most severely affected organ, showed only a 6.09-fold increase in caspase-3 activity (P < 0.005, n = 3; Fig. 5E). As expected, the administration of Jo2 antibody resulted in a drastic increase in the serum alanine transaminase (ALT) levels (control vs. Jo2 = 39.33 ± 7.02 vs. 272.50 ± 88.39 U/L, n = 3, P < 0.05; Fig. 5F). Serum n-DNA levels were also elevated in Jo2-treated animals (control vs. Jo2 = 1.00 ± 0.75 vs. 7.64 ± 1.75 A.U., n = 5, P < 0.005; Fig. 5G). In this system, serum fortilin levels were found to be 2.63-fold elevated in Jo-2 treated animals vs. their control (86.18 ± 27.71 vs. 226.31 ± 48.51 ng/mL, n = 4, P < 0.005; Fig. 5H). These findings in the well-characterized mouse model of apoptosis-induced liver injury suggest that serum fortilin levels represent a viable marker of in vivo apoptosis.
Fig. 5.
Serum fortilin levels increase in response to Jo2-antibody-induced apoptosis in the liver. Abbreviations: PBS, phosphate-buffered saline; CTL, control; i.p., intraperitoneal injection; ALT, alanine aminotransferase; n-DNA, nucleosomal DNA; H&E, hematoxylin and eosin staining; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick-end labeling staining; *, P < 0.05; **, P < 0.01; ***, P < 0.005. A. C57BL/6J mice were injected with Jo2 antibody and sacrificed 5–9 h later. B. The livers of control and Jo2-treated mice. C. Hematoxylin & eosin (H&E) and TUNEL staining of the control and Jo2-treated livers. Size bar: 100 μm. D. TUNEL index (%) of the control and Jo2-treated livers. E. Caspase-3 activities of control and Jo2-treated organs. F. Serum alanine aminotransferase (ALT) levels in control and Jo2-treated mice. G. Serum n-DNA levels in control and Jo2-treated mice. H. Serum fortilin levels in control and Jo2-treated mice.
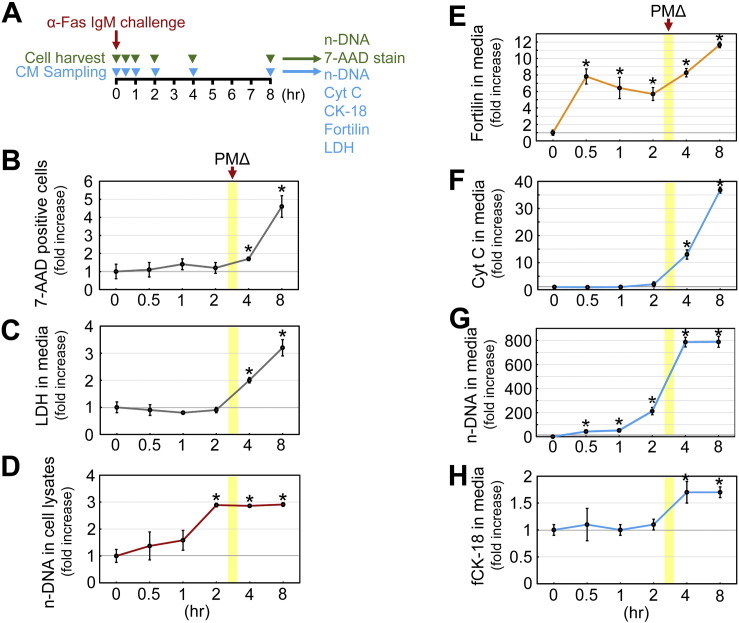
3.6. Fortilin excretion from the cell precedes the compromise of the plasma membrane integrity
To evaluate the possibility that the elevation of serum fortilin levels observed above was due solely to the passive release of fortilin from the damaged cells, we challenged Jurkat cells, a human T lymphocyte cell line, with anti-human Fas IgM (CH-11, 12.5 ng/mL), harvested aliquots of cells and conditioned media at times 0, 0.5, 1, 2, 4, and 8 h (Fig. 6A), and subjected the cells to (a) 7-aminoactinomycin D (7-ADD) staining to identify cells with plasma membrane damage (Fig. S3) and (b) a DNA fragmentation assay to monitor the progression of apoptosis. We also subjected the conditioned media to the assays for n-DNA, Cyt C, fCK-18, LDH, and fortilin (Fig. 6A). In this system, 7-ADD-positive Jurkat cells did not significantly increase until 4 h after the induction of apoptosis (Fig. 6B). Similarly, the LDH concentration in the media did not significantly increase until 4 h after apoptosis was induced (Fig. 6C). Taken together, these data suggest that plasma membrane integrity becomes compromised (PMΔ) between 2 and 4 h after the initiation of Fas-induced apoptosis in this system. DNA fragmentation within the cell began right after anti-Fas stimulation, progressed steadily, and was completed by 2 h after apoptosis was induced as quantified by DNA fragmentation assays performed on the cell lysates (Fig. 6D), suggesting that plasma membrane disruption occurring between 2 and 4 h after apoptosis induction coincided with the very late phase of apoptosis in this system. Using this system and fortilin ELISAs, we tested whether fortilin was actively released from apoptosing Jurkat cells before the plasma membrane disintegrated. Jurkat cells do not secrete fortilin into the media without apoptotic stimuli (Fig. S4). Strikingly, fortilin concentrations in the media drastically increased just 30 min after the induction of apoptosis, well before there were any detectable signs of plasma membrane disintegration (Fig. 6E; 0.5, 1, and 2 h), supporting the idea that fortilin is actively released from the apoptosing cells via exosomes [15]. While n-DNA exhibited release kinetics similar to those of fortilin (Fig. 6G), the concentrations of Cyt C and fCK-18 in the medium did not increase until 4 h had passed, suggesting that the release of those apoptosis biomarkers required the change to the plasma membrane (Fig. 6F & H). These data suggest that fortilin is actively released from apoptosing cells in the very early phase of apoptosis and its release does not require a compromise to the integrity of the plasma membrane detectable by the elevation of LDH and traversing of 7-AAD into the cells.
Fig. 6.
Fortilin is released in the very early phase of apoptosis, before the compromise to the plasma membrane integrity. Abbreviations: α-Fas IgM, anti-human Fas IgM (clone CH11); CM, conditioned media; n-DNA, nucleosomal DNA; 7-AAD, 7-aminoactinomycin D; Cyt C, cytochrome c; fCK-18, fragmented cytokeratin-18; LDH, lactate dehydrogenase; PMΔ, plasma membrane change; *, P < 0.05. A. Experimental design. Jurkat cells were challenged by 12.5 ng/mL of anti-human Fas IgM (clone CH11) in Roswell Park Memorial Institute (RPMI) media with 5% fetal bovine serum (FBS). Cells and conditioned media were harvested at indicated time points and subjected to the respective sets of assays as described in the panel. 7-AAD cannot pass through the intact plasma membrane. 7-AAD cannot enter into the live cell. B. 7-AAD staining to detect cells with change in plasma membrane integrity. C. LDH concentrations in the conditioned media. D. n-DNA concentration in the cell lysates that reflect the progression of apoptosis occurring within the cell. E. Fortilin concentrations in the conditioned media. F. Cyt C levels in the conditioned media. G. n-DNA concentration in the conditioned media. H. fCK-18 concentration in the conditioned media.
4. Discussion
There are three innovations in the current work. First, the present study is the first to report the development and characterization of an ELISA system capable of measuring fortilin in mouse and human sera. Our fortilin ELISA system both sensitively (detection limit = 0.4 ng/mL) and reliably (%CV = 8.6%) detected both human and mouse serum fortilins (Fig. 1). Second, it has been unknown whether fortilin circulates in the blood of normal subjects. We are the first to show that fortilin circulates in the blood of normal congenic C57BL6/J mice and apparently healthy humans (Fig. 1). Third, using the ELISA system, we demonstrate for the first time that serum fortilin is a unique biomarker of apoptosis (Fig. 2), distinct from and more sensitive and specific than the previously reported serum biomarkers of apoptosis [5], including Cyt C, n-DNA, and fCK-18 (Figs. 4, S5).
Renz and others reported that Cyt C not only translocates from the mitochondrial intermembrane space into the cytosol, but also is released into the extracellular space in response to apoptotic stimuli [8]. They found that the serum levels of Cyt C are elevated immediately after anti-cancer chemotherapy in patients with predominantly hematological malignancies [8]. When serum Cyt C levels were measured by quantitative Western blot analysis, more than a 2-fold increase in Cyt C levels was present in 8 out of 17 patients tested (47.1%) [8]. Other groups also reported that serum Cyt C levels are elevated in patients with un-treated malignant tumors, and that an elevated serum Cyt C level is an adverse prognostic marker [32], [33], [34]. However, Jemmerson and others reported that Cyt C release could occur from both apoptotic and necrotic cells [9]. In addition, Osaka and others found that there was a significant and positive correlation between serum Cyt C and LDH levels [32]. It is not entirely clear whether serum Cyt C becomes elevated solely and always due to apoptosis in vivo since, at least theoretically, any cell death stimuli that disrupt both mitochondria and the plasma membrane would release Cyt C into the extracellular space. In the current work, we found only 2 patients to have statistically significant elevation of Cyt C levels (Fig. 4A) after anti-cancer therapy, while 14 (77.8%) out of 18 patients had statistically significant increase in fortilin levels (Fig. 4A). The relative lack of sensitivity of Cyt C, compared to fortilin, could be explained by the results of Fig. 6, where we found that both Cyt C and fCK-18 require disruption of the plasma membrane to be released into the extracellular space (Fig. 6F & H). On the contrary, fortilin and n-DNA did not require such plasma membrane changes and were released from the cells in the very early phase of apoptosis (on and after 0.5 h; Fig. 6E & G), presumably through exosomes [15] and apoptotic bodies [35], respectively.
n-DNA are produced by the cleavage of chromosomal DNA by endonucleases activated during apoptosis and can be released into the circulation. Holdenrieder and others showed that patients with malignant tumors had higher levels of serum n-DNA than did healthy subjects and those with benign tumors and that serum n-DNA levels increased in response to anti-cancer therapy [36]. Despite the fact that the generation of n-DNAs is specific to apoptosis and that they appear to be released from the cell in an active process via apoptotic bodies [35] in the early phase of apoptosis (Fig. 6G), the serum n-DNA levels were found not to be as sensitive as serum fortilin levels in detecting apoptosis in the current cohort of patients—out of 17 samples tested, only 4 (23.5%) showed a statistically significant increase in n-DNA levels after anti-cancer therapy, while 2 (11.7%) had a statistically significant decrease (Fig. 4B). The apparently low sensitivity of n-DNA in human samples (Fig. 4B) may at least partly be due to the fact that circulating n-DNA is rapidly degraded by serum DNases within hours of its release into the circulation [7].
Cytokeratin-18 (CK-18) is a major component of intermediate filaments of epithelial cells and tumors derived from such cells [6]. During apoptosis, caspase-3 cleaves CK-18 into three fragments—NH2-terminal, 26-kDa; middle, 19-kDa; and COOH-terminal, 3-kDa. The M30 antibody recognizes a neo-epitope on the middle 19-kDa fragment, exposed only after caspase-3 cleavage of CK-18 [29]. Levels of the caspase-cleaved, 19-kDa, CK-18 fragment (fCK-18) were reported to be significantly elevated in patients with gastric cancer vs. healthy subjects [37], [38]. fCK-18 levels also increased significantly after cancer chemotherapy [39]. However, it was also reported that both caspase-cleaved and uncleaved/intact CK-18s are elevated (a) in patients with cancer compared to healthy subjects [4], [38] and (b) after cancer chemotherapy [34], [40], suggesting that the release of CK-18s relies on the disruption of the plasma membrane. In addition, CK-18 is present only in epithelial cells and may not be useful for the detection of apoptosis in other tissue types such as muscle, connective, and nervous tissues. In the current work, of 17 samples tested, only 2 (11.7%) showed a statistically significant increase in fCK-18 levels after anti-cancer therapy, while one (5.8%) had a statistically significant decrease (Fig. 4C), suggesting that serum fCK-18 levels are not as sensitive as serum fortilin levels in detecting apoptosis at least in the current cohort of patients with SCC undergoing anti-cancer therapy.
Fortilin has three key attributes that make it a preferred serum biomarker of apoptosis. First, it is released in the very early phase of apoptosis, well before the integrity of the plasma membrane is compromised (Fig. 6B, C, & E). In other words, fortilin is an authentic apoptosis marker, and its release into the extracellular space does not require plasma membrane damage, unlike Cyt C, fCK-18, and LDH (Fig. 6C, F & H). Second, it is detected in patients undergoing anti-cancer therapy more sensitively and consistently than are Cyt C, n-DNA, and fCK-18 (Figs. 2 & 4, Table 1), most likely due to its stability in the blood and its ability to be secreted from the cell without plasma membrane disruption. Third, our clinical study (Fig. 2, Fig. 3, & 4), animal experiments (Fig. 5), and cell-based experiments (Fig. 6) all support fortilin as a serum apoptosis biomarker (Fig. S5) superior to those previously reported.
In conclusion, our current work for the first time establishes serum fortilin as a viable apoptosis biomarker, which is secreted from apoptosing cells into the circulation in the very early phase of apoptosis and detectable by the ELISA. Further translational studies would define the precise roles of serum fortilin levels in the diagnosis and treatment of patients with the medical conditions in which apoptosis plays a key role.
The following are the supplementary data related to this article.
Mouse fortilin levels do not differ between males and females. Abbreviations: NS, not statistically significant. Fortilin levels were determined for mouse serum samples from 12-week-old C57BL/6J male (N = 15) and female (N = 15) mice, using a newly developed fortilin ELISA.
Human fortilin levels do not differ between males and females (A) or by age (B). Abbreviations: NS, not statistically significant. Fortilin levels were determined for 63 serum samples from apparently normal adult subjects, using a newly developed fortilin ELISA.
7-AAD staining of Jurkat cells stimulated by α-Fas IgM. Abbreviations: 7-AAD, 7-aminoactinomycin D. Size bar: 200 μm. Jurkat cells were challenged by 12.5 ng/mL of anti-human Fas IgM (clone CH11) in Roswell Park Memorial Institute (RPMI) media with 5% fetal bovine serum (FBS). Cells were harvested at indicated time points and subjected to the 7-AAD staining as described in the Materials and Methods section of the manuscript. 7-AAD cannot traverse through the intact plasma membrane. The cells with red fluorescence 7-AAD signal have disrupted plasma membrane. At least 200 cells were counted and the 7-ADD index was calculated as (the number of 7-AAD positive cells) / (the number of total cells) ∗ 100.
Jurkat cells do not secrete fortilin without anti-Fas IgM challenge. Abbreviations: NS, not statistically significant. 5 × 105 Jurkat cells were seeded at each cell of 6-well plates using PRMI media supplemented by 5% FBS. Next day, cells were washed once with PBS and re-suspended in 1 mL of fresh RPMI media with 5% FBS. At times 0, 6, and 12 h, 500 μL of cell suspension was harvested from each well in triplicates and subjected to centrifugation at 100 g for 5 min. The supernatant was transferred to a fresh microfuge tube and stored at − 80 °C for fortilin ELISA.
Comparison of fortilin with other apoptosis biomarkers and LDH. Abbreviations: Cyt C, cytochrome c; n-DNA, nucleosomal DNA; fCK-18, fragmented cytokeratin-18; LDH, lactate dehydrogenase. LDH is a cell death marker that is passively released through the damaged plasma membrane without apoptosis-specific modification. Although it is passively released from the cells unmodified, Cyt C can still be an apoptosis marker as Cyt C is released from the mitochondrial intermembrane space into the cytosol in the apoptosis-specific process. While it is passively released from the cells, fCK-18 is a caspase-cleaved product of the original cytoskeleton protein CK-18. Based on the data described in Fig. 6F and H, both Cyt C and fCK-18 rely on the compromise in plasma membrane integrity for their release into extracellular space. n-DNA is an apoptosis-specific degradation product of nuclear DNA by the caspaseactivated DNAse (CAD) and released before plasma membrane changes occur detectable by 7-AAD and LDH-release (Fig. 6G). Fortilin is unique because it does not undergo apoptosis-specific modification and is released in the very early phase of apoptosis, most likely via exosomes (Fig. 6E).
Disclosure of conflicts of interest
There is no conflict of interest for any of the contributing authors.
Acknowledgments
We thank Ms. Heather W. Foster for her editorial support in manuscript preparation, Dr. David Konkel for critically editing the manuscript and Ms. Glenda C. Brents for her help with the graphical abstract. This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch (supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health), a grant from the National Cancer Institute (5R01CA127971, to M.S.M.), a grant from the National Heart Lung and Blood Institute (R01HL117247 to K.F.), and a grant from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Graduate Program (Grant No. PHD/0041/2549, to P.S.).
References
- 1.Reed J.C. Dysregulation of apoptosis in cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 2.Dupont-Versteegden E.E. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp. Gerontol. 2005;40:473–481. doi: 10.1016/j.exger.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz L.M. Atrophy and programmed cell death of skeletal muscle. Cell Death Differ. 2008;15:1163–1169. doi: 10.1038/cdd.2008.68. [DOI] [PubMed] [Google Scholar]
- 4.Ulukaya E., Yilmaztepe A., Akgoz S., Linder S., Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007;56:399–404. doi: 10.1016/j.lungcan.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Ward T.H., Cummings J., Dean E., Greystoke A., Hou J.M., Backen A., Ranson M., Dive C. Biomarkers of apoptosis. Br. J. Cancer. 2008;99:841–846. doi: 10.1038/sj.bjc.6604519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caulin C., Salvesen G.S., Oshima R.G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamkovich S.N., Cherepanova A.V., Kolesnikova E.V., Rykova E.Y., Pyshnyi D.V., Vlassov V.V., Laktionov P.P. Circulating DNA and DNase activity in human blood. Ann. N. Y. Acad. Sci. 2006;1075:191–196. doi: 10.1196/annals.1368.026. [DOI] [PubMed] [Google Scholar]
- 8.Renz A., Berdel W.E., Kreuter M., Belka C., Schulze-Osthoff K., Los M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001;98:1542–1548. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- 9.Jemmerson R., LaPlante B., Treeful A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death Differ. 2002;9:538–548. doi: 10.1038/sj.cdd.4400981. [DOI] [PubMed] [Google Scholar]
- 10.Gross B., Gaestel M., Bohm H., Bielka H. cDNA sequence coding for a translationally controlled human tumor protein. Nucleic Acids Res. 1989;17:8367. doi: 10.1093/nar/17.20.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M., Jung Y., Lee K., Kim C. Identification of the calcium binding sites in translationally controlled tumor protein. Arch. Pharm. Res. 2000;23:633–636. doi: 10.1007/BF02975253. [DOI] [PubMed] [Google Scholar]
- 12.Graidist P., Yazawa M., Tonganunt M., Nakatomi A., Lin C., Phongdara A., Fujise K. Fortilin binds Ca2 + and blocks Ca2 +-dependent apoptosis in vivo. Biochem. J. 2007;408:181–191. doi: 10.1042/BJ20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarm F.R. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell. Biol. 2002;22:6209–6221. doi: 10.1128/MCB.22.17.6209-6221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashiwakura J.I., Ando T., Matsumoto K., Kimura M., Kitaura J., Matho M.H., Zajonc D.M., Ozeki T., Ra C., Macdonald S.M., Siraganian R.P., Broide D.H., Kawakami Y., Kawakami T. Histamine-releasing factor has a proinflammatory role in mouse models of asthma and allergy. J. Clin. Invest. 2012;122:218–228. doi: 10.1172/JCI59072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amzallag N., Passer B.J., Allanic D., Segura E., Thery C., Goud B., Amson R., Telerman A. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J. Biol. Chem. 2004;279:46104–46112. doi: 10.1074/jbc.M404850200. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald S.M., Rafnar T., Langdon J., Lichtenstein L.M. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 17.Fujita T., Felix K., Pinkaew D., Hutadilok-Towatana N., Liu Z., Fujise K. Human fortilin is a molecular target of dihydroartemisinin. FEBS Lett. 2008;582:1055–1060. doi: 10.1016/j.febslet.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graidist P., Phongdara A., Fujise K. Antiapoptotic protein partners fortilin and MCL1 independently protect cells from 5-FU-induced cytotoxicity. J. Biol. Chem. 2004;279:40868–40875. doi: 10.1074/jbc.M401454200. [DOI] [PubMed] [Google Scholar]
- 19.Koide Y., Kiyota T., Tonganunt M., Pinkaew D., Liu Z., Kato Y., Hutadilok-Towantana N., Phongdara A., Fujise K. Embryonic lethality of fortilin-null mutant mice by BMP-pathway overactivation. Biochim. Biophys. Acta. 2009;1790:326–338. doi: 10.1016/j.bbagen.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F., Zhang D., Fujise K. Characterization of fortilin, a novel anti-apoptotic protein. J. Biol. Chem. 2001;276:47542–47549. doi: 10.1074/jbc.M108954200. [DOI] [PubMed] [Google Scholar]
- 21.Tulis D.A., Mnjoyan Z.H., Schiesser R.L., Shelat H.S., Evans A.J., Zoldhelyi P., Fujise K. Adenoviral gene transfer of fortilin attenuates neointima formation through suppression of vascular smooth muscle cell proliferation and migration. Circulation. 2003;107:98–105. doi: 10.1161/01.cir.0000047675.86603.eb. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D., Li F., Weidner D., Mnjoyan Z.H., Fujise K. Physical and functional interaction between MCL1 and fortilin. The potential role of MCL1 as a fortilin chaperone. J. Biol. Chem. 2002;277:37430–37438. doi: 10.1074/jbc.M207413200. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Fujita T., Zhang D., Doan H., Pinkaew D., Liu Z., Wu J., Koide Y., Chiu A., Jun-Lin C., Chang J.Y., Ruan K.H., Fujise K. The physical and functional antagonism between p53 and fortilin, an anti-apoptotic molecule. J. Biol. Chem. 2011;286:32575–32585. doi: 10.1074/jbc.M110.217836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marriott H.M., Bingle C.D., Read R.C., Braley K.E., Kroemer G., Hellewell P.G., Craig R.W., Whyte M.K., Dockrell D.H. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Invest. 2005;115:359–368. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steimer D.A., Boyd K., Takeuchi O., Fisher J.K., Zambetti G.P., Opferman J.T. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerne K., Erman A., Veranic P. Analysis of cytotoxicity of melittin on adherent culture of human endothelial cells reveals advantage of fluorescence microscopy over flow cytometry and haemocytometer assay. Protoplasma. 2013;250:1131–1137. doi: 10.1007/s00709-013-0489-8. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 28.Koide Y., Kiyota T., Tonganunt M., Pinkaew D., Liu Z., Kato Y., Hutadilok-Towatana N., Phongdara A., Fujise K. Embryonic lethality of fortilin-null mutant mice by BMP-pathway overactivation. Biochim. Biophys. Acta. 2009;1790:326–338. doi: 10.1016/j.bbagen.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leers M.P., Kolgen W., Bjorklund V., Bergman T., Tribbick G., Persson B., Bjorklund P., Ramaekers F.C., Bjorklund B., Nap M., Jornvall H., Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J. Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.John K., Wielgosz S., Schulze-Osthoff K., Bantel H., Hass R. Increased plasma levels of CK-18 as potential cell death biomarker in patients with HELLP syndrome. Cell Death Dis. 2013;4:e886. doi: 10.1038/cddis.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakinuma C., Takagaki K., Yatomi T., Nakamura N., Nagata S., Uemura A., Shibutani Y. Acute toxicity of an anti-Fas antibody in mice. Toxicol. Pathol. 1999;27:412–420. doi: 10.1177/019262339902700404. [DOI] [PubMed] [Google Scholar]
- 32.Osaka A., Hasegawa H., Yamada Y., Yanagihara K., Hayashi T., Mine M., Aoyama M., Sawada T., Kamihira S. A novel role of serum cytochrome c as a tumor marker in patients with operable cancer. J. Cancer Res. Clin. Oncol. 2009;135:371–377. doi: 10.1007/s00432-008-0479-y. [DOI] [PubMed] [Google Scholar]
- 33.Osaka A., Hasegawa H., Tsuruda K., Inokuchi N., Yanagihara K., Yamada Y., Aoyama M., Sawada T., Kamihira S. Serum cytochrome c to indicate the extent of ongoing tumor cell death. Int. J. Lab. Hematol. 2009;31:307–314. doi: 10.1111/j.1751-553X.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 34.Barczyk K., Kreuter M., Pryjma J., Booy E.P., Maddika S., Ghavami S., Berdel W.E., Roth J., Los M. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int. J. Cancer. 2005;116:167–173. doi: 10.1002/ijc.21037. [DOI] [PubMed] [Google Scholar]
- 35.Turiak L., Misjak P., Szabo T.G., Aradi B., Paloczi K., Ozohanics O., Drahos L., Kittel A., Falus A., Buzas E.I., Vekey K. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J. Proteome. 2011;74:2025–2033. doi: 10.1016/j.jprot.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Holdenrieder S., Stieber P., Bodenmuller H., Busch M., Fertig G., Furst H., Schalhorn A., Schmeller N., Untch M., Seidel D. Nucleosomes in serum of patients with benign and malignant diseases. Int. J. Cancer. 2001;95:114–120. doi: 10.1002/1097-0215(20010320)95:2<114::aid-ijc1020>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Oyama K., Fushida S., Kinoshita J., Okamoto K., Makino I., Nakamura K., Hayashi H., Inokuchi M., Nakagawara H., Tajima H., Fujita H., Takamura H., Ninomiya I., Kitagawa H., Fujimura T., Ohta T. Serum cytokeratin 18 as a biomarker for gastric cancer. Clin. Exp. Med. 2013;13:289–295. doi: 10.1007/s10238-012-0202-9. [DOI] [PubMed] [Google Scholar]
- 38.Yaman E., Coskun U., Sancak B., Buyukberber S., Ozturk B., Benekli M. Serum M30 levels are associated with survival in advanced gastric carcinoma patients. Int. Immunopharmacol. 2010;10:719–722. doi: 10.1016/j.intimp.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Bilici A., Ustaalioglu B.B., Ercan S., Seker M., Yilmaz B.E., Orcun A., Gumus M. The prognostic significance of the increase in the serum M30 and M65 values after chemotherapy and relationship between these values and clinicopathological factors in patients with advanced gastric cancer. Tumour Biol. 2012;33:2201–2208. doi: 10.1007/s13277-012-0481-5. [DOI] [PubMed] [Google Scholar]
- 40.Demiray M., Ulukaya E.E., Arslan M., Gokgoz S., Saraydaroglu O., Ercan I., Evrensel T., Manavoglu O. Response to neoadjuvant chemotherapy in breast cancer could be predictable by measuring a novel serum apoptosis product, caspase-cleaved cytokeratin 18: a prospective pilot study. Cancer Investig. 2006;24:669–676. doi: 10.1080/07357900600981307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mouse fortilin levels do not differ between males and females. Abbreviations: NS, not statistically significant. Fortilin levels were determined for mouse serum samples from 12-week-old C57BL/6J male (N = 15) and female (N = 15) mice, using a newly developed fortilin ELISA.
Human fortilin levels do not differ between males and females (A) or by age (B). Abbreviations: NS, not statistically significant. Fortilin levels were determined for 63 serum samples from apparently normal adult subjects, using a newly developed fortilin ELISA.
7-AAD staining of Jurkat cells stimulated by α-Fas IgM. Abbreviations: 7-AAD, 7-aminoactinomycin D. Size bar: 200 μm. Jurkat cells were challenged by 12.5 ng/mL of anti-human Fas IgM (clone CH11) in Roswell Park Memorial Institute (RPMI) media with 5% fetal bovine serum (FBS). Cells were harvested at indicated time points and subjected to the 7-AAD staining as described in the Materials and Methods section of the manuscript. 7-AAD cannot traverse through the intact plasma membrane. The cells with red fluorescence 7-AAD signal have disrupted plasma membrane. At least 200 cells were counted and the 7-ADD index was calculated as (the number of 7-AAD positive cells) / (the number of total cells) ∗ 100.
Jurkat cells do not secrete fortilin without anti-Fas IgM challenge. Abbreviations: NS, not statistically significant. 5 × 105 Jurkat cells were seeded at each cell of 6-well plates using PRMI media supplemented by 5% FBS. Next day, cells were washed once with PBS and re-suspended in 1 mL of fresh RPMI media with 5% FBS. At times 0, 6, and 12 h, 500 μL of cell suspension was harvested from each well in triplicates and subjected to centrifugation at 100 g for 5 min. The supernatant was transferred to a fresh microfuge tube and stored at − 80 °C for fortilin ELISA.
Comparison of fortilin with other apoptosis biomarkers and LDH. Abbreviations: Cyt C, cytochrome c; n-DNA, nucleosomal DNA; fCK-18, fragmented cytokeratin-18; LDH, lactate dehydrogenase. LDH is a cell death marker that is passively released through the damaged plasma membrane without apoptosis-specific modification. Although it is passively released from the cells unmodified, Cyt C can still be an apoptosis marker as Cyt C is released from the mitochondrial intermembrane space into the cytosol in the apoptosis-specific process. While it is passively released from the cells, fCK-18 is a caspase-cleaved product of the original cytoskeleton protein CK-18. Based on the data described in Fig. 6F and H, both Cyt C and fCK-18 rely on the compromise in plasma membrane integrity for their release into extracellular space. n-DNA is an apoptosis-specific degradation product of nuclear DNA by the caspaseactivated DNAse (CAD) and released before plasma membrane changes occur detectable by 7-AAD and LDH-release (Fig. 6G). Fortilin is unique because it does not undergo apoptosis-specific modification and is released in the very early phase of apoptosis, most likely via exosomes (Fig. 6E).