Abstract
Background and importance
Liquid embolization using Onyx® of brain arteriovenous malformations (AVMs) is sometimes limited by micro catheter retention by Onyx® cast during the disengagement process. Use of the new detachable tip micro catheter is presented in this report.
Clinical presentation
Two feeding arteries of a previously ruptured brain AVM in a 62-year-old man were embolized by Onyx 18 prior to surgery using the new Apollo™ micro catheter with the detachable-tip (30 mm) was utilized. The arterial feeders were accessed without difficulty by micro catheter, guided by 0.010" microwire. Onyx reflux around the distal end of the micro catheter was necessary for effective embolization of arteriovenous fistulous components of brain AVM. The micro catheter was successfully retracted after embolization in both feeding arteries, with distal end detachment seen in one but not the other arterial embolization. No arterial thrombosis, vasospasm, dissection, or rupture was seen at respective sites of disengagement.
Conclusion
Use of the new micro catheters with detachable-tip design allowed prolonged Onyx® injection times, safe micro catheter disengagement, without any limitations in accessing target arterial feeders.
Keywords: Arteriovenous malformation, detachable-tip, embolization, micro catheter, Onyx, liquid embolic agent
Introduction
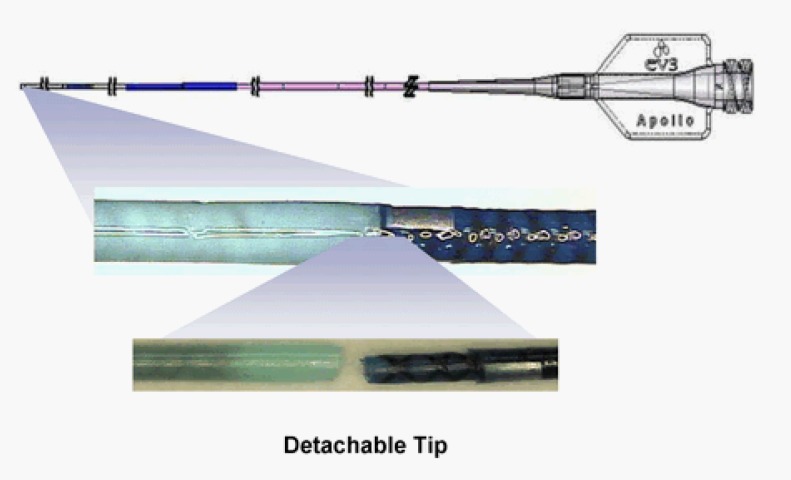
Brain arteriovenous malformations (AVMs) are managed with endovascular embolization prior to surgical resection or stereotactic radiosurgery [1, 2]. Onyx® liquid embolic system (eV3, Inc., Irvine, CA) comprising ethylene-vinyl alcohol copolymer, dimethyl-sulfoxide (DMSO) and micronized tantalum powder is frequently used in the management of AVMs. Use of this liquid embolic system allows for slow and controlled embolization. However, technical difficulties encountered during the procedure include catheter retention by the Onyx® cast and risk of vessel injury from forced catheter disengagement. The new micro catheter with detachable tip [Apollo™ (eV3; Irvine, CA)] is designed for use in these circumstances and helps prevent potential complications ( Figure 1). This technical report illustrates use of the new Apollo™ detachable tip micro catheter in a case of pre-surgical embolization of brain AVM.
Figure 1. Apollo™ detachable tip micro catheter (source: www.eV3.net).
Case presentation
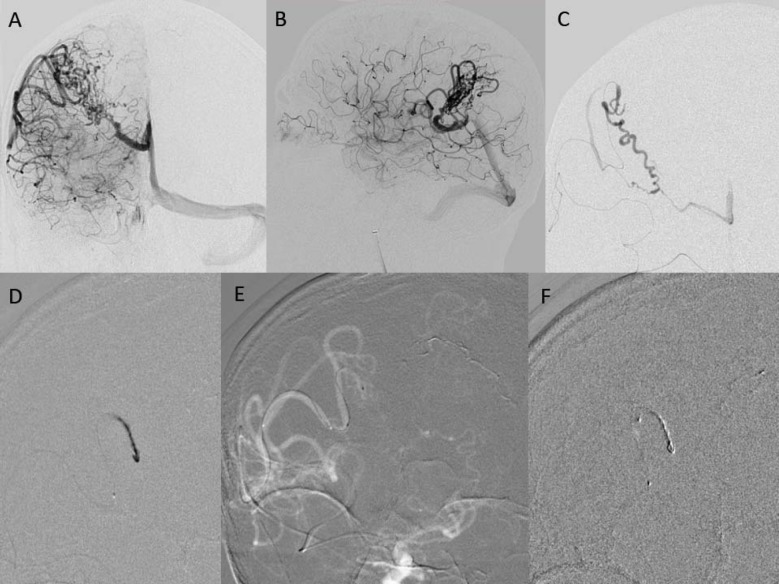
A 62-year-old man presented with symptoms of headache and visual disturbance. Initial neuroimaging studies (CT scan head) revealed intra-parenchymal hemorrhage in the parieto-occipital region of right cerebral hemisphere. Further diagnostic evaluation included a cerebral angiogram that identified a 36 mm × 16 mm AVM with major contribution from the cortical branches (M4 division) of the right middle cerebral artery (MCA). Additional minor feeders to the inferior aspect of the nidus were identified from the distal (A2 segment) portion of the right anterior cerebral artery (ACA). A pre-nidal aneurysm and fistulous components were also noted. Venous outflow via the vein of Galen into the straight sinus was observed, with mild strictures within the vein of Galen. The vasculature in the parieto-occipital area was not well visualized and appeared to be displaced by the hematoma ( Figure 2). With the morphologic characteristics, the AVM was classified as Spetzler and Martin grade III [3].
Figure 2. (A.) Pre-embolization antereoposterior view. (B.) Pre-embolization lateral view. (C.) Intra-nidal injection. (D.) Micro catheter Onyx injection. (E.) Detachable tip in feeder. (F.) Withdrawal and tip detachment.
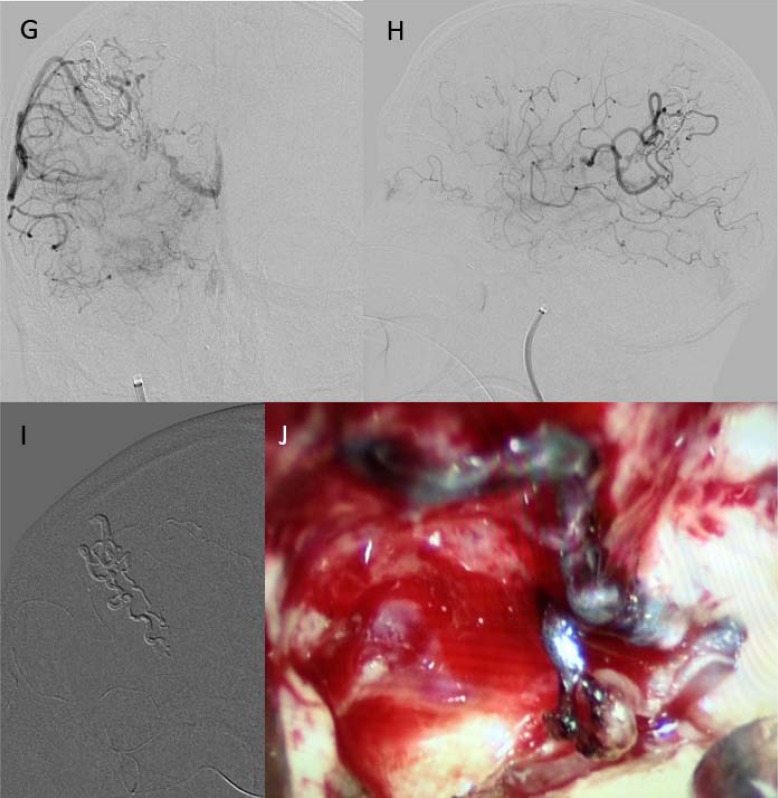
Staged embolization of the AVM was planned and Onyx® 18 was used as the agent (owing to its lower viscosity and better penetration). Initial embolization was performed for two arterial feeders from the right MCA. Initial attempt to access the feeder using Echelon™ micro catheter (eV3, Inc., Irvine, CA) was not successful due to prominent tortuosity of feeding arteries. The feeding artery was successfully accessed using Headway® Duo micro catheter (MicroVention Inc., Aliso Viejo, CA) and Synchro2™ (Boston Scientific, Natick, MA) 0.014 in microwire. After injection of Onyx into the brain AVM feeder with pre-nidal aneurysm, considerable difficulty was encountered in micro catheter disengagement. For the second session, more difficulty in accessing the remaining feeding arteries, and post Onyx injection disengagement of micro catheter was anticipated. Remaining components of the AVM included the fistulous component with rapid transit; therefore, requirement of considerable Onyx cast was anticipated for anterograde flow arrest around the distal end of microcatheter. Under a compassionate use exemption application from the Food and Drug Administration (FDA) and institutional review board acknowledgment, permission to use the new Apollo™ micro catheter (eV3, Inc., Irvine, CA) was obtained. At the second session, the previously noted nidal aneurysm was no longer visualized, and the fistulous component of the AVM via prominent arterial feeders arising from the superior division of the right MCA was visualized. Two residual major right MCA feeders of the AVM were targeted for embolization. At completion of second session, approximately 80% of total AVM was successfully embolized (Figure 3).
Figure 3. (G.) Post-embolization AP view. (H.) Post-embolization Lateral view. (I.) Onyx cast in nidus. (J.) Post-surgical resection.
Endovascular technique
A 5F Simmons-2 catheter was utilized to gain the right internal carotid artery (ICA) access to the Neuron™ Max (Penumbra, Inc., Alameda, CA) sheath was then advanced into the right ICA just distal to the cervico-petrous junction. The Apollo™ micro catheter with 30 mm detachable tip was selected and prepared for injection of Onyx®. Synchro® 10 guidewire (Stryker, Fremont, CA) was utilized to advance the Apollo™ micro catheter. The microwire was initially advanced through the petrous, cavernous, and clinoid segments of the right ICA followed by distal navigation of the Apollo™ micro catheter over the guidewire. Using similar technique, the superior distal hemispheric branch of the right MCA was catheterized. The tip of the micro catheter was left at the proximal segment of the arterial feeder so that reflux would occlude this feeder without affecting the ability to detach the tip. Angiography was performed to ensure that no normal arterial branches were supplied by the feeder which predominantly contributed to fistulous component of AVM with venous drainage was via the vein of Galen into the straight sinus. After DMSO was injected within the dead space of the micro catheter, Onyx® was injected at a rate of 0.1 mL/min to fill the arteriovenous fistulous component. The embolic agent was released from the tip of the micro catheter under free-flow conditions, filling the directly dependent nidus compartment in an anterograde and later retrograde manner until it flowed to the tip of the micro catheter [4]. The goal was to form an attenuated cast of Onyx around the tip of the micro catheter over a short distance. Injection of the agent was interrupted temporarily (2 min) for precipitation, followed by small volumes of the Onyx injection per cycle until there was enough reflux and attenuated cast formation. The injections were stopped if the reflux was larger than determined earlier and/or if any segment of draining veins was embolized. At the disengagement stage, intermittent gradual increase in tension was applied when the tip safely detached and embedded within the Onyx® cast at the expected location. A second Apollo™ micro catheter with 30 mm detachable tip was advanced into the other hemispheric branch of the right MCA using similar methods and pedicle was embolized. In this instance, the micro catheter was retracted and withdrawn using standard technique, but did not result in detachment of the tip. No arterial thrombosis, vasospasm, dissection, or rupture was seen at sites of disengagement. Full surgical resection of the AVM with the catheter-tip was subsequently performed on the next day with minimal blood loss.
Discussion
An advisory was released by the FDA revealed more than 100 reports of microcatheter breakage from entrapment during embolization procedures. [5]. In more than half of these reported cases, removal of the catheter was not possible, leading to partial catheter and Onyx plug implantation. Frequency of catheter entrapment associated with liquid embolization is reported as 4% [6], and several methods of catheter retrieval have been previously reported [7, 8]. Nonetheless, catheter entrapment is an undesirable procedural risk associated with serious complications such as hemorrhage and death [5]. The Apollo™ detachable tip micro catheter is a novel catheter designed to address the issue of catheter entrapment. It is a DMSO-compatible device with a soft, detachable distal tip, allowing for prolonged Onyx injection times. It has a stainless steel proximal coil for structural support and a Nitinol distal braiding for high kink resistance. The Apollo™ micro catheter is available with detachable distal tip lengths of 15, 20, and 30 mm. The catheter has a typical proximal diameter of 2.7 F, distal outer diameter of 1.5 F, inner diameter of 0.013 in., and a total length of 165 cm (www.eV3.net).
The detachable tip micro catheters are likely advantageous in embolization of arteriovenous fistulas where substantial cast around the distal end of the catheter is required to create flow arrest and prevent rapid transit of Onyx into the venous system. This arrangement avoids substantial cast deposition and obliteration of the arterial component of the fistula. Another scenario is AVM embolization with pre-nidal aneurysm that is traversed by the micro catheter. Reflux of liquid embolic agent is necessary for obliteration of such pre-nidal aneurysms. In these circumstances, a micro catheter with detachable tip may be valuable. Additionally, the detachable tip micro catheter allows for slow and prolonged injection times permitting complete embolization of AVMs, and simultaneously avoid potential procedural complications. Safety and feasibility of detachable tip micro catheters has previously been reported based on use of the SONIC micro catheter (BALT, Montmorency, France) [9]. However, in the United States, prior documentation of this catheter use the form of an abstract [10] and this report provides summary on catheter usage and procedural outcome. Further information on failure of tip-detachment process, compatibility of catheter with other embolic agents such as N-butyl cyanoacrylate (NBCA), will remain to be seen.
Conclusion
Use of Onyx® allows for slower and more controlled AVM embolization. However, this could result in the retention of micro catheter by Onyx® cast and increase the risk of vessel injury from any forced disengagement process. The ApolloTM microcatheter was designed to address this technical difficulty and help minimize procedural risk. Microcatheters with detachable tip capability have great potential for use in embolization procedures.
REFERENCES
- Barr JC, Ogilvy CS. Selection of treatment modalities or observation of arteriovenous malformations. Neurosurg Clin N Am. 2012 Jan;23(1):63–75. doi: 10.1016/j.nec.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Radvany MG, Gregg L. Endovascular treatment of cranial arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012 Jan;23(1):123–31. doi: 10.1016/j.nec.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–83. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- Natarajan SK, Ghodke B, Britz GW, Born DE, Sekhar LN.Neurosurgery 2008June626:1213–25.1April14 [DOI] [PubMed] [Google Scholar]
- FDA Safety Communication: Catheter Entrapment with the ev3 Onyx Liquid Embolic System http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm310121.htm
- Weber W, Kis B, Siekmann R, Kuehne D. AJNR Am J Neuroradiol. 2007 Feb;28(2):371–7. [PMC free article] [PubMed] [Google Scholar]
- Santillan A, Zink W, Knopman J, Riina H, Gobin YP. Interv Neuroradiol. 2009 Dec;15(4):453–5. doi: 10.1177/159101990901500414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Turner R, 4th, Gonugunta V, Rasmussen PA, Woo HH, Fiorella D. Neurosurgery. 2008;63(1 Suppl 1):ONSE89. doi: 10.1227/01.neu.0000335018.68369.e8. [DOI] [PubMed] [Google Scholar]
- Maimon S, Strauss I, Frolov V, Margalit N, Ram Z. Brain arteriovenous malformation treatment using a combination of Onyx and a new detachable tip microcatheter, SONIC: short-term results. AJNR Am J Neuroradiol. 2010;31(5):947–54. doi: 10.3174/ajnr.A1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk AS, Chaudry M, Turner RD. Initial US experience with detachable tip microcatheter for arteriovenous malformations. J NeuroIntervent Surg. 2012;4:A26–7. (Abstract) [Google Scholar]