Abstract
The liver is a very complex organ with a large variety of functions, making it an attractive organ for gene replacement therapy. Many genetic disorders can be corrected by delivering gene products directly into the liver using viral vectors. In this chapter, we will describe gene delivery via portal vein administration in mice and dogs to correct the blood coagulation disorder hemophilia B. Although there are multiple delivery routes for both viral and non-viral vectors in animals, portal vein administration delivers vectors directly and efficiently into the liver. Complete correction of murine hemophilia B and multi-year near-correction of canine hemophilia B have been achieved following portal vein delivery of adeno-associated viral (AAV) vectors expressing factor IX from hepatocyte-specific promoters. Peripheral vein injection can lead to increased vector dissemination to off-target organ such as the lung and spleen. Below, we will describe portal vein injection delivery route via laparotomy.
Keywords: Liver, Gene therapy, Portal vein, Viral vectors, AAV
1 Introduction
The goal of an in vivo gene transfer protocol for correction of human disease is optimal delivery and expression of the therapeutic gene in the target organ while minimizing dissemination to other organs, thereby increasing efficacy and minimizing possible immune reactions or other toxicities. Gene transfer to hepatocytes is very effective not only for correction of liver disease but also for systemic delivery of therapeutic proteins. For example, hepatocytes are the normal site of synthesis for several coagulation factors; and therefore, hepatic gene transfer has resulted in high therapeutic levels of factor VIII (FVIII) and factor IX (FIX) in several animal models, including mice, dogs, and nonhuman primates [1 – 6]. Stable therapeutic expression for many years, in some cases for more than a decade, in hemophilic dogs has been observed (unpublished data and refs. 2, 7–9). Importantly, hepatocytes are capable of producing and secreting high levels of biologically active coagulation factors into the blood. Furthermore, optimal liver gene transfer using adeno-associated viral (AAV), lentiviral, and also other vectors can induce immune tolerance to the transgene product, in part via a regulatory T cell response [10 – 13] (see Note 1). Finally, liver gene transfer for correction of the hemophilia B has been successfully translated to human treatment in recent clinical trials [14]. In this chapter, we will describe gene transfer for correction of murine and canine hemophilia B using recombinant AAV vectors via portal vein delivery (see Note 2).
AAV vectors have a single-stranded or self-complementary (sc) DNA genome and are devoid of viral coding sequences [15]. Use of a strong hepatocyte-specific enhancer/promoter combination is recommended for liver gene transfer [14, 16]. There are multiple serotypes of AAV with distinct tissue tropism. For example, AAV8 can be effectively delivered to hepatocytes by peripheral vein injection such as the tail vein of a mouse [14, 17, 18]. Other serotypes, including AAV2 and AAV5, require infusion more directly into the blood supply of the liver, which can be accomplished by administration into the portal vein, mesenteric vein, or hepatic artery [5, 6, 16, 19, 20]. In mice, injections into the splenic capsule may be similarly suitable (but only at vector doses of 1011 vector genomes [vg] or higher, as the spleen may sequester too much of the vector at lower doses). Capsid variants such as AAV2 devoid of surface-exposed tyrosine residues further improve gene transfer [21]. Use of cDNAs optimized for mammalian codon usage can further improve therapeutic expression [14, 18, 22, 23]. Generally, resulting expression levels are vector dose dependent. For AAV2, a single portal vein infusion of 1012 vg/kg to FIX-deficient mice or dogs resulted in long-term correction of hemophilia B, without eliciting any types of toxicity [5–7].
Prior to approval of a gene therapy protocol for clinical studies in humans suffering from genetic disorders, the approach has to be thoroughly investigated in a suitable animal model. Mice have been extensively used as a model for hepatic gene transfer via portal vein injection to treat numerous genetic disorders, such as hemophilia A and B, α1 antitrypsin deficiency, and OTC (ornithine transcarbamylase) deficiency [13]. Mice have the advantage of being easily accessible, having a high reproduction rate, and offering the ability to perform batch surgeries without a need for a separate dedicated facility. Recombinant DNA technology allowed the hemophilia research community to generate a large number of different knockout mice and transgenic mice, lacking FVIII or FIX or expressing nonfunctional forms of the protein [24, 25]. Therefore, one can test the risk of an immune response to the therapeutic gene product as a function of the underlying mutation, using gene mutations described in humans with hemophilia. However, there are also certain disadvantages such as limited ability to study the long-term effects of the gene transfer and lack of scale up to an animal of a size more similar to that of humans. Using an alternative large animal model, a single injection of AAV-FIX into the portal circulation, on canine hemophilia B models (Chapel Hill dogs), resulted in sustained expression of canine FIX for over 10 years at levels between 1 and 10 % [ 5, 6, 8, 26].
When choosing between mouse and canine models of hemophilia B for hepatic-based gene therapy studies, several issues need to be considered. The Chapel Hill strain of hemophilia B dogs has a missense mutation that leads to the absence of detectible FIX activity or antigen [27, 28]. Recombinant AAV-mediated gene therapy in these hemophilia B dogs has been successful for multiple years without adverse events, albeit at low but steadily improving levels of expression [5, 6, 26]. Thus the success of gene therapy can be better assessed in dogs since transgene expression seems to be lasting longer than the life span of mice. Hemophilia B dogs have on average 5 spontaneous bleeds/year in soft tissues or joints, a bleeder phenotype mirrors that found in human hemophilia B [7, 29]. This severe bleeding diathesis also provides a metric for detecting a change in phenotype following gene therapy. In general, hemophilia B mice do not bleed spontaneously, so assessing change in bleeder phenotype is more difficult. To date, inhibitor formation in this outbred hemophilia B dog strain has not been noted with portal vein approach. In some of the mouse strains, inhibitor formation to the coagulation proteins has been noted but newer mouse models may help understand this issue better [25] (see Notes 3–5). Also, dogs tolerate multiple blood draws better than mice (see Note 6). Frequent blood sampling is essential in these studies since the goal to express coagulation proteins in the systemic circulation over time. It is also helpful to have large volumes of plasma (~1,000 ml or more) drawn over time to determine the extent of posttranslational modification of transgene-expressed FIX relative to wild-type FIX. In contrast to rodents, the results of experiments in the hemophilia B dogs have been more predictive of outcomes in humans than those performed in other species. We have shown that FIX can be given subcutaneously or via the airways in our hemophilia B dogs, administration routes that could reduce or obviate the need for needles and venipuncture in hemophilia B patients and justify extrahepatic expression in patients with severe liver disease in whom a portal vein approach may be contraindicated [30]. As a direct result, human trials of subcutaneous and inhalational administration of recombinant FIX are being considered. FIX is not bioavailable by these routes in mice. Also, FIX infused into hemophilia B dogs had a comparable half-life to that found in humans [31 – 33], whereas mice had a markedly shorter half-life [19]. Had either the extravascular administration or half-life studies of FIX only been performed in mice, human trials may not have been pursued. Collecting semen from dogs, a procedure that is extremely difficult in mice, is used to assess the risk of germline transmission of gene therapy vectors [34]. These differences are the basis for many investigators, and advisory boards regard these dogs as an essential national resource for preclinical testing and long-term follow up of new treatments for the hemophilia B (see MASAC Recommendations #137 and #160, http://www.hemophilia.org/research/masac/masac_all.htm).
2 Materials
2.1 Rodent Surgery
Rodent survival surgeries do not require a separate facility. Surgeries can be performed in a regular procedure room in the areas that can be easily sanitized. All instruments used for surgery should be sterilized by autoclaving 270 °F for 10 min prior to use. For multiple surgeries during single session, instruments must be disinfected between animals using hot bead sterilizer.
2.1.1 Instrument Kit
2 scissors.
2 retractors.
Forceps.
1 ml syringe.
30Ga needles.
Sterile Q-tips.
Sterile gauze.
Sterile, absorbable haemostatic material.
2.1.2 Additional Equipment
Animal clippers, blade#40.
Anesthesia machine for rodents, e.g., SurgiVet.
Isoflurane, oxygen tank.
Purelube, ophthalmic ointment.
Sterile PBS.
Water-recirculating heating pad.
Sterile surgical gloves, gown, and face mask.
2.2 Canine Surgery
In contrast to rodents, survival surgeries in dogs require a separate dedicated surgical facility that meets or exceeds requirements for performing survival surgery. All instruments used for surgery must be sterilized by autoclaving using standardized protocols.
2.2.1 Standard “Vet Pack” Instrument Kit for Major Abdominal Surgery
Vet Pack (abdominal pack).
4 towel clamps.
1 operating scissors.
1 curved mayo scissors.
1 curved Metzenbaum scissors.
1 thumb forceps with teeth.
1 thumb forceps without teeth.
1 needle holder.
4 curved mosquito forceps.
4 straight mosquito forceps.
4 curved Kelly forceps.
4 Rochester-Carmalt forceps.
1 Haight Rib Spreader.
2 #3 blade handles.
2 Allis tissue forceps.
2 Babcocks.
2 Peans.
1 small suction tip.
1 large suction tip.
1 spay hook.
8 surgical towels.
3 × 3 gauze squares.
1 female catheter.
1 sponge forceps.
1 stainless steel bowl.
1 Weitlaner.
1 catheter introducer.
2.2.2 Additional Equipment
Animal clippers.
Anesthesia machine for larger animals, e.g., SurgiVet.
Isoflurane, oxygen tank.
Ophthalmic ointment.
Sterile PBS.
Heating pad.
Sterile surgical gloves, gowns, and appropriate face mask.
3 Methods
3.1 Rodent Surgery
3.1.1 Preparation of the Surgical Area
Surgical area must be disinfected prior to the surgery using any of the approved (appropriate) disinfectants in your facility.
Heating pad should be sanitized and placed on the cleaned surgical area.
Place sterile surgical wrap on the disinfected surface of the water-recirculating heating pad.
Open sterile instruments, gauze, Q-tips on the sterile surgical wrap.
3.1.2 Preparation of the Animal
For hemophilia B mice administer 200 μl of normal mouse plasma IV via tail vein 30 min before the surgery (see Notes 7 and 8).
Place mouse into induction chamber of the anesthesia machine and administer via inhalation 5 % of isoflurane in 21 % oxygen carrier (flow of 2 L/min).
Once unconscious the mouse is removed from the chamber, abdomen quickly shaved from xiphoid down to the groin, purelube is applied to the both eyes to prevent corneal drying and placed on its back with the face inside the nose cone part of anesthesia equipment in the non-sterile area.
Clean and aseptically prepare surgical site using an appropriate scrubbing technique: starting in the middle, going in circle, gradually enlarging circular pattern. Use three alternating scrubs of Betadine solution and 70 % ethanol.
Move the animal to the clean surgical area and place on top of a heating pad to minimize hypothermia.
Use sterile scissors to cut a small hole in the sterile drape and cover animal with the drape only exposing abdomen.
3.1.3 Preparation of the Surgeon
Rodent surgeries do not require sterile gowning. Surgeon must wear clean lab. coat, face mask, hair cover, and sterile surgical gloves.
3.2 Surgical Procedure
Once animal has been shaved, scrubbed, and moved to the sterile surgical area and covered with the sterile surgical drape while exposing abdomen, make a small (<1 in.) skin incision from the bladder up to the level of the xiphoid.
Repeat same opening technique for the muscle layer.
Retract the skin and muscle layers on both the right and left sides. Place tissue retractors to hold them in place.
Place a piece of sterile gauze over the left retractor and saturate gauze with the sterile PBS.
Using a sterile Q-tip, carefully move intestines onto the gauze and roll pancreas over to expose the portal vein (Fig. 1a). Portal vein is located on the ventral (bottom) part of the pancreas.
Once portal vein is exposed, keep applying slight traction with the sterile Q-tip on the pancreas near the vein bed to create tissue tension for the insertion of the needle (Fig. 1b).
Begin inserting needle into the body of the pancreas ~2 mm below the vein, keep advancing it into the vein until needle tip will become visible through the vein wall (Fig. 1c). Slowly depress the plunger and release the vector solution into the vein (Fig. 1d).
Using sterile dry Q-tip, apply pressure next to the needle insertion place, slowly retracting the needle and rolling Q-tip to cover the hole from the needle to prevent backflow of blood from the vein.
Hold pressure on the vein for at least 30 s. Cut a small piece of absorbable haemostatic material and place on injection site before replacing intestines back into the abdomen and prepare for suturing.
Close the abdominal wall using 4-0 silk, non-cutting taper point or round needle, continuous pattern. Place second layer of sutures on the skin, using Ethilon 4-0 suture material with cutting-edge needle and interrupted suture pattern.
Disconnect anesthesia and administer analgesic caprofen at 5 mg/kg or buprenorphine at 0.05–0.1 mg/kg.
Postsurgical care: Mice are kept on the heating pad for the entire duration of the surgery and postsurgical recovery to avoid hypothermia. Administration of normal mouse plasma (200 μl) is repeated approximately 30 min after surgery. Animals should be visually inspected frequently for bleeding, wound healing, and possible infection in the first 48 h and daily after that.
Sutures should be removed 10–14 days after surgery.
Fig. 1.
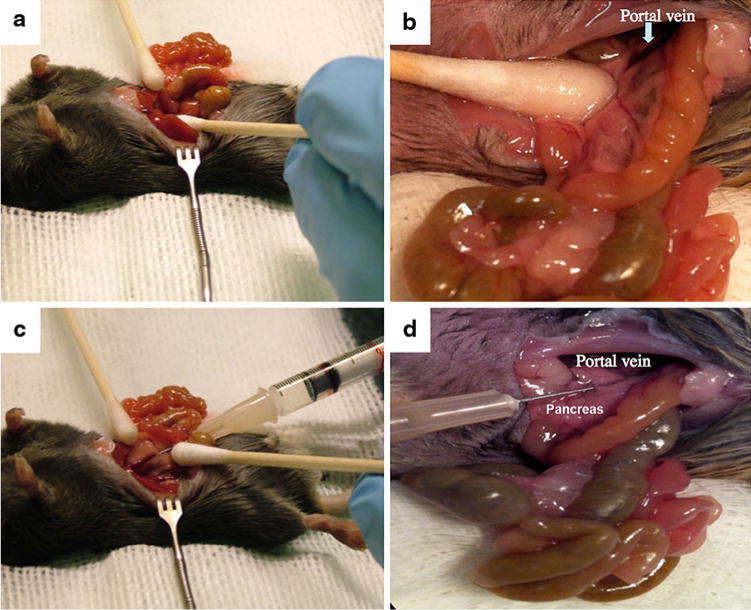
Portal vein delivery of gene therapy vector to a mouse. (a) Displacement of intestinal tract and pancreas to gain access to portal vein. (b) Exposure of portal vein. The area directly caudal to the portal vein must be flat enough to allow a needle to enter the vessel without inhibiting the syringe. A Q-tip is used to “retract” the pancreas to put tension on the vessel. (c) Insertion of needle through the multiple layers to enter the portal vein. One should see the bevel of the needle in the vessel. (d) Vector administration. Blanching of the liver should be obvious when depressing the plunger while observing the liver
3.3 Canine Surgery
3.3.1 Preparation of the Surgical Area
Surgical area must be disinfected prior to the surgery using any of the approved (appropriate) disinfectants in your facility.
Heating pad should be sanitized and placed on the cleaned surgical area.
All personnel must wear surgical clothing, hair cover, face mask, shoe covers, and, if in contact with the sterile field, sterile surgical gloves.
3.3.2 Preparation of the Animal
General anesthesia: Typically, dogs are premedicated with Atropine SQ (0.06 mg/kg) and then induced with propofol (“Propoflo28” at 3.2 mg/kg to 7.6 mg/kg IV over 60–90 s) followed by immediate intubation and transition to isoflurane to effect (~2 %). Nitrous oxide (50 %) is occasionally used during induction. Anesthesia is evaluated by direct observation of heart rate, respiratory rate, blood pressure, oxygen saturation, end-tidal CO2, body temperature, and persistence or absence of palpebral, corneal, and withdrawal reflexes. A computer-based system and hand-written notes are used to record events that occur perioperatively.
Once the dog is anesthetized and intubated, ophthalmic ointment is applied to both eyes to prevent corneal drying.
Fur is shaved from the surgical field, generally from the lower rib cage to the lower abdomen. The shaved skin is cleaned with Betadine solution and 70 % ethanol. The dog is covered with sterile surgical drapes.
3.3.3 Surgeon Preparation
Survival surgeries in dogs require surgical hand scrubbing and sterile gowning and gloving. The surgeon must wear shoe covers, a face mask, and hair cover as do all of the operation room attendees and assistants.
3.4 Surgical Procedure
Prior to making an incision, canine FIX levels are raised to at least 10–20 % by infusing an appropriate amount of normal canine plasma. One needs to know the weight and hematocrit of the dog. The total blood volume is estimated at 40 ml/lb (18.18 ml/kg).
-
Therefore,
Combined equation: (wt kg × 18.18 ml/kg) × (100 − hematocrit) = Total Plasma Volume in ml.
Then, to raise FIX levels to 10 % in a dog with 900 cc calculated plasma volume, 100 cc of normal plasma is infused with FIX at 1 unit/ml.
Additional normal plasma can be given if the surgeon feels hemostasis is inadequate. The whole blood clotting time can be given a quick estimate of whether or not FIX levels are above 5 % [35]. Meticulous attention must be paid to controlling bleeding as incisions are made. There is often a subcutaneous vein of substantial size just beneath the skin in the midline incision site that requires attention.
A skin incision is made from the xiphoid down to the lower abdomen (~10 cm) and then the abdominal cavity is opened via its muscle layers.
A baseline liver biopsy is taken for use as control tissue if a follow up biopsy is performed to determine vector copy number, gene expression or if there is cellular toxicity. A small wedge of liver tissue is encircled with suture material, and the tissue is processed for molecular and histological studies.
The spleen is carefully exteriorized (Fig. 2, panel 1), and a 3–5 French balloon-tipped catheter is prepared by inflating the balloon to check for leaks (~0.5–1.0 cc, Fig. 2, panel 2).
An appropriately sized branch of the splenic vein is isolated, and circumferential sutures are positioned proximally and distally over about 2 cm; this branch of the splenic vein is punctured and the balloon-tipped catheter is inserted and advanced into the portal vein, usually between 10 and 20 cm (Fig. 2, panels 2–5). The position of the catheter in the portal vein is confirmed visually or by palpation.
The distal port of the balloon-tipped catheter is aspirated to confirm blood can be withdrawn even when the balloon is securely inflate and occluding the portal vein. This may require a “trial and error” approach until positioning is optimized.
When the catheter position is optimized, a small amount (~1 cc) of vector is infused while the balloon is inflated. Infusion rates are adjusted as the blood pressure tolerates. In general, AAV vectors are well tolerated and can be infused over 15 or 30 min.
After the vector is infused, the catheter is removed, the branch of the splenic vein is ligated proximally and distally, and the spleen is returned to the abdomen being careful not to injure it (Fig. 2, panel 6).
The surgical incision is closed in layers using suture material (Fig. 2, panels 7 and 8). Each layer is carefully examined for bleeding sites. Extra time spent achieving hemostasis at this point may prevent reoperation for small but significant bleeders.
Discontinue anesthesia and extubate when appropriate gag reflex is documented. Care must be taken to avoid trauma to the airway that can result in hemorrhage and airway obstruction.
The dog receives an intramuscular injection of procaine penicillin (or other prophylactic antibiotic as prescribed by the attending veterinarian) alone with postoperative analgesia and is allowed to recover under observation. The dog and its incision site are observed daily, and immediate steps are taken to treat infection or bleeding as is appropriate. In general, FIX is given daily for 7 days postoperatively or longer if needed. The exogenous plasma FIX disappears to undetectable levels (<0.1 %) within 21–28 days [35].
Fig. 2.
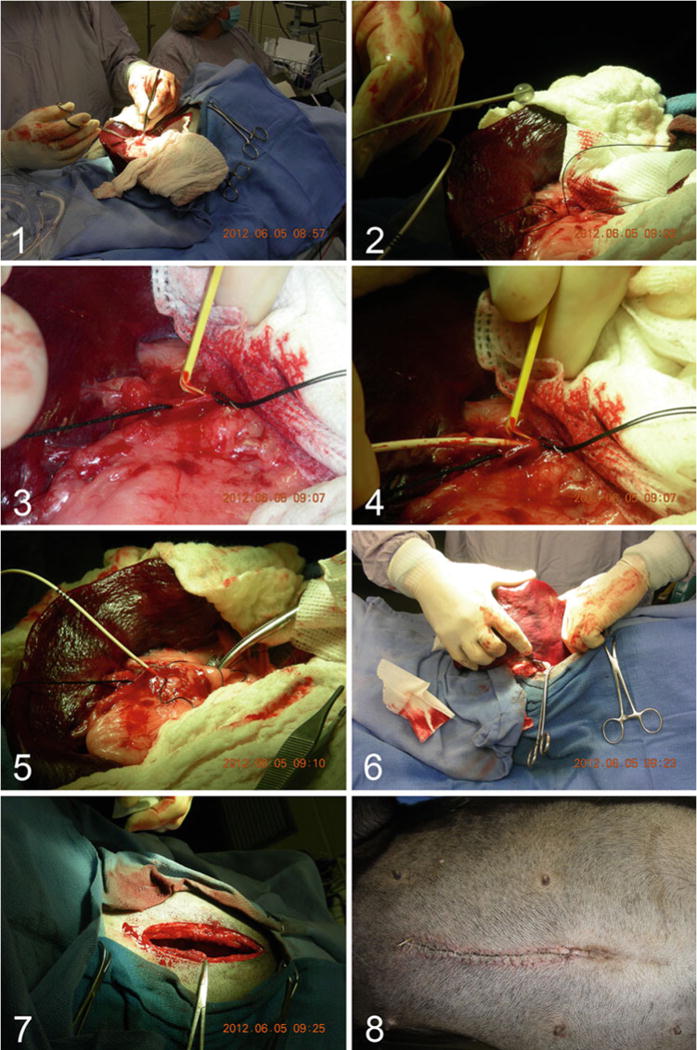
Portal vein delivery of gene therapy vector to a hemophilic dog. Panel 1: The spleen is exteriorized via a midline laparotomy incision. Panel 2: A 3–5 French balloon-tipped catheter is prepared by inflating the balloon to check for leaks. Panel 3: A small splenic vein is isolated and a venotomy is performed. Panel 4: The balloon-tipped catheter is inserted in the venotomy site and passed antegrade to the hepatic portal vein, usually 10–20 cm. Panel 5: With the balloon inflated, the gene therapy vector is administered to the liver. Panel 6: The spleen is returned to the abdomen. Panels 7 and 8: The laparotomy incision is repaired with careful attention to bleeding vessels
Footnotes
C57BL/6 mice typically show the highest level of transduction with AAV vectors in the liver, resulting in high systemic expression of FIX, while BALB/c mice show slightly lower transduction efficiency. Other strains, such as C3H mice, may show substantially reduced transduction efficiency and thus require higher vector doses to achieve similar levels of transgene expression.
Portal vein has access to two thirds of the liver, which is a normal site of expression of FIX. Following hepatic gene transfer, FIX can be detected in the circulation by ELISA from 1 week following gene transfer and persist for >1 year or even the life span of the mouse. Antibodies specific to human and canine FIX (that do not cross-react with murine FIX) are used for antigen capture and are commercially available.
Portal vein infusion of AAV-FIX in mice can give weak antibody response to the viral particles, which can appear in the peripheral blood days after gene transfer. Mice typically produce immunoglobulins IgG1 or IgG2a. Specific assays have been developed to detect anti-AAV antibodies. Enzyme-linked immunosorbent assay (ELISA) allows fast and accurate detection of various inhibitory and not inhibitory antibodies.
Presence of inhibitory antibodies can be detected by Bethesda assay, where hemophilic mouse plasma is mixed with normal mouse plasma 1:1 ratio. This mixture is incubated for 2 h at 37 °C. After addition of FIX-deficient human plasma and CaCl, time of clot formation is measured with fibrometer. Bethesda inhibitor titer is equivalent of the dilution of mouse plasma sample, calculated from a standard curve obtained from twofold serial dilution of normal human plasma.
Coagulation of plasma samples from hemophilic mice is determined by measuring aPPT, activated partial thromboplastin time using fibrometer. Range of aPPT in hemophilia B mice is 55–100 s, 25–30 s in normal mice.
Blood collection in mice can be performed via tail or retro-orbital bleeding. Mice need to be anesthetized for blood collection. Retro-orbital bleeding yields 50–150 μl of blood collected in to heparinized glass capillaries and can be used for ELISA assays. For Bethesda and APPT, tail bleed is a preferred method; blood is collected into citrate or oxalate containing tubes.
In this chapter, hepatic gene transfer to hemophilic mice is described. The identical procedure can also be performed in hemostatically normal mice to measure gene transfer or correct other disorders. Non-hemophilic mice will not require presurgical administration of normal mouse plasma.
Vector doses of 1010–1011 vg/mouse are typical for AAV2 and AAV5 expressing FIX. Higher doses may be required for FVIII expression in hemophilia A mice. Other serotypes or improvements in vector design (capsid, expression cassette, and so on) may allow for reduced vector doses.
References
- 1.Sarkar R, Xiao W, Kazazian HH., Jr A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J Thromb Haemost. 2003;1:220–226. doi: 10.1046/j.1538-7836.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 3.Markusic DM. Liver-directed adeno-associated viral gene therapy for hemophilia. J Genet Syndr Gene Ther. 2012;S1:009. doi: 10.4172/2157-7412.S1-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 6.Snyder RO, Miao C, Meuse L, Donahue BA, Lin H-F, Stafford DW, Patel S, Thompson A, Nichols T, Bellinger D, Read M, Brinkhous KM, Kay MA. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 7.Nichols TC, Raymer RA, Franck HW, Merricks EP, Bellinger DA, DeFriess N, Margaritis P, Arruda VR, Kay MA, High KA. Prevention of spontaneous bleeding in dogs with haemophilia A and haemophilia B. Haemophilia. 2010;16(Suppl 3):19–23. doi: 10.1111/j.1365-2516.2010.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatino DE, Lange AM, Altynova ES, Sarkar R, Zhou S, Merricks EP, Franck HG, Nichols TC, Arruda VR, Kazazian HH., Jr Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman BE, Martino AT, Sack BK, Cao O, Liao G, Terhorst C, Herzog RW. Nonredundant roles of IL-10 and TGF-beta in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol Ther. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–3265. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 16.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 17.Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CY, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, Sleep S, Gray JT, Nienhuis AW, Davidoff AM. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Takabe K, Bidlingmaier SM, C R, III, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci U S A. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mingozzi F, Schuttrumpf J, Arruda VR, Liu Y, Liu YL, High KA, Xiao W, Herzog RW. Improved hepatic gene transfer by using an adeno-associated virus serotype 5 vector. J Virol. 2002;76:10497–10502. doi: 10.1128/JVI.76.20.10497-10502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, Jayandharan GR, Ling C, Zolotukhin I, Ma W, Zolotukhin S, Srivastava A, Zhong L. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ, Herzog RW. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One. 2012;7:e37671. doi: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, Samulski RJ, Monahan PE. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 24.Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, Ertl HC, High KA, Herzog RW. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatino DE, Nichols TC, Merricks E, Bellinger DA, Herzog RW, Monahan PE. Animal models of hemophilia. Prog Mol Biol Transl Sci. 2012;105:151–209. doi: 10.1016/B978-0-12-394596-9.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margaritis P, Roy E, Aljamali MN, Downey HD, Giger U, Zhou S, Merricks E, Dillow A, Ezban M, Nichols TC, High KA. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113:3682–3689. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JP, Brinkhous KM, Brayer GD, Reisner HM, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog R, Arruda VR, Fischer TH, Read MS, Nichols TC, High KA. Absence of circulating factor IX antigen in hemophilia B dogs of the UNC-Chapel Hill colony. Thromb Haemost. 2000;84:352–354. [PMC free article] [PubMed] [Google Scholar]
- 29.Russell KE, Olsen EH, Raymer RA, Merricks EP, Bellinger DA, Read MS, Rup BJ, Keith JC, Jr, McCarthy KP, Schaub RG, Nichols TC. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune-tolerant hemophilia B dogs. Blood. 2003;102:4393–4398. doi: 10.1182/blood-2003-05-1498. [DOI] [PubMed] [Google Scholar]
- 30.Russell KE, Read MS, Bellinger DA, Leitermann K, Rup BJ, McCarthy KP, Keith JC, Jr, Khor SP, Schaub RG, Nichols TC. Intratracheal administration of recombinant human factor IX (BeneFix) achieves therapeutic levels in hemophilia B dogs. Thromb Haemost. 2001;85:445–449. [PubMed] [Google Scholar]
- 31.Brinkhous KM, Sigman JL, Read MS, Stewart PF, McCarthy KP, Timony GA, Leppanen SD, Rup BJ, Keith JC, Jr, Garzone PD, Schaub RG. Recombinant human factor IX: replacement therapy, prophylaxis, and pharmacokinetics in canine hemophilia B. Blood. 1996;88:2603–2610. [PubMed] [Google Scholar]
- 32.Shapiro AD, Ragni MV, Valentino LA, Key NS, Josephson NC, Powell JS, Cheng G, Thompson AR, Goyal J, Tubridy KL, Peters RT, Dumont JA, Euwart D, Li L, Hallen B, Gozzi P, Bitonti AJ, Jiang H, Luk A, Pierce GF. Recombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119:666–672. doi: 10.1182/blood-2011-07-367003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth DA, Kessler CM, Pasi KJ, Rup B, Courter SG, Tubridy KL. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98:3600–3606. doi: 10.1182/blood.v98.13.3600. [DOI] [PubMed] [Google Scholar]
- 34.Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, Herzog RW, Nichols TC, Biegel JA, Razavi M, Dake M, Huff D, Flake AW, Couto L, Kay MA, High KA. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- 35.Nichols TC, Franck HWG, Franck C, Raymer RA, Merricks EP. Sensitivity of whole blood clotting time and activated partial thromboplastin time for canine factor IX. J Thromb Haemost. 2012;10:474–476. doi: 10.1111/j.1538-7836.2011.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


