Abstract
Heart failure is a growing epidemic caused by cardiomyocyte depletion. Current therapies prolong survival by protecting remaining cardiomyocytes, but are unable to overcome the fundamental problem of regenerating lost cardiomyocytes. A number of strategies for promoting heart regeneration have emerged from decades of intensive study. While some of these remain confined to basic research, others are beginning to be tested in humans. Here, we review strategies for cardiac regeneration and summarize progress of related clinical trials.
Introduction
Heart failure is a growing, world-wide epidemic. Current heart failure therapies reduce heart failure morbidity and mortality by blocking chronic neurohumoral activation. However, these therapies are unable to reverse heart failure and do not address its fundamental cause, the loss of cardiomyocytes (Figure 1).
Figure 1. Cardiomyocyte loss in Myocardial Infarction.

Myocardial infarction causes loss of ~1 billion cardiomyocytes in the adult heart. After the acute event, there is progressive fibrosis and myocyte loss in the infarct border zone, and remaining cardiomyocytes undergo hypertrophy. Standard therapy is designed to preserve remaining cardiomyocytes, with variable efficacy.
Conventional wisdom has long held that adult mammalian cardiomyocytes have exited from the cell cycle and are not added to the mature heart. However, multiple independent lines of evidence now show that new cardiomyocytes are born in the post-natal heart. Histological examination of human hearts demonstrated the existence of cardiomyocytes with mitotic figures (1). However, inferring the extent of new cardiomyocyte formation from histological data is problematic for a number of technical reasons. Chief among these are the infrequency of cardiomyocyte proliferation compared to non-cardiomyocytes, the difficulty of definitively distinguishing proliferative events in cardiomyocytes versus non-cardiomyocytes (2), and the tendency of cardiomyocytes to become polyploid or multinucleated, particularly in response to stress (reviewed in ref. 3). The challenge of measuring cardiomyocyte proliferation has been overcome through a number of innovative labeling approaches. A seminal study by Bergmann and colleagues used the spike in atmospheric carbon-14 that occurred as a by-product of above ground nuclear testing as a tracing reagent to show that human cardiomyocytes are born in the post-natal heart (4). They estimated that 0.5–1% of cardiomyocytes turn over annually, so that roughly 50% of cardiomyocytes are replenished over a human lifespan. Cardiomyocyte proliferation in adult mouse heart was independently confirmed using multi-isotope imaging mass spectroscopy in combination with genetic labeling of pre-existing cardiomyocytes (5). This study showed that new cardiomyocytes are born from pre-existing cardiomyocytes at about 0.76% per year in young adult mice. After myocardial infarction (MI), cardiomyocyte proliferation increased, as 3.2% of cardiomyocytes in the infarct border zone had undergone productive cell division over an 8-week period. Collectively, these findings overturn the long-standing axiom that the post-natal heart is non-regenerative and demonstrate new cardiomyocyte addition to the adult mammalian heart.
The existence of innate regenerative capacity in the adult mammalian heart has ignited intense interest. Augmenting or supplementing endogenous repair mechanisms to replace the ~ 1 billion cardiomyocytes in an MI (6) is a tall order. Current strategies under investigation to regenerate the myocardium fall into four general categories: (1) Inducing pre-existing cardiomyocytes to re-enter the cell cycle to generate new cardiomyocytes; (2) Delivering cardiac progenitors cells or their derivatives, isolated and expanded ex vivo, to the heart; (3) Enhancing the activity of endogenous cardiac progenitor cells; and (4) Direct reprogramming of non-cardiomyocytes into cardiomyocytes. Here, we review pre-clinical and clinical data on these cardiac regeneration strategies and highlight the advantages and challenges of each strategy.
1. Cardiomyocyte cell-cycle re-entry
Fetal cardiomyocytes actively proliferate, while adult cardiomyocytes largely do not. One attractive strategy to replenish cardiomyocytes lost in heart disease is to induce mature cardiomyocytes to re-enter the cell cycle (Figure 2).
Figure 2. Cardiac regeneration through stimulation of adult cell cycle re-entry.
Existing cardiomyocytes (blue nuclei) proliferate to replenish lost cardiomyocytes (green nuclei). Cardiomyocyte refreshment through stimulation of endogenous pathways may allow newly formed cardiomyocytes to integrate and recruit their own vasculature.
In rodents, cardiomyocytes exit the cell cycle in the first post-natal week (7), and this correlates with the loss of functional cardiac regenerative capacity (8). In humans, the timing of cardiomyocyte cell cycle withdrawal is uncertain, but initial studies suggest that human cardiomyocytes continue to cycle beyond the immediate neonatal period and well into childhood (9). It will be interesting to determine if there is indeed a protracted period of proliferative competence in infant human cardiomyocytes, and if so how this relates to the regenerative capacity of the human heart and current timing and strategies for repair of congenital heart disease.
In mature cardiomyocytes, cell cycle genes such as Cyclin A, Cyclin B, and CDC2 are down-regulated, while cyclin-dependent kinase inhibitors are actively expressed (reviewed in 10). These cell cycle regulators are under transcriptional and epigenetic control in cardiomyocytes. For instance, the cell cycle inhibitors Ink4a/b are repressed by polycomb repressive complex 2 (PRC2), and inactivation of the PRC2 catalytic subunit EZH2 caused inappropriate fetal upregulation of Ink4a/b, reduction of cardiomyocyte proliferation, and myocardial hypoplasia (11). Rb/p130-dependent heterochromatin formation at E2F-regulated cell cycle genes also stimulated cardiomyocyte cell cycle withdrawal, and inactivation of Rb and p130 led to reduced heterochromatin formation, cell cycle gene de-repression, and adult cardiomyocyte cell cycle reentry (12).
Forced expression of individual cell cycle regulators has been evaluated as a means to stimulate cardiomyocyte cell cycle re-entry (reviewed in ref. 10). In some cases, this strategy successfully stimulated cardiomyocyte proliferation but caused extensive, lethal cardiac pathology (13). Cardiomyocyte-specific, transgenic expression of Cyclin D2 was more promising, as it was not deleterious and successfully stimulated adult cardiomyocyte DNA synthesis (14). Initial infarct size after MI was no different between control and Cyclin D2 transgenics, and infarct size progressively decreased over time, consistent with effective regeneration (14). These data show that overexpression of some cell cycle regulators may have salutary effects in heart disease.
Coordinated activation of pro-mitogenic gene programs may have greater success than overexpression of individual cell cycle regulators. This goal might be achieved through the re-deployment of developmental regulatory circuits. The YAP transcriptional co-activator is a major target of the Hippo signaling pathway, a highly conserved pathway that governs cell proliferation and organ size (15). Mutations in the Hippo/YAP pathway that enhance YAP transcriptional activity stimulated fetal cardiomyocyte proliferation and caused profound cardiac overgrowth (16–18). In the adult heart, YAP activating mutations likewise promoted proliferation of mature cardiomyocytes, albeit less robustly than in the fetal heart, and improved myocardial recovery after MI (19, 20).
MicroRNAs, which like transcription factors also coordinately regulate multiple genes, may also offer a means to coordinately activate a mitogenic program in adult cardiomyocytes. MicroRNAs are attractive as therapeutic targets, because small RNAs or their antagonists can be efficiently delivered as synthetic small molecules or adeno-associated virus. A screen of microRNAs that enhance neonatal cardiomyocyte proliferation identified miR-590 and miR-199a, and subsequent testing showed that these stimulate adult cardiomyocyte proliferation and enhance myocardial recovery after MI (21). Gain and loss of function approaches also showed that the miR-17-92 cluster promotes cardiomyocyte proliferation (22). On the other hand, the miR-15 family suppresses cardiomyocyte proliferation, and its inhibition by antagomirs stimulated cardiomyocyte proliferation and improved outcome in a murine MI model (23).
Paracrine signaling regulates developmental cardiomyocyte proliferation. Activation of tyrosine kinase receptor signaling pathways through delivery of insulin-like growth factor 1 (IGF1) and fibroblast growth factor (FGF) activated post-natal cardiomyocyte proliferation improved outcome after MI (24, 25). Neuregulin (NRG1) signaling through ErbB2/ErbB4 promotes fetal cardiomyocyte proliferation and differentiation as well as adult cardiomyocyte survival (reviewed in ref. 26). NRG1 was reported to drive adult cardiomyocyte proliferation (27), although this result awaits independent confirmation. As a result of its multifaceted and positive effects on heart function in animal models, recombinant human NRG1 (rhNRG1) has entered clinical trials for heart failure (Table 1). An initial clinical trial found that rhNRG1 infusion for 11 days is well tolerated in patients. The infusion acutely improved cardiac function, and the effect was sustained for up to 3 months (28). Larger, randomized, multi-center studies are underway. The extent to which cardiomyocyte proliferation contributes to these salutary effects of NRG1 is currently unknown.
Table 1. Clinical trials in cardiac regeneration.
This table summarizes the larger trials that have been performed. A comprehensive list of trials can be found in ref. 31.
| Trial Name/ Phase |
Reagent | Delivery Method and Timing |
Patient Number (Intervent ion/Contr ol) |
Indication | Follow up |
Outcome | Design | Reference |
|---|---|---|---|---|---|---|---|---|
| NRG1/Phase I | rhNRG1 | Intravenous infusion for 11 days | 15 | Stable chronic heart failure | 3 mo | EF +3.9, P<0.001 | non-randomized, open-label | (28) |
| MAGIC/Phase II | Skeletal muscle myoblast | Myocardial injection/during CABG surgery. | 63/34 | Heart failure (EF<3 5%) | 6 mo | EF unchanged Reduced LV remodeling More arrhythmic events |
multicenter, randomized, double-blind | (104) |
| SCIPIO/Phase I | c-kit+ CPCs | Intracoronary injection/113 d after CABG | 16/7 | Ischemic CMP | 1 yr | EF +12.3, P<0.0007 Scar −30% |
randomized, open-label | (45) |
| CADUCEUS/Phase I | CDCs | Intracoronary injection/1.5–3 mo post MI | 17/8 | Acute MI | 1 yr | EF unchanged Scar −12.3% |
randomized, open-label | (50) |
| REPAIR-AMI/Phase II | BMCs | Intracoronary injection/3–7 d postMI | 101/103 | Acute MI | 2 yr | EF unchanged Reduced combined endpoints (death+MI; death+MI+HF hospitalization) |
randomized, double-blind | (60) |
| HEBE/Phase II | BMCs | Intracoronary injection/3–8 d postMI | 69/66/65 BMC/PBC/Std |
Acute MI | 4 mo | EF unchanged | randomized, open-label | (64) |
| LateTIME/Phase II | BMCs | Intracoronary injection/14–21 d postMI | 55/26 | Acute MI | 6 mo | EF unchanged | randomized, double-blind | (65) |
| Jannsens/Phase II | BMCs | Intracoronary injection/1 day post MI | 33/34 | Acute MI | 4 mo | EF unchanged Scar −28% |
randomized, double-blinded, | (63) |
| BM-MNC/Phase II | BMCs | Intracoronary injection. 5–7 days; 3–4 | 133/67 | Acute MI | 4 mo | EF unchanged | open-label | (66) |
CABG, coronary artery bypass grafting, PBC, peripheral blood mononuclear cells, Std, standard therapy (no placebo)
Overall, stimulating cardiomyocyte cell cycle re-entry is an attractive strategy for cardiac regeneration as it appears to be the major endogenous mechanism for regeneration in both lower vertebrates (29, 30) and mammals (5, 8) (Figure 2). Likely this is because it leverages endogenous, developmental mechanisms for functional heart growth. These innate developmental programs generate new, autologous cardiomyocytes and ensure their mechanical, electrical, and vascular integration into the myocardium.
While promising, much work remains to advance these studies towards clinical translation. We must confirm that proposed interventions stimulate bona fide cardiomyocyte cell cycle re-entry in mature, adult heart, and we must place added emphasis on measuring the amount of newly generated myocardium to address the critical question of whether the proposed manipulation could achieve sufficient cardiomyocyte expansion to be therapeutically meaningful. The molecular mechanisms that limit adult cardiomyocyte proliferation remain poorly understood, and greater insights would likely enable far more robust mitogenic stimulation than presently achievable. We must show that the interventions enhance long-term myocardial outcome after myocardial insults such as MI. Finally, and perhaps most importantly, we must show that these proposed treatments are safe and not detrimental to the heart or other organs. A major concern for pro-proliferative therapies is their oncogenic potential. Strategies to selectively target cardiomyocytes are needed, and mitogenic therapies will need to be rigorously evaluated for oncogenesis before they can be translated to the clinical setting.
2. Cell therapy
Transplantation of many different cell types has been proposed as a means to augment cardiac repair and regeneration (Figure 3). Early work considered transplantation of differentiated cells such as skeletal myoblasts. It was hoped that these muscle cell progenitors would differentiate into cardiac muscle, but they did not. Rather the grafts formed myotubes that did not electrically integrate with host myocardium. Nevertheless, functional improvement by skeletal myoblasts in pre-clinical studies motivated their testing in patients. A number of clinical trials collectively suggested limited efficacy and elevated arrhythmia risk, perhaps as a result of poor electrical integration (reviewed in ref. 31). While disappointing, this experience laid a critical foundation for subsequent work in cardiac cell therapy, and underscored the need for effective graft integration to avoid arrhythmogenesis.
Figure 3. Cardiac regeneration through cell therapy.
A. Embryonic- or iPSC-derived cardiomyocytes (green cells) are produced in vitro and delivered to myocardium, possibly with paracrine factors and engineered scaffolds that enhance engraftment and functional integration. B. Resident and non-resident cardiac progenitors (purple cells) are isolated, expanded in vitro, and delivered to myocardium. Although initially envisioned to improve myocardial outcome by engraftment and cardiogenic activity, benefit observed from cardiac progenitor therapy currently undergoing clinical trials suggests that benefit comes from paracrine activity of injected cells, which do not stably engraft in host myocardium.
More recent work has focused on progenitor cells with cardiomyogenic activity, or on their differentiated derivatives. These cells can be classified into those originating from pluripotent stem cells (embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs)), and those arising from adult progenitor cells located in the heart (resident cardiac progenitor cells (CPCs)) or in non-cardiac sites (non-resident CPCs).
Pluripotent Stem Cells
ESCs have a nearly unlimited capacity for self-renewal and can be efficiently differentiated into cardiomyocytes (32). Undifferentiated ESCs formed teratomas when injected into immunocompatible host hearts (33), a complication that will need to be excluded in pluripotent cell-based therapies. Injection of differentiated, murine ESC-derived cardiomyocytes into immunocompatible hearts yielded stable intracardiac grafts that improved cardiac function after MI (34). Similarly, human ESC-derived cardiomyocytes formed stable grafts that improved function of infarcted rat heart (35), and their electrical integration with host myocardium was recently demonstrated in a guinea pig myocardial injury model (36). Co-delivery of paracrine factor cocktails and bio-engineered microenvironments enhanced survival, vascularization, and maturation of ESC-derived cardiomyocytes (35, 37).
These experiments suggest that transplantation of human ESC-derived cardiomyocytes may be therapeutically viable. However, aside from important ethical considerations, there are a number of challenges and relative drawbacks that need to be considered. One fundamental challenge is the relative immaturity of current ESC-derived cardiomyocytes. While these cells contract and generate force, their immaturity likely reduces their efficacy and host integration. ESC-based therapies will require immunosuppression to avoid graft rejection. Finally, the safety (lack of teratoma formation or arrhythmogenesis) and longevity of ESC-based grafts will need to be carefully demonstrated.
In most respects, iPSCs behave like ESCs, and thus offer their conceptual advantages. At the same time, iPSCs side-step the ethical issues that surround ESCs. Because it is possible to generate autologous iPSCs, iPSCs would also circumvent the need for immunosuppression. However, production of such cells will require months of preparation, precluding their deployment for acute or subacute illnesses such as MI. Furthermore, the uniform manufacture of iPSC-derived cardiomyocytes from individual patients is a major logistical and regulatory hurdle for clinical use of iPSC-derived cells.
Resident Cardiac Progenitor Cells
Several resident CPCs have been reported, including c-kit+ CPCs, Isl1+ CPCs, Sca1+ CPCs, side-population CPCs, and cardiosphere-derived cells (CDCs). The inter-relationships and overlaps between populations isolated by different groups are often uncertain, posing a hurdle to integrating the diverse studies in this area. Here we will focus on c-kit+ CPCs and CDCs as illustrative examples of the potential benefits, challenges, and controversies associated with this therapeutic strategy.
The receptor tyrosine kinase c-kit (CD117) is highly expressed on hematopoietic stem cells, as well as mature circulating cells, mast cells, some endothelial cells, and immature cardiomyocytes (38). The Anversa group reported that c-kit+ cells isolated from the heart are self-renewing, clonogenic, and multipotent, capable of differentiating into cardiomyocytes, smooth muscle cells, and endothelial cells in vitro (39). When injected into injured hearts, c-kit+ CPCs formed functional blood vessels and cardiomyocytes in the newly regenerated myocardium (39, 40), and indeed were found to be necessary and sufficient for cardiomyocyte regeneration and myocardial repair (41).
The cardiogenic activity of c-kit+ CPCs is highly controversial. Most c-kit+ cells in the heart originate from the bone marrow (42), and other groups reported that c-kit+ CPCs from adult mouse heart lack cardiomyogenic potential (38, 43). However, these groups did report cardiomyogenic activity in neonatal c-kit+ CPCs, suggesting that cardiogenic activity of c-kit+ cardiac cells may developmentally regulated. Concerns over data integrity led to the recent retraction of a prominent manuscript on cardiac renewal by the Anversa lab (44), raising further questions on their studies of c-kit+ CPC cardiogenic activity.
Based on pre-clinical studies showing beneficial effects of c-kit+ CPCs on myocardial outcome after MI, these cells were tested in humans with ischemic heart failure who underwent coronary artery bypass graft surgery in a randomized, open-label, phase I study (SCIPIO; Table 1). Four months after surgery, autologous c-kit+ CPCs, expanded from myocardial tissue harvested during surgery, were administered by intracoronary infusion (45). No adverse events related to CPC treatment were noted. CPC-treated patients had slightly better left ventricular ejection fraction (LVEF) than untreated controls at 4 months (36% vs. 29%). The difference was sustained in interim analysis at 2 years (46). This beneficial effect is unlikely to arise from c-kit+ CPC cardiomyogenic activity, but rather may arise through paracrine effects (see Mechanism of Action section below).
CDCs are a second resident CPC population that has been intensively studied. Primary cardiospheres are phase-bright cell clusters isolated from myocardial explants by their spontaneous detachment from the growth surface (47). Replated cardiospheres expand to yield CDCs. CDCs were self-renewing, clonogenic, and multipotent, differentiating into cardiomyocytes, smooth muscle cells, and endothelial cells (48). Cardiac CDC delivery reduced scar size and improved heart function in rodent and porcine models of ischemic and non-ischemic heart disease (47). Injected CDCs survived in host myocardium up to 20 days after MI, and some cells differentiated into cardiomyocytes. CDCs also functioned by secreting paracrine factors, such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin-like growth factor (IGF)-1 (49).
The salutary effects of CDCs in pre-clinical models led to a clinical trial. Autologous CDCs, expanded from endomyocardial biopsy specimens, were delivered by intracoronary infusion 1.5–3 months after MI in a randomized Phase I trial (CADUCEUS; Table 1). No adverse events related to CDC delivery were detected. CDC-treated patients had reduced scar mass and regional contractile dysfunction but no changes in global measures of left ventricular function, with up to 1 year of followup (50, 51).
Non-resident Cardiac Progenitor Cells
Cardiac progenitor cells do not necessarily reside in the heart. In mice transplanted with LacZ-expressing bone marrow, LacZ+ cardiomyocytes and endothelial cells appeared in the heart at a low frequency (0.02% and 3%, respectively) (52). In humans, female hearts transplanted into male recipients contained XY cardiomyocytes, although the extent of chimerism varied widely in different studies (0.04%–10%) (53–55). Hearts of sex-mismatched bone marrow transplant patients also contained cardiomyocytes with donor genetic material, pointing to bone marrow as a source of non-resident CPCs capable of de novo cardiomyocyte formation in humans (56).
Anversa and colleagues tested hematopoietic lineage-negative, c-kit+ bone marrow cells for myocardial repair. They reported that injection of these bone marrow cells into the infarct border zone in mice formed new myocardium that occupied 68% of the infarcted portion of the ventricle (57). However, the differentiation of transplanted c-kit+ bone marrow cells into cardiomyocytes could not be reproduced by two other groups (58, 59). Fusion of transplanted cells with host cardiomyocytes, microscopy artifacts, and data selection may be the reasons why the groups reached such divergent conclusions. Nevertheless, enthusiasm for cardiac regeneration from an autologous source as accessible as the bone marrow led to a number of clinical trials in patients with ischemic and non-ischemic heart disease (reviewed in ref. 31.) These trials have tested unfractionated bone marrow mononuclear cells or fractionated subsets, primarily mesenchymal stromal cells (MSCs), hematopoietic stem cells (HSCs), and endothelial progenitor cells (EPCs). These trials established that bone marrow-derived cell preparations can be safely delivered via direct intramyocardial or intracoronary injection. Administration of these cells had at most modest intermediate-term benefit in some studies (60–63) and no benefit in others (64–66) (Table 1). Larger phase II clinical trials are underway to further assess the efficacy of bone marrow-derived progenitor cells in heart failure. Overall these data from numerous groups have tempered the initial excitement that bone marrow cells might drive robust cardiac regeneration.
Mechanism of Action
How does cell therapy enhance outcome in heart disease? Most forms of cell therapy were initiated with the goal of replenishing lost cardiomyocytes through differentiation of injected cells into cardiomyocytes. Pluripotent stem cell-derived cardiomyocytes do form stable myocardial grafts that likely functionally contribute to contractile force generation. However, most injected adult progenitor cells fail to efficiently engraft in the heart. For example, after direct intramyocardial injection of c-kit+ CPCs into infarcted mouse heart, 25% of cells present at 5 minutes remained at 24 hours, 7.6% remained at 7 days, and 2.8% remained at 35 days (67). Similarly low engraftment rates were found for CDCs (49) and bone marrow-derived progenitors (68). Engraftment is even lower after intracoronary injection compared to intramyocardial injection (69). Considering the number of cells delivered to patients (millions to hundreds of millions), the low rate of engraftment, the cardiomyocyte deficit (hundreds of millions), and the low frequency of progenitor cell-derived cardiomyocytes in most studies, it is clear that the number of cardiomyocytes formed directly from injected cells is too low to account for their reported functional benefits.
These observations led to the hypothesis that transplanted progenitor cells secrete paracrine factors that promote cardiac repair and regeneration (70). Adult stem cells release a host of soluble factors (reviewed in ref. 70), which may act on cardiomyocytes to enhance survival (71–73) and augment proliferation (74). Paracrine factors secreted by injected progenitor cells may also act on non-cardiomyocytes to promote myocardial neovascularization (71, 75–77), increase activity of resident cardiac stem cells (74, 78), and favorably modify the extracellular matrix (71, 79). Indeed, progenitor cell conditioned media injection itself enhanced outcome after MI in some studies (73, 77, 80, 81). Further efforts are required to understand the beneficial actions of injected progenitor cells, and to pinpoint the responsible paracrine factor(s).
In summary, there has been tremendous excitement about the potential regenerative activity of cardiac progenitors. For pluripotent stem cell-derived cardiomyocytes, the challenge will be to obtain adequate quantities of mature cardiomyocytes, to effectively integrate them with endogenous myocardium, and to ensure that these therapeutic goals can be reached with minimal risk of teratoma formation. iPSCs offer a means to achieve immunocompatibility and to side-step ethical concerns linked to embryonic stem cells, but logistical and regulatory barriers to their clinical deployment will need to be overcome. Thousands of heart failure patients have already received adult progenitor cell therapy. Adult progenitor cells can be delivered safely, at least at current low engraftment rates, but efficacy has been modest at best and inconsistent between studies. However, these are still early days and a number of critical questions need to be answered before the ultimate therapeutic potential of cardiac progenitor cell therapy will be known. The optimal cell type, delivery route, dose, and frequency will need to be determined. Cell engraftment will need to be markedly improved. Much more needs to be learned about the mechanisms of action of injected progenitor cells, so that their beneficial effects can be amplified and refined. If the major activity of injected cell comes from elaboration of paracrine factors, then focused efforts on defining and delivering the optimal paracrine factor cocktails may circumvent many of the logistical hurdles of cell therapy and thereby accelerate therapeutic translation (see following section).
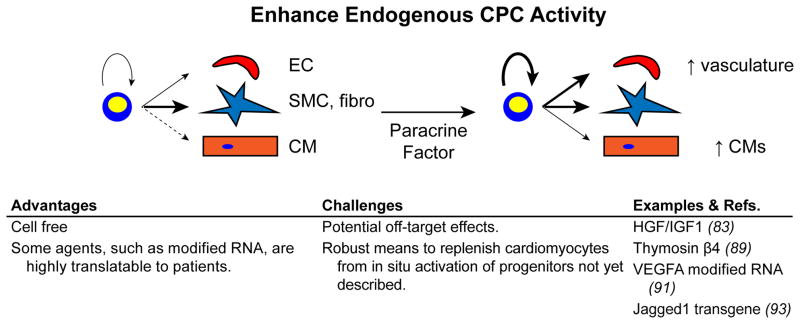
3. Enhancing the activity of endogenous cardiac progenitor cells
While cell therapy seeks to isolate, amplify, differentiate, and then transplant progenitor cells or their progeny, an alternative strategy is to enhance the regenerative activity of resident cardiac progenitor cells in situ (Figure 4). Current data indicates that cardiac progenitors do not substantially differentiate into cardiomyocytes during normal homeostasis. However, myocardial injury stimulated increased birth of cardiomyocytes from non-myocytes (82). Pharmacological or genetic augmentation of this process may enhance cardiac repair and regeneration.
Figure 4. Modulating resident cardiac progenitor activity in situ.
Resident cardiac progenitors could be recruited for cardiac regeneration by treatment with paracrine factors that enhance their amplification and mobilization, and that redirect their fate decisions.
Urbanek, Anversa, and colleagues performed an early study in this direction. They showed that c-kit+ CPCs express receptors for hepatocyte growth factor (HGF) and insulin-like growth factor 1 (IGF1) (83). HGF acted as a chemotactic factor that enhanced migration of c-kit+ cells towards the injection site, while IGF1 increased survival and proliferation of the cells. The combination of HGF and IGF1 thus mobilized and amplified resident c-kit+ CPCs and improved myocardial outcome after MI.
Another cardiac progenitor population is located within the epicardium, a specialized epithelial sheet that covers the heart (84). Epicardial cells play a central role in heart development by undergoing EMT to form epicardium-derived cells (EPDCs), multipotent cells that differentiate into most cardiac fibroblasts and coronary smooth muscle cells. EPDCs also contribute less frequently to the endothelial and cardiomyocyte lineages (85, 86). In addition, the myocardial-epicardial interface is a crucial signaling center that coordinates myocardial growth and coronary vessel development (87, 88). While the epicardium is largely quiescent in the adult heart, it is reactivated by myocardial injury and modulates the myocardial injury response (77).
As a result of the multiple roles of epicardium in cardiac development, the partial restoration of these roles in heart injury, and its accessibility on the surface of the heart, there has been great interest in modulating epicardial function to enhance myocardial repair and regeneration. Although normally epicardium does not contribute to the cardiomyocyte lineage of the adult heart (77), systemic treatment with the peptide thymosin β4 from 7 days prior to 14 days after LAD ligation resulted in de novo generation of cardiomyocytes from epicardium (89). Notably, this switch in epicardial fate was not observed in the absence of MI, nor was it observed when thymosin β4 “priming” prior to the MI was omitted (90). Although the number of cardiomyocytes formed through this mechanism (6.6% of peri-infarct cardiomyocytes) was too low to substantively enhance myocardial function (89), this study established the principle that paracrine factors modulate epicardial progenitor cardiogenic activity following MI.
Effective paracrine factor activity often depends on precise regulation its dose, location, timing, and duration. Standard methods of administration using peptides, DNA plasmids, or viral vectors, may not possess the pharmacodynamics profiles required for optimal paracrine factor activity. Our group collaborated with Chien, Zangi, and colleagues to explore the use of modified RNA as an alternative delivery method with unique kinetics (91). Modified RNA, synthesized by in vitro transcription in the presence of substituted nucleotides, efficiently transfects many primary cell types, including cardiac cells, but avoids innate immune system activation and cytotoxicity. Modified RNA achieves “pulse-like” kinetics, with maximal expression at ~18 hours and minimal residual expression by 72 hours. VEGF modified RNA injected into the murine heart at the time of MI reduced infarct size and enhanced neovascularization, leading to improved heart function at 3 weeks and significantly improved survival for over one year. In contrast, VEGF delivered by DNA plasmid injection reduced survival compared to controls, likely due to sustained VEGF expression that promoted the formation of excessively permeable vessels. VEGF modified RNA markedly amplified EPDCs and mobilized them so that they actively penetrated the myocardium. Moreover, VEGF redirected EPDC differentiation away from the smooth muscle and fibroblast lineages and towards the endothelial lineage, thereby enhancing perfusion of the infarct border zone.
The cell free regenerative paradigm advanced by this VEGF modified RNA study, in which paracrine signals delivered at the right time and place modulates progenitor cell activity, and its implementation using modified RNA, will likely apply to cardiogenic activity of CPCs. For example, the Notch signaling pathway reportedly stimulates differentiation of c-kit+ CPCs towards the myocyte lineage, but maintains the newly formed cells in a proliferative state (92). Transgenic overexpression of the Notch ligand Jagged1 on cardiomyocytes reduced cardiac fibrosis and promoted cardiac precursor expansion (93). Given the cost and logistical difficulties in translating cell-based progenitor therapy, in situ enhancement of cardiac progenitor activity is certainly an attractive strategy.
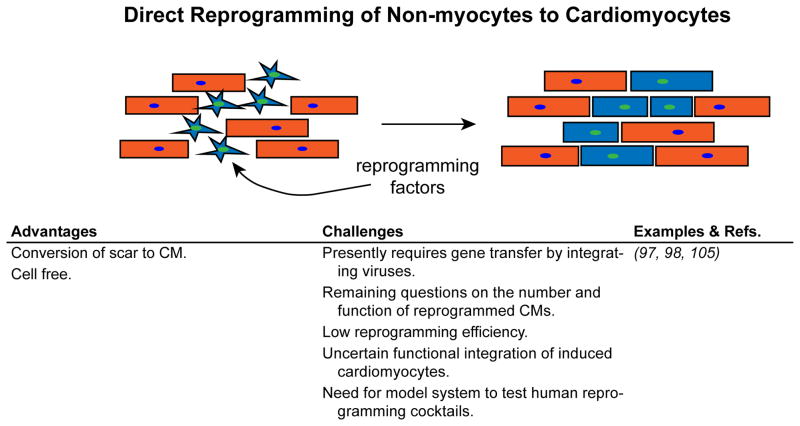
4. Direct reprogramming of non-cardiomyocytes into cardiomyocytes
The discovery that expression of MyoD in fibroblasts stimulated their transdifferentiation into skeletal muscle established the paradigm of cellular reprogramming. This paradigm has been expanded in recent years in the wake of Yamanaka’s startling discovery that fibroblasts can be transcriptionally reprogrammed to pluripotency (94). Subsequently, conversion of one differentiated cell type to another without proceeding through a pluripotent intermediate – direct reprogramming – was reported for several cell types including cardiomyocytes (Figure 5). Retroviral delivery of Tbx5, Mef2c, and Gata4, three central cardiac transcription factor genes, successfully direct reprogramming of murine fibroblasts into “induced cardiomyocyte-like cells” (iCMs) (95). Subsequent studies demonstrated fibroblast to cardiomyocyte reprogramming in vivo in the context of MI, associated with reduced infarct size and improved cardiac function (96). Genetic lineage tracing suggested that directly reprogramming generated roughly one third of cardiomyocytes in the infarct border zone. 38–58% of iCMs had mature sarcomere structure by immunofluorescent staining, while the remaining iCMs appeared less mature. The Olson lab concurrently reported similar findings (97). Other groups have subsequently reported fibroblast conversion to cardiomyocytes using alternative sets of reprogramming factors (98, 99) and extended these results to human fibroblasts (100–103). Together, these studies provide proof-of-concept that fibroblasts can be directly reprogrammed into cardiomyocytes.
Figure 5. Refreshing cardiomyocytes by cellular reprogramming.
Proliferating fibroblasts of the injured heart may be recruited to replenish cardiomyocytes by delivering reprogramming factors that induce them to transdifferentiate into cardiomyocyte-like cells (blue).
Direct reprogramming of cardiac fibroblasts to iCMs raises the tantalizing possibility that the same cells that cause deleterious scarring in MI can be repurposed to replenish lost cardiomyocytes. The approach would obviate many of the difficulties faced by progenitor cell therapy, such as immunocompatibility, manufacture and delivery of a large number of cells, and achieving stable engraftment, differentiation, and functional integration. However, this approach comes with a new set of hurdles. Identification of fibroblast to iCM reprogramming in vivo currently relies on genetic lineage tracing approaches, which have numerous caveat and pitfalls such as cell fusion and imperfect regulation of Cre activity that could potentially result erroneous classification of a cardiomyocyte as having arisen from reprogramming. Thus, we need independent approaches to quantitate direct reprogramming in vivo. The efficiency of reprogramming and the maturity and function of iCMs need to be improved. Mechanical and electrical integration of reprogrammed cardiomyocytes needs to be further assessed, and it will be important to determine if reprogramming has pro-arrhythmic consequences, e.g. due to the presence of less mature or poorly integrated iCMs. It appears that human cells require different sets of reprogramming factors than mouse cells (100–102); if true, then appropriate animal models will need to be developed for pre-clinical testing of cardiac reprogramming protocols targeted for use in humans. At present, all direct reprogramming approaches have used integrating viruses, which are associated with risk of oncogenesis and other consequences of genomic disruption. Development of optimized and preferably non-integrating methods for direct reprogramming will be essential to begin clinical translation of this promising regenerative strategy.
Summary and comments
The past decade has seen a sea change in cardiac biology. Rather than seeing the heart as a terminally differentiated organ devoid of regenerative capacity, the mainstream view has shifted to accept that the heart possesses intrinsic, albeit inadequate, regenerative mechanisms. This conceptual reorientation has opened the door to innovative approaches to augment or supplement these innate regenerative abilities. As a result, there are now multiple strategies for cardiac regeneration under active development, each with its own advantages and challenges. Time will tell which strategy, or combination of strategies, will achieve robust cardiac regeneration in humans.
Acknowledgments
This study was supported by funding from the American Heart Association and the National Heart, Lung, and Blood Institute (2R01HL094683 and 1R01HL116461), and by charitable donations from Gail Federici Smith, Edward Marram, and Karen Carpenter.
References
- 1.Beltrami A, Urbanek K, Kajstura J, Yan S, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami C, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Ang K, Shenje L, Reuter S, Soonpaa M, Rubart M, Field L, Galinanes M. Limitations of conventional approaches to identify myocyte nuclei in histologic sections of the heart. Am J Physiol Cell Physiol. 2010;298:C1603–9. doi: 10.1152/ajpcell.00435.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme M, Murry C. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann O, Bhardwaj R, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz B, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senyo S, Steinhauser M, Pizzimenti C, Yang V, Cai L, Wang M, Wu T, Guerquin-Kern JL, Lechene C, Lee R. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laflamme M, Murry C. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 7.Soonpaa M, Field L. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–6. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 8.Porrello E, Mahmoud A, Simpson E, Hill J, Richardson J, Olson E, Sadek H. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollova M, Bersell K, Walsh S, Savla J, Das L, Park S, Silberstein L, Dos Remedios CG, Graham D, Colan S, Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahuja P, Sdek P, Maclellan W. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He A, Ma Q, Cao J, Von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou D, Cahan P, Daley G, Kong S, Orkin S, Seidman C, Seidman J, Pu W. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res. 2012;110:406–415. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sdek P, Zhao P, Wang Y, Huang C, Ko C, Butler P, Weiss J, Maclellan W. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol. 2011;194:407–423. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agah R, Kirshenbaum L, Abdellatif M, Truong L, Chakraborty S, Michael L, Schneider M. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100:2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassink R, Pasumarthi K, Nakajima H, Rubart M, Soonpaa M, De La Riviere AB, Doevendans P, Field L. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78:18–25. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong W, Guan K. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–793. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson R, Martin J. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Gise A, Lin Z, Schlegelmilch K, Honor L, Pan G, Buck J, Ma Q, Ishiwata T, Zhou B, Camargo F, Pu W. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin M, Kim Y, Sutherland L, Qi X, Mcanally J, Schwartz R, Richardson J, Bassel-Duby R, Olson E. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heallen T, Morikawa Y, Leach J, Tao G, Willerson J, Johnson R, Martin J. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin M, Kim Y, Sutherland L, Murakami M, Qi X, Mcanally J, Porrello E, Mahmoud A, Tan W, Shelton J, Richardson J, Sadek H, Bassel-Duby R, Olson E. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Huang Z, Seok H, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu W, Liao R, Wang D. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112:1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porrello E, Mahmoud A, Simpson E, Johnson B, Grinsfelder D, Canseco D, Mammen P, Rothermel B, Olson E, Sadek H. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch S, Plank D, Witt S, Glascock B, Schaefer E, Chimenti S, Andreoli A, Limana F, Leri A, Kajstura J, Anversa P, Sussman M. Cardiac-specific IGF-1 expression attenuates dilated cardiomyopathy in tropomodulin-overexpressing transgenic mice. Circ Res. 2002;90:641–648. doi: 10.1161/01.res.0000013780.77774.75. [DOI] [PubMed] [Google Scholar]
- 25.Engel F, Schebesta M, Duong M, Lu G, Ren S, Madwed J, Jiang H, Wang Y, Keating M. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odiete O, Hill M, Sawyer D. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 28.Jabbour A, Hayward C, Keogh A, Kotlyar E, Mccrohon J, England J, Amor R, Liu X, Li X, Zhou M, Graham R, Macdonald P. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi K, Holdway J, Werdich A, Anderson R, Fang Y, Egnaczyk G, Evans T, Macrae C, Stainier D, Poss K. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte J. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanganalmath S, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burridge P, Keller G, Gold J, Wu J. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussbaum J, Minami E, Laflamme M, Virag J, Ware C, Masino A, Muskheli V, Pabon L, Reinecke H, Murry C. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 34.Min J, Yang Y, Sullivan M, Ke Q, Converso K, Chen Y, Morgan J, Xiao Y. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 35.Laflamme M, Chen K, Naumova A, Muskheli V, Fugate J, Dupras S, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill E, Ueno S, Yuan C, Gold J, Murry C. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 36.Shiba Y, Fernandes S, Zhu W, Filice D, Muskheli V, Kim J, Palpant N, Gantz J, Moyes K, Reinecke H, Van Biber B, Dardas T, Mignone J, Izawa A, Hanna R, Viswanathan M, Gold J, Kotlikoff M, Sarvazyan N, Kay M, Murry C, Laflamme M. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden L, Mortisen D, Sussman E, Dupras S, Fugate J, Cuy J, Hauch K, Laflamme M, Murry C, Ratner B. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tallini Y, Greene K, Craven M, Spealman A, Breitbach M, Smith J, Fisher P, Steffey M, Hesse M, Doran R, Woods A, Singh B, Yen A, Fleischmann B, Kotlikoff M. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beltrami A, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 40.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins R, Lecapitaine N, Cascapera S, Beltrami A, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler R, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellison G, Vicinanza C, Smith A, Aquila I, Leone A, Waring C, Henning B, Stirparo G, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 42.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel R, Keating A, Li R. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaruba M, Soonpaa M, Reuter S, Field L. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero M, Fiorini C, D’Alessandro DA, Michler R, Del Monte F, Hosoda T, Perrella M, Leri A, Buchholz B, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Bolli R, Chugh A, D’Amario D, Loughran J, Stoddard M, Ikram S, Beache G, Wagner S, Leri A, Hosoda T, Sanada F, Elmore J, Goichberg P, Cappetta D, Solankhi N, Fahsah I, Rokosh D, Slaughter M, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Chugh A, Beache G, Loughran J, Mewton N, Elmore J, Kajstura J, Pappas P, Tatooles A, Stoddard M, Lima J, Slaughter M, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith R, Barile L, Cho H, Leppo M, Hare J, Messina E, Giacomello A, Abraham M, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 48.Davis D, Zhang Y, Smith R, Cheng K, Terrovitis J, Malliaras K, Li T, White A, Makkar R, Marban E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chimenti I, Smith R, Li T, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makkar R, Smith R, Cheng K, Malliaras K, Thomson L, Berman D, Czer L, Marban L, Mendizabal A, Johnston P, Russell S, Schuleri K, Lardo A, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malliaras K, Makkar R, Smith R, Cheng K, Wu E, Bonow R, Marban L, Mendizabal A, Cingolani E, Johnston P, Gerstenblith G, Schuleri K, Lardo A, Marban E. Intracoronary Cardiosphere-Derived Cells After Myocardial Infarction: Evidence of Therapeutic Regeneration in the Final 1-Year Results of the CADUCEUS Trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson K, Majka S, Wang H, Pocius J, Hartley C, Majesky M, Entman M, Michael L, Hirschi K, Goodell M. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayes-Genis A, Salido M, Sole Ristol F, Puig M, Brossa V, Camprecios M, Corominas J, Marinoso M, Baro T, Vela M, Serrano S, Padro J, Bayes De Luna A, Cinca J. Host cell-derived cardiomyocytes in sex-mismatch cardiac allografts. Cardiovasc Res. 2002;56:404–410. doi: 10.1016/s0008-6363(02)00597-7. [DOI] [PubMed] [Google Scholar]
- 54.Laflamme M, Myerson D, Saffitz J, Murry C. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 55.Quaini F, Urbanek K, Beltrami A, Finato N, Beltrami C, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 56.Deb A, Wang S, Skelding K, Miller D, Simper D, Caplice N. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 57.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S, Li B, Pickel J, Mckay R, Nadal-Ginard B, Bodine D, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 58.Balsam L, Wagers A, Christensen J, Kofidis T, Weissman I, Robbins R. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 59.Nygren J, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann B, Jacobsen S. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 60.Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey D, Hamm C, Suselbeck T, Tonn T, Dimmeler S, Dill T, Zeiher A, Schachinger V. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 61.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu J. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 62.Yousef M, Schannwell C, Kostering M, Zeus T, Brehm M, Strauer B. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 63.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van De Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 64.Hirsch A, Nijveldt R, Van Der Vleuten PA, Tijssen J, Van Der Giessen WJ, Tio R, Waltenberger J, Ten Berg JM, Doevendans P, Aengevaeren W, Zwaginga J, Biemond B, Van Rossum AC, Piek J, Zijlstra F. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2011;32:1736–1747. doi: 10.1093/eurheartj/ehq449. [DOI] [PubMed] [Google Scholar]
- 65.Traverse J, Henry T, Ellis S, Pepine C, Willerson J, Zhao D, Forder J, Byrne B, Hatzopoulos A, Penn M, Perin E, Baran K, Chambers J, Lambert C, Raveendran G, Simon D, Vaughan D, Simpson L, Gee A, Taylor D, Cogle C, Thomas J, Silva G, Jorgenson B, Olson R, Bowman S, Francescon J, Geither C, Handberg E, Smith D, Baraniuk S, Piller L, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre S, Vojvodic R, Skarlatos S, Gordon D, Ebert R, Kwak M, Moye L, Simari R. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, Erne P, Zuber M, Auf Der Maur C, Jamshidi P, Gaemperli O, Windecker S, Moschovitis A, Wahl A, Buhler I, Wyss C, Kozerke S, Landmesser U, Luscher T, Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 67.Hong K, Li Q, Guo Y, Patton N, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schachinger V, Aicher A, Dobert N, Rover R, Diener J, Fichtlscherer S, Assmus B, Seeger F, Menzel C, Brenner W, Dimmeler S, Zeiher A. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 69.Hofmann M, Wollert K, Meyer G, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp W, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 70.Gnecchi M, Zhang Z, Ni A, Dzau V. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 72.Fidelis-De-Oliveira P, Werneck-De-Castro JP, Pinho-Ribeiro V, Shalom B, Nascimento-Silva JH, Costa RH, Souza E, Cruz I, Rangel R, Goldenberg R, Campos-De-Carvalho AC. Soluble factors from multipotent mesenchymal stromal cells have antinecrotic effect on cardiomyocytes in vitro and improve cardiac function in infarcted rat hearts. Cell Transplant. 2012;21:1011–1021. doi: 10.3727/096368911X623916. [DOI] [PubMed] [Google Scholar]
- 73.Nagnagnaggnecchi M, He H, Liang O, Melo L, Morello F, Mu H, Noiseux N, Zhang L, Pratt R, Ingwall J, Dzau V. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 74.Hatzistergos K, Quevedo H, Oskouei B, Hu Q, Feigenbaum G, Margitich I, Mazhari R, Boyle A, Zambrano J, Rodriguez J, Dulce R, Pattany P, Valdes D, Revilla C, Heldman A, Mcniece I, Hare J. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gnecchi M, He H, Noiseux N, Liang O, Zhang L, Morello F, Mu H, Melo L, Pratt R, Ingwall J, Dzau V. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 76.Kinnaird T, Stabile E, Burnett M, Lee C, Barr S, Fuchs S, Epstein S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 77.Zhou B, Honor L, He H, Ma Q, Oh J, Butterfield C, Lin R, Melero-Martin JM, Dolmatova E, Duffy H, Gise A, Zhou P, Hu Y, Wang G, Zhang B, Wang L, Hall J, Moses M, Mcgowan F, Pu W. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loffredo F, Steinhauser M, Gannon J, Lee R. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 80.Huang C, Gu H, Yu Q, Manukyan M, Poynter J, Wang M. Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One. 2011;6:e29246. doi: 10.1371/journal.pone.0029246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Timmers L, Lim S, Hoefer I, Arslan F, Lai R, Van Oorschot AA, Goumans M, Strijder C, Sze S, Choo A, Piek J, Doevendans P, Pasterkamp G, De Kleijn DP. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Hsieh P, Segers V, Davis M, Macgillivray C, Gannon J, Molkentin J, Robbins J, Lee R. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 84.Von Gise A, Pu W. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res. 2012;110:1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou B, Ma Q, Rajagopal S, Wu S, Domian I, Rivera-Feliciano J, Jiang D, Von Gise A, Ikeda S, Chien K, Pu W. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai C, Martin J, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup W, Denton C, Mcculloch A, Chen J, Evans S. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavine K, Ornitz D. Fibroblast growth factors and Hedgehogs: at the heart of the epicardial signaling center. Trends Genet. 2008;24:33–40. doi: 10.1016/j.tig.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 88.Li P, Cavallero S, Gu Y, Chen T, Hughes J, Hassan A, Bruning J, Pashmforoush M, Sucov H. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smart N, Bollini S, Dube K, Vieira J, Zhou B, Davidson S, Yellon D, Riegler J, Price A, Lythgoe M, Pu W, Riley P. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou B, Honor L, Ma Q, Oh J, Lin R, Melero-Martin JM, Von Gise A, Zhou P, Hu T, He L, Wu K, Zhang H, Zhang Y, Pu W. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J Mol Cell Cardiol. 2012;52:43–47. doi: 10.1016/j.yjmcc.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zangi L, Lui K, Von Gise A, Ma Q, Ebina W, Ptaszek L, Spater D, Xu H, Tabebordbar M, Gorbatov R, Sena B, Nahrendorf M, Briscoe D, Li R, Wagers A, Rossi D, Pu W, Chien K. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey K, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Nemir M, Metrich M, Plaisance I, Lepore M, Cruchet S, Berthonneche C, Sarre A, Radtke F, Pedrazzini T. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 95.Ieda M, Fu J, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau B, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian L, Huang Y, Spencer C, Foley A, Vedantham V, Liu L, Conway S, Fu J, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song K, Nam Y, Luo X, Qi X, Tan W, Huang G, Acharya A, Smith C, Tallquist M, Neilson E, Hill J, Bassel-Duby R, Olson E. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jayawardena T, Egemnazarov B, Finch E, Zhang L, Payne J, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau V. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Addis R, Ifkovits J, Pinto F, Kellam L, Esteso P, Rentschler S, Christoforou N, Epstein J, Gearhart J. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110:12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu J, Stone N, Liu L, Spencer C, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau B, Srivastava D. Direct Reprogramming of Human Fibroblasts toward a Cardiomyocyte-like State. Stem Cell Reports. 2013;1:235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nam Y, Song K, Luo X, Daniel E, Lambeth K, West K, Hill J, Dimaio J, Baker L, Bassel-Duby R, Olson E. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110:5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Islas J, Liu Y, Weng K, Robertson M, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima R, Willerson J, Potaman V, Schwartz R. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci U S A. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menasche P, Alfieri O, Janssens S, Mckenna W, Reichenspurner H, Trinquart L, Vilquin J, Marolleau J, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege A. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 105.Qiao H, Zhang H, Yamanaka S, Patel V, Petrenko N, Huang B, Muenz L, Ferrari V, Boheler K, Zhou R. Long-term improvement in postinfarct left ventricular global and regional contractile function is mediated by embryonic stem cell-derived cardiomyocytes. Circ Cardiovasc Imaging. 2011;4:33–41. doi: 10.1161/CIRCIMAGING.110.957431. [DOI] [PMC free article] [PubMed] [Google Scholar]