Abstract
Perchlorate, an environmental contaminant, disrupts normal functioning of the thyroid. We previously showed that perchlorate disrupts behavior and gonad development, and induces external morphological changes in a vertebrate model organism, the threespine stickleback. Whether perchlorate alters these phenotypes via a thyroid-mediated mechanism, and the extent to which the effects depend on dose, are unknown. To address these questions, we chronically exposed stickleback to control conditions and to three concentrations of perchlorate (10, 30 and 100 ppm) at various developmental stages from fertilization to reproductive maturity. Adults chronically exposed to perchlorate had increased numbers of thyroid follicles and decreased numbers of thyrocytes. Surprisingly, T4 and T3 levels in larval, juvenile, and adult whole fish chronically exposed to perchlorate did not differ from controls, except at the lowest perchlorate dose, suggesting a non-monotonic dose response curve. We found no detectable abnormalities in external phenotype at any dose of perchlorate, indicating that the increased number of thyroid follicles compensated for the disruptive effects of these doses. In contrast to external morphology, gonadal development was altered substantially, with the highest dose of perchlorate causing the largest effects. Perchlorate increased the number both of early stage ovarian follicles in females and of advanced spermatogenic stages in males. Perchlorate also disrupted embryonic androgen levels. We conclude that chronic perchlorate exposure may not result in lasting adult gross morphological changes but can produce lasting modifications to gonads when compensation of T3 and T4 levels occurs by thyroid follicle hyperplasia. Perchlorate may therefore affect vertebrate development via both thyroidal and non-thyroidal mechanisms.
Keywords: 11-Ketotestosterone, Dose response curve embryonic androgen levels, Endocrine disruption, Gasterosteus aculeatus, Steroid biogenesis
1. Introduction
Thyroid hormones (THs) are important for nearly every aspect of vertebrate growth and development (Power et al., 2001; Jugan et al., 2010). Consequently, environmental contaminants affecting TH production and use have wide-ranging impacts. Contaminants that interfere with the vertebrate hypothalamic–pituitary–thyroid (HPT) axis have clear deleterious effects, particularly in aquatic organisms (Power et al., 2001; Milnes et al., 2006; Carr and Patiño, 2011). A large number of abnormal phenotypes have been ascribed to thyroid-disrupting chemicals, including altered body size and shape (Lawrence et al., 2005; Bernhardt et al., 2011), disrupted immune function (Capps et al., 2004), modified bone development (Stevens et al., 2000; Bernhardt et al., 2011), and changes in behavior and gonad masculinization or feminization in threespine stickleback (Gasterosteus aculeatus) and zebrafish (Danio rerio), respectively (Bernhardt et al., 2006; Mukhi and Patiño, 2007; Bernhardt and von Hippel, 2008; Sharma and Patiño, 2013). The biological mechanisms of contaminant-induced phenotypic abnormalities are largely unknown, however. In particular, interactions of the HPT axis with other endocrine axes and developmental pathways are still poorly understood in many vertebrates, although it is clear that early developmental disruption of thyroid signaling would be deleterious in mammalian (Préau et al.,, 2014) and non-mammalian vertebrates (Morvan Dubois et al., 2006; Préau et al., 2014). Consequently, the full suite of developmental and reproductive abnormalities caused by HPT disruption is still unknown for most contaminants. In addition, the dose–response curves, critical developmental windows of exposure, and physiological endpoints to assess the effects of many HPT disruptors have not been well defined in any vertebrate.
To better understand the biological mechanisms of contaminant-induced abnormalities we examined the hormonal and morphological effects of a known HPT antagonist, perchlorate (), on thyroidal, musculoskeletal, and reproductive development in a vertebrate model species, the threespine stickleback, at environmentally relevant concentrations (Urbansky, 1998; Goleman et al., 2002). Perchlorate is a persistent, widespread environmental contaminant and a potent endocrine disruptor with documented pathological effects on TH production in humans (US EPA, 2002; Blount et al.,, 2006b). The perchlorate anion acts at the thyroid follicle to block iodide uptake via competitive inhibition of the sodium-iodide symporter (NIS, alias SLC5A5; Eskandari et al., 1997; Wolff, 1998), thereby impairing sequestration of iodide, an ion necessary for synthesis of TH. Perchlorate appears in ground and surface waters throughout the United States (Trumpolt et al., 2005) at levels up to 4000 ppm (Urbansky, 1998). Recent studies detected perchlorate in breast milk of pregnant and lactating women (Kirk et al., 2005; Pearce et al., 2007), and perchlorate was ubiquitously detectable in the urine of 22,000 pregnant women in Wales and Italy (Pearce et al., 2010). Amazingly, all 2800 participants in a recent study had perchlorate in their urine, with levels ranging from 0.00019 to 0.160 ppm; children had the highest calculated exposure (Blount et al., 2006a, 2006b).
Perchlorate produces deleterious effects in other vertebrates similar to those seen in humans. For example, perchlorate negatively affects amphibian health – and can lead to death – at environmentally relevant concentrations of 200–500 ppm (Goleman et al., 2002). Perchlorate exposure in teleost fishes leads to a wide array of altered phenotypes, some of which suggest disruption to development outside of the HPT axis (Crane et al., 2005; Bernhardt et al., 2006; Bernhardt and von Hippel, 2008; Sharma and Patiño, 2013). Effects of chronic perchlorate exposure on stickleback include abnormal lateral plate development, decreased swimming performance, slower growth rates, and reduced pigmentation (Bernhardt et al., 2006, 2011; Bernhardt and von Hippel, 2008). Perchlorate also appears to act via an unknown mechanism to alter gonad development and sex determination in teleosts, a finding not predicted to occur solely via thyroid disruption (Bernhardt et al., 2006; Mukhi and Patiño, 2007; Sharma and Patiño, 2013); the HPT axis, however, has been implicated in effects on reproductive development and function in teleosts (Carr and Patiño, 2011; Flood et al., 2013). Developmental exposure to perchlorate skews the sex ratio towards female in zebrafish (Mukhi and Patiño, 2007; Sharma and Patiño, 2013), a species in which various strains are reported to have different genetic bases for sex determination (Bradley et al., 2011; Anderson et al., 2012). Perchlorate masculinizes the gonad in male and female stickleback, in addition to increasing the gonadal-somatic index in male stickleback (Bernhardt et al., 2006). In some cases, perchlorate exposure causes genotypically female stickleback to become functional hermaphrodites, leading us to hypothesize that perchlorate has androgenic effects (Bernhardt et al., 2006).
Multiple lines of evidence suggest that the effects of perchlorate could be widespread throughout the body because TH receptors occur in most cells (Hulbert, 2000; Power et al., 2001). Perchlorate-induced changes in thyroid hormone production therefore have the potential for widespread disruption of numerous tissues regulated by the HPT axis. In addition, studies of the specific effects of perchlorate on circulating or whole body TH concentration are often contradictory. For example, Mukhi et al. (2005) found no significant effect of 12 weeks of ammonium perchlorate exposure on whole-body T4 concentrations in zebrafish, but in a subsequent study these authors reported that T4 concentrations were significantly decreased while T3 concentrations remained unchanged after 16 weeks of exposure (Mukhi and Patiño, 2007). A similar study in mosquitofish (Gambusia affinis), however, demonstrated no dose-dependent relationship in T4 levels in response to various levels of perchlorate exposure after 2, 10 or 30 days (Bradford et al., 2005). Conflicting results across studies of HPT target pollutants may occur because the widespread effects of TH toxins interact with the specifics of experimental conditions such as length of exposure, age, species and genotype of the animal exposed, and dose of toxin to produce discordant results (reviewed by Carr and Patiño (2011)). Because the shape of the dose–response curve for perchlorate has not been rigorously determined across various life stages or broadly across vertebrate phylogeny, and because different studies have used various end points as indicators of HPT function, it is difficult to determine the scope of perchlorate’s effects on vertebrate development.
Taken together, these results indicate that two key hormone signaling pathways, the HPT axis and the hypothalamic–pituitary–gonadal (HPG) axis, which regulates production of reproductive steroids, are likely disrupted by perchlorate (Bernhardt et al., 2006; Carr and Patiño, 2011; Sharma and Patiño, 2013). Establishing a perchlorate dose–response curve for a variety of phenotypes related to the HPT and HPG axes is an important step in understanding whether early developmental exposure to perchlorate affects vertebrates solely through thyroidal mechanisms. We hypothesized that perchlorate may alter development of thyroid tissues as well as reproductive tissues through cross-talk among signaling pathways or through interference with normal cell physiology during development. In particular, sodium-iodide symporters (NIS) are located in the gonads of vertebrates (Bermudez, 2008; Russo et al., 2011), and a reasonable mechanistic hypothesis is that perchlorate directly affects gonad tissues through interactions with these symporters. Because perchlorate has been shown to be behaviorally and morphologically masculinizing in stickleback, we investigate possible effects of perchlorate on androgen levels.
To better understand the effects of perchlorate on the HPT and HPG axes, we performed a large, replicated, and long-running experiment on developing stickleback at a variety of chronic and environmentally relevant perchlorate exposures. We examined intermediate and end-point effects of perchlorate exposure by mapping changes of endocrine profiles as well as alterations to morphology and gonadal development. We studied stickleback because it is a widely used aquatic model of ecology, behavior, evolution, endocrine disruption, and genetics (Bell and Foster, 1994; Gravenmier et al., 2005; Cresko et al., 2007; von Hippel, 2010). This small fish is a differentiated gonochorist with genotypic sex determination (Hahlbeck et al., 2004; Kitano et al., 2007; Lewis et al., 2008). Stickleback live in a variety of marine, brackish and freshwater habitats throughout much of the Northern Hemisphere (Bell and Foster, 1994), including in polluted sites. Stickleback are therefore a good model species for investigations of environmental contaminants that might affect human sexual differentiation and development and thyroid function.
In the current study, we found no abnormalities in external phenotype at any dose of perchlorate, but we did discover a significant increase in the number of thyroid follicles in stickleback. Surprisingly, whole fish T4 and T3 levels were not different from controls, except at the lowest dose (10 ppm). Perchlorate induced an increase of early stage follicular ovarian follicles in females, and enlargement of the testes and increased occurrence of mature stage sperm in males with increasing dose in both juveniles and adults. Perchlorate also disrupted embryonic androgen levels. We conclude that perchlorate-induced perturbations to the thyroid throughout development may not result in lasting adult gross morphological changes if the chronic exposure leads to compensation by thyroid follicle hyperplasia. Life-long exposure to perchlorate does, however, produce modifications to the adult gonad. Our data suggest that perchlorate exposure during development affects several multiple endocrine axes and development of thyroid and gonad tissues and that the effects on the gonad may be independent of whole-body concentrations of T3 and T4.
2. Methods
2.1. Study species and experimental design
Adult anadromous stickleback were collected during the 2010 and 2011 breeding seasons (May through July) in Rabbit Slough (61.5595°N, 149.2583°W) in south-central Alaska. These wild-caught stickleback were stripped of eggs and sperm for mass-cross in vitro fertilizations using protocols described in Cresko et al. (2007) to produce approximately 2250 embryos per replicate, totaling approximately 800,000 embryos. Mass crosses were performed in 1 L Pyrex jars with a ratio of one male per six females. Stickleback were maintained in 113.6 L glass aquaria filled with 98.4 L of fortified reverse osmosis (RO) water from hatching through sexual maturity. Nominal water quality parameters in every tank/treatment included 6 ppt salinity and zero (immeasurable) ammonia. Temperature and photoperiod were manipulated to simulate ambient seasonality. Water temperatures ranged from 12.5–19.6 °C according to season. Approximately 40 mL of Bactapur (Aquatic Ecosystems) was added to tanks with initial fry introductions to enhance denitrifying bacteria colonization in biofilters.
Approximately 1700 L of RO water was delivered on a weekly basis to support the static/renewal experimental design (MatSu Water, Anchorage, AK). This RO water was mixed in stock tanks to produce 6 ppt salinity, and nominal 99.5% sodium perchlorate hydrate (Sigma Aldrich, St. Louis, MO) concentrations of zero, 10, 30, or 100 mg/L (ppm). Trace iodide (0.06 ppm) was added according to treatment during tank maintenance. All stock and experimental tanks were continually aerated using 15 cm diameter biofilters (Aquatic Ecosystems). We measured ammonia, iodide, nitrate, perchlorate, pH, temperature, salinity, and specific conductivity. Ammonia levels were monitored using a commercial test kit (API). Ammonia was typically measured at two-week intervals unless detectable concentrations were recorded, at which time additional Bactapur was added and/or water exchanges were performed. Subsequent measurements were performed (up to multiple times daily) until ammonia concentrations returned to non-detectable levels. Iodide concentrations were measured at 1–2 week intervals using a commercially available iodine/iodide multitest (Seachem, Madison, GA). Perchlorate concentrations were measured using an Orion Perchlorate Probe (Thermo Electron Corp). Perchlorate concentrations were measured weekly with supplemental measurements (up to multiple times daily) as adjustments were made to regain nominal treatment concentrations following water changes. Perchlorate levels were adjusted such that they never deviated more than 10% from target values. Temperature, salinity, and pH were monitored using a commercial multiparameter probe (YSI). Temperature and pH readings were conducted at 7–10 day intervals with supplemental measurements (up to daily) recorded following pH variations or water changes. Nominal salinity concentrations were 6 ppt, and measurements were conducted weekly. Specific conductivity measurements were performed weekly, with as needed supplementation.
Fish were chronically exposed to control (no perchlorate) and three nominal perchlorate concentrations (10, 30 and 100 ppm), and sampled at the following days post fertilization (dpf): 0 through 8, 28, 31, 44, 56, 84, 112, 140, 168, 196, 224, 252, 280, 308, or 336. 0 dpf means the fish were exposed to perchlorate from fertilization until 3 h post fertilization. Thyroid hormones were measured from fish at sampling periods from day 0 to 336 as listed above. In a separate trial in the 2011 breeding season in which we targeted embryonic and larval developmental androgen levels, fish were collected from each experimental treatment for measurement of developmental whole body testosterone (T) and 11-ketotestosterone (11-KT) levels at 0–7 dpf, 28 dpf, and 56 dpf.
2.2. Hormone assays
On each sampling day, fish were euthanized with neutral pH MS222 (Sigma–Aldrich, St. Louis, MO) and pooled by age and treatment in numbers sufficient to attain three samples of 0.5 g fresh weight (usually requiring 5–20 individuals per sample depending on the age of fish). Samples were stored at −80 °C for later analysis of whole body concentrations of THs (T4 and T3) and androgens (11-KT and T).
THs were extracted from whole-body homogenates, and the final extract was stored dry at −80 °C until the day of the assay, as outlined in Crane et al., (2004), with the following modifications. Each sample was reconstituted with 330 μL of EIA buffer (0.1 M PBS, 0.15 M NaCl, 0.1% BSA, pH 7.4). T4 was assayed in technical duplicate using 15 μL per well (Total T4 EIA, MP Biomedicals, Santa Ana, CA) and T3 was assayed in technical triplicate using 20 μL per well (Total T3 EIA, MP Biomedicals, n = 5–18 pools (biological replicates) of age matched fish (mass equal to 1 g)). Both EIAs were validated using tests of parallelism and standard addition. Intra-assay variation was 3.6% for T4 and 6.9% for T3. Inter-assay variation was 13.0% and 12.2% for T4 and T3, respectively.
Androgens were extracted with an excess of diethyl ether from whole-body homogenates that had been sonicated in PBS. Extracts were evaporated under a nitrogen stream and stored dry at −80 °C. Samples assayed for T were reconstituted with 200 μL of extraction buffer and run in triplicate using 50 μL per well (Testosterone EIA, Neogen). Samples assayed for 11-KT were reconstituted with 800 μL of EIA buffer and run in technical duplicate using 50 μL per well (11-ketotestosterone EIA, Cayman Chemicals, Lexington KY), n = 3–5 pools (biological replicates) of age-matched fish (mass equal to 1 g). Both EIAs were validated using tests of parallelism and standard addition for both fish and embryo homogenates. Intra-assay variation was 5.5% for T and 4.6% for 11-KT. Inter-assay variation was 18.8% and 9.8% for T and 11-KT, respectively.
While the ELISA kit for 11-KT has been commercially validated for use with teleost serum, it had not been validated for whole fish homogenate. To verify that the ELISA kit was measuring 11-KT accurately in stickleback tissue homogenates, which contain lipids and other substances that have the potential for interference with ELISA assays, stickleback adult and embryo tissues were analyzed at the Endocrine Technology Support Core Lab (ETSC) at the Oregon National Primate Research Center (ONPRC). Adult fish tissue, as well as a pool of approximately 80 embryos each aged 3 dpf, were prepared by euthanizing the animals, blotting off excess liquid, and flash freezing in liquid nitrogen before shipping animals on dry ice to ETSC. Three methods for extraction of hydrophobic lipids and sample purification were utilized prior to measuring the concentration of hormone in the adult and embryo samples. In addition, a monkey serum pool from Rhesus Macaque (Macaca mullata), with or without an 11-KT spike, was used as a control. The three methods were (1) radioimmunoassay (RIA) after methanol extraction without Strata-X SPE column purification (Phenomenex, Torrence, CA), (2) RIA after methanol extraction and reverse phase liquid chromatography using a C-18 reverse column, and (3) RIA after methanol extraction, C-18 reverse column purification, and Strata-X column purification. A standard dilutions test was also performed in duplicate at 1:1, 1:2, 1:4 and 1:8, and the results were found to be parallel across dilutions. 11-KT values of 10.9, 2.2, and 20.4 pg/ml were measured for the pooled embryonic tissue using the three methods, respectively. These results corresponded well with the value recorded using ELISA on untreated 3 dpf stickleback embryos (8.2 pg/ml ± 3.1). Similarly, adult 11-KT values determined by RIA were 36.0, 19.5, and 29.4 pg/ml using the three methods, again corresponding well with the ELISA results for control adults (21.08 pg/ml ± 8.8). We conclude that 11-KT results using ELISA are repeatable using RIA methods, and are therefore likely not significantly affected by lipid or impurity interference in whole body assays.
2.3. Histology preparation
A total of 128 stickleback ages 1–12 months post fertilization from each of the treatment groups (0, 10, 30, 100 ppm) were euthanized and immediately fixed in Bouin’s solution at 4 °C for 48 h. After Bouin’s fixation, fish were serially washed in ethanol and then stored in 70% ethanol at 4 °C. Samples were subsequently paraffin-embedded and sectioned horizontally in the head and neck for thyroid visualization, and in cross-section in the abdomen to visualize the gonads at 16 μm resolution, and stained with hematoxylin and eosin (H&E).
2.4. Thyroid pathohistology
Histological preparations of stickleback thyroid follicles were analyzed for thyroid follicle morphology in 51 individuals (n = 31 exposed to 100 ppm perchlorate; n = 20 control fish, with at least 4 individuals at each time point sampled up to 1 year). The whole throat was cryosectioned, and all slides containing thyroid follicles were viewed at 100× magnification. The single slide containing the largest number of follicles for that individual was chosen for follicle quantification and morphometric analysis of two sections (technical replicates) on that slide. The sections were imaged using a Nikon Eclipse T3200 inverted light microscope with an Olympus DP71 camera and Olympus imaging software. Images were analyzed using Image J software and an Intuos 4 tablet (Wacom Co., Vancouver WA) to trace and quantify thyroid follicle number, follicle area, follicle width, thyrocyte number, thyrocyte height, and thyrocyte width. Morphometric analyses to quantify size and shape were performed on five of the largest follicles on the sections, and data averaged to a single value per fish. Representative images of thyrocytes were photographed at 100× and 600× from both 100 ppm and control fish. For thyroid analysis and morphometrics, fish 9–100 dpf were considered larvae, 101–250 dpf were considered juveniles, and fish more than 250 dpf were considered adults.
2.5. Gonad and kidney pathohistology
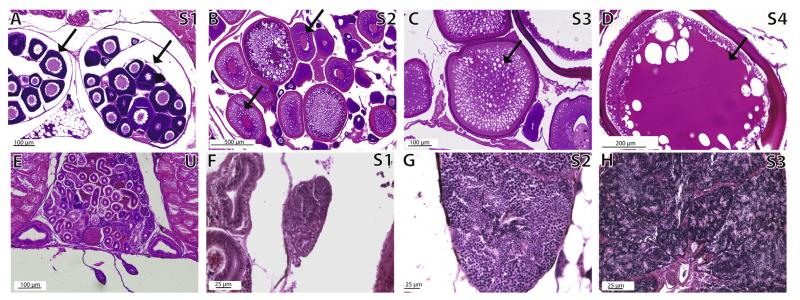
At least six females from each age class (larvae 9–100 dpf; juveniles 101–250 dpf; adults 251–336 dpf) with two technical replicates from the left and right ovaries (two sections/slide, two slides for each fish = four gonad sections) were evaluated for gonadal maturity. Age class designations were determined following definitions outlined in Parichy et al. (2009). Female gonads, which in stickleback have group synchronous follicular development (Wallace and Selman, 1979; Sokolowska and Kulczykowska, 2006; John Baker personal comm.), were analyzed using light microscopy by tallying the number of follicles per ovary in each stage in the four sections analyzed per fish. Ovarian follicles were staged based on Sokolowska and Kulczykowska (2006), and collapsed into four categories: early follicles (corresponding to their stages 1–2, defined by small, round, previtellogenic oogonia), mid (their stages 3–4, defined by beginning of vitellogenesis, vacuolization, enlarged ovarian follicles), late (their stages 5–6, defined by cytoplasm densely filled with vacuoles, yolk vesicles, beginning of secondary yolk), and mature (their stages 7–8, defined by cytoplasm full of yolk stained dark red, yolk sometimes in densely packed globules, largest ovarian follicles) (Fig. 6). We could not unequivocally determine if adult females had spawned prior to harvesting, but the recent spawning of an individual would appear in the histology preparations as the absence of stage 4 (mature) ovarian follicles.
Fig. 6.
Ovarian and testicular stages in control stickleback fish. Panels A–D show ovarian follicle developmental stages S1–S4. Follicles representative of the stage are denoted with a black arrow. Panel E shows a representative undifferentiated gonad (U) in an adult (>300 dpf) male. Panels F–H show spermatogenic stage progression S1–S3. Staging schema condensed from Sokolowska and Kulczykowska (2006).
At least three males from each age class (larvae 9–100 dpf, juveniles 101–250 dpf, adults 251–336 dpf) were evaluated histologically for gonadal maturity. Because lobules within a stickleback testis are relatively synchronous in terms of spermatogenic stage, the testes of each fish were given a single score. Testes were designated as belonging to a single stage for each fish according to Sokolowska and Kulczykowska (2006), except that stages were collapsed into: stage 1 early spermatogenesis (their stages 6 and 7), stage 2 intermediate spermatogenesis (their stages 1 and 2), stage 3 late spermatogenesis and complete spermatogenesis (their stages 3–5). Three slides per male, with two sections per slide, were analyzed as technical replicates. For each individual we chose to analyze the slide with sections with the largest cross-sectional area in order to standardize the region of the testis from which we were measuring across individuals. Testis width and area were measured using NIH image J software (Rasband, 1997-2012) calibrated to a scale bar imaged simultaneously.
The height of kidney epithelial cells was measured using Image J software and designated per age class for those ages that had developed kidneys (juvenile, adult) as normal, hypertrophic, and hypotrophic based on kidney cell height (μm). Normal, hypertrophic and hypotrophic designations were determined by creating a size range based on a subset of kidneys per age group. Juvenile kidney epithelial cells were considered normal in the height range of 8–12 μm based on values for juvenile controls, and adult kidney epithelial cells were classified as normal in the height range of 9–15 μm based on values for adult controls. Hypotrophic kidney epithelial cells (<8 μm) were not found in any fish.
2.6. Statistical analysis
Statistical analyses of all data were performed using JMP PRO software (2013 SAS Institute, Cary, NC). Means presented are ± 1SE. Comparisons of means for continuous response variables were analyzed using either a one-way or two factor factorial analysis of variance (ANOVA) followed by Tukey’s HSD post hoc analysis of means for significant treatment effects, after testing for statistical assumptions. Analysis of count response variables utilized Generalized Linear Models (GLMs) using a Poisson error distribution and Log link function. Categorical response variables were analyzed using contingency analysis or logistic regression and standard error. Values were considered significantly different at an alpha level of p < 0.05 (see Fig. 7).
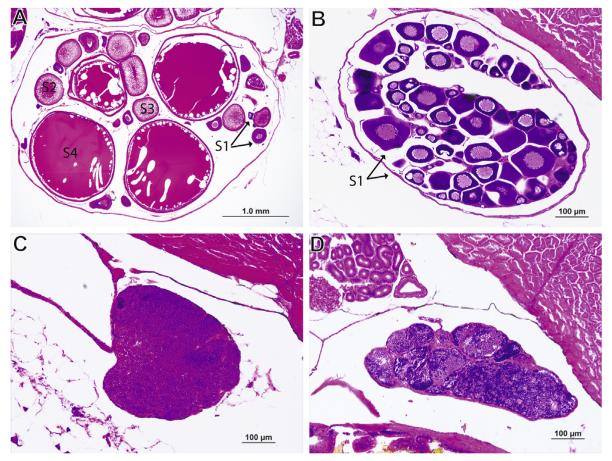
Fig. 7.
Representative images of the effects of perchlorate treatment on gonad development and reproductive stage in stickleback males and females. (A) Ovaries analyzed from adult control fish not treated with perchlorate (n = 4–6 individuals) contain ovarian follicles of all 4 stages of maturity. Follicles representative of each stage denoted with black arrows (B) Ovaries analyzed from adult fish treated with 100 ppm perchlorate (n = 4–6 individuals) contain predominantly stage 1 immature ovarian follicles, denoted with black arrows. (C) Testes from adult control fish not treated with perchlorate (n = 3–4 individuals) were found to be undergoing less spermiation and to be less mature than (D) testes from 100 ppm perchlorate treated fish (n = 3–4 individuals).
3. Results
3.1. TH levels do not change in response to chronic perchlorate treatment except at the lowest tested concentration
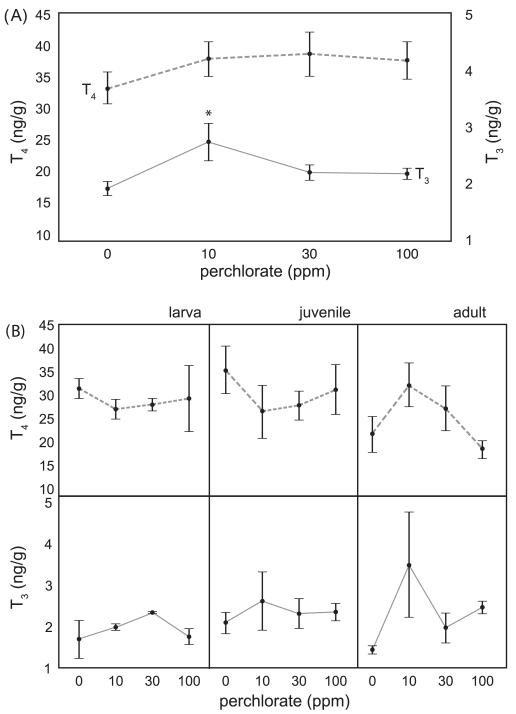
Perchlorate had no significant effect at any dose on whole body [T4] in one year old chronically exposed stickleback (F3, 162 = 0.66, P = 0.5720; Fig. 1A). Given our sample size, the statistical power to detect a biologically relevant difference of 5 pg/g was high (power = 0.82), and thus the absence of a significant effect is biologically meaningful. Subsequent tests had similar power. Median concentrations of T4 in adult fish (aged > 250dpf) ranged between 30 and 40 pg/g (Fig. 1A). In contrast, whole body adult [T3] was significantly affected by perchlorate treatment (F3, 151 = 3.29, P = 0.0231), but only the lowest dose, 10 ppm, elicited a statistically significant increase in whole body [T3] with respect to the control treatment (Tukey’s HSD LS Means test; Fig. 1A).
Fig. 1.
Effect of perchlorate on thyroid hormone levels. Mean concentrations of T4 and T3 in adult stickleback chronically exposed since fertilization to 0, 10, 30, or 100 ppm perchlorate over 336 days of development (error bars are ± 1 SE.). (A) Whole body thyroxine (T4, dashed line, left axis) and whole body triiodothyronine (T3, solid line, right axis) levels in adult stickleback chronically exposed to 0, 10, 30, or 100 ppm perchlorate. The asterisk (*) denotes significantly different [T3] from control. (B) Developmental T4 (top panels, dashed line) and T3 (bottom panels, solid line) concentrations in stickleback exposed to 0, 10, 30, or 100 ppm perchlorate for up to 336 days of development with ages grouped into category stages larval (up to 100 dpf), juvenile (100–250 dpf), and adult stickleback (older than 250 dpf), n = 5–18 pools (biological replicates) of age matched fish (mass equal to 1 g).
Perchlorate exposure also did not significantly alter whole body [T4] over the course of development (Fig. 1B; F3, 46 = 0.47, P = 0.7034); although [T4] varied across age (F3, 46 = 4.92, P = 0.0050) there was no significant interaction between treatment and age (F9, 46 = 1.13, P = 0.3613). Levels of T4 exhibited similar developmental trajectories across the 365 days of development except for 10 ppm perchlorate exposed fish (Fig. 1B). T4 levels in control fish and 100 ppm exposed fish increased slightly from larval to juvenile stages, and then fell in adults (Fig. 1B). In 30 ppm exposed fish T4 levels were fairly level across the three stages (Fig. 1B). In 10 ppm exposed fish, however, T4 levels were steady from larval to juvenile stages, and then increased in adult fish. This pattern in 10 ppm exposed fish is the inverse of the pattern seen in control fish and 100 ppm exposed fish (Fig. 1B).
Levels of T3 did not vary significantly over the course of development (Fig. 1B) with respect to the dose of perchlorate (two factor ANOVA; F3, 28 = 0.083, P = 0.5400) or the age of the fish (F2, 28 = 0.58, P = 0.5675), and there was no significant interaction between treatment and age (F6, 46 = 0.71, P = 0.6440). Fish exposed to 10 ppm perchlorate demonstrated altered developmental T3 levels, holding constant in the larval-to-juvenile transition, and increasing at adulthood. For both T4 and T3 therefore, this pattern is essentially an inversion of the normal TH developmental profile in which TH levels fell in adulthood after the larval-juvenile transition (Fig. 1B).
Chronic perchlorate exposure, irrespective of dose, did not have a discernible effect on gross morphology (Table 1). We found no significant differences among treatments in standard length, body mass, mean dorsal spine number, anal spine length, number of lateral plates, pigmentation, or frequency of exophthalmia. We also did not find a higher frequency of otherwise abnormal morphologies, or any intersex fish (Table 1).
Table 1.
External gross morphometric parameters of perchlorate treated and control fish (0 ppm). No morphometric analyses revealed a significant difference in any parameter based on perchlorate treatment. n = 24–36 per treatment. Juvenile age ranges from 84 to 250 dpf. Adult age ranges from 251 to 350 dpf. SL and mass are measured variables, dorsal spine and anal spine are averaged counts of number of spines present, Lt and Rt plates are total counts of lateral plates on the left (Lt) and right (Rt) sides of the body, pigment score is based on a predetermined rubric scoring melanocyte density (1 is least dense, 5 is most dense), occurrence of exophthalmia, intersex, are rated as Yes (Y), or No (N).
| Age class | (ppm) | SL (mm) | Mass (g) | Dorsal Spine | Anal spine | Rt plates | Lt plates | Pigment score different from controls |
Frequent exopthalmia |
Inter sex |
|---|---|---|---|---|---|---|---|---|---|---|
| Juvenile | 0 | 12.8 (2.0) | 0.03 (0.1) | 2.08 (0.3) | 0.75 (0.1) | 12.8 (2.5) | 12.8 (2.6) | - | N | N |
| Juvenile | 10 | 9.7 (1.7) | 0.06 (0.1) | 1.83 (0.3) | 0.59 (0.1) | 9.7 (2.0) | 9.7 (2.1) | N | N | N |
| Juvenile | 30 | 9.0 (1.5) | 0.04 (0.1) | 2.28 (0.2) | 0.8 (0.1) | 9.0 (1.8) | 9.0 (1.8) | N | N | N |
| Juvenile | 100 | 7.5 (1.5) | 0.09 (0.1) | 2.08 (0.2) | 0.69 (0.1) | 7.3 (1.8) | 7.5 (1.8) | N | N | N |
| Adult | 0 | 34.2 (2.1) | 0.64 (0.1) | 2.83 (0.3) | 1.0 (0.1) | 23 (2.4) | 24 (2.4) | - | N | N |
| Adult | 10 | 34.3 (1.8) | 0.57 (0.1) | 3.0 (0.3) | 1.0 (0.1) | 23 (2.1) | 23 (2.1) | N | N | N |
| Adult | 30 | 33.8 (2.0) | 0.57 (0.1) | 3.0 (0.3) | 1.0 (0.1) | 23 (2.3) | 23 (2.4) | N | N | N |
| Adult | 100 | 32.6 (1.5) | 0.76 (0.1) | 3.0 (0.3) | 1.0 (0.1) | 22 (2.4) | 23 (2.4) | N | N | N |
3.2. Perchlorate causes an increase in the number of small thyroid follicles and altered thyrocyte morphology
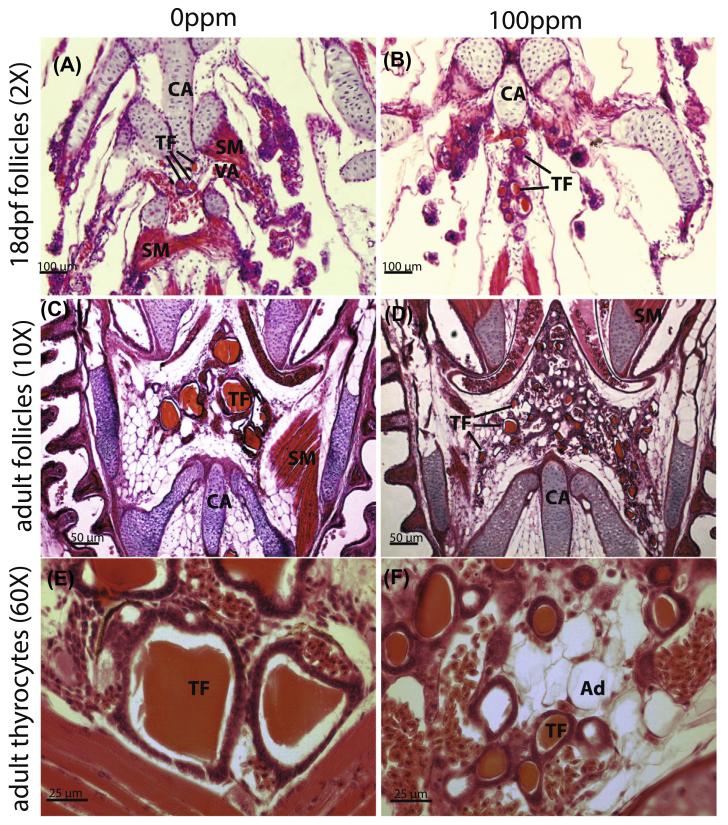
Histological examination of cryosectioned pharynges of stickleback (Fig. 2) revealed an interaction between perchlorate concentration and age on the development of thyroid follicles. Sections from treated and control fish were compared qualitatively (Fig. 2) and quantitatively analyzed using Image J software followed by appropriate statistical analysis (Fig. 3). We observed a significant increase in the number of thyroid follicles associated with increasing perchlorate concentration (F1, 47 = 44.17, P < 0.0001) and advancing developmental stage (F2, 47 = 10.24, P = 0.0002), as well as a significant interaction between these two factors of perchlorate dose and developmental stage (F2, 47 = 8.45, P = 0.0007). An increase in thyroid follicle number in the 100 ppm perchlorate treatment became evident as early as 18 dpf (Fig. 2A and B). Adults chronically exposed to perchlorate showed hyperplasia of small thyroid follicles (Fig. 2C and D). In particular, the average thyroid follicle area was affected by perchlorate treatment (F1, 47 = 8.80, P = 0.0047) and developmental stage (F2, 47 = 11.26, P = 0.0001), and, as with follicle number, a significant interaction existed between age and perchlorate treatment, which had the largest effect on follicle size in adult fish (F2, 47 = 7.99, P = 0.0010). Higher magnification (60×, Fig. 2E and F) revealed that the number of thyrocytes was decreased by perchlorate exposure (F1, 47 = 19.73, P = 0.0001) and developmental stage (F2, 47 = 14.26, P = 0.0001), and that age and treatment again interacted (F2, 47 = 10.87, P = 0.0001).
Fig. 2.
Perchlorate disrupts thyroid morphology. Horizontal paraffin section H&E stained thyroid follicles of 18 dpf (A and B) and adult (C–F) stickleback at 20× magnification (A–C), and 60× magnification (E and F) showing thyrocyte morphology in untreated (A, C and E) and treated (B, D and F) stickleback. TF = thyroid follicle; Ad = adipocyte; SM = smooth muscle; VA = ventral aorta; CA = cartilage. Perchlorate causes hyperplasia of small thyroid follicles. Perchlorate causes an increase in the number of thyroid follicles and a dramatic decrease in thyroid follicle width.
Fig. 3.
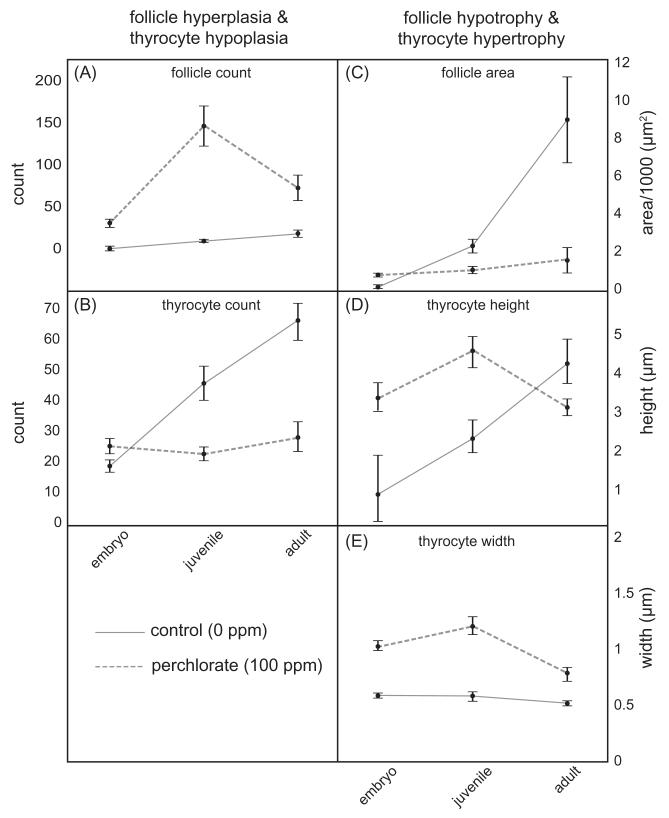
Morphometric analysis of the thyroid. (A) follicle count, (B) thyrocyte cell count, (C) follicle area, (D) thyrocyte height, and (E) thyrocyte width in larval (<100 dpf), juvenile (100–250 dpf), and adult (>250 dpf) stickleback exposed chronically to 0 ppm (control, 0 ppm, solid line) or 100 ppm perchlorate (perchlorate, 100 ppm, dashed line). n = 4–14 individuals for each treatment and age class. A significant interaction and model effect of perchlorate and age by one-way ANOVA (P < 0.05) was detected for each morphological character measured.
Thyroid morphologies varied with developmental stage due to perchlorate treatment (Fig. 3). Fish chronically treated with perchlorate had significantly more thyroid follicles as juveniles and adults, and the relationship between perchlorate treatment and age exhibited an interaction. Compared to controls, treated fish had more follicles early in development, and follicles increased in number more rapidly late in development. Although thyroid follicle area (μm2) in perchlorate-treated juveniles was not significantly different than in control fish, perchlorate-treated adults had substantially smaller thyroid follicles and demonstrated a faster increase in number of follicles as compared to controls (Fig. 3A and B). Average follicle area increased in a linear fashion in control fish (Fig. 3B), but was arrested in perchlorate treated fish (Fig. 3B).
Because follicles are three-dimensional structures, and the NIS is embedded in the layer of thyrocytes that surround the lumen of the follicle, we calculated an approximate surface area of all the thyroid follicles present in a fish. We made the simplifying assumption that the follicles are spheres, and calculated (follicle count) * (4πr2) where radius is the average radius of 5 follicles/fish. As expected, we found that the total surface area increases as fish get older (F2, 47 = 9.41, P = 0.0004). However, there was not an overall effect of perchlorate treatment (F1, 47 = 0.01, P = 0.9055), although there was a significant treatment-by-age interaction (F2, 47 = 13.89, P < 0.0001), indicating that the effects of perchlorate on surface area varied depending on embryo, juvenile or adult stages. To decipher this interaction effect, we examined the post hoc means contrasts using a Tukey’s HSD test. We found that follicles earlier in development, in larval fish, have approximately cover equal total surface area in both treated and control fish. In juvenile fish, however, perchlorate treatment caused a significant increase of over four fold in total thyroid surface area (P = 0.012) compared to controls. In adults, however, total estimated organ surface area was significantly reduced in treated fish by 3 to 4-fold (P < 0.0001), presumably due to the small size of the follicles in perchlorate treated adults compared to control adults. Thus, the lack of an overall main effect of perchlorate is due to equal and opposite offsetting effects within the larval and adult categories.
We also measured morphology of thyrocytes within the follicles. Thyrocytes of follicles from perchlorate treated fish were larger in height (F1, 47 = 3.58, P = 0.0007) and width (F1, 47 = 28.64, P = 0.0001). Both height and width of thyrocytes increased over developmental stage (height – F2, 47 = 7.79, P = 0.0012; width – F2, 47 = 4.83, P = 0.0124) and they showed a significant interaction between perchlorate treatment and developmental stage for height (F2, 47 = 13.99, P = 0.0001) but not for width (F2, 47 = 2.39, P = 0.1022). In juveniles, thyrocyte height and width were significantly larger in perchlorate treated fish when compared with controls (Fig. 3D and E). Thyrocytes of control fish grew larger in height than treated fish over the course of development, but maintained a steady width (Fig. 3D and E). In contrast, thyrocytes of perchlorate-treated fish were arrested in their development or even shrank as the fish aged (Fig. 3D and E). Overall, perchlorate appears to cause hyperplasia of small hypotrophic follicles that are lined by larger, fewer, hypertrophic epithelial thyrocytes that do not continue to grow normally over development as compared to controls (Figs. 2 and 3).
3.3. Chronic low doses of perchlorate dramatically increase embryonic androgen levels in the embryo
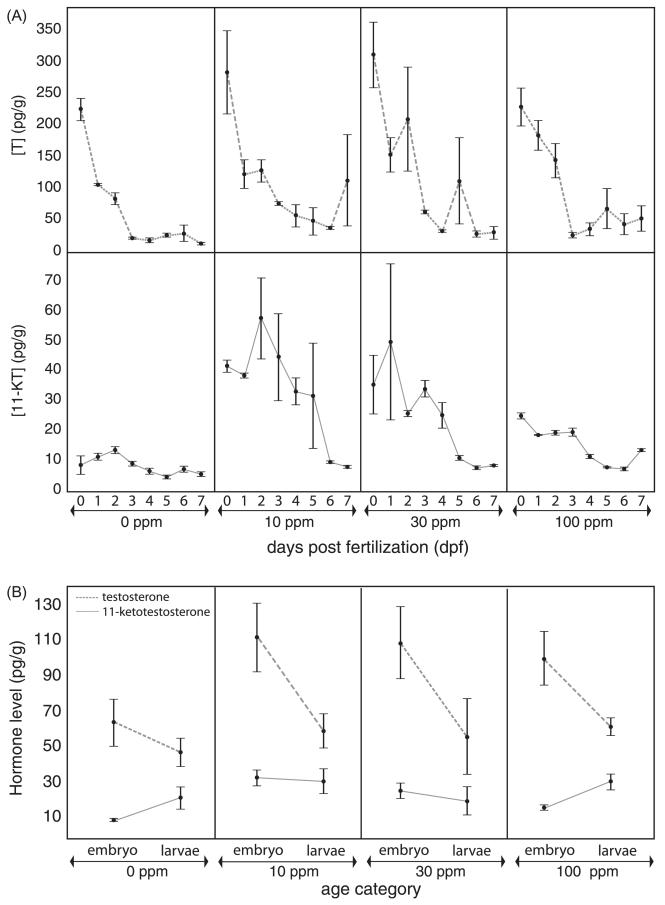
When considering androgen levels in embryos (chronically exposed to perchlorate and harvested at time points 0–7 days; Fig. 4A) as a nominal variable in a statistical analysis, unexpected results emerged. Both treatment (F3, 86 = 19.02, P < 0.0001) and age (F7, 86 = 6.94, P < 0.0001) were highly significant factors in the model, and the interaction effect between treatment and age (F21, 86 = 1.67, P = 0.0606) approached significance. Exposure to 10 ppm perchlorate for only a few hours (0 dpf, Fig. 4A) resulted in significant elevation of 11-KT concentration in the embryo, and exposure to 10 ppm and 30 ppm perchlorate elicited the greatest effect on 11-KT levels over the first 5 dpf of development, raising 11-KT levels 3-6× as compared to control embryos (Fig. 4A). The pattern weakened but was still detected at 28 dpf (larval averages shown in Fig. 4B). After 28 dpf, perchlorate had no measurable effect on whole body 11-KT concentration at any dose.
Fig. 4.
Androgen levels in perchlorate-treated and control stickleback. (A) Whole body [Testosterone] (T, pg/g) in embryonic stickleback at 0, 10, 30, and 100 ppm perchlorate exposure measured at 0, 1, 2, 3, 4, 5, 6, and 7 dpf. 0 dpf embryos have been exposed from fertilization until 3 h post fertilization. (B) Whole body [11-Ketotestosterone] (11-KT, pg/g) in development, with data pooled by biological life stage (embryos, 0–7 dpf, larvae, 8–56 dpf) n = 3–5 pools (biological replicates) of age matched fish (mass equal to 1 g). Results revealed a significantly different [11-KT] in embryos treated with 10 ppm and 30 ppm perchlorate. Dashed lines represent [T] and solid lines represent [11-KT] in embryos and larvae. Over the course of the first 56 dpf of development there is a significant interaction effect of perchlorate exposure and time post fertilization on 11-KT levels.
When fish were grouped into the life stages of embryos (0–7 dpf) or larvae (28 and 56 dpf) for analysis (Fig. 4B), overall levels of 11-KT were not significantly different between age categories (F1, 104 = 1.32, P = 0.2532), and no interaction existed between age and perchlorate dose (F3, 104 = 1.59, P = 0.1952). However, perchlorate treatments resulted in significantly elevated embryonic and larval 11-KT levels (F3, 104 = 3.09, P = 0.0303). Mean whole body 11-KT levels (solid line) were clearly highest in 10 ppm treated fish, reaching levels 5-fold higher than levels observed in control embryos, and 30 ppm treated fish also had significantly elevated 11-KT (Fig. 4B). Despite the lack of a significant interaction, the trajectory of 11-KT levels in control fish appears to be an increase from less than 10 pg/g to nearly 30 pg/g by the larval stage (up to 56 dpf). 100 ppm treated embryos demonstrated 11-KT increases similar in pattern and magnitude to controls (Fig. 4B, solid lines). In contrast, mean 11-KT levels in 10 ppm and 30 ppm exposed embryos remained constant from embryo to larva (10 ppm), or decreased slightly (30 ppm).
Levels of whole body T did not significantly vary as a function of perchlorate treatment (F3, 123 = 0.37, P = 0.7713), but tended to vary with age (F1, 123 = 3.52, P = 0.0628), between the embryonic and larval age categories (Fig. 4B). Mean [T] varied across life stage (Fig. 4B, dashed lines), with 10 ppm perchlorate treated embryos exhibiting twice the [T] of controls, an effect that was not evident in 10 ppm treated larval fish (Fig. 4B, dashed lines). Similar to the 11-KT results, a more detailed picture emerges from the independent analysis of the embryonic data with age (0–7 days) included in the model as a nominal variable (Fig. 4A). Both age (F7, 80 = 33.52, P < 0.0001) and perchlorate treatments (F3, 80 = 5.35, P = 0.0021) were significant main effects, but no significant interaction existed between them (F21, 80 = 1.04, P = 0.4327). Whole body [T] decreased over the 7 days of development, and an increased whole body [T] due to perchlorate treatment is particularly evident in 10 ppm and 30 ppm treated embryos less than 3 dpf (Fig. 4A). In control embryos, maternally derived T levels were near 75 pg/g for the first measured time points immediately after fertilization, and then fell over the course of development (Fig. 4B). Perchlorate exposure did not alter this trend at any dose (Fig. 4B). Overall, in control and 100 ppm exposed embryos, [T] fell over the course of the first 6 weeks of development (Fig. 4B), while 11-KT levels increased steadily (Fig. 4B). Doses of 10 and 30 ppm perchlorate did not affect this trend in developmental T concentrations, but did significantly alter developmental 11-KT concentrations (F3, 104 = 3.09, P = 0.0303) (Fig. 4).
3.4. High perchlorate exposure increases early stage ovarian follicle numbers, kidney hypertrophy, and spermatogenesis
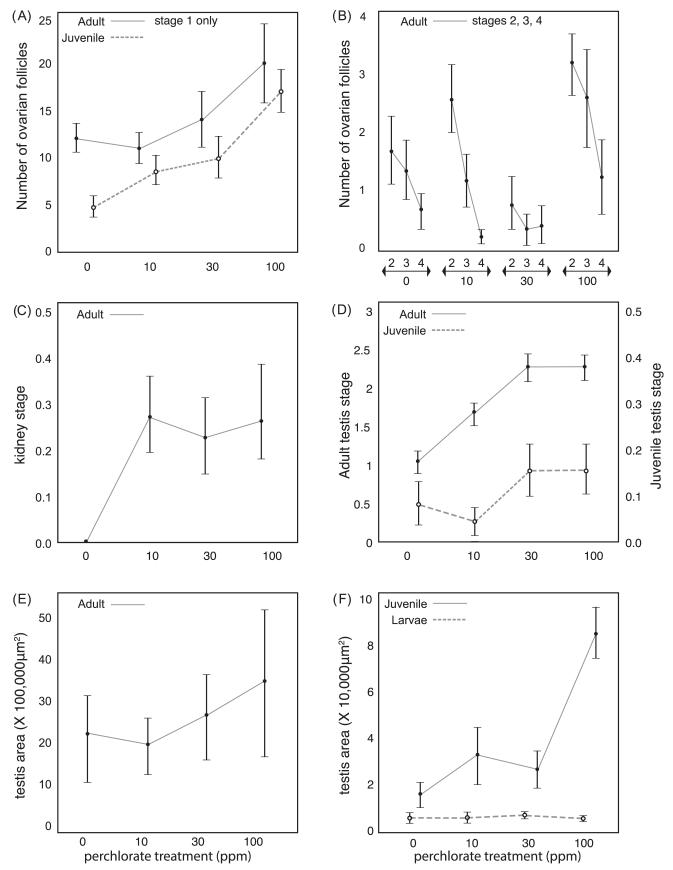
Concomitant to changes in the androgen profiles of stickleback, perchlorate exposure also resulted in several measurable abnormalities in gonadal morphology. We found a significant increase in ovarian follicle number with dose in both juvenile and adult ovaries (Fig. 5A and B). The effects of perchlorate on ovarian follicle number for each ovarian follicle stage were analyzed separately using a Generalized Linear Model (GLM) with a Poisson error distribution and log link function. Ovarian follicles only developed to stage-1 in juvenile fish, but the number of stage 1 ovarian follicles in juveniles showed a significant treatment effect (, P < 0.0001). The 100 ppm perchlorate treated juveniles had 3–4 times more stage 1 ovarian follicles than control juveniles (Fig. 5B). A similar GLM analysis of all four ovarian follicle stages in adult gonads (see Section 2 for staging criteria, stages 1, 2, 3 and 4) showed significant differences across perchlorate treatments (, P = 0.0173; 10.62, 0.0140; 19.17, 0.0173; 8.78, 0.0324 for the four stages, respectively). Adult females treated with 100 ppm perchlorate had 1.5 times more stage 1 ovarian follicles than adult control females (Fig. 5A). The number of mid, late, or mature ovarian follicles increased modestly, albeit non-significantly, in adults treated with 100 ppm perchlorate.
Fig. 5.
Gonad morphometrics in perchlorate exposed juvenile and adult stickleback. (A) Mean number of follicular ovarian follicles in early stage (stage 1) of oogenesis within the ovary of juvenile (dashed line) and adult (solid line) females chronically treated with 0 ppm, 10 ppm, 30 ppm, or 100 ppm perchlorate. n = 4–6 individuals at each concentration. (B) Mean number of follicular ovarian follicles in mid (stage 2), late (stage 3), or mature (stage 4) stages of oogenesis within the ovary of juvenile (dashed line) and adult (solid line) females chronically treated with 0 ppm, 10 ppm, 30 ppm, or 100 ppm perchlorate. n = 4–6 individuals at each concentration. (C) Kidney morphometric analysis for hypertrophy on a scale of 0 (normal size) to 1 (hypertrophy) in adult male stickleback treated (10 ppm, 30 ppm, or 100 ppm) or untreated (0 ppm) with perchlorate. Mean based on n = 3–4 individuals at each concentration. Tukey’s HSD post hoc analysis reveals a significant effect of perchlorate treatment on kidney hypertrophy. (D) Mean testis stage in juvenile (dashed line) and adult (solid line) stickleback males chronically treated with 0 ppm 10 ppm, 30 ppm, or 100 ppm perchlorate. n = 3–4 individuals at each concentration. Testes were staged based on morphological criteria to determine stage of spermatogenesis: stage 0 (no development), 1 (early spermatogenesis), 2 (mid spermatogenesis), 3 (late and completed spermatogenesis). Mean score by treatment in adults (>250 dpf) reveals a significant effect of perchlorate treatment on stage of spermatogenesis (Tukey’s HSD post hoc analysis). (E) Mean adult male stickleback testis area (μm2) measured using Image J software in fish chronically treated with 0, 10, 30, and 100 ppm perchlorate. (F) Mean testis area (μm2) measured using Image J software in larval (<100 dpf, dashed line) and juvenile (100–250 dpf, solid line) stickleback males. Analysis of mean testis area in juvenile stickleback reveals a significant effect of perchlorate treatment increasing with dose (Tukey’s HSD post hoc analysis).
In male stickleback, the kidney can be considered a reproductive organ due to its production of spiggin, the glue protein males use in nest construction. A Likelihood ratio contingency analysis revealed a statistically significant effect of perchlorate treatment on the stage of kidneys when considering all four treatment levels (, P < 0.0149, Fig. 5C), or when condensing all three perchlorate treatments (10, 30 and 100) into a single level and analyzing with respect to the controls (, P < 0.0013; Fisher’s Exact Test P = 0.0099). Doses of 10 and 100 ppm significantly increased mean kidney stage score to hypertrophic for 20–30% of all adult kidneys assessed (Fig. 5C).
Average spermatogenic stage of adult testes increased significantly with perchlorate dose. A logistic regression of testis stage as a nominal response variable on perchlorate treatment level (Fig. 5D) indicated a significant effect of perchlorate in adult stickleback (, P < 0.0001). The majority of perchlorate-exposed adult male testes were in advanced or near complete spermatogenesis, while the average control fish of a comparable age had testes in early stages (Fig. 5D). No differences in testis stage were detected in juveniles across perchlorate treatment levels (Fig. 5D; P = 0.2939).
No significant effect of perchlorate treatment on testis area as estimated per section (Fig. 5E and F) was seen in larvae (F3,17 = 0.51, P = 0.6839) or adults (F3, 21 = 0.35, P = 0.7901). However, juvenile testis area increased with perchlorate dose (F3, 10 = 14.13, P = 0.0006). A significant increase in testis area in juveniles exposed to 100 ppm as compared to controls was found by a Tukey’s post hoc test (Fig. 5E and F). Juvenile testes from fish exposed to 100 ppm perchlorate increased in area more than four-fold (Fig. 5F) while adult testes were enlarged 1.5-fold (Fig. 5E), continuing the trend that perchlorate appears to have more dramatic effects on juveniles versus adults in terms of morphology and endocrine profiles.
4. Discussion
Here we report results from the first study to associate endocrine profiles and morphological development in an organism that has been chronically exposed to perchlorate from embryo to adult. In this large well-replicated study, we subjected stickleback to three concentrations of perchlorate plus a control treatment, and analyzed a comprehensive set of hormonal and morphological variables that might be altered in response to changes to the HPT or HPG axes. We show that this common pollutant may have systemic, deleterious effects on vertebrate health and reproduction. An important implication of our findings is that the effects of perchlorate may be occurring via mechanisms that are beyond the previously documented mechanism of disrupting thyroid function through alterations of NIS function.
4.1. Perchlorate caused only small changes in TH profiles and external morphology
In contrast to our expectation that whole body TH profiles would be significantly reduced by perchlorate treatments, the only significant changes in TH levels we found were at the lowest dose of perchlorate (10 ppm, Fig. 1). Otherwise, TH levels were comparable to those reported for healthy stickleback and closely related fish (Crane et al., 2005; Lema et al., 2009; Kitano et al., 2010). Over the course of development, trajectories and slopes of [T4] in perchlorate-treated fish were not different than in control fish, with levels remaining steady or increasing slightly between 20 and 30 ng/g. This trend is consistent with other studies measuring TH over normal development in fish (Crane et al., 2004; Chang et al., 2012).
A significant effect of perchlorate was detected for [T3] primarily due to the 10 ppm exposure over development, which increased more rapidly than in controls and remained elevated into adulthood (Fig. 1). A small mean increase of [T3] of 1–2 ng/g in the adult may represent an increase in deiodinase activity converting T4 to T3 in response to perchlorate interference with TH production triggering a feedback loop for T3 production, as has been reported in Chinese minnow (Li et al., 2011). However, we did not find a concomitant decrease in T4 levels at 10 ppm or any other dose of perchlorate, suggesting that perchlorate is not limiting T4 availability for conversion to metabolically active T3 for use by tissues. The specific increase in [T3] may indicate an increased biological demand for T3, or a reduction in T3 breakdown and metabolism. Treatment with androgens elevates T3 levels in fish (Eales and MacLatchy, 1989), possible evidence that perchlorate exposure also stimulates androgenic steroid pathways during development.
The effects that we observed at the lowest perchlorate dose are consistent with other studies that have documented a widespread occurrence of low dose effects on vertebrates of several types of endocrine disruptors including bisphenol A, androgens, estradiol and other estrogenic compounds (Melnick et al., 2002; Vandenberg et al., 2012). Low dose effects, and non-monotonic dose response curves such as we report here for perchlorate, may be due to the evolution of high sensitivity to low doses of hormones in the hypothalamic-pituitary axis that improves the sensitivity of feedback loops (Weshons et al., 2003; Vandenberg et al., 2012). In concordance with the T3 and T4 hormone results, we found no significant changes in external morphological characters, including those characters that we had previously found to be affected by perchlorate in stickleback from the same study population. It is not clear why our current study did not replicate findings from our earlier study showing induced changes to gross anatomy by chronic perchlorate exposure in stickleback, but our earlier work occurred under different experimental conditions which included use of plastic tanks and natural outdoor lighting and ambient temperatures of the Alaskan summer (Bernhardt et al., 2011). These disparate results underscore the problem that responses to endocrine disruptors may have a strong environmental component, such as interaction with other chemicals.
4.2. Evidence for compensation by the HPT in response to perchlorate exposure
Our finding that perchlorate, an inhibitor of iodine uptake into the thyroid, caused little or no change to TH levels might initially suggest that perchlorate does not affect the stickleback thyroid. An alternative explanation is that, at all levels of perchlorate exposure (except 10 ppm), the HPT was able to make compensatory changes to the levels of THs. In humans, the effects of perchlorate exposure on circulating TH levels, thyroid stimulating hormone (TSH) levels, and thyroid function depend on a multitude of factors including sex, age, and background iodide state (Jugan et al., 2010), suggesting that healthy humans may also be able to compensate to perchlorate exposure through feedback loops in the HPT axis. In quail, initial changes in TH levels due to 0.5–50 ppb aluminum perchlorate exposure returned to control values after 8 weeks of chronic ingestion, explained by the authors as evidence for compensatory mechanisms such as increased iodide sequestration (McNabb, 2004). TH levels therefore may not be a reliable sole endpoint for identifying perchlorate effects in vertebrates. In addition, single time point measurements may miss key changes, and can serve as only a snapshot of TH levels.
To support the hypothesis of HPT compensation in response to perchlorate, we employed pathohistology to measure changes at the site of TH production, the thyroid follicular cell. Recent studies in zebrafish (Sharma and Patiño, 2013) and fathead minnows (Crane et al., 2005) demonstrated that thyrocyte height and morphology may be reliable indicators of perchlorate compensation in fish, more so than whole body or circulating TH levels which can yield contradictory results (Jugan et al., 2010). In striking contrast to the absence of effects on TH concentration and external morphology, we found that larval (as early as 18 dpf) stickleback exposed to 100 ppm perchlorate experienced a significant increase in number (hyperplasia) of thyroid follicles with enlarged thyrocytes when compared to control fish (Fig. 2). In adult perchlorate-treated stickleback, thyroid follicles also increased in number, were densely packed, and appeared much smaller than control follicles. These morphological changes to the thyroid follicles may play a role in compensation to perchlorate treatment. Supporting this idea we found that in juveniles, perchlorate treatment leads to a significant increase of almost four fold in thyroid follicle surface area over control fish, which could increase the efficiency of and potential for iodide uptake and TH export. Interestingly we found an effect of approximately the same magnitude in adults, but in the opposite direction with the total surface area in control fish much higher than in perchlorate treated fish.
Other thyroid morphological traits affected by chronic 100 ppm perchlorate exposure at some point in development included thyroid follicle number, average follicle area in sections, thyrocyte number, and thyrocyte width. Thyroid follicle development in control fish consisted of steady incremental growth or increase in all parameters, except for thyrocyte width, which remained constant over development in control fish. In contrast, growth and development of follicle area and thyrocyte number were arrested or decreased under perchlorate treatment conditions. Developmental growth factors such as Wnt signaling cascades or other paracrine signaling mechanisms responsible for cell proliferation and tissue growth are plausible candidates for localized proliferation, often in response to perturbation or injury (Berge et al., 2008; Minear et al., 2010), and may be responding to perchlorate treatment. We also measured an average increase from 2 to 3 μm in thyrocyte height in juvenile fish, and no change in adult fish exposed to 100 ppm perchlorate (Fig. 3D). Doubling in thyrocyte height in zebrafish from an average of 3 to an average of 8 μm (Sharma and Patiño, 2013) was found in response to perchlorate doses comparable to those used in our study. Increased thyrocyte height is a commonly used endpoint indicating HPT disruption, and is thought to be directly proportional to degree of TSH stimulation in response to perchlorate (Carr and Patiño, 2011). Although the increase in juvenile thyrocyte height was small, we did detect a significant increase in thyrocyte width, which may be a stickleback-specific morphological response, and likely indicates changes in growth signals in response to perchlorate (Fig. 3).
Our general finding is that perchlorate leads to changes in thyroid development that result in an overall increase in the number of thyroid follicles, each of which is smaller than the follicles in untreated fish. The smaller size of the thyroid follicles is likely a result of colloid depletion in the inner lumen of thyroid follicles (Carr and Patiño, 2011). These findings provide an intriguing mechanistic hypothesis. Because NIS are localized on the basolateral cell surface of thyrocytes, the increased surface area of more numerous but smaller follicles may allow for increased NIS expression along the surface of the thyrocytes. Thus, more of the exposed thyrocyte surface may be lined with NIS, increasing the potential for overcoming the interruption to iodide transport caused by perchlorate. Supporting this idea is the finding that the human NIS gene is expressed in thyroid follicular cells, and NIS gene expression in mammals is upregulated at low doses of perchlorate (McDougal et al., 2011). Furthermore, previous studies found that the iodide-concentrating function is compromised at perchlorate doses that do not affect circulating thyroid hormone levels (Lawrence et al., 2005), suggesting that NIS function is highly sensitive to perchlorate and that compensatory mechanism can adjust for perchlorate toxicity.
Our data are consistent with this surface area compensation hypothesis. We estimated total surface area of all thyroid follicles combined in larval, juvenile, and adult stickleback. In the larval fish, perchlorate had no effect on total follicle surface area. However, we found that in juveniles (101–250 dpf), perchlorate treated fish had significantly more total thyroid follicle surface area, at a time when thyroid hormones are of paramount importance for the larval to juvenile transition and overall body growth (Power et al., 2001). In adult stickleback exposed to perchlorate, however, the reverse was true, and the thyroid follicle surface area of treated fish was significantly smaller than of control fish; follicles from perchlorate treated fish remained so small that although they were more abundant than follicles in control fish, their total surface area was smaller. This reversal of effects between juvenile and adult stages is curious. Thyroid hormone production may be less important in adult vs. juvenile fish, and by the time adulthood is reached, compensation may be occurring by increased receptor capacity rather than by increased ligand production. Alternatively, the compensation mechanism may be exhausted in adults simply because the number of follicles that can be created is finite, regardless of their size. Regardless, the potential for increased number of NIS, via either increased surface area in juveniles, or increased TH receptors in adults, could contribute as a compensatory mechanism responsible for maintenance of TH levels upon exposure to chronic, high doses of perchlorate. Measurement of NIS density and thyroid hormone receptor expression levels under the treatment conditions, could address these possibilities.
4.3. Perchlorate effects on embryonic and larval 11-KT suggest disrupted cross talk between the HPT and HPG axes during early development
We were surprised to find a measurable and significant effect of low doses of perchlorate on embryonic and larval (through 56 dpf) androgen levels (Fig. 4). 11-KT is the key androgen in teleost fishes for stimulating male reproductive physiology (Borg et al., 1993; Lokman et al., 2007), and is synthesized from T. Stimulation of biosynthetic pathways for 11-KT may be expected to cause decreases in T temporally linked to increases in 11-KT, which we found over the first 3 days of exposure in untreated controls and 100 ppm perchlorate exposed fish. This inverse correlation between T and 11-KT was disrupted, however, in 10 and 30 ppm exposed embryos and larvae. Remarkably, perchlorate exposure caused a significant increase in levels of 11-KT in embryonic stickleback within hours of exposure, a result that we hypothesize is based on conversion of maternally deposited precursors. However, we did not detect a decrease in the levels of T, the immediate precursor of 11-KT, in embryos, suggesting possible stimulation of the steroid biogenesis pathway upstream of the T-to-11-KT conversion. Perchlorate has a significant effect on embryonic 11-KT levels most obviously in the first 3 dpf, a time of organogenesis and cell proliferation prior to any testes development or sex determination.
We hypothesize that a possible interaction with perchlorate and thyroid hormones may cause cross talk between the HPT and HPG axes during larval development and possibly throughout development. In embryonic stages, however, when any hormones and conversion enzymes that are present in the embryo must be maternally deposited, the effects of perchlorate on [11-KT] must occur through direct action somewhere in the steroid synthesis pathway in the embryo, or via a direct biochemical oxidation of T to 11-KT. Numerous endocrine disruptors, including DDT, atrazine, and flame retardants, affect the function of steroid biosynthesis enzymes in vertebrates (Sanderson, 2006). Our data suggest a similar action of perchlorate. Further experiments are needed to clarify the mechanism responsible for changes in embryonic 11-KT levels. We hypothesize that early perchlorate exposure during a “critical window” leads to alteration of androgen levels that may have effects on important reproductive developmental stages such as primary germ cell differentiation (Lewis et al., 2008) and development and function of HPT and HPG axes later in development (Sharma and Patiño, 2013). Our finding that perchlorate alters the adult gonad supports this hypothesis (Fig. 5). This hypothesis could also explain our previous result of some perchlorate-exposed genotypic females developing into functional hermaphrodites (Bernhardt et al., 2006).
Perchlorate has subtler and less significant effects on T levels over the course of development as compared to its effects on 11-KT (Fig. 4A and B). In control and 100 ppm treated stickleback, whole body T levels declined over the first 56 dpf, presumably as maternally deposited stores of hormone were utilized and endogenous production was minimal. 11-KT levels increased steadily as T was converted to the metabolically active 11-KT when gonads formed and differentiated (Fig. 4B). In 10 and 30 ppm treated fish, however, T levels fell, but 11-KT levels failed to rise or even fell to levels lower than those found in embryos (Fig. 4B). This U-shaped dose response curve is due to the abnormally high levels of 11-KT in the earliest stage embryos treated with 10 ppm and 30 ppm perchlorate. These abnormal androgen levels may have consequences for proper reproductive development. Although perchlorate has only a minor effect on T levels under all perchlorate treatment conditions (Fig. 4), the changes we report in 11-KT levels yield a significant change in the T:11-KT ratio, perhaps a more important biological measure in fish than T levels alone, since 11-KT is often the most potent fish androgen (Borg, 1994). Because we were forced to pool males and females for these assays, a more pronounced sex-specific effect on androgens may have been masked. Even with pooling of both sexes, it is clear that perchlorate increases early embryonic 11-KT levels, and may also have an impact on T in embryos. Perchlorate’s androgenic effect is most pronounced in embryos less than 7 dpf, however, before the gonads develop. Future studies should also examine the effect of perchlorate on embryonic 5α-Dihydrotestosterone levels, which is also a potent fish androgen, exogenous exposure of which can cause abnormal ovarian folliculogenesis and intersex in female fathead minnows (Margiotta-Casaluci and Sumpter, 2011).
4.4. Perchlorate significantly affects gonadogenesis of both sexes
Endocrine disruption by exogenous pollutants often perturbs gonad size (Mukhi and Patiño, 2007; Bernhardt et al., 2011; Sharma and Patiño, 2013), shape (Wester, 2003), and reproductive development and behavior (Woodling et al., 2006; Bernhardt and von Hippel, 2008). We previously found that perchlorate masculinizes both male and female stickleback (Bernhardt et al., 2006), leading to the hypothesis that perchlorate can have androgenic effects in some organisms mediated through the thyroid itself. If this hypothesis were true, masculinization in the present study should have been mitigated via the compensation of TH levels by changes in thyroid morphology. Our data, however, support a non-thyroidally mediated, direct effect of perchlorate on gonad development. We found that chronic perchlorate exposure caused abnormal testis development even in the absence of changes in whole body [T4] (Fig. 4).
Testis area is related to spermatogenic potential and male reproductive state, and testis development and growth correlate with circulating androgen levels in teleost fishes (Borg et al., 1993; Weltzien et al., 2002). In halibut, circulating androgen levels are directly proportional to stage of testis development and spermatogenic stage (Weltzien et al., 2002). We found that perchlorate causes juvenile testis enlargement as dose increases (Fig. 5E and F), confirming our previous findings that perchlorate is a masculinizing agent in stickleback (Bernhardt et al., 2006; Bernhardt and von Hippel, 2008).
Based on the masculinization effect of perchlorate on both males and females, we previously hypothesized that perchlorate has an androgenic effect (Bernhardt et al., 2006), a phenomenon that would be quite rare for endocrine disrupting contaminants. Consistent with this hypothesis we found elevated androgen levels in early development at the lowest dose of perchlorate (10 ppm), but not at the highest dose, which was the dose that affected testis development most significantly. These results point to an incongruity between effects of perchlorate on androgen levels and testis development (Figs. 4 and 5) and warrant additional experimentation to determine how perchlorate might alter androgens and testis development independently, such as through different rates of androgen utilization and metabolism. Similar to our findings of thyroid morphology and TH levels, these effects on gonadogenesis show that perchlorate has complex effects that implicate more than just competitive inhibition of the NIS. Because the NIS is expressed in male Leydig cells and female reproductive tract in mammals (Russo et al., 2011; Riesco-Eizaguirre et al., 2014), we hypothesize that perchlorate may be affecting reproductive development via interactions with NIS in these tissues.
Our finding that the lowest dose of perchlorate had the greatest effect on embryonic androgen levels, whereas the highest dose affected testis development most significantly, argues that perchlorate is altering these two pathways independently. Another possible explanation is that perchlorate stimulates not only androgen production, but also androgen degradation, and at high perchlorate concentrations degradation is faster than production. An inverse correlation between androgen concentration and testis development, however, is not supported by studies in other adult teleosts, where highest testis mass correlates directly to the highest levels of circulating androgens (Weltzien et al., 2002). To our knowledge, no study has correlated embryonic and juvenile androgen levels with testis development.
Surprisingly, we also found that perchlorate affects the synchrony of ovarian follicle development in female juveniles and adults (Fig. 5A and B). Specifically, perchlorate induced a significant (3 to 4-fold) increase in the number of early stage ovarian follicles as dose increases in juvenile fish. Adults also showed a significant increase in early stage ovarian follicles, accompanied by a trend towards increased total numbers of ovarian follicles in later stages raised in 100 ppm perchlorate. Stickleback females have group synchronous development of ovarian follicles that occurs continuously throughout the breeding season, and it is common to find simultaneously all identified stages of ovarian follicles present in the ovary of an adult female (Wallace and Selman, 1979, 1981; Sokolowska and Kulczykowska, 2006). Environmental contaminants with effects on steroidogenesis affect the synchrony of ovarian follicle development in white suckers (Woodling et al., 2006), presumably through estrogenic effects on the gonad. Although we cannot fully know if an individual had recently spawned, we found no significant difference in late stage ovarian follicle number based on perchlorate treatment. Stickleback spawn all late stage ovarian follicles synchronously (John Baker, pers. comm.), and therefore if perchlorate affected spawning as well as stage 1 ovarian follicle number we would have seen a difference in number of late stage ovarian follicles between treated and control fish, which we did not (Fig. 5). Instead, perchlorate treatment appears mainly to affect proliferation of early stage ovarian follicles. The ovarian follicles presumably undergo apoptosis prior to maturation, which would explain why we did not see an increase in late stage ovarian follicles in perchlorate treated females.
Proliferation and early stage oogenesis signals are poorly understood in all vertebrates (Lubzens et al., 2010), but gonadotropes (Lyman-Gingerich and Pelegri, 2007; Lubzens et al., 2010), estradiol (Kirk, 2003; Miura et al., 2007; Lubzens et al., 2010), 17-20Beta-dihydroxy-4-pregnen-3-one (Miura et al., 2007), and IGF-1 (Lokman et al., 2007), all increase oogonial proliferation in teleosts, suggesting numerous pathways stimulate oogenesis in fish and that these pathways are susceptible to perturbation by exogenous chemicals. Perchlorate can now be added to this list, although the underlying mechanism remains to be determined. The near dose-dependence of ovarian follicle proliferation in stickleback again stands in contrast to our findings that androgen and T3 levels are affected by perchlorate in an inverse dose dependent manner, and argues for independent molecular mechanisms for the effects of perchlorate on gonad development versus thyroid development. One possibility is that perchlorate might increase female T levels, which is consequently aromatized, leading to higher estrogen levels and thereby stimulating growth of more ovarian follicles. Another possibility is that perchlorate acts on NIS-expressing granulosa cells to decrease aromatase and estrogen, thereby slowing oocytes maturation and leading to accumulation of early stage ovarian follicles. More studies are necessary to determine if these are plausible hypotheses.
Our findings also suggest that more experiments are necessary to understand relationships between maternally deposited androgens and gonad development, especially the process of testis growth and spermatogenesis in juvenile vertebrates. Adult testis area and spermatogenic stage followed the same trend as found in juvenile stickleback of increasing as dose increases(Fig. 5), and thus supporting our previous findings that perchlorate has androgenic effects on stickleback males (Bernhardt et al., 2006; Bernhardt and von Hippel, 2008). Our finding that kidney size also increases significantly and in relation to dose (Fig. 5C) further supports an androgenic role for perchlorate. However, kidney hypertrophy did not occur at any dose in juvenile stickleback, suggesting that reproductive maturity may be necessary for perchlorate to induce this effect. One might expect that kidney hypertrophy would lead to increased spiggin production, however we may have sampled fish in the key time point when the height of the cells of the collecting duct are increased in preparation for spiggin production (Wootton, 1984) but prior to the actual increase in production. We have previously found no effect of perchlorate exposure on spiggin levels (unpublished data), nor did Katsiadaki and colleagues (pers. comm.), raising the possibility that the increase in kidney cell height in the current study (Fig. 5c) may be unrelated to spiggin production.
5. Conclusion
Experiments reported here show that perchlorate exposure during development can cause abnormal growth and proliferation of three tissues: thyroid, gonad, and kidney. Our results raise the hypothesis that perchlorate affects vertebrate health and development via both thyroidal and non-thyroidal mechanisms in positive correlation with dose, non-monotonic, and inverse dose-dependent manners. Additional testing of more components of the HPT axis such as pituitary hormones, thyroid hormone binding proteins, and possible local changes in T4/T3 ratios that could affect specific tissues, is necessary to rule out that our findings are entirely thyroidally mediated. Stickleback that were chronically exposed to perchlorate demonstrated hyperplasia of the thyroid, ovary, testis, and kidney. This finding is striking because these tissues all have different functions and arise from different developmental precursor cell populations. Perchlorate exposure during development may affect the HPT, HPG, and possibly global growth signaling pathways such as IGF-1 production or paracrine acting factors such as Wnt signaling. Interestingly, the effects of perchlorate on whole body TH levels appear to follow a horseshoe-shaped dose response curve, while responses of the gonad to perchlorate follow a standard linear dose response curve, further suggesting that perchlorate independently affects multiple pathways. We conclude that perchlorate exposure during development can cause abnormal growth and proliferation in numerous tissue systems, possibly through unrelated mechanisms.
Acknowledgments
We thank Poh Kheng Loi for expertise in histology preparations, and Ruth Bremiller, Yi-Lin Yan, Christoff G. Furin, and John Baker for helpful discussion and technical expertise. We also thank Dr. K.-Y. Francis Pau of the Endocrine Technology & Support Lab at Oregon National Primate Research Center Oregon Health & Science for invaluable help with hormone analysis. Funding was provided by NIH Grant number 1RO1ES017039-01A1. Fish were collected under Alaska Department of Fish and Game permit SF-2010-029 and SF-2011-025, and all research protocols were approved by the UAA Institutional Animal Care and Use Committee (IRB reference # 159870-1) and the University of Oregon Animal Care and Use Committee (protocol #10-16R).
References
- Anderson JL, Rodríguez Marí A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait J. multiple sex-associated regions and a putative sex chromosome in Zebrafish revealed by rad mapping and population genomics. PLoS ONE. 2012;7(7):e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Foster SA. Introduction to the evolutionary biology of the threespine stickleback. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford, New York, Tokyo: 1994. pp. 1–27. [Google Scholar]
- Ten Berge D, Brugmann S, Helms J, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development (Cambridge, England) 2008;135(19):3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez DS. Thyroid-Gonad Axis of the American alligator (Alligator mississippiensis): An Examination of Physiological and Morphological Endpoints (Dissertation Thesis) University of Florida Doctoral; 2008. pp. 1–135. [Google Scholar]
- Bernhardt RR, von Hippel FA. Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour. 2008;145(4-5):537–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, von Hippel F, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environ. Toxicol. Chem. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, O’Hara TM. Chronic perchlorate exposure causes morphological abnormalities in developing stickleback. Environ. Toxicol. Chem. 2011;30(6):1468–1478. doi: 10.1002/etc.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ. Health Perspect. 2006a;114(12):1865–1871. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Valentin-Blasini L, Osterloh JD, Mauldin JP, Pirkle JL. Perchlorate exposure of the US population, 2001-2002. J. Eposure Sci. Environ. Epidemiol. 2006b;17(4):400–407. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fishes. Comp. Biochem. Physiol. 1994;109:219–245. [Google Scholar]
- Borg B, Antonopoulou E, Andersson E, Carlberg T, Mayer I. Effectiveness of several androgens in stimulating kidney hypertrophy, a secondary sexual character, in castrated male three-spined sticklebacks Gasterosteus aculeatus. Can. J. Zool. 1993;71(11):2327–2329. [Google Scholar]
- Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in Eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ. Sci. Technol. 2005;39(14):5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JW. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Genes, Genomics, Genetics) 2011;1:3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capps T, Mukhi S, Rinchard JJ, Theodorakis CW, Blazer VS, Patiño R. Exposure to perchlorate induces the formation of macrophage aggregates in the trunk kidney of zebrafish and mosquitofish. J. Aquat. Anim. Health. 2004;16(3):145–151. [Google Scholar]
- Carr JA, Patiño R. The hypothalamus–pituitary–thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen. Comp. Endocrinol. 2011;170(2):299–312. doi: 10.1016/j.ygcen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in TH Levels during zebrafish development. Zoolog. Sci. 2012;29(3):181–184. doi: 10.2108/zsj.29.181. [DOI] [PubMed] [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of THs in the fathead minnow Pimephales promelas. Gen. Comp. Endocrinol. 2004;139(1):55–60. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows Pimephales promelas. Environ. Health Perspect. 2005;113(4):396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresko WA, McGuigan KL, Phillips PC, Postlethwait JH. Studies of threespine stickleback developmental evolution: progress and promise. Genetica. 2007;129(1):105–126. doi: 10.1007/s10709-006-0036-z. [DOI] [PubMed] [Google Scholar]
- Eales JG, MacLatchy DL. The relationship between T3 production and energy balance in salmonids and other teleosts. Fish Physiol. Biochem. 1989;7(1):289–293. doi: 10.1007/BF00004719. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272(43):27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- Flood DE, Fernandino JI, Langlois VS. Thyroid hormones in male reproductive development: evidence for direct crosstalk between the androgen and thyroid hormone axes. Gen. Comp. Endocrinol. 2013;192:2–14. doi: 10.1016/j.ygcen.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ. Toxicol. Chem. 2002;21(3):590–597. [PubMed] [Google Scholar]
- Gravenmier JJ, Johnston DW, Santore RC, Arnold WR. Acute toxicity of copper to the threespine stickleback Gasterosteus aculeatus. Environ. Toxicol. 2005;20(2):150–159. doi: 10.1002/tox.20089. [DOI] [PubMed] [Google Scholar]
- Hahlbeck E, Griffiths R, Bengtsson BE. The juvenile three-spined stickleback (Gasterosteus aculeatus L.) as a model organism for endocrine disruption. Aquat. Toxicol. 2004;70(4):287–310. doi: 10.1016/j.aquatox.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol. Rev. Camb. Philos. Soc. 2000;75(4):519–631. doi: 10.1017/s146479310000556x. [DOI] [PubMed] [Google Scholar]
- Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and TH physiology. Biochem. Pharmacol. 2010;79(7):939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Kirk C. Environmental endocrine disrupters dysregulate estrogen metabolism and Ca2+ homeostasis in fish and mammals via receptor-independent mechanisms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003;135(1):1–8. doi: 10.1016/s1095-6433(02)00366-5. [DOI] [PubMed] [Google Scholar]
- Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ. Sci. Technol. 2005;39(7):2011–2017. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- Kitano J, Mori S, Peichel C. Sexual dimorphism in the external morphology of the threespine stickleback (Gasterosteus aculeatus) Copeia. 2007;2007(2):336–349. [Google Scholar]
- Kitano J, Lema SC, Luckenbach JA, Mori S, Kawagishi Y, Kusakabe M, Swanson P, Peichel CL. Adaptive divergence in the TH signaling pathway in the stickleback radiation. Curr. Biol. 2010;20(23):2124–2130. doi: 10.1016/j.cub.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JE, Lamm SH, Pino S, Richman K, Braverman LE. The effect of short-term low-dose perchlorate on various aspects of thyroid function. Thyroid. 2005;10:659–663. doi: 10.1089/10507250050137734. [DOI] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin-subunit, glycoprotein-subunit, and TH receptors and in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J. Endocrinol. 2009;202(1):43–54. doi: 10.1677/JOE-08-0472. [DOI] [PubMed] [Google Scholar]
- Lewis ZR, McClellan M, Postlethwait JH, Cresko WA, Kaplan RH. Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus) J. Morphol. 2008;269(8):909–921. doi: 10.1002/jmor.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zha J, Yang L, Li Z, Wang Z. Regulation of iodothyronine deiodinases and sodium iodide symporter mRNA expression by perchlorate in larvae and adult Chinese rare minnow (Gobiocypris rarus) Mar. Pollut. 2011;63:350–355. doi: 10.1016/j.marpolbul.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Lokman PM, George KA, Divers SL, Algie M, Young G. 11-Ketotestosterone and IGF-I increase the size of previtellogenic ovarian follicles from shortfinned eel, Anguilla australis, in vitro. Reproduction. 2007;133(5):955–967. doi: 10.1530/REP-06-0229. [DOI] [PubMed] [Google Scholar]
- Lubzens E, Young G, Bobe J, Cerdà J. Oogenesis in teleosts: how fish eggs are formed. Gen. Comp. Endocrinol. 2010;165(3):367–389. doi: 10.1016/j.ygcen.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Lyman-Gingerich J, Pelegri F. Maternal factors in fish oogenesis and embryonic development. Fish Ovarian Follicle. 2007:141–174. [Google Scholar]
- Margiotta-Casaluci L, Sumpter JP. 5α-Dihydrotestosterone is a potent androgen in the fathead minnow (Pimephales promelas) Gen. Comp. Endocrinol. 2011;171(3):309–318. doi: 10.1016/j.ygcen.2011.02.012. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Jones KL, Fatuyi B, Gray KJ, Blount B, Valentin-Blasini, Fisher JW. The effects of perchlorate on thyroidal gene expression are different from the effects of iodide deficiency. J. Toxicol. Environ. Health A. 2011;74(14):917–926. doi: 10.1080/15287394.2011.573740. [DOI] [PubMed] [Google Scholar]
- McNabb FMA. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol. Sci. 2004;82(1):106–113. doi: 10.1093/toxsci/kfh247. [DOI] [PubMed] [Google Scholar]
- Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho S, Brown T, Moore J, Leakey J, Haseman J, Kohn M. Summary of the national toxicology program’s report of the endocrine disruptors low-dose peer review. Environ. Health Perspect. 2002;110(4):427–431. doi: 10.1289/ehp.02110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin IL, Moore BC, Guillette LI., Jr. Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ. Res. 2006;100(1):3–17. doi: 10.1016/j.envres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci. Transl. Med. 2010;2(29):29ra30–29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- Miura C, Higashino T, Miura T. A progestin and an estrogen regulate early stages of oogenesis in fish. Biol. Reprod. 2007;77(5):822–828. doi: 10.1095/biolreprod.107.061408. [DOI] [PubMed] [Google Scholar]
- Morvan Dubois G, Sebillot A, Kuiper GGJM, Verhoelst CHJ, Darras VM, Visser TJ, Demeneix BA. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol. Sci. 2007;96(2):246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev. Dyn. 2009;238(12):2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN, Leung AM, Blount BC, Bazrafshan HR, He X, Pino S, Valentin-Blasini L, Braverman LE. Breast milk iodine and perchlorate concentrations in lactating boston-area women. J. Clin. Endocrinol. Metab. 2007;92(5):1673–1677. doi: 10.1210/jc.2006-2738. [DOI] [PubMed] [Google Scholar]
- Pearce EN, Alexiou M, Koukkou E, Braverman LE, He X, Ilias I, Alevizaki M, Markou KB. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J. Clin. Endocrinol. Metab. 2010;95(7):3207–3215. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson BT, Einarsdottir IE, Canario AV, Sweeney GE. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;130(4):447–459. doi: 10.1016/s1532-0456(01)00271-x. [DOI] [PubMed] [Google Scholar]
- Préau L, Fini JB, Morvan Dubois G, Demeneix B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Biophys. Acta (early online) 2014 doi: 10.1016/j.bbagrm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2012. < http://www.imagej.nih.gov/ij/>. [Google Scholar]
- Riesco-Eizaguirre G, Leoni SG, Mendila M, Estevez-Cebrero MA, Gallego MI, Redondo A, Hardisson D, Santisteban P, De La Vieja A. NIS mediates iodide uptake in the female reproductive tract and is a poor prognostic factor in ovarian cancer. J. Clin. Endocrinol. Metab. 2014 doi: 10.1210/jc.2013-4249. jc.2013-4249. [DOI] [PubMed] [Google Scholar]
- Russo D, Scipioni A, Durante C, Ferretti E, Gandini L, Maggisano V, Paoli D, Verrienti A, Costante G, Lenzi A, Filetti S. Expression and localization of the sodium/iodide symporter (NIS) in testicular cells. Endocrine. 2011;40(1):35–40. doi: 10.1007/s12020-011-9469-y. [DOI] [PubMed] [Google Scholar]
- Russo D, Scipioni A, Durante C, Ferretti B, Gandini L, Maggisano V, Paoli D, Verrienti A, Costante G, Lenzi A, Filetti S. Expression and localization of the sodium/iodide symporter (NIS) in testicular cells. Endocrine. 2011;40:35–40. doi: 10.1007/s12020-011-9469-y. [DOI] [PubMed] [Google Scholar]
- Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol. Sci. 2006;94(1):3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Sharma P, Patiño R. Regulation of gonadal sex ratios and pubertal development by the thyroid endocrine system in zebrafish (Danio rerio) Gen. Comp. Endocrinol. 2013;184:1–9. doi: 10.1016/j.ygcen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Sokolowska E, Kulczykowska E. Annual reproductive cycle in two free living populations of three-spined stickleback (Gasterosteus aculeatus L.): patterns of ovarian and testicular development. Oceanologia. 2006;48(1):103–124. [Google Scholar]
- Stevens DA, Hasserjian RP, Robson H, Siebler T, Shalet SM, Williams GR. Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of paraTH-related peptide and its receptor during endochondral bone formation. J. Bone Miner. Res. 2000;15(12):2431–2442. doi: 10.1359/jbmr.2000.15.12.2431. [DOI] [PubMed] [Google Scholar]
- Trumpolt CW, Crain M, Cullison GD, Flanagan SJP, Siegal L, Lathrop S. Perchlorate: sources, uses, and occurrences in the environment. Rem. J. 2005;16(1):65–89. [Google Scholar]
- Urbansky ET. Perchlorate chemistry: implications for analysis and remediation. Bioremediat. J. 1998;2(2):81–95. [Google Scholar]
- U.S. EPA . Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization (External Review Draft) 2002. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office; Washington, DC: 2002. NCEA-1-0503. [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto A, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel FA, editor. Tinbergen’s Legacy in Behaviour: Sixty Years of Landmark Stickleback Papers. Vol. 539. Brill Academic Publishers; Leiden, The Netherlands: 2010. [Google Scholar]
- Wallace R, Selman K. Physiologic aspects of oogenesis in two species of sticklebacks, Gasterosteus aculeatus, L., and Apeltes quadracus (Mitchill) J. Fish Biol. 1979;14(6):551–564. [Google Scholar]
- Wallace R, Selman K. Cellular and dynamic aspects of ovarian follicle growth in teleosts. Am. Zool. 1981;21:325–343. [Google Scholar]
- Weltzien FA, Taranger GL, Karlsen Ø, Norberg B. Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;132(3):567–575. doi: 10.1016/s1095-6433(02)00092-2. [DOI] [PubMed] [Google Scholar]
- Weshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003;111(8):994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester PW. Identification of endocrine disruptive effects in the aquatic environment: a partial life cycle assay in zebrafish. Nat. Ins. Pub. Health Environ. 2003 [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998;50(1):89–106. [PubMed] [Google Scholar]
- Woodling JD, Lopez EM, Maldonado TA, Norris DO, Vajda AM. Intersex and other reproductive disruption of fish in wastewater effluent dominated Colorado streams. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;144(1):10–15. doi: 10.1016/j.cbpc.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Wootton RJ. A Functional Biology of Sticklebacks. University of California Press; Berkeley and Los Angeles, CA: 1984. [Google Scholar]