Abstract
Human schistosomiasis is a common parasitic disease endemic in many tropical and subtropical countries. One barrier to achieving long-term control of this disease has been re-infection of treated patients when they swim, bathe, or wade in surface fresh water infested with snails that harbor and release larval parasites. Because some snail species are obligate intermediate hosts of schistosome parasites, removing snails may reduce parasitic larvae in the water, reducing re-infection risk. Here, we evaluate the potential for snail control by predatory freshwater prawns, Macrobrachium rosenbergii and M. vollenhovenii, native to Asia and Africa, respectively. Both prawn species are high value, protein-rich human food commodities, suggesting their cultivation may be beneficial in resource-poor settings where few other disease control options exist. In a series of predation trials in laboratory aquaria, we found both species to be voracious predators of schistosome-susceptible snails, hatchlings, and eggs, even in the presence of alternative food, with sustained average consumption rates of 12% of their body weight per day. Prawns showed a weak preference for Bulinus truncatus over Biomphalaria glabrata snails. Consumption rates were highly predictable based on the ratio of prawn: snail body mass, suggesting satiation-limited predation. Even the smallest prawns tested (0.5–2g) caused snail recruitment failure, despite high snail fecundity. With the World Health Organization turning attention toward schistosomiasis elimination, native prawn cultivation may be a viable snail control strategy that offers a win-win for public health and economic development.
Keywords: predation, biological control, functional response, predator, schistosoma, mansoni, haematobium
1. Introduction
Human schistosomiasis is a common parasitic disease of humans that is relatively easy to treat, but hard to control. Today, public health campaigns in endemic regions in the tropics and subtropics focus on mass drug administration using the oral drug, praziquantel. While praziquantel has a high cure rate, re-exposure to infected snails in the environment leads to rapid reinfection of treated patients in endemic areas (Fenwick et al., 2006; Fenwick and Webster, 2006; King et al., 2006; Tchuem Tchuente et al., 2013; Webster et al., 2013). In addition to drug treatment, a complementary approach is to control the populations of snails that serve as intermediate hosts. Snails are infected when parasite eggs, released in human urine or feces, are washed into the surface fresh water. A few weeks after infection, snails release larval schistosomes into the water where they can infect people, completing the lifecycle. Breaking this lifecycle through snail control was an approach used extensively for schistosomiasis control prior to the advent of the drug praziquantel. Snail control aims to reduce the number of parasitic larvae in the water, effectively reducing reinfection prevalence and intensity. Traditional approaches to snail control using molluscicide application and habitat modification can be expensive to implement and hard to maintain over the long-term. In some areas, researchers have documented a negative relationship between natural aquatic snail predators and the density of schistosome-susceptible snails, such as occurs in regions of Lake Malawi, where overfishing pressure may have caused snail populations to increase after predators were removed (Evers et al., 2006; Madsen and Stauffer, 2011). This suggests that in some ecological situations, control of snails through predator introductions could offer an effective snail control strategy that is affordable and sustainable and that may complement ongoing drug distribution campaigns. Here, we evaluate the potential for snail control by predatory freshwater prawns in the genus Macrobrachium.
Macrobrachium vollenhovenii, is a freshwater prawn native to rivers and streams throughout West Africa. We hypothesized that M. vollenhovenii would be an effective snail predator because this species shares similar habitats with medically important snails, grows to a large size, and because the congener M. rosenbergii (an Asian species) consumes snails as a preferred food (Lee et al., 1982; Roberts and Kuris, 1990). In Senegal, the construction of the Diama Dam to block tidal influence in the Lower Senegal River created a large freshwater irrigation system that offered abundant habitat to medically important snails, hosts of both Schistosoma mansoni and S. haematobium. This was associated with a severe and persistent outbreak of schistosomiasis involving both species, with increased snail abundance resulting from expanded low-flow, freshwater snail habitat after dam completion as well as probable immigration of infected agricultural workers to the region (Southgate, 1997; Sow et al., 2002). The Diama Dam also presumably blocked the migration of native M. vollenhovenii prawns to their estuarine breeding grounds. Although other impacts of the dam were not investigated, local fishermen reported that prawns were once common, but declined sharply after dam construction. Since prawns have been shown in laboratory studies to be voracious and effective predators of Biomphalaria glabrata (Lee et al., 1982; Roberts and Kuris, 1990), we speculate that a loss of prawns above Diama Dam may have contributed to the increase in snail intermediate hosts in the Lower Senagal River Basin, and therefore, an increase in schistosomiasis transmission. If so, restoration of M. vollenhovenii to the Senegal River might contribute to schistosomiasis control in that region or other similar regions of the world where schistosomiasis has increased after dam construction (Steinmann et al., 2006).
Roberts and Kuris (1990) published a series of laboratory trials that built on earlier work (Lee et al., 1982) demonstrating that M. rosenbergii – the most commonly aquacultured species of freshwater prawn worldwide – can consume B. glabrata snails. Roberts and Kuris concluded that prawn cultivation may offer a valuable complementary strategy for schistosomiasis control activities. Yet, to date, biological control using crustacean snail-predators has not been widely applied within schistosomiasis control programs. One of the major barriers to adoption is the lack of safe and effective native species for biological control. Introducing exotic species into habitats where they have never been previously naturalized can cause unwanted effects (Barbaresi and Gherardi, 2000; Fishar, 2006; Lodge et al., 2012). Nevertheless, there are a few examples where the introduction of exotic crustacean predators was successful in controlling schistosomiasis. For example, in Kenya, the introduction of a previously naturalized exotic crustacean, the Louisiana crayfish Procambarus clarkii, to village impoundments significantly reduced the prevalence and intensity of S. haematobium in schoolchildren for at least two years (Mkoji et al., 1999). Some additional evidence is emerging to suggest that invasions of this species throughout the Nile Delta may influence the rates of schistosomiasis transmission there (Khalil and Sleem, 2011). We argue that native predator augmentation would be similarly beneficial for schistosomiasis control programs while minimizing unwanted non-target effects associated with exotic introductions.
Here, we examine the long-term (days) consumption rates and characterize the functional response of two prawn species: M. vollenhovenii and M. rosenbergii feeding on two species of snails Biomphalaria glabrata (a host of S. mansoni) and Bulinus truncatus (a host of S. haematobium). Our goals were: 1) to assess the capacity for prawns – especially the African native M. vollenhovenii for which there were no previous data – to control laboratory populations of Biomphalaria and Bulinus snails, hosts for human schistosomes in Africa and the Americas; 2) to compare the predation rates and preferences of small juvenile prawns versus large adult prawns and between Malaysian and African prawn species; and 3) to characterize the functional response of prawns when offered varying sizes and densities of snails, as would be found in natural populations. Finally, we aimed to synthesize this information to guide the development of a new strategy for sustainable schistosomiasis control and elimination through restoration or stocking of river prawns in schistosomiasis-endemic areas, especially throughout Africa where the highest schistosomiasis transmission rates are found today.
2. Methods
2.1 Animals
Uninfected, laboratory-reared Biomphalaria glabrata, strain NMRI, and Bulinus truncatus, subsp. truncatus, were supplied by the Schistosomiasis Resources Center (BEI Resources, Manassas, VA). Laboratory-reared M. rosenbergii juvenile prawns were supplied by the Aquaculture Department at Kentucky State University and delivered by airfreight to the University of California Santa Barbara. Captive populations of M. vollenhovenii prawns were not available, so wild-caught prawns were collected from the Lobe River, Cameroon (Gulf Aquatics-Cameroon, Duoung, Cameroon) and delivered by air freight in December 2011 to Kentucky State University’s Aquaculture Department. At Kentucky State, the prawns were captively bred and the first generation juvenile prawns were delivered by airfreight to UC Santa Barbara in June 2012. Prawns and snails were housed in closed, recirculating freshwater tanks at UC Santa Barbara’s Marine Biotechnology Laboratory. The tank system had both mechanical and biological filtration, continuous aeration, and 20% weekly water exchanges using conditioned tap water. Between experiments, prawns and snails were housed in holding tanks: a 400L common holding tank for prawns, and four 25L holding tanks for snails. Both during and between experiments, prawns were fed a commercial shrimp crumble diet with 40% protein content (Rangen Corporation, Buhl, Idaho) at a rate of 3–5% body weight per day, five days per week, and snails were fed organic romaine lettuce rinsed in DI water, ad libitum. In some trials, snails also fed on the shrimp diet (see below for details). Experiments were conducted in individual, clear polyethylene tanks with plastic lids, filled to 6L with conditioned tap water, and connected by PVC and vinyl plumbing in a closed recirculating freshwater tank system. Tanks each had a single simulated prawn habitat refuge (a section of PVC pipe). Prawns were fasted, and all snails removed, for at least 24 hours between trials to prevent cross-trial carryover effects.
2.2 Measuring consumption in terms of snail number versus snail biomass
In all experiments, consumption was measured and reported in terms of snail number, snail biomass, or both. For the most part, we focused on the number of snails eaten by prawns because of its relevance to biological control of parasite transmission: the number (not biomass) of infected snails determines transmission risk to humans throughout a transmission season. This is due to the several-month average lifespan of schistosome-susceptible snails, along with the long pre-patency and patency periods and the fact that small snails can be more susceptible to infection (Barbosa, 1963; Loreau and Baluku, 1987; Niemann and Lewis, 1990; Pfluger, 1980; Pfluger et al., 1984; Woolhouse and Chandiwana, 1990a; Woolhouse and Chandiwana, 1990b). Thus, we measured consumption mainly in terms of the number of snails consumed/time or, where appropriate, the number consumed/gram-prawn-biomass/time. A second benefit of focusing on the number of snails consumed was to allow comparison with other studies, since we used the classic Holling’s Type II functional response equation, which defines two aggregate parameters that govern the overall shape and asymptotic maximum of the functional response curve, typically expressed in terms of prey number over a range of prey densities (not biomass). In a few cases, it was logical to report consumption in terms of both snail number and biomass (e.g., in the preference trials) or to focus on biomass alone. For example, for comparisons of consumption patterns among prawn species, we focused solely on biomass as a measure of consumption because this is the most important factor from the predator’s perspective. Digestion is the most common limiting factor regulating consumption rates among predators, including crustaceans (Jeschke et al., 2002; Konan et al., 2010). We therefore hypothesized that over relatively long time spans (days), prawns would satiate based on the total biomass consumed, compared with the capacity of their gastrointestinal tracts, and that they would satiate more quickly when feeding on large versus small snails, making the biomass of snails a more tractable and consistent predictor of satiation than the number of snails consumed, in this specific case. In all statistical tests, we used an alpha level of 0.05.
2.3 Experiment 1 – Preference trials
Preference for Biomphalaria or Bulinus spp. was assessed for five replicate M. rosenbergii prawns (size range: 9.6 – 77g) offered equal numbers of each snail species (first in a ratio of 20:20 snails, then repeated with a ratio of 40:40 snails). Similar trials were repeated to assess size-preferences using Biomphalaria single-species assemblages, examining preferences of large and small M. rosenbergii prawns (size range: 0.5 – 77g) consuming snails in three size classes: small (4mm ± 2mm shell diameter), medium (8mm ± 2mm), and large (12mm± 2mm) at equal densities (1:1:1).
At the start of each preference trial, all snails were weighed and measured. At each daily observation point, all remaining snails were again weighed, measured, and returned to the tanks until all of the snails had been consumed. Data were analyzed using a selectivity index calculated for each replicate prawn at each observation point excluding those observation points at which no snails remained (Equation 1). The selectivity index (SI) was calculated as follows:
| Equation 1 |
where ni / ntot was the fraction of snails consumed that were species (or size class) i and oi / otot was the fraction offered that were species (or size class) i during that observation period. Under conditions of random selection (no preference), the expected value of this index is 1. Selectivity indices were calculated by number of snails and by biomass of snails. Bootstrapped confidence intervals for this index were calculated in R.2.15.2 (CRAN http://cran.us.r-project.org/) using 999 bootstrap replicates in the “boot” function of the R base package.
2.4 Experiment 2 – Predation rates and functional response
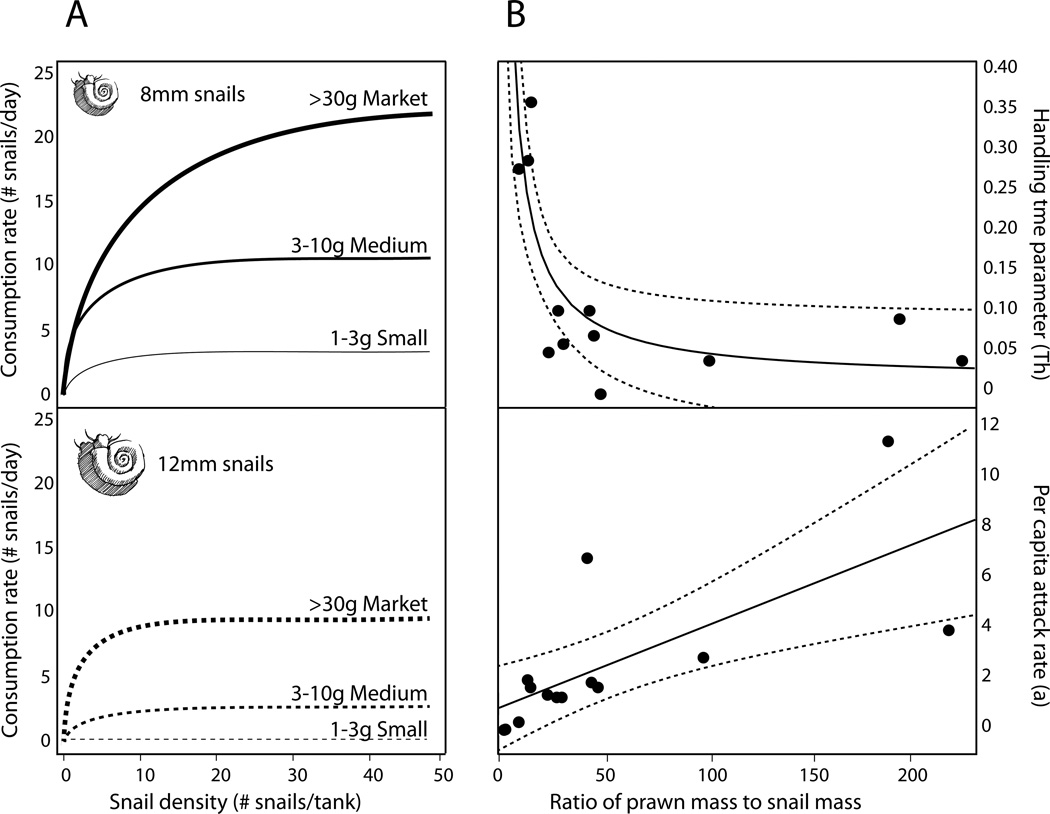
In order to quantify the daily consumption rates and characterize the functional response, prawns were housed individually and offered varying densities of snails, with replacement every 12 hours, for 72 hours. During all trials, prawns were fed their normal ration of pelleted shrimp diet calculated by dry weight and fed daily at 3–5% of prawn wet weight per day. Snails were size-sorted so that prawns were offered small (4mm ± 2mm shell length), medium (8mm ± 2mm), or large (12mm± 2mm) Biomphalaria glabrata, or one-size-class (5–10mm shell length) Bulinus truncatus. M. rosenbergii were offered either 6, 12, 24, or 48 snails/tank, and M. vollenhovenii 12 and 24 snails/tank for comparison. Prawns of a range of body masses were tested, based on availability: M. rosenbergii ranged from 0.3 to 130g and M. vollenhovenii ranged from 0.3 to 20g.
For some analyses, prawns were split into size classes, with a cut-off above 30 grams body mass signifying a full “market” size (target size at harvest)(New and Valenti, 2000). There were five size classes for prawns: XS (0.3–1g), S (1–3g), M (3–10g), L (10–30g), Market (>30g). A total of 3 to 6 (median 5) replicate prawns of each species/size-class were tested in each snail-size/-species/-density combination, and each replicate 12-hour trial was repeated 4–6 consecutive times, for a total of 48–72 hours of observation with replacement of consumed snails every 12 hours. Trials were conducted within three banks of nine 10L tanks, each bank with a common sump, pump, and filtration. In order to complete all replicates, batches of trials were conducted longitudinally through time, with the order of the snail-size/-species/-density combinations randomized through time and among tanks. One to three control tanks containing snails but no prawns were monitored during each trial. The number of snails offered, the number consumed, and the number of empty shells was recorded every 12 hours.
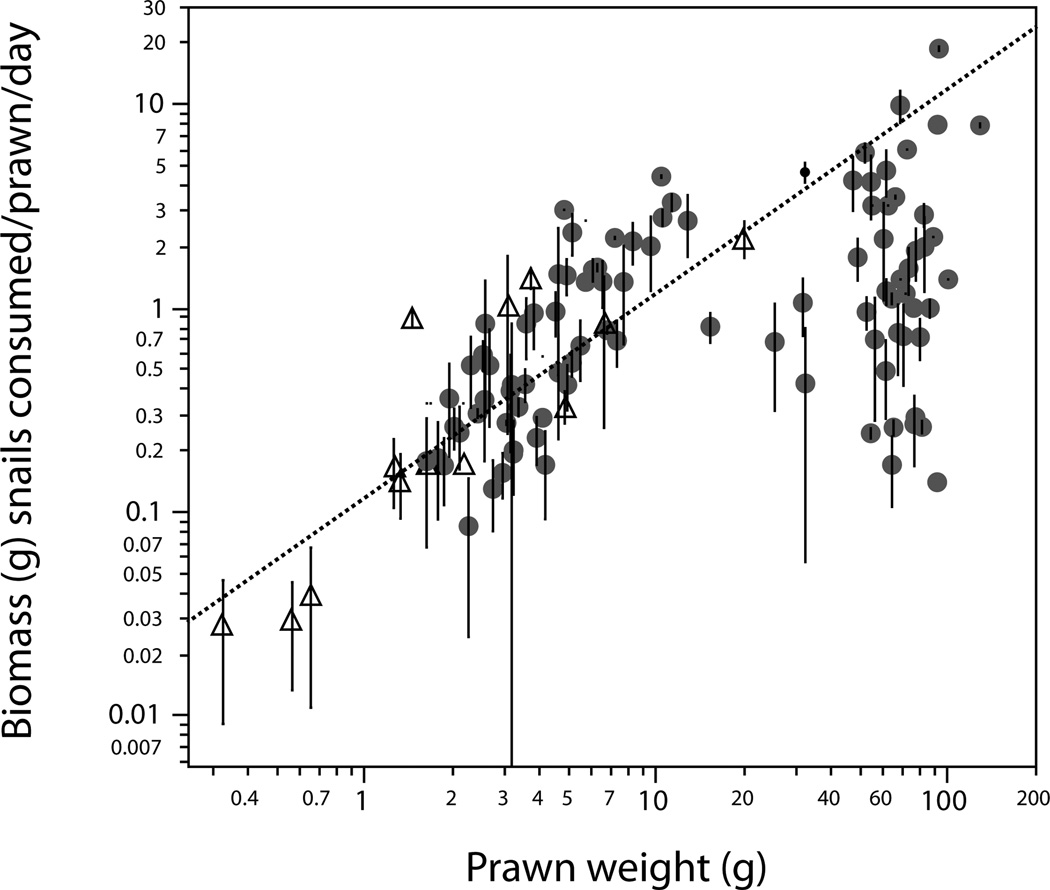
We compared predation rates of the two prawn species within their overlapping size range (2–26g). We assumed that predation rates would follow from metabolic requirements of growth, which for Macrobrachium spp. follow a Von Bertalanffy growth curve (Etim and Sankare, 1998; Gabche and Hockey, 1995) and thus there would be a predictable relationship between prawn size and consumption rate. We thus calculated the biomass of snails consumed (per gram prawn) in each trial and used a cube-root transformation to linearize the relationship, based on the the Von Bertalanffy growth equations and published length–weight relationships for each species (King et al., 2005; Sandbach, 1976). This allowed a simple comparison of consumption by size patterns between the two prawn species via a comparison of the slopes of the regression lines. To assess the differences among species, we compared the slope of the regression line for prawn length (size) versus linearized consumption rate using ANCOVA, with snail length and snail density as additional covariates. After finding no differences between consumption rates for the two prawn species (see results, below and Fig 1; ANCOVA on linearized data: p= 0.53), the data were pooled across species and the remainder of the analyses of consumption rates and functional response were based on the full range of trials for M. rosenbergii (0.5 to 130g) and M. vollenhovenii (0.3 to 20g) together.
Figure 1.
Comparing consumption rates for two prawn species: M. rosenbergii (filled circles) and M. vollenhovenii (open triangles). The dashed line represents the “line of equivalence,” or a constant 12% consumption rate over all body sizes which was the average overall consumption rate (it is not a regression line). Points above the line represent trials in which prawns ate more than the average of 12% of their body weight daily and those falling below the line ate less than 12%. Each point and vertical bar represents the average and standard error of multiple consecutive consumption trials with a single prawn. Trials where prawns were offered single-species populations of either B. glabrata (three size classes) or Bu. truncatus (one size class) were combined to generate this figure.
The effects of prawn size and snail size on the (log-transformed) consumption rates were tested using a linear mixed model implemented in JMP version 10 (SAS Institute Inc., Cary NC, USA) with individual prawns included as a random effect (due to the fact that individual prawns were measured repeatedly). Trials in which prawns were molting were excluded because prawns typically fast during the molt (Roberts and Kuris, 1990).
In order to quantify the functional responses to changing snail density, we estimated a classic Holling’s Type II functional response curve (Equation 2) for each prawn-species/-size and snail-species/-size combination:
| Equation 2 |
where Ne is the number of snails eaten, No is the number (density) offered per tank, a is the per capita attack rate, and Th is the “handling time” parameter. Parameters were estimated from the data by non-linear least squares fitting of equation 2 in JMP version 10. Confidence bounds for each parameter were determined using bootstrapping, via 999 bootstrapped replicates in the “boot” function of the R base package.
2.5 Experiment 3a – Regulation of laboratory populations of Biomphalaria glabrata snails by very large prawns
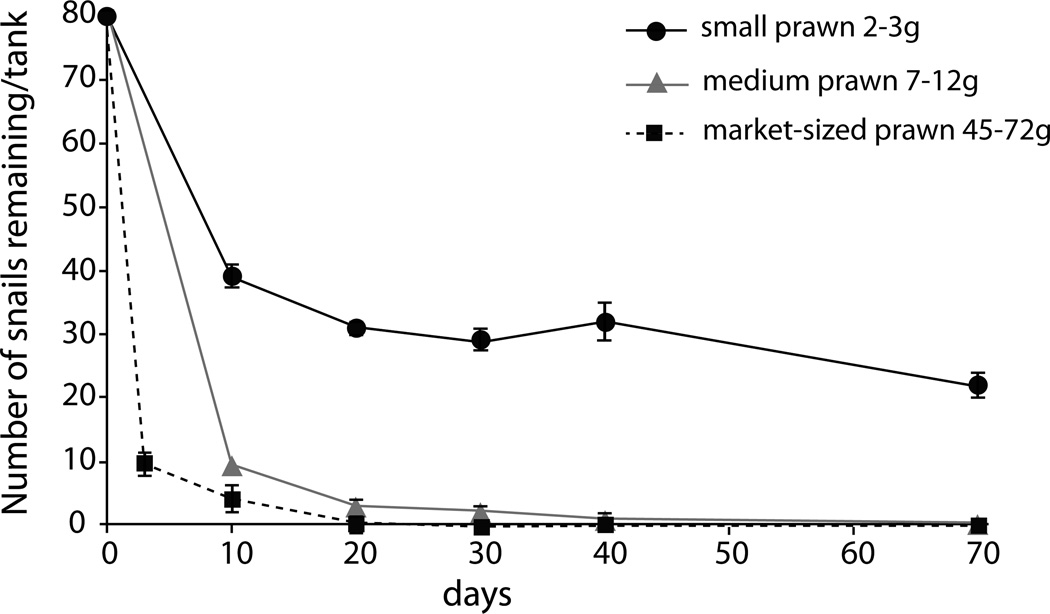
Here, we built on the work of Roberts and Kuris (1990), who showed that dense laboratory populations of B. glabrata could be consumed by predation in 20 days by individually housed medium sized (3–10g) prawns. We repeated their experiments using very large market-sized prawns to compare the rate at which the largest prawns (>30g) could eliminate densely-populated mixed-sized populations of snails in the laboratory. Three large prawns (45, 56, and 72 g) were individually housed with 80 B. glabrata snails each, 20 snails from each of four size classes (4mm, 8mm, 12mm, and 16mm, all ± 2mm) after Roberts and Kuris (1990). Two control tanks in the same flow-through Equation 2 system were set up with a founder population of 10 adult snails per tank (>10mm) but no prawns. All snails were weighed and measured at the start and monitored until no snails remained in the prawn tanks.
2.6 Experiment 3b – Regulation of laboratory populations of Biomphalaria and Bulinus snails by small prawns
Although Macrobrachium spp. prawns can consume high numbers of snails, our results and previous studies (Roberts and Kuris, 1990) suggested that there exists a size refuge for large adult snails faced with very small prawn (<2g) predators. Yet, we suspected that small prawns would consume eggs and hatchling snails, leading to snail population regulation despite the size refuge. Due to the difficulty in handling and weighing very small hatchling snails less than 1mm in diameter, rather than directly manipulating snail densities, we opted to address this question by monitoring snail population recruitment rates among laboratory snail populations through time in the presence or absence of very small prawns.
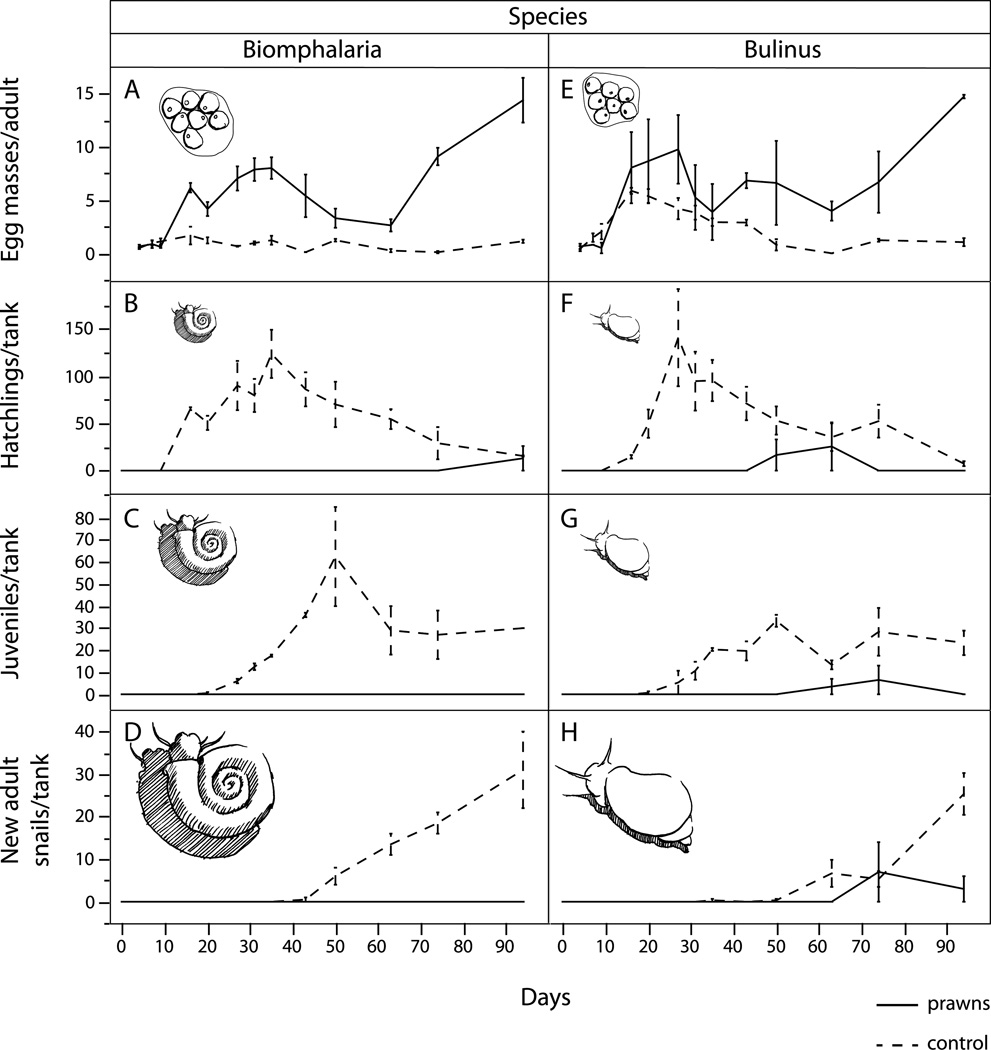
To test whether small M. vollenhovenii prawns could achieve population regulation despite their inability to consume adult snails, snail populations were established and monitored for 90 days. Ten large (>10mm shell length) adult snails of either B. glabrata or B. truncatus were added to each tank as founder populations. Snails were weighed and measured at the start and fed lettuce ad libitum throughout the study. Snails were left to acclimate and lay eggs for 7 days at the start of the experiment. Then, prawns were introduced into half the tanks (at a density of 3 prawns per tank, each prawn <2g) and all tanks were monitored every 5–7 days until the termination of the experiment. At each observation point, all remaining adult snails were counted, and any dead snails were removed and replaced to simulate an open population of adult snails at equilibrium densities, where any loss of an adult snail would likely lead to an opportunity for replacement by an immigrant snail into that habitat. The number of egg masses and the number of recruit snails in the hatchling, juvenile, and adult size classes – which were defined as <4mm, 4–8mm, and >8mm shell length respectively for Biomphalaria and <3mm, 3–6mm, and >6mm shell length respectively for Bulinus – were counted at each observation point. The population trajectories for snail populations in the presence and absence of prawns were plotted over time and the means were compared at the midpoint and endpoint using a repeated measures ANOVA.
2.7 Experiment 3c – Comparing the effect of prawns with the effects of food supplementation & manual removal of juvenile snails
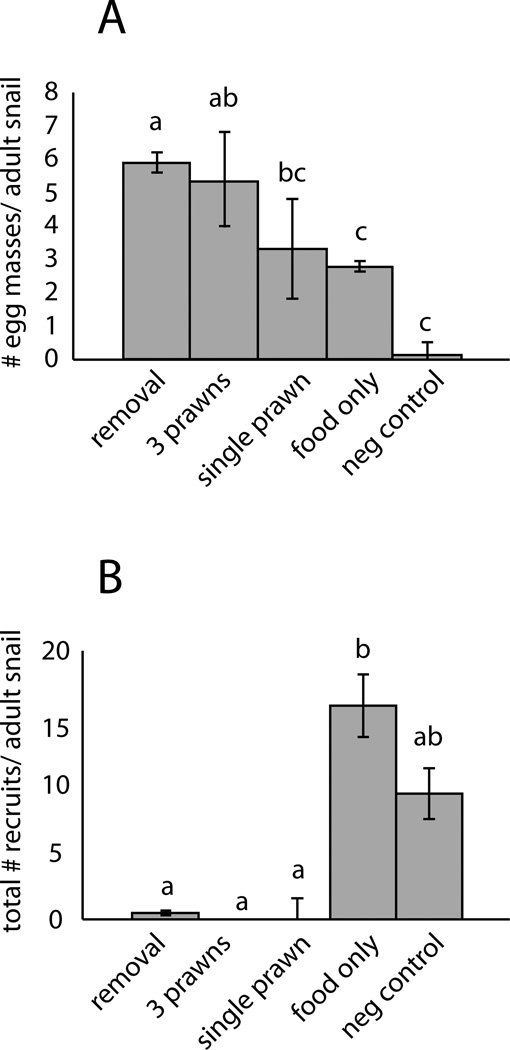
Because it was noted that snail fecundity remained very high in the presence of prawns, a second experiment was conducted to isolate a number of possible competing influences on the number of egg masses that accumulated in tanks over time: effects of shrimp feed supplementation (which the snails consumed), loss of recruit snails via predation – which was hypothesized to increase egg numbers via reduced egg cannibalism by recruit snails (ie. a cannibalism effect) or reduced competition for food resources between adult and juvenile snails (ie. a crowding effect) – or other effects associated with prawn presence such as chemical/visual cues that could have influenced hatching rates. Single-species laboratory populations of Biomphalaria snails were again established in nine tanks, as before, using 10 large (>10mm shell length) snails as founders in each tank, and four treatments were applied as follows. Eight tanks were offered equal weights of shrimp feed plus lettuce ad libitum added daily and one tank served as a negative control (with only lettuce fed ad libitum, as in the previous experiment). In four of the eight tanks given shrimp feed supplementation, a single prawn (0.5 to 1.5g) was added. In two of the remaining four tanks without prawns, all recruit snails that hatched from the eggs were manually crushed (to simulate predation) and removed weekly. All tanks were again monitored every 5–7 days for 63 days, recording the number of egg masses, the number of original founding adults remaining alive (replaced if dead or missing), and the number and sizes of snail recruits. The population trajectories were plotted over time and the means compared at the midpoint and endpoint via repeated measures ANOVA tests.
3. Results
3.1 Comparing prawn species
No differences in consumption patterns were detected among the two prawn species, M. vollenhovenii and M. rosenbergii, despite these two species originating from different continents, Africa and Asia respectively (Fig 1; ANCOVA on linearized data: p= 0.53). This suggests that our results may be generalizable among both of these prawn species. Although no other species were tested here, there are other large-bodied species with similar morphology among the more than 240 species in the genus Macrobrachium distributed throughout the world (De Grave and Fransen, 2011), many of which occur where schistosomiasis is endemic, and tests of snail predation among many of these species may be warranted.
3.2 Predation rates
Prawns consumed, on average, 12% (CI = 10.7 – 14.1%) of their body weight in snail biomass per day. Predation rates varied considerably among individual prawns on individual days, ranging from 0% to more than 90% of their body weight in snail tissue killed daily (snail tissue = snail wet weight excluding shell weight). Although prawn predation behavior was not formally recorded in these experiments, many instances of prawn predation on snails were directly observed. Prawns were often observed attacking snails by first apprehending the snails using their first pair of pareiopods (walking legs) and then lifting the snails to their mouthparts and crushing the shells using their mandibles, consuming the tissue and discarding (though sometimes consuming) the leftover shell. In most cases, small prawns would not attack snails that were more than 1/3 their own body weight, although small prawns were occasionally directly observed killing very large snails by picking at snail tissue through the shell aperture without crushing the shell.
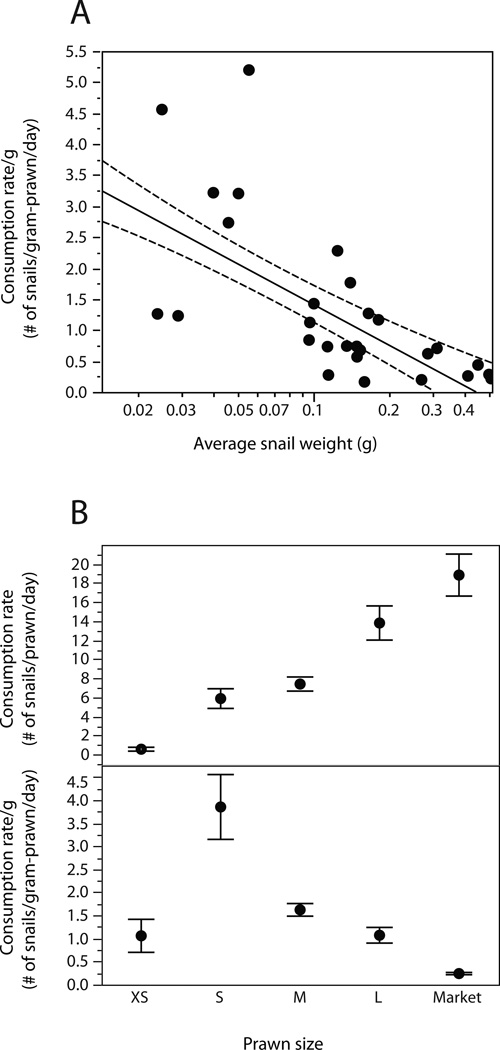
Prawn size (p=0.0003) and snail size (p<0.0001) were significantly associated with consumption rates based on the linear mixed effects model results. The interaction between prawn size and snail size was not significant (p=0.26). Larger prawns generally consumed the most snails and depleted high density snail populations faster than smaller prawns (Fig 2, 3). Large M. rosenbergii prawns presented with dense, mixed-sized snail populations (experiment 3a) consumed all sizes of snails presented to them, and completely eliminated snails from the tanks within an average of 10.4 days (range 7 – 21 days, Fig 2). This extends previous findings by Roberts and Kuris for 7–12g M. rosenbergii prawns that could deplete the same number of snails within c.a. 20 days (Fig 2). However, on a gram per gram basis, prawn size class was significantly, non-linearly related to consumption rates with smaller sized prawns having higher killing efficiency per gram (Fig. 3, p=0.0004 by Wilcoxon test). Because prawns are territorial and can be stocked at much higher densities when they are smaller, this suggests that prawns of small to intermediate mass (1–30g) may be optimal for snail control. Prawns of all sizes could consume more small snails than large ones when presented with single-sized populations of snails, likely due to satiation effects (Fig 3).
Figure 2.
Consumption and elimination of high-density mixed-sized populations of B. glabrata snails (80 snails/tank) by small M. rosenbergii prawns (2–3g; circles), medium M. rosenbergii prawns (7–12g, triangles) and very large M. rosenbergii prawns (>30g, squares). Data for small and medium prawns are from Roberts and Kuris (1990); data for very large prawns are from this study.
Figure 3.
The effect of (A) snail size and (B) prawn size on snail consumption rates by prawns. XS = <1g prawns; S= 1–3g prawns; M= 3–10g prawns; L=10–30g prawns; Market = >30g prawns. Data from both M. rosenbergii and M. vollenhovenii preying on snails of either B. glabrata or B. truncatus were combined to generate this figure.
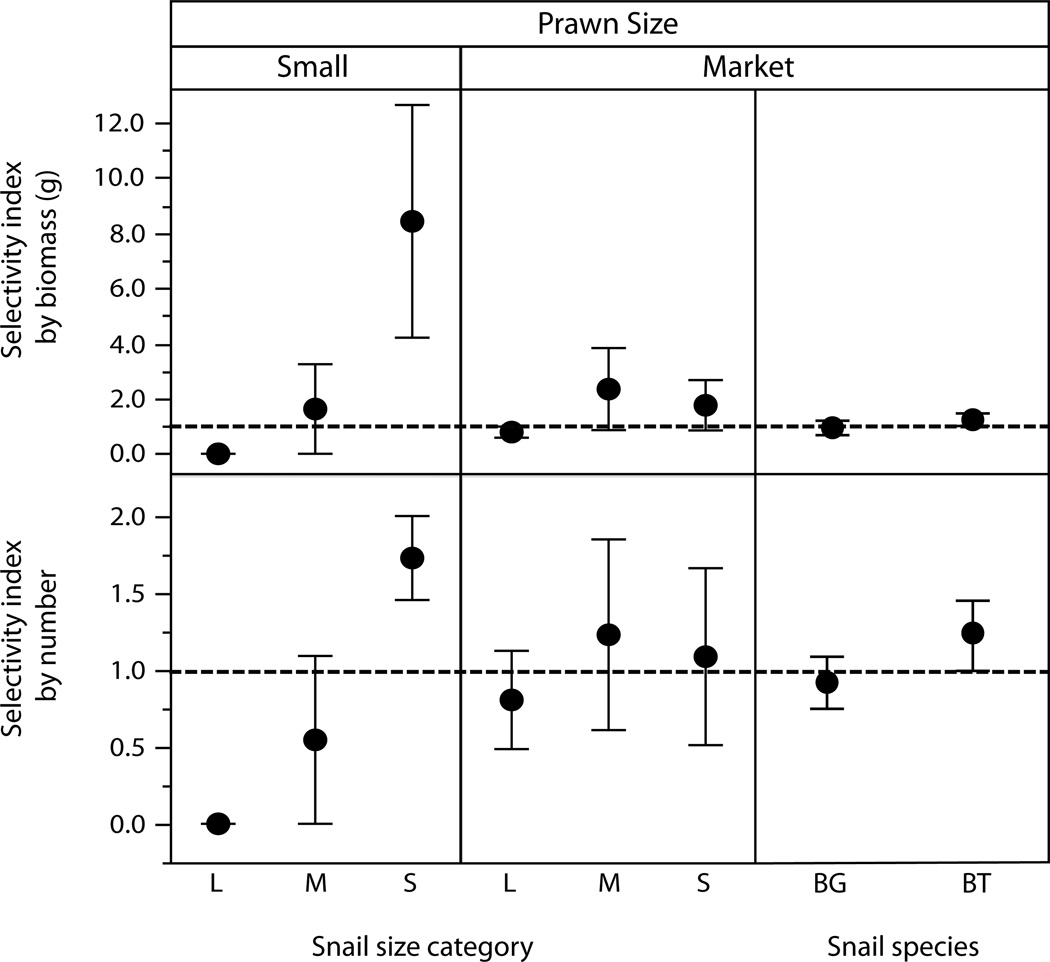
3.3 Preference
By comparing the observed and expected selectivity indices and their confidence intervals, calculated for each snail species (or size category), we assessed whether any preferences could be detected. Significant preferences were demonstrated if the entire confidence interval was greater than one, while indices with confidence intervals entirely less than one demonstrated significant avoidance. Small, but not large, prawns preferred small snails and avoided large ones (Fig 4). The average wet weight of the largest B. glabrata snails was an order of magnitude larger, 0.31 ± SE 0.01g (range: 0.16 – 1.06g), than the wet weight of the smallest size class, 0.022 ± SE 0.001g (range: 0.01 to 0.05g). However, it should be noted that even the smallest snail size class offered here (4mm ± 2mm shell length) was still of a sufficient size to be susceptible to schistosome infection (Anderson et al., 1982). In contrast, the largest, market-sized prawns showed no clear size preferences in the snails they would attack and kill (Fig 4).
Figure 4.
Results of preference trials for Small (<3g) and Market sized (>30g) M. rosenbergii prawns when offered either single species populations of B. glabrata snails of three size classes (S = 4mm ± 2mm, M = 8mm ± 2mm, L = 12mm ± 2mm) or two snail species (BT = B. truncatus, BG = B. glabrata) in equal ratios. Outcomes are similar whether expressed in terms of biomass of snails consumed (top graph) or the number of snails consumed (bottom graph).
When M. rosenbergii prawns were offered mixed-species assemblages of snails, there was a marginally significant preference exhibited for B. truncatus over B. glabrata, (Fig 4). To rule out any effect of snail size on inter-species preference measurements, we investigated selectivity indices for B. truncatus versus B. glabrata on a subset of the data with only size-matched snails (medium B. truncatus versus medium B. glabrata). In this case, the selectivity indices for B. truncatus remained significantly higher than for B. glabrata (sign test: p=0.04). When considering only size-matched snails, an odds ratio of attack probabilities for Biomphalaria compared with Bulinus was 0.37 (CI = 0.17 – 0.85) showing some factors other than size differences may be contributing to the preferences observed. The observed preference could theoretically alter the effectiveness of control for Bulinus versus Biomphalaria snails and therefore S. mansoni versus S. haematobium carried by each snail species, respectively.
3.4 Functional Response
For each prawn-species-size/snail-species-size category, the relationship between snail density and the number of snails consumed was fit to equation 2 using non-linear least squares regression. A Type II, or saturating functional response, fit the data well (Fig 5). Both attack rate a and handling time Th, were highly predictable given the ratio of prawn to snail body mass, with attack rate directly proportional to, and handling time inversely proportional to, the ratio of the two masses. Below a 3:1 ratio of prawn to snail body mass, prawns usually attacked zero snails, demonstrating a threshold of ~30% of their own body weight above which snails were perceived as too large to attack. These data suggest a predictable size refuge for the largest snails in the face of predation risk by small prawns, which was observed commonly in our experiments and in previous studies (Roberts and Kuris, 1990).
Figure 5.
(A) A subset of best fit functional response curves, based on least squares fitting of Holling’s disc equation for prawns of different size (Small = 1–3g prawns, Medium = 3–10g prawns, Market = >30g prawns) consuming B. glabrata snails of different size classes (8mm or 12mm ± 2mm shell length). (B) The relationship between the parameters governing the attack rate and handling time and prawn:snail body mass ratio, showing a predictable functional response. Data for M. vollenhovenii and M. rosenbergii preying on either B. glabrata or B. truncatus were combined to generate this figure.
3.5 Regulation of laboratory populations of Biomphalaria and Bulinus snails
In experiments 3b, small prawns prevented recruitment in snail populations for at least 90 days by consuming small snails, hatchlings, and/or eggs despite their inability (or unwillingness) to attack and consume large snails (>10 mm shell length). Population growth rates remained near zero in the presence of prawns. In contrast, the earliest recruits in the control tanks for all experiments appeared within 10 days after stocking. The population growth rate in control tanks was high, with peak abundances seen at 10–30 days for both Biomphalaria and Bulinus populations (Fig 6). After day 30, recruitment began to wane and by 90 days, very few hatchlings were seen in either prawn or control tanks. By the end of the 90-day experiment, the prawn tanks had few new snails beyond those originally stocked as founders, whereas the snail populations in control tanks had increased by an order of magnitude and the adult egg-laying population had quadrupled (for Biomphalaria) or tripled (for Bulinus). However, given that tanks with prawns had far fewer snails overall, it was surprising to find that the number of egg masses was higher in the presence of prawns. By week 3, a difference could be easily detected in the number of egg masses in prawn tanks and by the end of the 90-day experiment, prawn tanks contained an order of magnitude more egg masses on average than control tanks for both Biomphalaria (p=0.02) and Bulinus (p=0.008). In other words, small prawns led to an increase in egg laying or egg survival by adult snails, but nevertheless prevented eggs from recruiting into the snail population.
Figure 6.
Results of 90-day population regulation experiments using very small M. vollenhovenii (0.5–2g) prawns. Shown are the trajectories over time for snail eggs (A,E), hatchlings (B, F), juveniles (C, G), and new adults, not including the founding population (D,H) in prawn-treatment (solid lines) and control tanks (dashed lines). Small prawns strongly suppressed recruitment, despite their inability to consume adult snails, even with an increased standing biomass of snail eggs. Snails began to hatch at day 10 and then passed through successive size cohorts until they reached adult size by day 40–50.
Manual removal of snail recruits (by crushing weekly, Experiment 3c) resulted in similar outcomes compared with the effect of prawns, in terms of both snail population growth and standing egg numbers (Fig 7). That is, recruitment of hatchling snails was blocked by either the addition of prawns or manual snail recruit removal, but egg masses accumulated in both treatments at a similar rate, which was much higher than in controls. This suggests that the main mechanism by which prawn treatments led to higher numbers of snail eggs was by reduction of the density of snail recruits, so that small prawns, paradoxically, had a positive effect on eggs (Fig 8). Tanks with 1 rather than 3 prawns had intermediate numbers of eggs, suggesting a dose-response relationship between the number of prawns (and hence the rate of recruit removal by predation) and the standing stock of snail eggs in the tanks. Despite the fact that snails in all the tanks were fed lettuce ad libitum, egg masses accumulated faster in the tanks with shrimp feed supplementation, even without prawns, demonstrating an additional, direct effect of high quality (protein-and calorie-rich) food supplementation on snail fecundity.
Figure 7.
Effect of five treatments on (A) snail fecundity (egg masses/adult snail) and (B) recruitment (new snail recruits/adult snail) in laboratory populations of Biomphalaria pfeifferi snails. “Removal” = manual snail recruit removal (by crushing weekly) plus 0.15g shrimp diet added daily; “3 prawns” = 3 M. vollenhovenii prawns (0.5–2g each) housed/tank plus 0.15g shrimp diet added daily, “single prawn” = 1 M. vollenhovenii prawn (0.5–2g) housed/tank plus 0.15g of shrimp diet added daily, food only = 0.15g shrimp diet added daily (no prawns or recruit removal), and neg control = a negative control whereby snails were only fed lettuce ad libitum. Bars sharing a letter are not significantly different.
Figure 8.
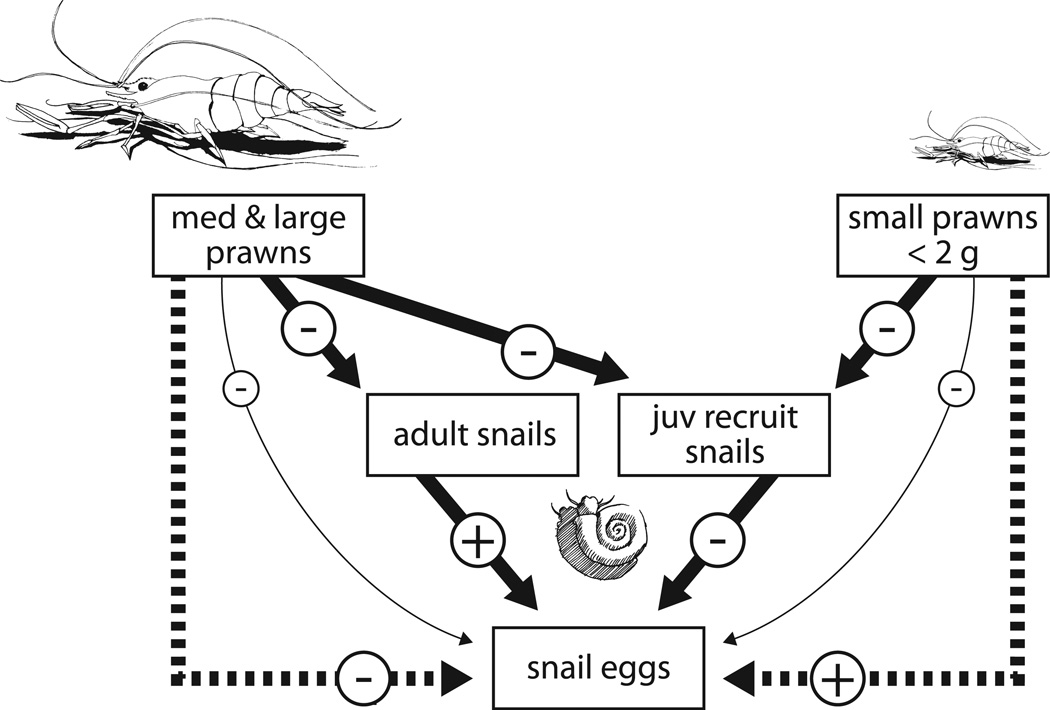
Proposed trophic interactions between large or small prawns and adult snails, snail recruits, and snail eggs. Large prawns have negative direct and indirect effects on all snail life stages. On the other hand, small prawns (<2g) are limited in their ability to kill adult snails. A negative effect of juvenile recruit snails on eggs (due to competitive or egg-cannibalistic effects) led to positive indirect effects of small prawns on snail eggs, despite a strong negative effect on snail recruitment.
4. Discussion
The results of our experiments indicate that M. vollenhovenii and M. rosenbergii prawns are voracious predators of snails, hatchlings, and eggs, even in the presence of alternative food sources (e.g., a commercial pelleted shrimp diet offered at a dry weight of 3–5% of prawn wet weight per day). Prawns consumption rates were predictable based on prawn size, snail size, and snail density. These data fill a critical gap in knowledge concerning the rate at which prawns can consume snails and their functional response to increasing or decreasing snail density, important parameters for predicting the regulatory effect of prawns on natural snail populations. The results presented here will provide information which can be useful, once validated with field data, to investigate the efficacy of using prawns as biological control agents to reduce parasite transmission to people.
4.1 Satiation-limitation caused predictable functional responses among prawns of all sizes
During the functional response experiments performed here, all prawns consumed many more small than large snails when presented with single-sized populations at all densities. These data suggest that digestion and satiation are limiting factors in ingestion rates. Satiation-limitation caused a predictable saturating functional response in the face of increasing snail densities, with saturation determined by the total biomass consumed, rather than the total number consumed. Satiation- or digestion-limitation is the most common type of limitation among predators, and many other crustaceans follow this pattern (Konan et al., 2010). Based on our data, the parameters controlling the attack rate a and “handling time” Th, two aggregate parameters that govern the overall shape and asymptotic maximum of the functional response curve, were highly predictable based on the ratio of prawn-mass:snail-mass (Fig 5). These data will facilitate parameterization of mechanistic models of schistosomiasis transmission that include biologically-realistic trophic interactions between predators and snail prey, not previously addressed in schistosomiasis models to date. Including trophic interactions in schistosomiasis models will allow calculation of the optimal predator (prawn) stocking and harvesting rate needed for effective biological control.
4.2 Preferences for Bulinus over Biomphalaria were detected among prawns
The slight preference for B. truncatus snails compared with B. glabrata demonstrated in the laboratory may be explained, in part, by the differing shell morphology of the two snail species: Bulinus have thinner shells with relatively larger apertures, compared with Biomphalaria, perhaps making it easier for prawns to access snail tissue by flipping the snails over and reaching through the aperture. Other behavioral differences and other unmeasured determinants of preference may be at work as well, such as visual or chemical cues or differences in shell crush resistance or defensive behavior by the snails.
4.3 Prawns suppressed snail recruitment and fecundity, but the smallest prawns caused paradoxical trophic interactions in laboratory snail populations
Large prawns rapidly consumed all sizes of snails presented to them, strongly suppressing snail numbers, egg numbers, and recruitment rates, with the potential to rapidly extinguish dense snail populations within weeks. Large snails in the presence of small prawns, on the other hand, experienced a size refuge which caused complex trophic interactions that regulated snail fecundity and recruitment in unexpected ways (Fig 8). Paradoxically, small prawns caused a striking increase in the standing stock of snail eggs in tanks, even though a separate experiment showed they efficiently consumed eggs, when tested in isolation, separated from other snails (see supplemental figure 1). Because this paradoxical effect could, theoretically, reduce prawn efficacy for snail control, we speculate a bit further on why this occurs in our laboratory studies. One explanation may be that competition for limiting resources such as food, oxygen or micronutrients (Chernin and Michelson, 1957; Coelho et al., 1975; Loreau and Baluku, 1987; Mishkin and Jokinen, 1986) between juvenile and adult snails led to lower fecundity in the adults when juveniles were not removed by predation or crushing. Alternatively, it is possible that juvenile Biomphalaria and Bulinus snails, like many aquatic snails, are egg cannibals; that is, in tanks with prawns, where many small snails were removed, a reduction of egg cannibalism by snails may have caused a trophic cascade, leading to a high standing stock of snail eggs (Fig 8).
The applicability of this laboratory finding to field conditions remains to be determined; it is unclear whether removal of snail recruits by predation will lead to an increase in snail eggs in the presence of small prawns in natural settings. It may be that egg cannibalism by juvenile snails or competition between juveniles and adults are more likely in the laboratory setting, where low habitat complexity and high snail densities facilitate high egg-encounter rates and strong competition for resources. On the other hand, both cannibalism and resource limitation have been shown to be common features among many terrestrial and aquatic animals in the wild (Fox, 1975; Hairston et al., 1960; Polis, 1981; Wise, 2006) and other natural snail populations have been shown to be regulated by egg-cannibalistic interactions, suggesting a possible importance of these complex trophic interactions in the field (Baur, 1988). Field studies may distinguish whether cannibalism or competition or both are mechanisms underlying the observed patterns and whether these mechanisms are at work under field conditions.
4.4 Considering Macrobrachium prawns for biological control of schistosomiasis
Based on our laboratory findings, aquaculture-based restocking of native Macrobrachium prawns warrants serious consideration as a biological control strategy for schistosomiasis, although future field trials to validate our results are recommended before drawing conclusions about feasibility in the field. Although the idea that river prawns may control schistosomiasis has been suggested in the past (Jordan, 1985; Lee et al., 1982; Roberts and Kuris, 1990), the idea was mostly dismissed based on the argument that fishing of the prawns by the local native human populations might interfere with their effectiveness as control agents (Jordan, 1985). We argue exactly the opposite: it is precisely because prawns are a valuable human food commodity that their re-introduction might offer the most effective, feasible, and economically sustainable option for biological control in some rural locations. Our results support this hypothesis by demonstrating that young, growing prawns (too small for harvest) have the most efficient snail-killing ability. Maximal snail consumption per gram prawn was seen in the mid-sized prawns. The pattern that intermediate-sized prawns consumed more than the largest or smallest prawns can be explained by two factors: 1) at the smallest sizes, prawns are limited by lack of strength or insufficient mouth part size and are unable or unwilling to attack large snails, reducing their consumption rates overall; however, 2) at the largest sizes, prawns approach their asymptotic maximum size and thus growth slows and molting occurs less frequently, reducing consumption. Removal of the largest prawns through harvest might actually increase snail control because the threat of large prawns cannibalizing smaller ones would be reduced.
If our results are validated in the field, it is then plausible that a regional scheme could be devised whereby revenue generated by fishing or harvest of the largest prawns (>30g) could finance aquaculture and re-introduction of smaller prawns into the cohort at schistosomiasis transmission sites. Therefore, maintaining a captive prawn broodstock for mass rearing of larval prawns, releasing them into net pens or “free range” (in the open waterways) along rivers and canals near transmission sites, could have simultaneous economic and disease control benefits for local communities.
Biological control of snail populations using predators has received little attention by the public health community since the drug praziquantel became widely available in the 1980’s. There have been limited successes using molluscivorous fish predators for biological control of schistosome-susceptible snails in the past, such as Serranochromis macrocephala in Central Africa (Adewale and Afolayan, 2004), snail-eating cichlids in Lake Malawi (Slootweg et al., 1994), and Oreochromis niloticus (larger adults only) in Cameroon (Etim and Sankare, 1998). Prawns have benefits over other biological control agents such as fish because prawns can penetrate dense vegetation, or exit the water for brief periods (Baumgartner, 2003), allowing them access to snails where other fish predators are excluded. In addition, prawns fetch a higher price per kilogram than fish in most markets as a human food commodity, increasing the likelihood that their cultivation would be financially feasible and scalable. In Senegal, for example, the price of large prawns is more than twice the price of fish in the local markets. Lastly, M. vollenhovenii prawns have the advantage that they are a native inhabitant of the river in large areas of Africa where schistosomiasis is endemic. It is interesting to note that the M. vollenhovenii used in our experiments were collected from the Lobe River, situated in the South Region of Cameroon, where these native prawns are abundant (Brummett et al., 2008; Gabche and Hockey, 1995) and schistosomiasis prevalence is relatively low (Ratard et al., 1990). It is not clear if prawns or other factors, such as low human population density or differences in rainfall, water flow, ecology, and the predominant schistosome and snail species in the South, are responsible for the relatively low schistosomiasis rates there (Greer et al., 1990). Nevertheless, it is possible that others of the more than 240 named species in the genus Macrobrachium distributed throughout the world (De Grave and Fransen, 2011) could be tested for their snail control benefits and, if proven effective, cultivated where their native ranges overlap with schistosomiasis endemic regions, especially where their populations have been recently reduced by dams.
We recommend further consideration of prawns for biological control of schistosomiasis. Our studies show that prawns are effective at controlling snails under laboratory conditions and if this is also true in the field setting, they may offer a novel, ecologically restorative and sustainable control strategy for schistosomiasis in endemic regions. Large-scale field trials to introduce prawns into net enclosures at transmission sites in Senegal are underway to test whether our laboratory results are scalable and applicable under field conditions. A meta-analysis of schistosomiasis rates in Africa showed that the risk of infection was elevated for people living near dams and irrigation schemes compared with those far from these schemes throughout much of Sub-Saharan Africa (Steinmann et al., 2006). Although speculative, we suppose many other dam sites have similarly excluded some freshwater prawns upriver, as apparently occurred in Senegal, and these dams may offer wider opportunities for testing whether prawn restoration or prawn aquaculture at transmission sites can decrease schistosomiasis. Additionally, although fine-scale data on prawn densities are scarce, investigating the distribution of schistosomiasis rates in regions where prawn populations are naturally abundant and not excluded by dams could be useful.
Ultimately, bio-economic models of the prawn-snail-schistosome system would be valuable for understanding the utility of aquaculture versus fisheries restoration (e.g. fish/prawn ladders installed on dams) and for comparing the cost-effectiveness of using prawns in combination with other disease control measures already commonly employed (e.g., sanitation improvement, molluscicides to control snails, and chemotherapy to treat human patients). With the World Health Organization now turning its attention toward schistosomiasis elimination (WHO, 2011), prawns may offer a simple and affordable transmission control solution in rural poor communities where few alternatives exist and drug treatment is failing to achieve long-term disease reductions. If scale-up of this approach proves effective in field trials, developing and promoting native prawn cultivation may offer a win–win for poverty alleviation and public health at schistosome-endemic sites throughout the world.
Supplementary Material
Highlights.
We measured predation by two Macrobrachium spp. prawns on medically-relevant snails in lab aquaria
Prawns are voracious snail predators, consuming c.a. 12% body weight daily in snails
Consumption rates are predictable given prawn: snail body mass ratio
Small prawns <2g cause snail population recruitment failure despite a size refuge for large snails
We concluded that prawns may offer a valuable biological control option for schistosomiasis
Acknowledgements
The authors thank G Galin for assistance with artwork, C Wood and G DeLeo for useful comments on early manuscript drafts, and J Aman, M Lin, and A Wood for assistance with animal care. SHS was supported by Award Number K08AI082284 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale IO, Afolayan A. Purification and catalytic properties of glutathione transferase from the Hepatopancreas of crayfish Macrobrachium vollenhovenii (Herklots) Journal of Biochemical and Molecular Toxicology. 2004;18:332–344. doi: 10.1002/jbt.20044. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Mercer JG, Wilson RA, Carter NP. Transmission of Schistosoma mansoni from man to snail: experimental studies of miracidial survival and infectivity in relation to larval age water temperature, host size and host age. Parasitology. 1982;85(Pt 2):339–360. doi: 10.1017/s0031182000055323. [DOI] [PubMed] [Google Scholar]
- Barbaresi S, Gherardi F. The invasion of the alien crayfish Procambarus clarkii in Europe, with particular reference to Italy. Biological Invasions. 2000;2:259–264. [Google Scholar]
- Barbosa FS. Survival in the field of Australorbis glabratus infected with Schistosoma mansoni . J Parasitol. 1963;49:149. [PubMed] [Google Scholar]
- Baumgartner L. Fish passage through a Deelder lock on the Murrumbidgee River, Australia. NSW Fisheries Final Report Series. 2003:34. [Google Scholar]
- Baur B. Population regulation in the land snail Arianta arbustorum: density effects on adult size, clutch size and incidence of egg cannibalism. Oecologia. 1988;77:390–394. doi: 10.1007/BF00378049. [DOI] [PubMed] [Google Scholar]
- Brummett RE, Youaleu JLN, Tian AM, Kenmengne MM. Traditional Fisheries of Rainforest Rivers in Campo-Ma'an Area of Southern Cameroon. In: Bojang F, editor. Nature and Faune. Accra, Ghana: Food and Agricultural Organization of the United Nations; 2008. [Google Scholar]
- Chernin E, Michelson EH. Studies on the biological control of schistosome-bearing snails. III. The effects of population density on growth and fecundity in Australorbis glabratus. Am J Hyg. 1957;65:57–70. doi: 10.1093/oxfordjournals.aje.a119856. [DOI] [PubMed] [Google Scholar]
- Coelho PM, Gazzinelli G, Pelegrino J, Pereira LH. Aspects of the crowding effect in Biomphalaria glabrata (Say, 1818) evaluated by 59-Fe uptake. Rev Inst Med Trop Sao Paulo. 1975;17:129–134. [PubMed] [Google Scholar]
- De Grave S, Fransen CHJM. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda) Zool Meded. 2011;85:195–589. [Google Scholar]
- Etim L, Sankare Y. Growth and mortality, recruitment and yield of the freshwater shrimp, Macrobrachium vollenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fahe reservoir, Cote d'Ivoire, West Africa. Fish Res. 1998;38:211–223. [Google Scholar]
- Evers BN, Madsen H, McKaye KM, Stauffer JR., Jr The schistosome intermediate host, Bulinus nyassanus, is a 'preferred' food for the cichlid fish, Trematocranus placodon, at Cape Maclear, Lake Malawi. Ann Trop Med Parasitol. 2006;100:75–85. doi: 10.1179/136485906X78553. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Rollinson D, Southgate V. Implementation of human schistosomiasis control: Challenges and prospects. Advances in Parasitology. 2006 doi: 10.1016/S0065-308X(05)61013-5. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Current Opinion in Infectious Diseases. 2006;19:577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- Fishar MD. Red swamp crayfish (Procambarus clarkii) in River Nile, Egypt: Case study. In: Agency EEA, editor. Biodiversity Monitoring and Assessment Project (BioMap) Egypt: Ministry of State for Environmental Affairs; 2006. [Google Scholar]
- Fox L. Cannibalism in natural popualtions. Annual Reviews of Ecology and Systematics. 1975;6:87–106. [Google Scholar]
- Gabche CE, Hockey HUP. Growth and Mortality of the Giant African River Prawn Macrobrachium-Vollenhovenii (Herklots, Crustacea, Palaemonidae) in the Lobe River, Cameroon - a Preliminary Evaluation. J Shellfish Res. 1995;14:185–190. [Google Scholar]
- Greer GJ, Mimpfoundi R, Malek EA, Joky A, Ngonseu E, Ratard RC. Human schistosomiasis in Cameroon. II. Distribution of the snail hosts. Am J Trop Med Hyg. 1990;42:573–580. doi: 10.4269/ajtmh.1990.42.573. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Smith FE, Slobodkin LB. Community Structure, Population Control, and Competition. Am Nat. 1960;94:421–425. [Google Scholar]
- Jeschke E, Kopp M, Tollrian R. Predator funcitonal responses: discriminating between handling and digesting prey. Ecol Monogr. 2002;72:95–112. [Google Scholar]
- Jordan P. Schistosomiasis: the St. Lucia project. Cambridge, UK: Cambridge University Press; 1985. [Google Scholar]
- Khalil M, Sleem SH. Can the freshwater crayfish eradicate schistosomiasis in Egypt and Africa? Journal of American Science. 2011;7:457–462. [Google Scholar]
- King C, Dickman K, Tisch D. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis - why it matters now. Trends in Parasitology. 2006;22:575–582. doi: 10.1016/j.pt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Konan KM, Adepo-Gourene AB, Ouattara A, Nyingy WD, Gourene G. Morphometric variation among male populations of freshwater shrimp Macrobrachium vollenhovenii Herklots, 1851 from Cote d'Ivoire Rivers. Fish Res. 2010;103:1–8. [Google Scholar]
- Lee PG, Rodrick GE, Sodeman WA, Blake NJ. The giant malaysian prawn, Macrobrachium rosenbergii a potential predator for controlling the spread of schistosome vector snails in fish ponds. Aquaculture. 1982;28:293–301. [Google Scholar]
- Lodge D, Deines A, Gherardi F, Yeo D, Arcella T, Baldridge A, Barnes M, Chadderton W, Feder J, Gantz C, Howard G, Jerde C, Peters B, Peters J, Sargent L, Truner C, Wittmann M, Zeng Y. Global Introductions of Crayfishes: Evaluating the Impact of Species Invasions on Ecosystem Services. Annual Reviews of Ecology, Evolution, and Systematics. 2012:43. [Google Scholar]
- Loreau M, Baluku B. Growth and demography of populations of Biomphalaria pfeifferi (Gastropoda, Planorbidae) in the laboratory. Journal of Molluscan Studies. 1987;53:171–177. [Google Scholar]
- Madsen H, Stauffer JR. Density of Trematocranus placodon (Pisces: Cichlidae): a predictor of density of the schistosome intermediate host, Bulinus nyassanus (Gastropoda: Planorbidae), in Lake Malawi. Ecohealth. 2011;8:177–189. doi: 10.1007/s10393-011-0737-3. [DOI] [PubMed] [Google Scholar]
- Mishkin EM, Jokinen EH. Effects of environmental calcium on fecundity and cercarial production of Biomphalaria glabrata (Say) infected with Schistosoma mansoni Sambon. J Parasitol. 1986;72:885–890. [PubMed] [Google Scholar]
- Mkoji GM, Hofkin BV, Kuris AM, Stewart-Oaten A, Mungai BN, Kihara JH, Mungai F, Yundu J, Mbui J, Rashid JR, Kariuki CH, Ouma JH, Koech DK, Loker ES. Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. Am J Trop Med Hyg. 1999;61:751–759. doi: 10.4269/ajtmh.1999.61.751. [DOI] [PubMed] [Google Scholar]
- New MB, Valenti W. Freshwater prawn culture: the farming of Macrobrachium rosenbergii. Oxford: Blackwell Science Ltd; 2000. [Google Scholar]
- Niemann GM, Lewis FA. Schistosoma mansoni: influence of Biomphalaria glabrata size on susceptibility to infection and resultant cercarial production. Exp Parasitol. 1990;70:286–292. doi: 10.1016/0014-4894(90)90110-x. [DOI] [PubMed] [Google Scholar]
- Pfluger W. Experimental epidemiology of schistosomiasisI. I. The prepatent period and cercarial production of Schistosoma mansoni in Biomphalaria snails at various constant temperatures. Z Parasitenkd. 1980;63:159–169. doi: 10.1007/BF00927532. [DOI] [PubMed] [Google Scholar]
- Pfluger W, Roushdy MZ, El Emam M. The prepatent period and cercarial production of Schistosoma haematobium in Bulinus truncatus (Egyptian field strains) at different constant temperatures. Z Parasitenkd. 1984;70:95–103. doi: 10.1007/BF00929579. [DOI] [PubMed] [Google Scholar]
- Polis The evolution and dynamics of intraspecific predation. Annu Rev Ecol Syst. 1981;12:225–251. [Google Scholar]
- Ratard RC, Kouemeni LE, Bessala MM, Ndamkou CN, Greer GJ, Spilsbury J, Cline BL. Human schistosomiasis in Cameroon. I. Distribution of schistosomiasis. Am J Trop Med Hyg. 1990;42:561–572. doi: 10.4269/ajtmh.1990.42.561. [DOI] [PubMed] [Google Scholar]
- Roberts JK, Kuris AM. Predation and control of laboratory populations of the snail Biomphalaria glabrata by the freshwater prawn Macrobrachium rosenbergii. Ann Trop Med Parasitol. 1990;84:401–412. doi: 10.1080/00034983.1990.11812486. [DOI] [PubMed] [Google Scholar]
- Sandbach F. The history of schistosomiasis research and policy for its control. Medical History. 1976;20:259–275. doi: 10.1017/s0025727300022663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slootweg R, Malek EA, McCullough FS. The biological control of intermediate hosts of schistosomiasis by fish. Rev Fish Biol Fish. 1994;4:67–90. [Google Scholar]
- Southgate VR. Schistosomiasis in the Senegal river basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J Helminthol. 1997;71:125–132. doi: 10.1017/s0022149x00015790. [DOI] [PubMed] [Google Scholar]
- Sow S, de Vlas SJ, Engels D, Gryseels B. Water-related disease patterns before and after the construction of the Diama dam in northern Senegal. Ann Trop Med Parasitol. 2002;96:575–586. doi: 10.1179/000349802125001636. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Momo SC, Stothard JR, Rollinson D. Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon. Acta Trop. 2013;128:275–283. doi: 10.1016/j.actatropica.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC, Rollinson D. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop. 2013;128:292–302. doi: 10.1016/j.actatropica.2012.09.010. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva: World Health Organization; 2011. Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. [Google Scholar]
- Wise DH. Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu Rev Entomol. 2006;51:441–465. doi: 10.1146/annurev.ento.51.110104.150947. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Chandiwana SK. The Epidemiology of Schistosome Infections of Snails: Taking the Theory into the Field. Parasitology Today. 1990a;6:65–70. doi: 10.1016/0169-4758(90)90211-l. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Chandiwana SK. Population biology of the freshwater snail Bulinus globosus in the Zimbabwe Highveld. Journal of Applied Ecology. 1990b;27:41–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.