Abstract
BACKGROUND AND PURPOSE
Modulation of the sphingosine 1-phosphate receptor is an approved treatment for relapsing multiple sclerosis because of its anti-inflammatory effect of retaining lymphocytes within the lymph nodes. Here, we evaluated the potential of an agonist at this receptor, FTY720 (fingolimod), to activate the promyelinating pathways within the brain to encourage remyelination and neuroprotection.
EXPERIMENTAL APPROACH
In this study, we used the cuprizone model in male C57BL/6 mice and tested the promyelinating and neuroprotective effects of FTY720 after acute and chronic toxin-induced experimental demyelination. We used histological, immunohistochemical and gene expression methods.
KEY RESULTS
The midline of the corpus callosum was severely demyelinated after acute and chronic cuprizone-induced demyelination. Robust endogenous remyelination was evident after acute, but impaired after chronic, demyelination. FTY720 treatment modestly accelerated myelin recovery after acute but not chronic cuprizone exposure. Markers of gliosis (astrocyte and microglia activation) were not affected by FTY720 treatment. Remarkably, the accumulation of amyloid precursor protein-positive spheroids in axons was less distinct in FTY720-treated animals, indicating that this compound alleviated ongoing axonal damage.
CONCLUSIONS AND IMPLICATIONS
We show that even during endogenous remyelination, axonal degeneration continued at a low level, accumulating over time. This continuous neurodegenerative process was ameliorated by FTY720 treatment. FTY720 preserved CNS integrity by direct interaction with brain resident cells, the actions of which are still to be defined.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Enzymesb |
| Sphingosine 1-phosphate (S1P) receptors | Ppap2b, phosphatidic acid phosphatase 2B |
| S1P1 receptors | Sgpp1, sphingosine 1-phosphate phosphatase 1 |
| S1P5 receptors | Sphk2, sphingosine kinase 2 |
| LIGANDS |
|---|
| FTY720 (fingolimod) |
| FTY720 –P, FTY720 phosphate |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b Alexander et al., 2013a,b)
Introduction
Demyelination is a characteristic histopathological finding in multiple sclerosis (MS). In addition, axonal and neuronal loss (i.e. neuronal degeneration) is an equally important disease characteristic (Back et al., 2005; Geurts and Barkhof, 2008; Geurts et al., 2009). Remyelination, one of the most efficient endogenous repair processes within the CNS, maintains the structural integrity of the axon, restores axonal conduction properties and functio laesa following demyelination. For example, it has been found that proper conduction is restored with remyelination (Smith et al., 1979) and that demyelinated, but not remyelinated, lesions are characterized by acute axonal damage (Kornek et al., 2000). Furthermore, experimental inhibition of remyelination in rodents results in a significant increase in the extent of axonal degeneration and loss (Irvine and Blakemore, 2008), and extensive remyelination at the histopathological level correlates with functional recovery (Duncan et al., 2009). The presence of shadow plaques, representing fully remyelinated lesions, demonstrate that complete repair of MS plaques is, in principle, possible (Prineas et al., 1984), although it is common to observe only limited repair at the edge of lesions (Prineas and Connell, 1979). It is not clear why, in some patients, remyelination is widespread while in others it is sparse, and understanding why a relatively robust regenerative process should lose momentum is an important prerequisite for developing an effective therapeutic approach (Kipp et al., 2012).
FTY720 (fingolimod), a sphingosine 1-phosphate (S1P) receptor agonist, is the first oral disease-modifying therapeutic agent to be approved for the treatment of MS. FTY720 is rapidly converted in vivo to the active S-fingolimod-phosphate (FTY720-P) which binds to S1P receptors. This action inhibits emigration of lymphocytes from the lymph nodes preventing entry into the blood and thus infiltration into the CNS (Brinkmann et al., 2010; Kipp and Amor, 2012). S1P receptors belong to the endothelial differentiation gene receptor family which is composed of eight different GPCRs. Preclinical studies have demonstrated that FTY720 exerts beneficial functions in studies of several experimental autoimmune encephalomyelitis (EAE) models (Fujino et al., 2003; Foster et al., 2007; Papadopoulos et al., 2010; Choi et al., 2011; Anthony et al., 2014). In addition, clinical trials revealed that several aspects of MS disease activity, for example brain volume loss and disability progression, are suppressed in patients treated with FTY720 (Kappos et al., 2010; Radue et al., 2012).
As mentioned, the efficacy of FTY720 in immune-mediated diseases has been attributed to lymphocyte sequestration in secondary lymphoid organs and consequent inhibition of lymphocyte trafficking to target organs via the blood stream (Mandala et al., 2002). Notably, the efficacy of FTY720 in MS and the related animal models may in part be due to additional, direct effects within the brain (Van Doorn et al., 2010; Choi et al., 2011). In this context, it is important to note that S1P receptors are expressed by various brain intrinsic cell types, such as oligodendrocytes (Jaillard et al., 2005), neurons (Kimura et al., 2007) and astrocytes (Wu et al., 2008; Van Doorn et al., 2010), and therefore have the potential to regulate neuron and/or glia cell morphology, migration, process extension, cell growth, differentiation, apoptosis and/or survival. The observation that S1P receptor activity can regulate oligodendrocyte physiology was first reported over a decade ago, when S1P was shown to stimulate Ca2+ responses in an oligodendrocyte cell line (Fatatis and Miller, 1996). Furthermore, S1Ps regulate MAPK signalling in primary cultured oligodendrocytes (Hida et al., 1999). It is thus a very attractive concept to postulate that FTY720 might exert additional beneficial effects on MS disease progression independent of its effect on T and B cells.
Various animal models are available to study operant pathways during myelin repair and to elaborate how pharmacological intervention can modulate these pathways. One frequently used model in remyelination research is that based on the toxin cuprizone (Acs et al., 2009; Kipp et al., 2009; 2011). In this model, the capacity for remyelination depends on the initial exposure to cuprizone. After 5 (acute demyelination) or 12 weeks (chronic demyelination) of treatment with cuprizone, several regions, such as the white matter tract corpus callosum (CC), are severely demyelinated. While the myelin status of the CC significantly recovers after acute demyelination, endogenous remyelination is impaired and/or delayed after chronic cuprizone exposure (Kipp et al., 2009; Lindner et al., 2009). Furthermore, axonal damage occurs in acute and chronic lesions. In this study, we have used the cuprizone model in mice to investigate the effects of FTY720 on remyelination and axonal damage by means of immunohistochemical analyses and gene expression studies.
Methods
Animals, induction of demyelination and FTY720 treatment
All animal care and experimental procedures were formally approved by the Review Board for the Care of Animal Subjects of the district government (North Rhine-Westphalia, Germany). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 72 animals were used in the experiments described here.
C57BL/6 mice (Le Genest-Saint-Isle, Janvier, France) were bred and maintained in a pathogen-free environment with a maximum of five animals per cage. Animals underwent routine cage maintenance once a week and microbiological monitoring according to the Federation of European Laboratory Animal Science Associations recommendations. Food and water were available ad libitum. Demyelination was induced by feeding 8-week-old (19–21 g) male mice a diet containing 0.2% cuprizone [bis(cyclohexanone)oxaldihydrazone; Sigma-Aldrich Inc., St Louis, MO, USA] mixed into a ground standard rodent chow. Acute or chronic cuprizone-induced demyelination was performed by feeding animals for 5 or 12 weeks respectively (Kipp et al., 2011). After cuprizone-induced demyelination, animals were randomly assigned to experimental groups. Animals were allowed to recover for 11 days after acute and for 28 days after chronic toxin-induced demyelination by feeding normal chow. During this recovery period, animals were treated daily (between 0800 and 1000 h) by oral gavage (volume 100 μL) with either vehicle (H2O) or FTY720 (0.3 mg·kg−1 body weight). Two independent experiments were performed with at least four animals per experimental group if not stated otherwise.
Tissue preparation
Tissue preparation was performed as previously described (Kipp et al., 2011; Clarner et al., 2012). For histological and immunohistochemical studies, mice were anaesthetized with ketamine (100 mg·kg−1; i.p.) and xylazine (10 mg·kg−1; i.p.), transcardially perfused with ice-cold PBS followed by transcardial perfusion with 3.7% paraformaldehyde solution (pH 7.4). After overnight post-fixation in the same fixative, brains were dissected, embedded in paraffin and then coronal sectioned into 5 μm sections at the levels 255–285 according to the mouse brain atlas by Sidman et al. (http://www.hms.harvard.edu/research/brain/atlas.html). Prior to transcardial perfusion, blood was sampled by the left ventricle and further processed for the quantification of FTY720 and FTY720-P levels as published previously (Brinkmann et al., 2002). For gene expression studies and quantification of FTY720-P levels, tissues were dissected after PBS perfusion, immediately frozen in liquid nitrogen and kept at −80°C until used.
Gene expression analyses
Gene expression levels were measured using the reverse transcription real time-PCR technology (BioRad, Munich, Germany), SensiMix Plus SYBR and Fluorescein (Quantace, Bioline, Luckenwalde, Germany) and a standardized protocol as described previously by our group (Buschmann et al., 2012; Clarner et al., 2012). Primer sequences and individual annealing temperatures are shown in Table 1. Relative quantification was performed using the ΔΔCt method which results in ratios between target genes and the housekeeping reference gene hypoxanthine guanine phosphoribosyltransferase. Melting curves and gel electrophoresis of the PCR products were routinely performed to determine the specificity of the PCR reaction (data not shown).
Table 1.
Sequences of primers used in the study
| Primers | Sequence (5′→3′) | Base pairs | Primer annealing temperature | |
|---|---|---|---|---|
| Sphk2 | s | CCATGCCCGTGAGCTGGTGC | 218 | 65°C |
| as | CGACAACCTGCTCAAACCCGCC | |||
| Ppap2b | s | GCCTCTTCATGGCGGCTCTGC | 159 | 65°C |
| as | CGATGACGATCCCCACCGCA | |||
| Sgpp1 | s | TACGGGCTGATTCTCATTCCC | 142 | 60°C |
| as | GGTCCACCAATGGGTAGAAGA |
Immunohistochemistry (IHC)
For IHC, sections were rehydrated, and if necessary unmasked by Tris/EDTA buffer (pH 9.0) or citrate (pH 6.0) heating, washed in PBS and incubated overnight (4°C) with the primary antibody diluted in blocking solution (serum of species in which the secondary antibody was raised). Anti-proteolipid protein-1 (PLP, 1:5000, AbD Serotec, Bio-Rad, Pucheim, Germany) antibody was used to detect intact myelin; anti-oligodendrocyte transcription factor (OLIG2, 1:2000, Merck Millipore, Darmstadt, Germany) antibody was used to detect oligodendrocytes, whereas anti-glial fibrillary acidic protein (GFAP, 1:12000, EnCor Biotechnologie Inc, Gainesville, FL, USA) antibody was used to visualize astrocytes. Anti-ionized calcium-binding adaptor molecule-1 (IBA-1, 1:10000, Wako Chemicals GmbH, Neuss, Germany) antibody was used to detect microglia/macrophages and anti-amyloid precursor protein (APP, 1:5000, Merck Millipore) antibody was used to detect acute axonal damage. The next day, slides were subsequently incubated with biotinylated secondary antibodies for 1 h, followed by peroxidase-coupled avidin-biotin complex (ABC kit, Vector Laboratories, Peterborough, UK) and treated with 3,3-diaminobenzidine (DAKO, Hamburg, Germany) as a peroxidase substrate. Luxol fast blue (LFB)/periodic acid-Schiff (PAS) stains were performed following established protocols (Acs et al., 2009). Stained and processed sections were digitalized using a Nikon ECLIPSE E200 microscope (Nikon, Nikon Instruments, Düsseldorf, Germany) equipped with a DS-Vi1 camera. The open source program ImageJ was used to determine chromogen intensity in experimental groups.
Immunofluorescence labelling
To assess axonal densities in the chronic groups, the antibody to 200 kD neurofilament heavy (NF200, 1:50, Abcam, Cambridge, UK) was used followed by fluorescence detection. To this end, sections were incubated with fluorescent anti-rabbit secondary antibodies (Alexa Fluor 568 goat IgG; Invitrogen, Darmstadt, Germany; 1:500) diluted in blocking solution. Sections were then incubated with Hoechst 33342 (Invitrogen; 1:1000) diluted in PBS for the staining of cell nuclei. Stained and processed sections were digitalized using a Leica DMI 6000B fluorescence microscope working station equipped with a DFC 365FX camera (Leica, Wetzlar, Germany). The open source program ImageJ was used to determine fluorescence intensity in experimental groups. To detect cellular distribution of APP+ spheroids, we performed immunofluorescence double labelling as published previously (Baertling et al., 2010). In these experiments, we used a goat polyclonal antibody raised against a peptide mapping within an internal region of NF-H (E-15, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:100). For this analysis, processed sections were documented with the confocal laser microscope working station Zeiss LSM 7 Duo (Oberkochen, Germany).
Quantification of (immuno)-histochemical parameters
In order to evaluate demyelination in PLP- or LFB-stained sections, the magnitude of myelination was scored in the midline of the CC (CCm) between 100 and 0. To assess myelination in the cortex, the somatosensory area of the cortex was defined as the region of interest, and staging was performed in PLP-stained sections as published previously (Clarner et al., 2012). A score of 100 is equivalent to the myelin status of a control untreated mouse, whereas zero is equivalent to a totally demyelinated region of interest. Two independent readers, who were unaware of the treatments, performed the scoring and the results were averaged (Acs et al., 2009; Kipp et al., 2011). Quantification of axonal spheroids (APP staining) as well as oligodendrocyte (OLIG2 staining), astrocyte (GFAP staining) and microglia (IBA-1 staining) cell numbers was performed by manually counting the number of positive structures in the region of interest. Counting was performed using a Nikon Eclipse 55i (Nikon) and a 40x objective. Numbers are expressed as cells·mm−2. As another parameter for astrocyte and microglia activation, we determined the area covered by IBA-1 or GFAP staining, respectively, using the freely available ImageJ software. Results are expressed as relative area of the CCm (stained area in relation to non-stained area in percentage). Finally, hypertrophy of microglia cells was analysed by quantifying the mean area covered per microglia cell in IBA-1-stained sections using the area measurement tool integrated in the Nikon NIS Elements software (Version 3.22.14, Nikon).
Data analysis
All data are given as arithmetic means ± SEM. Differences between groups were statistically tested by anova followed by a Tukey's post hoc multiple-range test using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) except for differences between myelin scoring (PLP-stained and LFB/PAS) in cuprizone-treated animals. There, differences between groups were analysed with the non-parametric Kruskal–Wallis test followed by Dunn's post hoc test. To test whether hypertrophy of microglia cells was different between vehicle and FTY720-treated animals, a two-tailed Student's t-test was performed. P values < 0.05 were considered to be statistically significant.
Results
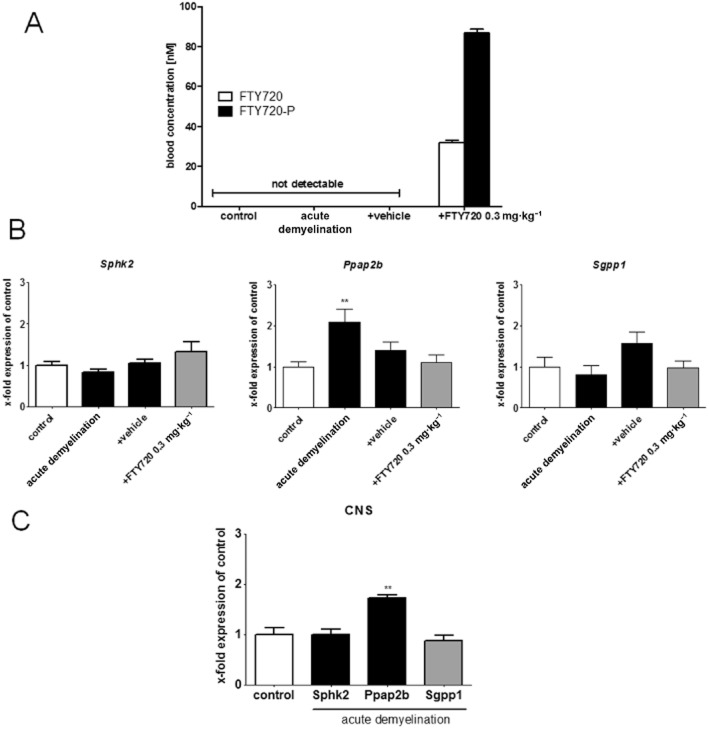
FTY720 is an orally available S1P receptor modulator and a pro-drug that is mainly phosphorylated by sphingosine kinase type 2 (Sphk2) to the biologically active metabolite FTY720-P (Kaneider et al., 2004). In the first set of experiments, we analysed the potency of FTY720 to boost remyelination in the cuprizone model in mice. To this end, animals were fed cuprizone for 5 weeks to induce acute demyelination. During the 11 days of recovery period, animals received either vehicle or FYT720 in a concentration of 0.3 mg·kg−1 body weight. To confirm adequate drug absorption, blood levels of FTY720 and its active phosphorylated metabolite FTY720-P were quantified in placebo- and vehicle-treated animals. As demonstrated in Figure 1, both FTY720 entities were not detectable in control and vehicle-treated animals, whereas a significant increase was evident in FTY720-treated animals. In treated animals, FTY720 and FTY720-P blood levels were 31.8 and 86.6 nM respectively (Figure 1A). We, furthermore, analysed the expression of genes known to be involved in FTY720 phosphorylation (i.e. Sphk2) and dephosphorylation [i.e. sphingosine 1-phosphate phosphatase 1 (Sgpp1) and phosphatidic acid phosphatase type 2B (Ppap2b)]. Gene expression analyses revealed that the key FTY720-metabolizing enzymes Sphk2 and Sgpp1 were stably expressed in the liver (Figure 1B) and brains (Figure 1C). The expression of Ppap2b, the second phosphatase involved in the equilibrium between FTY720 and FTY720-P (Mechtcheriakova et al., 2007), was slightly induced in both compartments (P < 0.01).
Figure 1.
Daily FTY720 treatment results in stable phosphorylated FTY720 (FTY720-P) blood levels. (A) Blood levels of phosphorylated and non-phosphorylated FTY720. (B) Expression levels of Sphk2, Ppap2b and Sgpp1 in liver tissues. (C) Expression levels of Sphk2, Ppap2b and Sgpp1 in CNS tissues after acute demyelination. Note constant expression of the two key FTY720-metabolizing enzymes Sphk2 and Sgpp1, paralleled by increased FTY720 and FTY720-P levels in FTY720-treated animals. **P < 0.01, significantly different from controls.
It is well known that cuprizone treatment does not affect the integrity of the blood–brain barrier (Kipp et al., 2009). In a separate set of experiments, we determined the ratio of FTY720/FTY720-P in blood and in brain tissue. To this end, control animals were treated with FTY720 for 5 days, blood and brain tissue harvested and FTY720/FTY720-P concentrations quantified. This analysis revealed that both molecular entities of FTY720 (FTY720 and FTY720-P) accumulated in the brains despite an intact blood–brain barrier. FTY720 levels were 12.4 times and FTY720-P levels 10.8 times higher in the brain, compared with those in the blood respectively (data not shown). In summary, the applied study protocol resulted in reproducible accumulation of active FTY720 (i.e. FTY720-P) within the brain parenchyma.
To address myelination capacity, we performed anti-PLP and LFB/PAS stains and evaluated staining intensity in the CCm (see Table 2). As demonstrated in Figure 2A by anti-PLP stain, severe demyelination (−70%) was evident in the CCm after acute cuprizone exposure. The magnitude of demyelination was more severe in LFB-stained sections (comparison of grey and white bars in Figure 2A). Both stains revealed significant recovery of myelination 11 days after acute cuprizone-induced demyelination. The extent of remyelination was, however, not significantly higher in FTY720- compared with vehicle-treated animals. We furthermore tested the ability of FTY720 to boost remyelination in the cortex which is as well affected in this animal model (Skripuletz et al., 2008). As demonstrated in Figure 2A (lower right side), significant cortical demyelination (−95%) was evident after acute cuprizone-induced demyelination. Remyelination in the cortex was delayed compared with the robust remyelination observed in the CC. However, the extent of remyelination was comparable in both treatment groups, suggesting that FTY720 did not affect remyelination in both brain areas. In line with these findings, oligodendrocyte numbers, as quantified in OLIG2-stained sections, were comparable in the CCm of FTY720- and vehicle-treated animals (compare Figure 2B).
Table 2.
Summary of corpus callosum myelination values (%), as determined in the study
| Control | Demyelination | +Vehicle | +FTY720 0.3 mg·kg−1 | |
|---|---|---|---|---|
| Acute model | ||||
| PLP | 94.2 ± 0.9 | 28.9 ± 1.9 | 86.4 ± 3.2 | 81.6 ± 4.0 |
| LFB | 96.9 ± 1.3 | 4.8 ± 1.2 | 50.9 ± 1.7 | 66.3 ± 2.7 |
| Chronic model | ||||
| PLP | 93.9 ± 0.8 | 5.1 ± 1.8 | 62.7 ± 6.3 | 74.5 ± 4.1 |
| LFB | 96.9 ± 1.3 | 2.5 ± 1.8 | 34.0 ± 4.3 | 40.2 ± 8.0 |
Data shown in the Table are means ± SEM (from n = 4 for control; n=8 for all other groups). After either acute or chronic cuprizone exposure, there was significant and profound demyelination, with remyelination on vehicle treatment. However, remyelination was not different between FTY720- and vehicle-treated groups. LFB, Luxol fast blue; PLP, proteolipid protein (myelin) 1.
Figure 2.
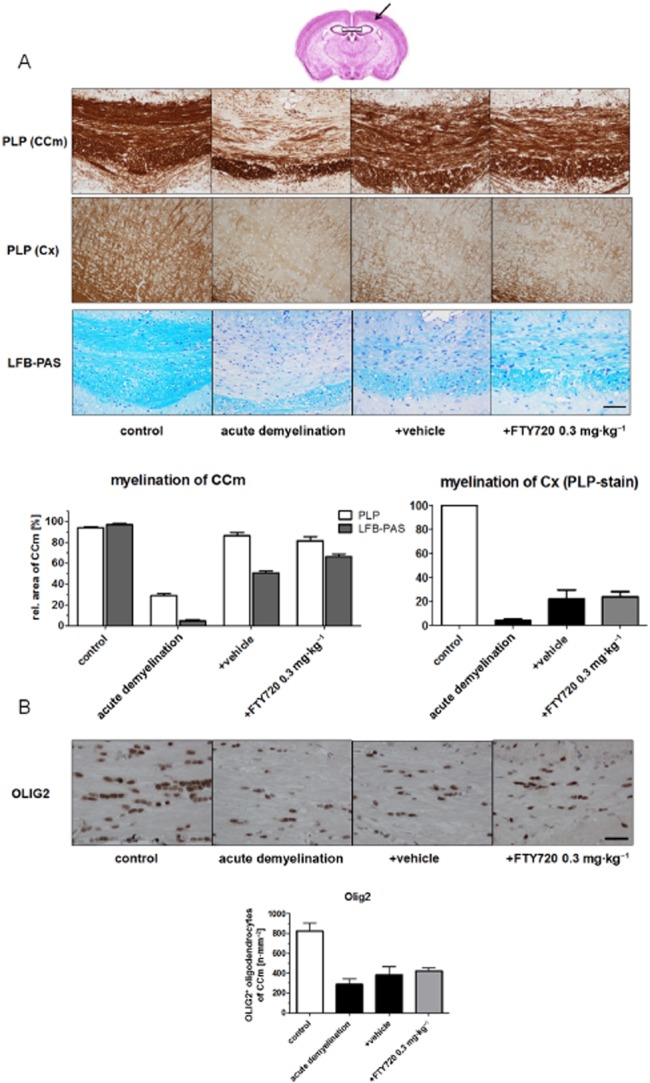
Remyelination parameters are not affected by FTY720 after acute cuprizone-induced demyelination. (A) Effects of FTY720 on myelin recovery after acute cuprizone-induced demyelination (5 weeks cuprizone exposure) demonstrated by anti-PLP (first and second row) and LFB/PAS stains (third row) in the CCm and cortex (Cx). The box in (A) indicates the CCm, the arrow in (A) indicates the somatosensory cortex. (B) Oligodendrocyte numbers estimated by anti-OLIG2 IHC in the CCm. Note that neither myelination nor oligodendrocyte numbers are different in FTY720- and vehicle-treated groups (scale bar A:100 μm; B: 30 μm).
We next evaluated the magnitude of astrocyte and microglia activation by measuring the relative area covered by positive immunoreactivity for anti-GFAP and anti-IBA-1. Furthermore, absolute cell numbers were quantified in the respective sections. As demonstrated in Figure 3A, both analyses revealed that severe astrocytosis was evident after acute demyelination. Astrocytosis was still clearly evident 11 days after cessation of acute demyelination, highlighting the important role of astrocytes for remyelination processes (Skripuletz et al., 2013). Comparably, the relative area covered by positive immunoreactivity for anti-IBA-1 as well as absolute numbers of microglia cells dramatically increased during the 5 weeks of cuprizone treatment protocol. In contrast to astrocytosis, microgliosis returned almost to control levels during the 11 days of recovery period (compare Figure 3A and B). Most importantly, FTY720 treatment did not affect either of the gliosis parameters during the observation period. Furthermore, the relative area covered per microglia cell, an indirect measurement of microglia hypertrophy, was comparable in FTY720- and vehicle-treated animals (P = 0.17; Figure 3B, lower right side). These results strongly suggest that the activation of astrocytes and microglia was not affected by FTY720 treatment, at least on the morphological level.
Figure 3.
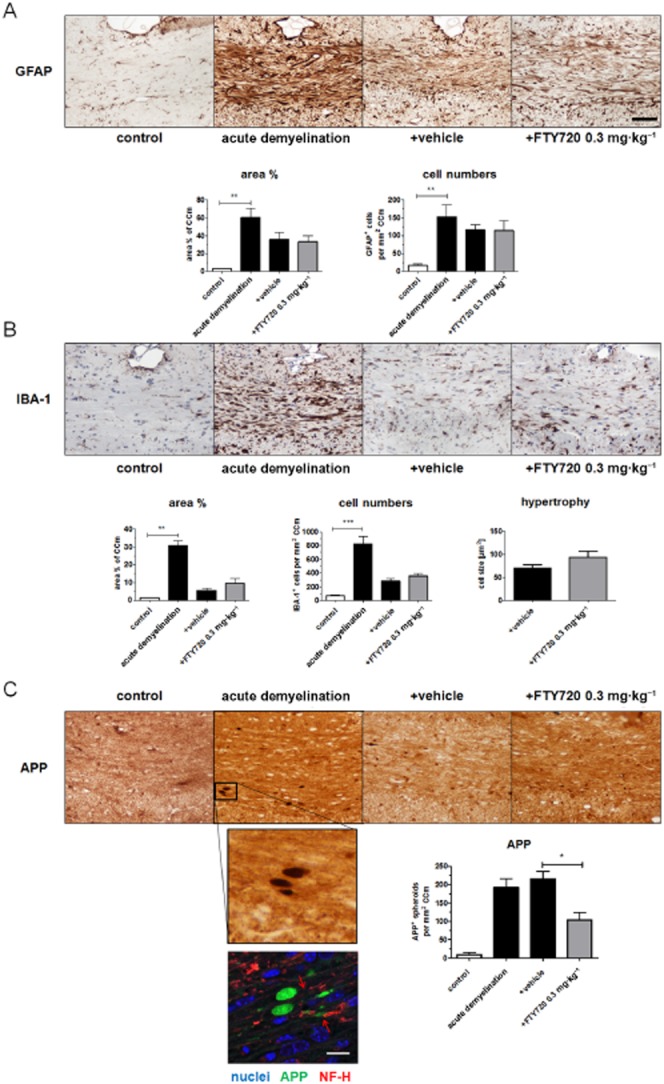
FTY720 ameliorates acute axonal damage after acute cuprizone-induced demyelination. (A/B) Glial response determined by evaluating anti-GFAP-stained sections for astrocytosis (A) and anti-IBA-1-stained sections for microgliosis (B) respectively. (C) Acute axonal damage determined by evaluating anti-APP-stained sections. Note that acute axonal damage is significantly lower in animals treated with FTY720 for 11 days after acute cuprizone-induced demyelination. Further note that APP+ spheroids do not colocalize to cell bodies but are flanked by axonal neurofilaments (red arrows in the lower part of C) (black scale bar: 100 μm; white scale bar: 10 μm). *P < 0.05; ** P < 0.01; ***P < 0.001, significantly different as indicated.
While it has been suggested that remyelination protects axons from demyelination-associated axon degeneration (Irvine and Blakemore, 2008), results of a recent study suggest that even after completed remyelination, axonal degeneration continues to progress at a low level (Manrique-Hoyos et al., 2012). To address this point, slides were processed for IHC using anti-APP antibodies which is a frequently used marker to detect acute, ongoing axonal damage (Bitsch et al., 2000; Herrero-Herranz et al., 2008). As demonstrated in Figure 3C, low numbers of APP+ spheroids were detectable in control animals, whereas a significant accumulation was evident after acute cuprizone-induced demyelination. Interestingly, despite significant myelin repair, numbers of APP+ spheroids were still increased 11 days after acute demyelination. Notably, numbers of APP+ spheroids were dramatically lower in FTY720-treated compared with vehicle-treated mice. Precisely, 215 spheroids·mm−2 were detectable in vehicle-treated animals compared with 104 spheroids·mm−2 in FTY720-treated animals (P < 0.01). Distinct isoforms of APP can be expressed by activated glia cells, among astrocytes (Gehrmann et al., 1995). To confirm that APP+ spheroids are indeed an accurate marker for axonal damage in this model, we performed immunofluorescence double labelling and analysed the stained sections by confocal laser scanning microscopy. As shown in Figure 3C (lower part), APP+ spheroids do not colocalize with cell nuclei and are regularly flanked by neurofilaments, indicating their axonal origin. In summary, our data clearly showed that ongoing axonal damage after a demyelinating event is effectively suppressed by FTY720 application.
Our results indicated that FTY720 might exert neuroprotective effects in acutely demyelinated lesions. In the next set of experiments, we investigated whether (i) acute axonal damage was still ongoing in chronic lesions; and (ii) whether FTY720 was able to ameliorate this chronic, neurodegenerative process. To this end, animals were fed cuprizone for 12 weeks to induce chronic lesions. Becasuse remyelination is limited after prolonged cuprizone exposure (Lindner et al., 2009; Zhou et al., 2012), animals were allowed to recover for 28 days in these experiments. As demonstrated in Figure 4A, severe demyelination was seen in the CCm after chronic cuprizone exposure. In line with findings from Lindner et al. (2009), significant remyelination was observed – but delayed – after chronic cuprizone exposure. Specifically, recovery occurred from 5 to 63% in PLP-stained and from 3 to 34% in LFB-stained sections. FTY720-treated animals did not show higher remyelination capacity in any of these stains in this chronic experimental setting. These findings are supported by quantification of oligodendrocyte cell numbers, where comparable amounts were detected in both experimental groups (Figure 4B). Mild astrogliosis and microgliosis were evident after chronic cuprizone exposure, although at lower levels as compared with acute lesions (data not shown).
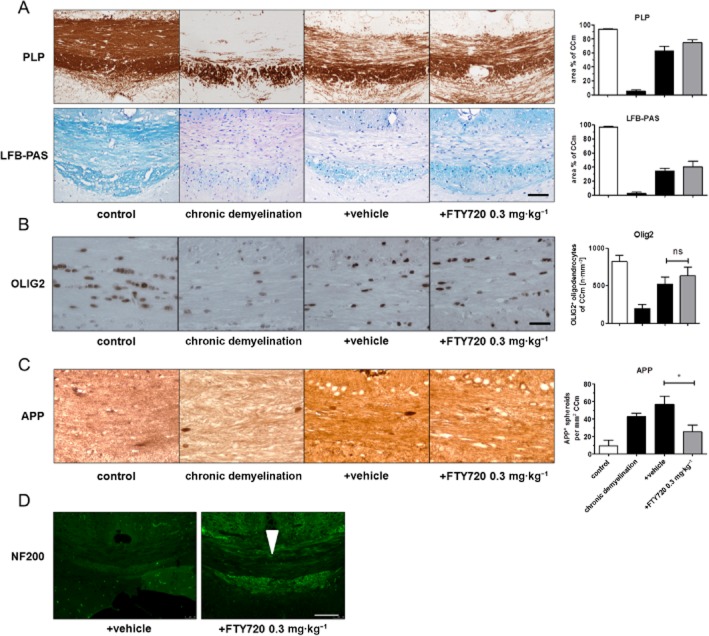
Figure 4.
FTY720 ameliorates acute axonal damage and axonal loss after chronic cuprizone-induced demyelination. (A) Effects of FTY720 on myelin recovery after chronic cuprizone-induced demyelination (12 weeks cuprizone exposure) demonstrated by anti-PLP and LFB/PAS stains. (B) Oligodendrocyte numbers estimated by anti-OLIG2 IHC in the CCm. (C) Acute axonal damage determined by evaluating anti-APP-stained sections. (D) Effects of FTY720 on axonal density after chronic cuprizone-induced demyelination demonstrated by anti-NF200 stains. Arrowhead indicates the CCm. Note that acute axonal damage is significantly lower and that axonal density is preserved in FTY720-treated animals after chronic cuprizone exposure (scale bar A and C: 100 μm; scale bar B: 30 μm; scale bar D: 200 μm). *P < 0.05, significant effect of FTY720.
APP+ spheroids were still present in chronic lesions, although fewer such signs of acute axonal damage were found, compared with active lesions (215 vs. 43 spheroids per mm2). Comparably, significant numbers of spheroids were detectable after 28 days of recovery. Most importantly, numbers of APP+ spheroids were significantly lower in FTY720-treated compared with vehicle-treated mice (Figure 4C). In line with this finding, NF-200 staining intensity was significantly increased in FTY720 compared with vehicle-treated animals (see Figure 4D) demonstrating that FTY720 preserved axonal integrity not just in acute, but also in chronic, lesions.
Discussion
FTY720 is the first oral disease-modifying therapy to be approved for the treatment of relapsing-remitting MS (Kipp and Amor, 2012). In line with observations from preclinical studies where FTY720 was effective in the autoimmune MS model EAE (Fujino et al., 2003; Papadopoulos et al., 2010; Choi et al., 2011), subsequent clinical trials have clearly demonstrated that FTY720 significantly reduced clinical and MRI disease activity in relapsing-remitting MS patients (Devonshire et al., 2012). Besides the reported anti-inflammatory effects, which address the focal aspect of the disease, it has been suggested that FTY720 also exerts protective effects directed against diffuse pathology. This assumption is based on the observation that FTY720 significantly reduced the loss of brain volume compared with placebo treatment. Subgroup analyses confirmed these effects, irrespective of the presence/absence of gadolinium-enhancing lesions, T2 lesion load, previous treatment status or level of disability (Radue et al., 2012). Furthermore, FTY720 significantly reduced the risk of disability progression over a 24-month follow-up period (Kappos et al., 2010). A third, pivotal phase III trial – the INFORMS study – is ongoing in patients with primary progressive MS. FTY720 might offer distinct advantages to patients with this type of MS that is characterized by prominent neurodegeneration and for which an effective therapy is sorely needed (Aktas et al., 2010).
As discussed in the introduction, S1P receptors, the designated interaction partners of phosphorylated FTY720, are expressed in the brain by a variety of cell types including oligodendrocytes (Jaillard et al., 2005), neurons (Kimura et al., 2007) and astrocytes (Wu et al., 2008; Van Doorn et al., 2010). Results of recent studies have shown that a disturbance and/or alterations in S1P signalling may contribute to neuropathological disorders, including MS. Fischer et al. were able to demonstrate that sphingosine kinase 1, one of the two kinases known to be able to activate FTY720, is up-regulated in activated astrocytes (Fischer et al., 2011). In the same study, they further observed an up-regulation of the S1P receptor subtype 3, which is in line with the findings from Van Doorn et al. (2010). A comparable dynamic alteration of S1P signalling in MS lesions has been observed by others including expression changes of astrocytic S1P1 receptors and oligodendrocytic S1P5 receptors (Van Doorn et al., 2010; Brana et al., 2014), a shift to a higher phospholipid and lower sphingolipid content in the normal appearing white and grey matter (Wheeler et al., 2008) or higher S1P concentrations in the cerebrospinal fluid of relapsing-remitting MS patients (Kulakowska et al., 2010). The neuroprotective spectrum of FTY720 has been illustrated in various animal models including models for MS (Al-Izki et al., 2011; Kim et al., 2011), Alzheimer's disease (Hemmati et al., 2013), ischaemic stroke (Hasegawa et al., 2010; Liu et al., 2013), spinal cord injury (Lee et al., 2009), Huntington's disease (Di Pardo et al., 2014) or Rett syndrome (Deogracias et al., 2012). Although it is not easy to separate anti-inflammatory effects from neuroprotective ones, it is obvious that FTY720 exerts beneficial effects beyond its immune-modulatory function. Preservation of axons, which is an intriguing aspect of neuroprotection, is frequently linked to remyelination (Smith et al., 1979; Kornek et al., 2000; Kipp et al., 2012). Indeed, results from several studies suggested that FTY720 might boost remyelination and by this regenerative function might exert neuroprotection. Oligodendrocytes express functionally active S1P receptors (Jung et al., 2007), FTY720 modulates human oligodendrocyte progenitor process extension (Miron et al., 2008b) and rescues human oligodendrocytes from serum and glucose deprivation-induced apoptosis (Miron et al., 2008a). Anti-apoptotic effects of FTY720 have also been described in oligodendrocyte progenitor cell cultures (Coelho et al., 2007). These results indicate that FTY720 modulates maturity- and species-specific oligodendrocyte membrane dynamics and survival responses that might be directly relevant for myelin integrity. This attractive concept gained momentum following the recent observations that FTY720 enhances remyelination in organotypic slice cultures (Miron et al., 2010). Contradictory to these promising in vitro results, remyelination was not affected by FTY720 treatment in two independent studies after acute cuprizone-induced demyelination (Hu et al., 2011; Kim et al., 2011) or focal demyelination induced by lysophosphatidylcholine injection into the rat spinal cord (Hu et al., 2011). As a consequence, the effects of FTY720 on myelin repair have been questioned. Our results are in line with previous studies using the cuprizone model as a remyelination tool (Kim et al., 2011). After acute cuprizone-induced demyelination, an experimental setting with robust endogenous remyelination capacity, FTY720 was only modestly able to accelerate this spontaneous remyelination process. While recovery of LFB staining intensity was slightly better in FTY720-treated animals, PLP immunoreactivity was comparable with that observed after acute cuprizone-induced demyelination (see Figure 2). In our study, we extended the analysis of remyelinating FTY720 effects by investigating myelin recovery after chronic cuprizone exposure, an experimental paradigm where endogenous remyelination is significantly impaired (Kipp et al., 2009; 2011; Lindner et al., 2009). In line with our findings after acute demyelination, remyelination occurred to the same magnitude in vehicle- and FTY720-treated animals. This prompts the conclusion that FTY720 does not have the capacity to induce remyelination in the cuprizone model when treatment is initiated after lesion formation. Whether this holds true for other experimental paradigms and, thus, is relevant for MS needs to be clarified in future studies.
Although remyelination per se is neuroprotective, the absence of promyelinating FTY720 effects does not necessarily exclude a neuroprotective property. Some important new aspects of the relationship between remyelination and acute axonal damage have recently been established using the cuprizone model. Following cessation of cuprizone treatment (5 weeks), animals showed an initial recovery of locomotor performance. However, long after remyelination was completed (approximately 6 months after the last demyelinating episode), locomotor performance again declined in remyelinated animals compared with age-matched controls (Manrique-Hoyos et al., 2012). This study clearly highlights that remyelination and axonal degeneration might occur independently from each other. In the current study, we demonstrated that 11 days after acute cuprizone-induced demyelination, acute axonal damage was still ongoing. This feature was significantly ameliorated by FTY720. Furthermore, we demonstrated that axonal degeneration is as well ongoing in chronic lesions, although to a lower extent. A greater extent of acute axonal damage in active, compared with chronic, lesions is a well-known phenomenon. In myelin oligodendrocyte glycoprotein-induced progressive EAE in mice, the highest incidence of acute axonal injury is present during active demyelination, whereas low but significant axonal injury was observed in inactive demyelinated plaques (Kornek et al., 2000). The patterns of axonal pathology in chronic active EAE are qualitatively and quantitatively comparable with those found in MS (Kuhlmann et al., 2002). Importantly, FTY720 also ameliorated this ‘slow-burning’ tissue destruction in chronically demyelinated lesions.
In our study, we have investigated the occurrence of acute axonal damage assessed by IHC for APP which is produced in neuronal perikarya and accumulates at sites of recent axon transection or damage. It is important to note that acute axonal damage is more likely to be a reversible process (Shriver and Dittel, 2006; Nikic et al., 2011), and thus, a potential target for therapy in MS. Our observation that FTY720 decreased the number of acutely damaged axons raises critical questions as to which underlying mechanisms and factors contribute to such a protective effect. Understanding these effects would help to understand how FTY720 reduces brain atrophy and prevents progression of clinical disability.
Acknowledgments
We would like to thank Sandra Vidal de la Torre, Helga Helten, Petra Ibold and Wouter Gerritsen for excellent technical assistance. This study was supported by a research grant from Novartis/Basel. We acknowledge scientific discussions with members of the Novartis team, particularly Anna Schubart and Paul Smith.
Glossary
- APP
amyloid precursor protein
- CCm
midline of the corpus callosum
- EAE
experimental autoimmune encephalomyelitis
- FTY720-P
phosphorylated fingolimod (FTY720)
- GFAP
glial fibrillary acidic protein
- IBA-1
ionized calcium-binding adaptor molecule 1
- IHC
immunohistochemistry
- LFB
Luxol fast blue
- MS
multiple sclerosis
- OLIG2
oligodendrocyte transcription factor 2
- PAS
periodic acid-Schiff
- PLP
proteolipid protein
- Ppap2b
phosphatidic acid phosphatase type 2B
- S1P
sphingosine 1-phosphate
- Sgpp1
sphingosine 1-phosphate phosphatase 1
- Sphk2
sphingosine kinase type 2
Author contributions
A. S. and T. S. performed the experiments, evaluated the data and wrote main parts of the manuscript. C. B. and S. A. supervised the study and advised during the manuscript preparation. T. C. participated in evaluation of immunohistochemical results. M. K. supervised the study, wrote the main parts of the manuscript, discussed the experimental setting and financed the study.
Conflict of interest
Markus Kipp received a research grant from Novartis/Basel, manufacturers of fingolimod, Gilenya™.
References
- Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57:807–814. doi: 10.1002/glia.20806. [DOI] [PubMed] [Google Scholar]
- Aktas O, Kury P, Kieseier B, Hartung HP. Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol. 2010;6:373–382. doi: 10.1038/nrneurol.2010.76. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Izki S, Pryce G, Jackson SJ, Giovannoni G, Baker D. Immunosuppression with FTY720 is insufficient to prevent secondary progressive neurodegeneration in experimental autoimmune encephalomyelitis. Mult Scler. 2011;17:939–948. doi: 10.1177/1352458511400476. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Sibson NR, Losey P, Meier DP, Leppert D. Investigation of immune and CNS-mediated effects of fingolimod in the focal delayed-type hypersensitivity multiple sclerosis model. Neuropharmacology. 2014;79:534–541. doi: 10.1016/j.neuropharm.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Baertling F, Kokozidou M, Pufe T, Clarner T, Windoffer R, Wruck CJ, et al. ADAM12 is expressed by astrocytes during experimental demyelination. Brain Res. 2010;1326:1–14. doi: 10.1016/j.brainres.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123(Pt 6):1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2014;40:564–578. doi: 10.1111/nan.12048. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Buschmann JP, Berger K, Awad H, Clarner T, Beyer C, Kipp M. Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J Mol Neurosci. 2012;48:66–76. doi: 10.1007/s12031-012-9773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarner T, Diederichs F, Berger K, Denecke B, Gan L, van der Valk P, et al. Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia. 2012;60:1468–1480. doi: 10.1002/glia.22367. [DOI] [PubMed] [Google Scholar]
- Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323:626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2012;109:14230–14235. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire V, Havrdova E, Radue EW, O'Connor P, Zhang-Auberson L, Agoropoulou C, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11:420–428. doi: 10.1016/S1474-4422(12)70056-X. [DOI] [PubMed] [Google Scholar]
- Di Pardo A, Amico E, Favellato M, Castrataro R, Fucile S, Squitieri F, et al. FTY720 (fingolimod) is a neuroprotective and disease-modifying agent in cellular and mouse models of Huntington disease. Hum Mol Genet. 2014;23:2251–2265. doi: 10.1093/hmg/ddt615. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A. 2009;106:6832–6836. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatatis A, Miller RJ. Sphingosine and sphingosine 1-phosphate differentially modulate platelet-derived growth factor-BB-induced Ca2 + signaling in transformed oligodendrocytes. J Biol Chem. 1996;271:295–301. doi: 10.1074/jbc.271.1.295. [DOI] [PubMed] [Google Scholar]
- Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS ONE. 2011;6:e23905. doi: 10.1371/journal.pone.0023905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Banati RB, Cuzner ML, Kreutzberg GW, Newcombe J. Amyloid precursor protein (APP) expression in multiple sclerosis lesions. Glia. 1995;15:141–151. doi: 10.1002/glia.440150206. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7:841–851. doi: 10.1016/S1474-4422(08)70191-1. [DOI] [PubMed] [Google Scholar]
- Geurts JJ, Stys PK, Minagar A, Amor S, Zivadinov R. Gray matter pathology in (chronic) MS: modern views on an early observation. J Neurol Sci. 2009;282:12–20. doi: 10.1016/j.jns.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati F, Dargahi L, Nasoohi S, Omidbakhsh R, Mohamed Z, Chik Z, et al. Neurorestorative effect of FTY720 in a rat model of Alzheimer's disease: comparison with memantine. Behav Brain Res. 2013;252:415–421. doi: 10.1016/j.bbr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Herrero-Herranz E, Pardo LA, Gold R, Linker RA. Pattern of axonal injury in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neurobiol Dis. 2008;30:162–173. doi: 10.1016/j.nbd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Hida H, Nagano S, Takeda M, Soliven B. Regulation of mitogen-activated protein kinases by sphingolipid products in oligodendrocytes. J Neurosci. 1999;19:7458–7467. doi: 10.1523/JNEUROSCI.19-17-07458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lee X, Ji B, Guckian K, Apicco D, Pepinsky RB, et al. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo. Mol Cell Neurosci. 2011;48:72–81. doi: 10.1016/j.mcn.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131(Pt 6):1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, et al. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–1667. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- Kaneider NC, Lindner J, Feistritzer C, Sturn DH, Mosheimer BA, Djanani AM, et al. The immune modulator FTY720 targets sphingosine-kinase-dependent migration of human monocytes in response to amyloid beta-protein and its precursor. FASEB J. 2004;18:1309–1311. doi: 10.1096/fj.03-1050fje. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Miron VE, Dukala D, Proia RL, Ludwin SK, Traka M, et al. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 2011;25:1509–1518. doi: 10.1096/fj.10-173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25:115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- Kipp M, Amor S. FTY720 on the way from the base camp to the summit of the mountain: relevance for remyelination. Mult Scler. 2012;18:258–263. doi: 10.1177/1352458512438723. [DOI] [PubMed] [Google Scholar]
- Kipp M, Clarner T, Dang J, Copray S, Beyer C. The cuprizone animal model: new insights into an old story. Acta Neuropathol. 2009;118:723–736. doi: 10.1007/s00401-009-0591-3. [DOI] [PubMed] [Google Scholar]
- Kipp M, Gingele S, Pott F, Clarner T, van der Valk P, Denecke B, et al. BLBP-expression in astrocytes during experimental demyelination and in human multiple sclerosis lesions. Brain Behav Immun. 2011;25:1554–1568. doi: 10.1016/j.bbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Kipp M, Victor M, Martino G, Franklin RJ. Endogeneous remyelination: findings in human studies. CNS Neurol Disord Drug Targets. 2012;11:598–609. doi: 10.2174/187152712801661257. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(Pt 10):2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Kulakowska A, Zendzian-Piotrowska M, Baranowski M, Kononczuk T, Drozdowski W, Górski J, et al. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci Lett. 2010;477:149–152. doi: 10.1016/j.neulet.2010.04.052. [DOI] [PubMed] [Google Scholar]
- Lee KD, Chow WN, Sato-Bigbee C, Graf MR, Graham RS, Colello RJ, et al. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma. 2009;26:2335–2344. doi: 10.1089/neu.2008.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci Lett. 2009;453:120–125. doi: 10.1016/j.neulet.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int J Neurosci. 2013;123:163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Manrique-Hoyos N, Jürgens T, Grønborg M, Kreutzfeldt M, Schedensack M, Kuhlmann T, et al. Late motor decline after accomplished remyelination: impact for progressive multiple sclerosis. Ann Neurol. 2012;71:227–244. doi: 10.1002/ana.22681. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakova D, Wlachos A, Sobanov J, Bornancin F, Zlabinger G, Baumruker T, et al. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 2007;581:3063–3068. doi: 10.1016/j.febslet.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Miron VE, Hall JA, Kennedy TE, Soliven B, Antel JP. Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am J Pathol. 2008a;173:1143–1152. doi: 10.2353/ajpath.2008.080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008b;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, et al. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176:2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Rundle J, Patel R, Marshall I, Stretton J, Eaton R, et al. FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J Neurosci Res. 2010;88:346–359. doi: 10.1002/jnr.22196. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Radue EW, O'Connor P, Polman CH, Hohlfeld R, Calabresi P, Selmaj K, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69:1259–1269. doi: 10.1001/archneurol.2012.1051. [DOI] [PubMed] [Google Scholar]
- Shriver LP, Dittel BN. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:999–1011. doi: 10.2353/ajpath.2006.050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, et al. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008;172:1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, et al. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136(Pt 1):147–167. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131(Pt 11):3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YX, Pannu R, Le TQ, Armstrong RC. Fibroblast growth factor 1 (FGFR1) modulation regulates repair capacity of oligodendrocyte progenitor cells following chronic demyelination. Neurobiol Dis. 2012;45:196–205. doi: 10.1016/j.nbd.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]